Abstract
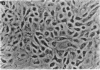
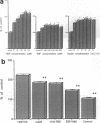
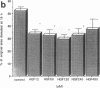
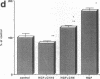
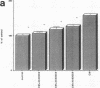
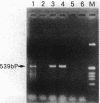
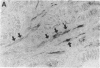
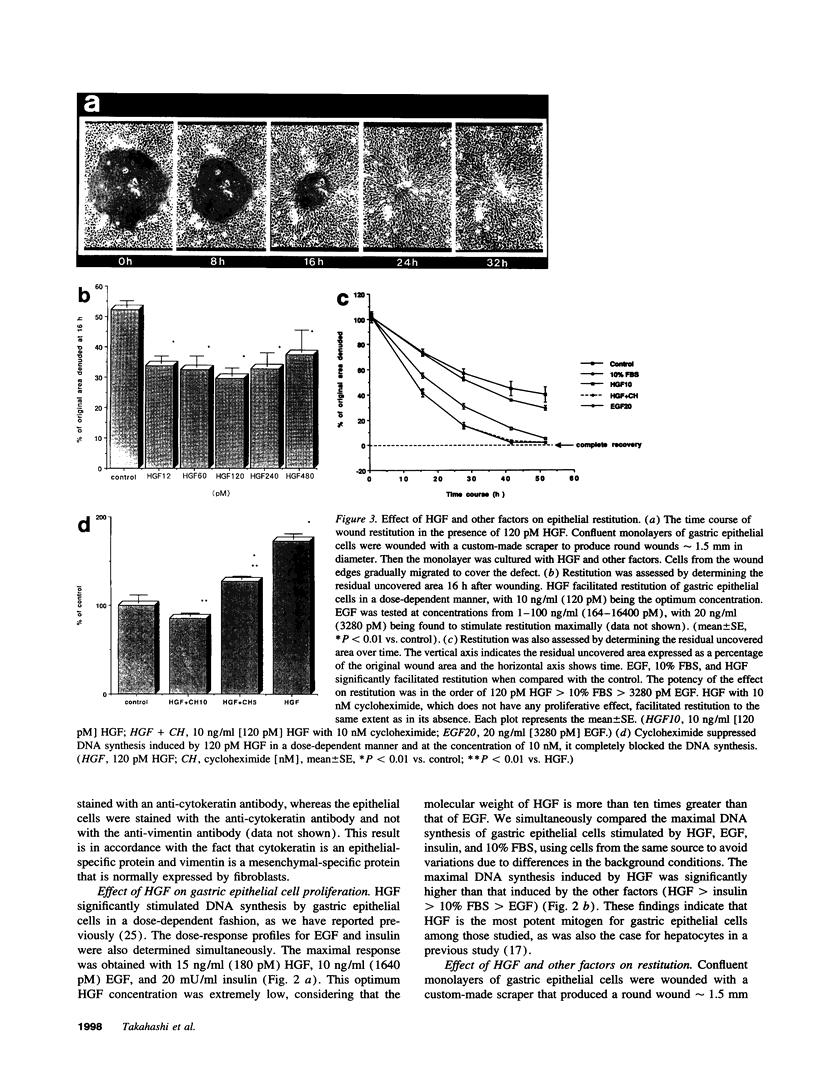
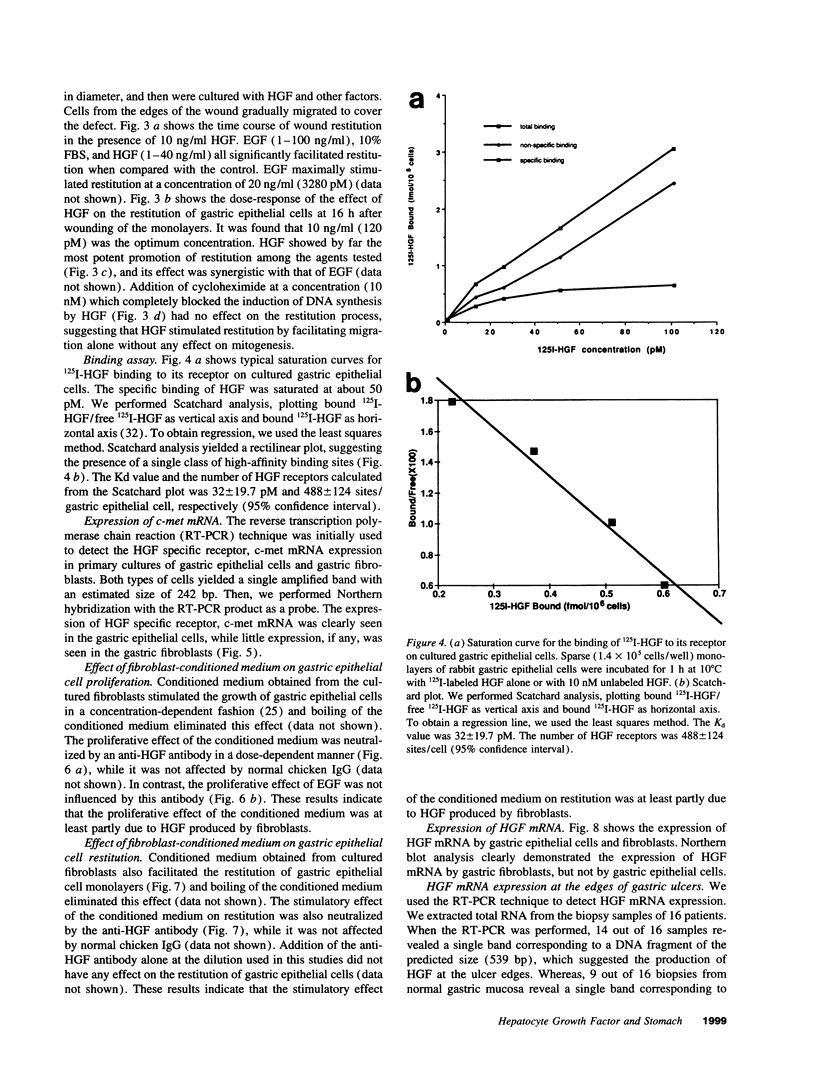
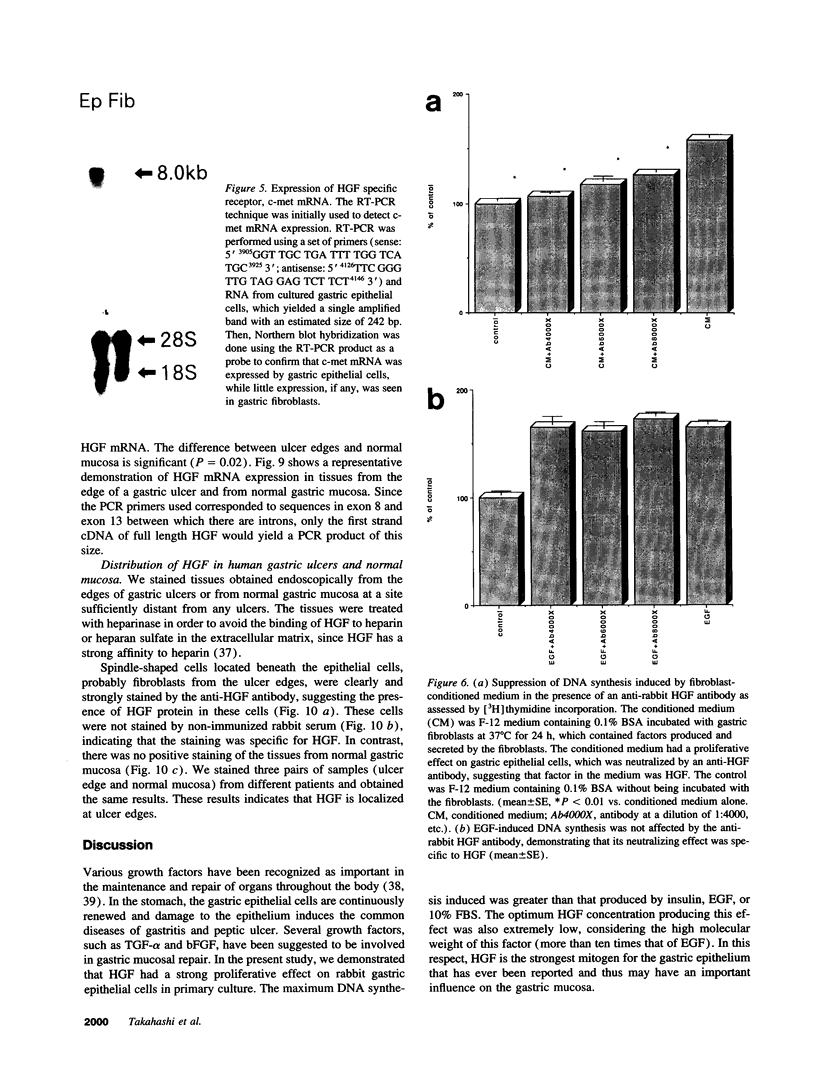
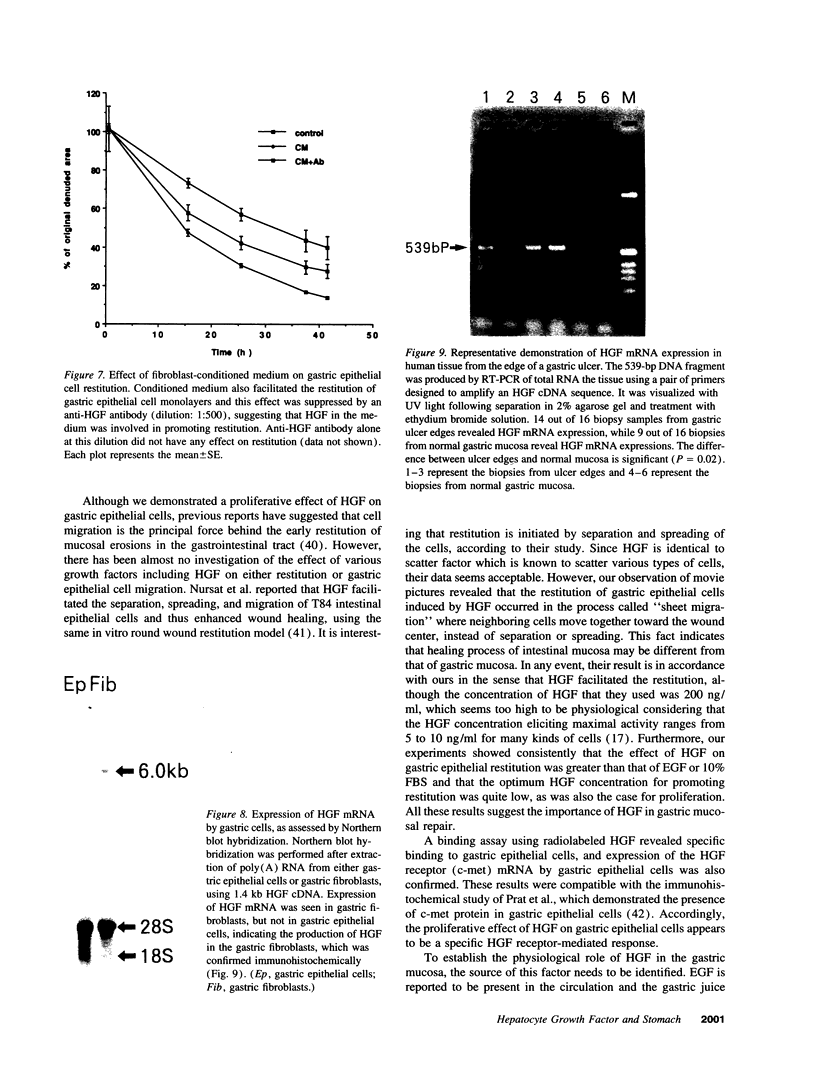
Various growth factors are suggested to be involved in gastric mucosal repair. Our previous studies have shown that exogenous hepatocyte growth factor (HGF) has a proliferative effect on gastric epithelial cells. In the present study, comparison of the maximum proliferative effects and the optimum concentrations of several growth factors revealed that HGF was the most potent mitogen for gastric epithelial cells, as is the case for hepatocytes. Restitution of gastric epithelial cell monolayers was assessed using a round wound restitution model. HGF was the most effective agent for facilitating gastric epithelial restitution among those tested. A binding assay revealed specific binding of HGF to its receptor on gastric epithelial cells. Northern blot analysis confirmed the expression of specific HGF receptor mRNA (c-met) by gastric epithelial cells but not by gastric fibroblasts. To investigate endogenous HGF production, we determined the effect of gastric fibroblast-conditioned medium on epithelial proliferation and restitution. The conditioned medium produced similar effects to HGF and its activity was neutralized by an anti-HGF antibody. In addition, expression of HGF mRNA was detected in gastric fibroblasts but not in gastric epithelial cells. Our immunohistochemical study confirmed these in vitro data by means of demonstrating the existence and localization of HGF at human native gastric mucosa. HGF was localized at fibroblasts under the epithelial cell layer around gastric ulcers. These results suggest that HGF may be a potent endogenous promotor of gastric epithelial cell proliferation and migration, and may contribute to gastric mucosal repair through a paracrine mechanism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp R. D., Barnard J. A., McCutchen C. M., Cherner J. A., Coffey R. J., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989 Sep;84(3):1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. C., Lee A. T., Karnes W. E., Avedian D., Martin M., Sorvillo J. M., Soll A. H. Paracrine control of gastric epithelial cell growth in culture by transforming growth factor-alpha. Am J Physiol. 1993 Feb;264(2 Pt 1):G390–G396. doi: 10.1152/ajpgi.1993.264.2.G390. [DOI] [PubMed] [Google Scholar]
- Chen M. C., Lee A. T., Soll A. H. Mitogenic response of canine fundic epithelial cells in short-term culture to transforming growth factor alpha and insulinlike growth factor I. J Clin Invest. 1991 May;87(5):1716–1723. doi: 10.1172/JCI115189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Di Renzo M. F., Narsimhan R. P., Olivero M., Bretti S., Giordano S., Medico E., Gaglia P., Zara P., Comoglio P. M. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991 Nov;6(11):1997–2003. [PubMed] [Google Scholar]
- Folkman J., Szabo S., Stovroff M., McNeil P., Li W., Shing Y. Duodenal ulcer. Discovery of a new mechanism and development of angiogenic therapy that accelerates healing. Ann Surg. 1991 Oct;214(4):414–427. doi: 10.1097/00000658-199110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi O., Nakamura T. Identification and change in the receptor for hepatocyte growth factor in rat liver after partial hepatectomy or induced hepatitis. Biochem Biophys Res Commun. 1991 Apr 30;176(2):599–607. doi: 10.1016/s0006-291x(05)80226-8. [DOI] [PubMed] [Google Scholar]
- Igawa T., Kanda S., Kanetake H., Saitoh Y., Ichihara A., Tomita Y., Nakamura T. Hepatocyte growth factor is a potent mitogen for cultured rabbit renal tubular epithelial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):831–838. doi: 10.1016/0006-291x(91)91493-v. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Tashiro K., Nakamura T. Marked increase of HGF mRNA in non-parenchymal liver cells of rats treated with hepatotoxins. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1229–1234. doi: 10.1016/0006-291x(89)92733-2. [DOI] [PubMed] [Google Scholar]
- Konturek J. W., Bielanski W., Konturek S. J., Bogdal J., Oleksy J. Distribution and release of epidermal growth factor in man. Gut. 1989 Sep;30(9):1194–1200. doi: 10.1136/gut.30.9.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Hashimoto K., Yoshikawa K., Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocyte growth factor. Exp Cell Res. 1991 Sep;196(1):114–120. doi: 10.1016/0014-4827(91)90462-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3(1-2):27–54. [PubMed] [Google Scholar]
- Matsumoto K., Tajima H., Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991 Apr 15;176(1):45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- McCormack S. A., Viar M. J., Johnson L. R. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992 Sep;263(3 Pt 1):G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Wallace J. L. The roles of ethanol and of acid in the production of gastric mucosal erosions in rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;38(1):23–38. doi: 10.1007/BF02892800. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3(1):67–85. doi: 10.1016/0955-2235(91)90014-u. [DOI] [PubMed] [Google Scholar]
- Noji S., Tashiro K., Koyama E., Nohno T., Ohyama K., Taniguchi S., Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990 Nov 30;173(1):42–47. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- Nusrat A., Parkos C. A., Bacarra A. E., Godowski P. J., Delp-Archer C., Rosen E. M., Madara J. L. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest. 1994 May;93(5):2056–2065. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota S., Terano A., Hiraishi H., Mutoh H., Nakada R., Hata Y., Shiga J., Sugimoto T. A monolayer culture of gastric mucous cells from adult rabbits. Gastroenterol Jpn. 1990 Feb;25(1):1–7. doi: 10.1007/BF02785323. [DOI] [PubMed] [Google Scholar]
- Polk W. H., Jr, Dempsey P. J., Russell W. E., Brown P. I., Beauchamp R. D., Barnard J. A., Coffey R. J., Jr Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology. 1992 May;102(5):1467–1474. doi: 10.1016/0016-5085(92)91703-7. [DOI] [PubMed] [Google Scholar]
- Prat M., Narsimhan R. P., Crepaldi T., Nicotra M. R., Natali P. G., Comoglio P. M. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991 Sep 30;49(3):323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- Rutten M. J., Dempsey P. J., Solomon T. E., Coffey R. J., Jr TGF-alpha is a potent mitogen for primary cultures of guinea pig gastric mucous epithelial cells. Am J Physiol. 1993 Aug;265(2 Pt 1):G361–G369. doi: 10.1152/ajpgi.1993.265.2.G361. [DOI] [PubMed] [Google Scholar]
- Rutten M. J., Harmon P., Campbell D. R. Insulin enhances epidermal growth factor- and transforming growth factor-alpha-stimulated growth in primary cultures of guinea pig gastric mucous epithelial cells. Scand J Gastroenterol. 1991 Sep;26(9):965–973. doi: 10.3109/00365529108996250. [DOI] [PubMed] [Google Scholar]
- Rutten M. J., Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol. 1983 Feb;244(2):G171–G182. doi: 10.1152/ajpgi.1983.244.2.G171. [DOI] [PubMed] [Google Scholar]
- Sato Y., Mukai K., Watanabe S., Goto M., Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986 Dec;125(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Silen W., Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol. 1985;47:217–229. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. A major advance in the use of growth factors to enhance wound healing. J Clin Invest. 1993 Dec;92(6):2565–2566. doi: 10.1172/JCI116868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Ota S., Terano A., Yoshiura K., Matsumura M., Niwa Y., Kawabe T., Nakamura T., Omata M. Hepatocyte growth factor induces mitogenic reaction to the rabbit gastric epithelial cells in primary culture. Biochem Biophys Res Commun. 1993 Mar 15;191(2):528–534. doi: 10.1006/bbrc.1993.1250. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Hagiya M., Nishizawa T., Seki T., Shimonishi M., Shimizu S., Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano A., Ivey K. J., Stachura J., Sekhon S., Hosojima H., McKenzie W. N., Jr, Krause W. J., Wyche J. H. Cell culture of rat gastric fundic mucosa. Gastroenterology. 1982 Dec;83(6):1280–1291. [PubMed] [Google Scholar]
- Tsao M. S., Zhu H., Giaid A., Viallet J., Nakamura T., Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993 Jul;4(7):571–579. [PubMed] [Google Scholar]
- Wang J. Y., Johnson L. R. Induction of gastric and duodenal mucosal ornithine decarboxylase during stress. Am J Physiol. 1989 Aug;257(2 Pt 1):G259–G265. doi: 10.1152/ajpgi.1989.257.2.G259. [DOI] [PubMed] [Google Scholar]
- Yanagita K., Nagaike M., Ishibashi H., Niho Y., Matsumoto K., Nakamura T. Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem Biophys Res Commun. 1992 Jan 31;182(2):802–809. doi: 10.1016/0006-291x(92)91803-x. [DOI] [PubMed] [Google Scholar]
- Yeomans N. D., Saint John D. J., de Boer W. G. Regeneration of gastric mucosa after aspirin-induced injury in the rat. Am J Dig Dis. 1973 Sep;18(9):773–780. doi: 10.1007/BF01070847. [DOI] [PubMed] [Google Scholar]
- Yoshinaga Y., Matsuno Y., Fujita S., Nakamura T., Kikuchi M., Shimosato Y., Hirohashi S. Immunohistochemical detection of hepatocyte growth factor/scatter factor in human cancerous and inflammatory lesions of various organs. Jpn J Cancer Res. 1993 Nov;84(11):1150–1158. doi: 10.1111/j.1349-7006.1993.tb02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura K., Ota S., Terano A., Takahashi M., Hata Y., Kawabe T., Mutoh H., Hiraishi H., Nakata R., Okano K. Growth regulation of rabbit gastric epithelial cells and protooncogene expression. Dig Dis Sci. 1994 Jul;39(7):1454–1463. doi: 10.1007/BF02088048. [DOI] [PubMed] [Google Scholar]
- Zarnegar R., Muga S., Rahija R., Michalopoulos G. Tissue distribution of hepatopoietin-A: a heparin-binding polypeptide growth factor for hepatocytes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1252–1256. doi: 10.1073/pnas.87.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]