Abstract
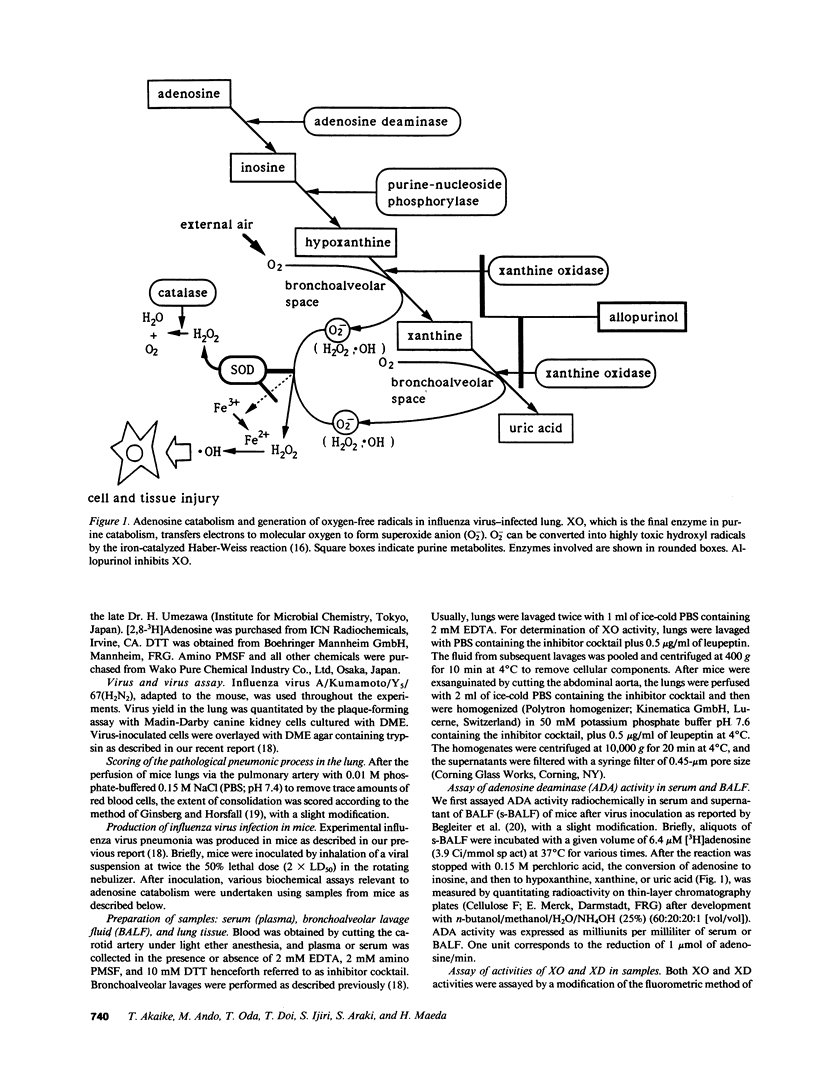
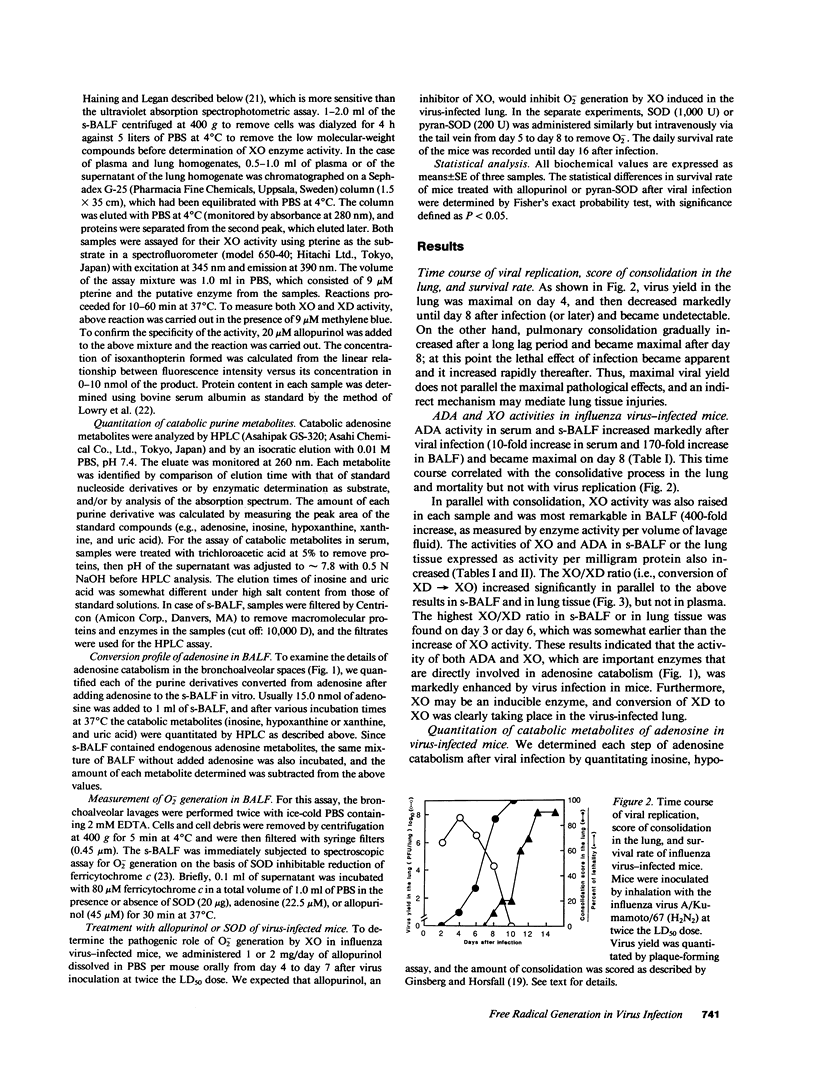
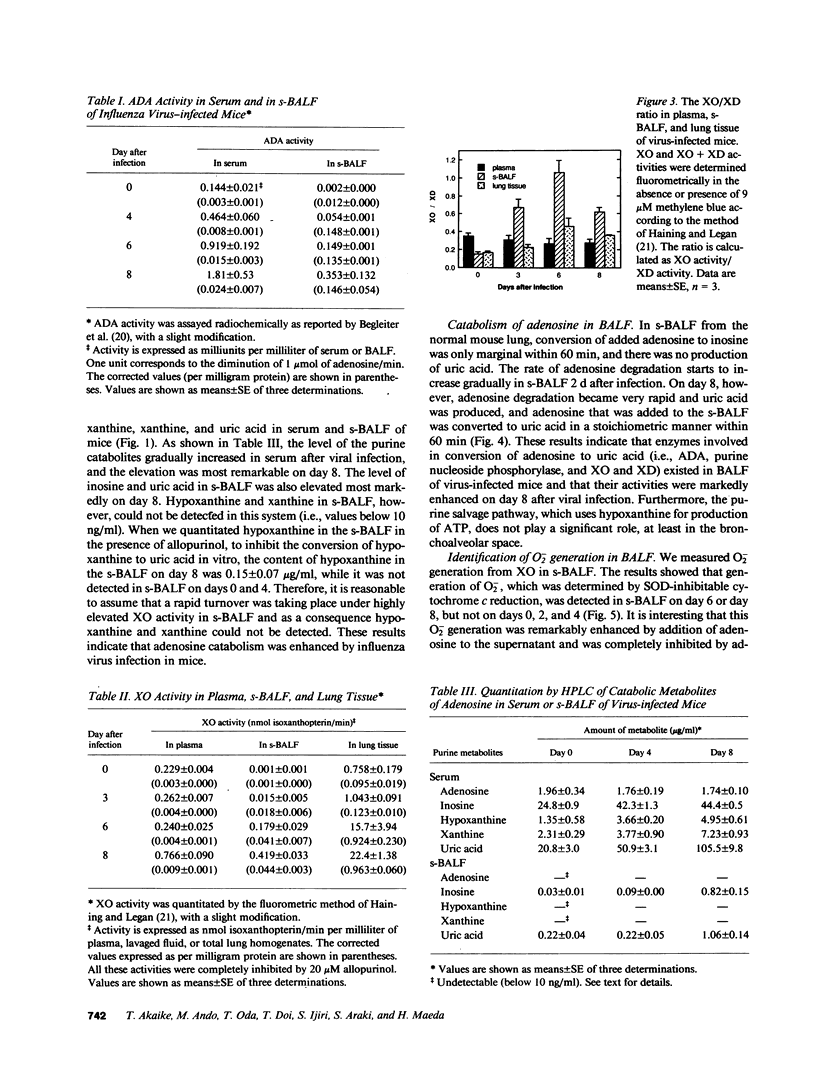
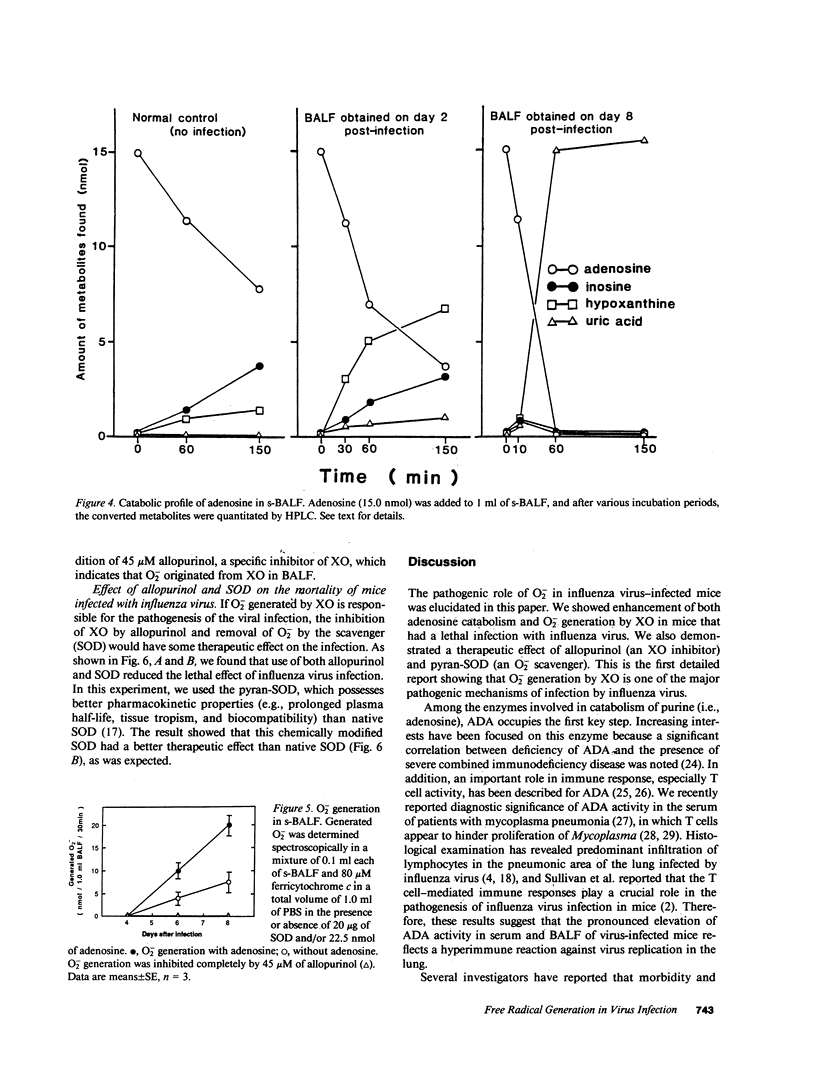
We evaluated various biochemical parameters in influenza virus-infected mice and focused on adenosine catabolism in the supernatant of bronchoalveolar lavage fluid (s-BALF), lung tissue, and serum (plasma). The activities of adenosine deaminase (ADA) and xanthine oxidase (XO), which generates O2-, were elevated in the s-BALF, lung tissue homogenate, and serum (plasma). The elevations were most remarkable in s-BALF and in lung tissue: We found a 170-fold increase in ADA activity and a 400-fold increase in XO activity as measured per volume of alveolar lavage fluid. The ratio of activity of XO to activity of xanthine dehydrogenase in s-BALF increased from 0.15 +/- 0.05 (control; no infection) to 1.06 +/- 0.13 on day 6 after viral infection. Increased levels of various adenosine catabolites (i.e., inosine, hypoxanthine, xanthine, and uric acid) in serum and s-BALF were confirmed. We also identified O2- generation from XO in s-BALF obtained on days 6 and 8 after infection, and the generation of O2- was enhanced remarkably in the presence of adenosine. Lastly, treatment with allopurinol (an inhibitor of XO) and with chemically modified superoxide dismutase (a scavenger of O2-) improved the survival rate of influenza virus-infected mice. These results indicate that generation of oxygen-free radicals by XO, coupled with catabolic supply of hypoxanthine from adenosine catabolism, is a pathogenic principle in influenza virus infection in mice and that a therapeutic approach by elimination of oxygen radicals thus seems possible.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Molla A., Ando M., Araki S., Maeda H. Molecular mechanism of complex infection by bacteria and virus analyzed by a model using serratial protease and influenza virus in mice. J Virol. 1989 May;63(5):2252–2259. doi: 10.1128/jvi.63.5.2252-2259.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M., Elmets C. A., Bickers D. R., Mukhtar H. A novel mechanism for the generation of superoxide anions in hematoporphyrin derivative-mediated cutaneous photosensitization. Activation of the xanthine oxidase pathway. J Clin Invest. 1989 Apr;83(4):1137–1143. doi: 10.1172/JCI113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballet J. J., Insel R., Merler E., Rosen F. S. Inhibition of maturation of human precursor lymphocytes by coformycin, an inhibitor of the enzyme adenosine deaminase. J Exp Med. 1976 May 1;143(5):1271–1276. doi: 10.1084/jem.143.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry B. E., Crapo J. D. Patterns of accumulation of platelets and neutrophils in rat lungs during exposure to 100% and 85% oxygen. Am Rev Respir Dis. 1985 Sep;132(3):548–555. doi: 10.1164/arrd.1985.132.3.548. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Glazer R. I., Israels L. G., Pugh L., Johnston J. B. Induction of DNA strand breaks in chronic lymphocytic leukemia following treatment with 2'-deoxycoformycin in vivo and in vitro. Cancer Res. 1987 May 1;47(9):2498–2503. [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P., Sterner G. Cell-mediated immune response following Mycoplasma pneumoniae infection in man. I. Lymphocyte stimulation. Clin Exp Immunol. 1974 May;17(1):29–41. [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Bacon P. A., Dieppe P. A., Halliwell B., Gutteridge J. M. Effect of a specific iron chelating agent on animal models of inflammation. Ann Rheum Dis. 1983 Feb;42(1):89–93. doi: 10.1136/ard.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda D., Németh I., Hencz P., Dénes K. Effect of allopurinol treatment in premature infants with idiopathic respiratory distress syndrome. Dev Pharmacol Ther. 1984;7(6):357–367. doi: 10.1159/000457187. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W., Taylor-Robinson D., Allison A. C. The role of thymus-dependent immunity in Mycoplasma pulmonis infections of mice. J Med Microbiol. 1972 Aug;5(3):327–336. doi: 10.1099/00222615-5-3-327. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S., HORSFALL F. L., Jr Quantitative aspects of the multiplication of influenza A virus in the mouse lung; relation between the degree of viral multiplication and the extent of pneumonia. J Exp Med. 1952 Feb;95(2):135–145. doi: 10.1084/jem.95.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Bianchi M., Gianera L., Landolfo S., Salmona M. Role of reactive oxygen intermediates in the interferon-mediated depression of hepatic drug metabolism and protective effect of N-acetylcysteine in mice. Cancer Res. 1985 Aug;45(8):3444–3447. [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Granger D. N., Höllwarth M. E., Parks D. A. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- Haining J. L., Legan J. S. Fluorometric assay for xanthine oxidase. Anal Biochem. 1967 Dec;21(3):337–343. doi: 10.1016/0003-2697(67)90308-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd J., Heath R. B. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect Immun. 1975 May;11(5):886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lasley R. D., Ely S. W., Berne R. M., Mentzer R. M., Jr Allopurinol enhanced adenine nucleotide repletion after myocardial ischemia in the isolated rat heart. J Clin Invest. 1988 Jan;81(1):16–20. doi: 10.1172/JCI113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A. S., Coltart D. J., Hearse D. J. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984 Oct;55(4):545–548. doi: 10.1161/01.res.55.4.545. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Nishikawa H., Suga M., Ando M., Tanaka F., Araki S. Serum adenosine deaminase activity with Mycoplasma pneumoniae. Chest. 1988 Dec;94(6):1315–1315. doi: 10.1378/chest.94.6.1315a. [DOI] [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989 May 26;244(4907):974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Rodell T. C., Cheronis J. C., Ohnemus C. L., Piermattei D. J., Repine J. E. Xanthine oxidase mediates elastase-induced injury to isolated lungs and endothelium. J Appl Physiol (1985) 1987 Nov;63(5):2159–2163. doi: 10.1152/jappl.1987.63.5.2159. [DOI] [PubMed] [Google Scholar]
- Sanfey H., Sarr M. G., Bulkley G. B., Cameron J. L. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand Suppl. 1986;548:109–118. [PubMed] [Google Scholar]
- Shimomura E., Suzuki F., Ishida N. Characterization of cells infiltrating the lungs of x-irradiated and nude mice after influenza virus infection. Microbiol Immunol. 1982;26(2):129–138. doi: 10.1111/j.1348-0421.1982.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Mayner R. E., Barry D. W., Ennis F. A. Influenza virus infection in nude mice. J Infect Dis. 1976 Jan;133(1):91–94. doi: 10.1093/infdis/133.1.91. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Osborne W. R., Wedgewood R. J. Adenosine deaminase activity in lymphocytes. Br J Haematol. 1977 Sep;37(1):157–158. [PubMed] [Google Scholar]
- Sultatos L. G. Effects of acute ethanol administration on the hepatic xanthine dehydrogenase/oxidase system in the rat. J Pharmacol Exp Ther. 1988 Sep;246(3):946–949. [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]


