Abstract
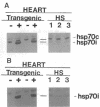
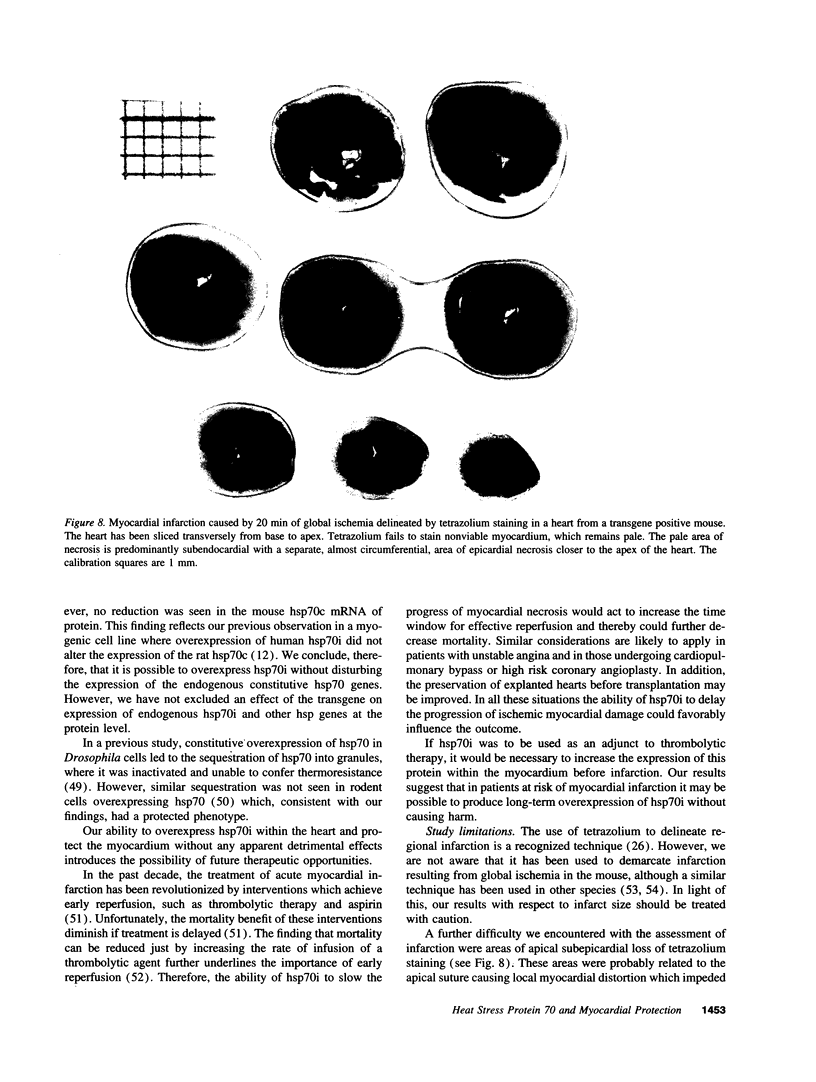
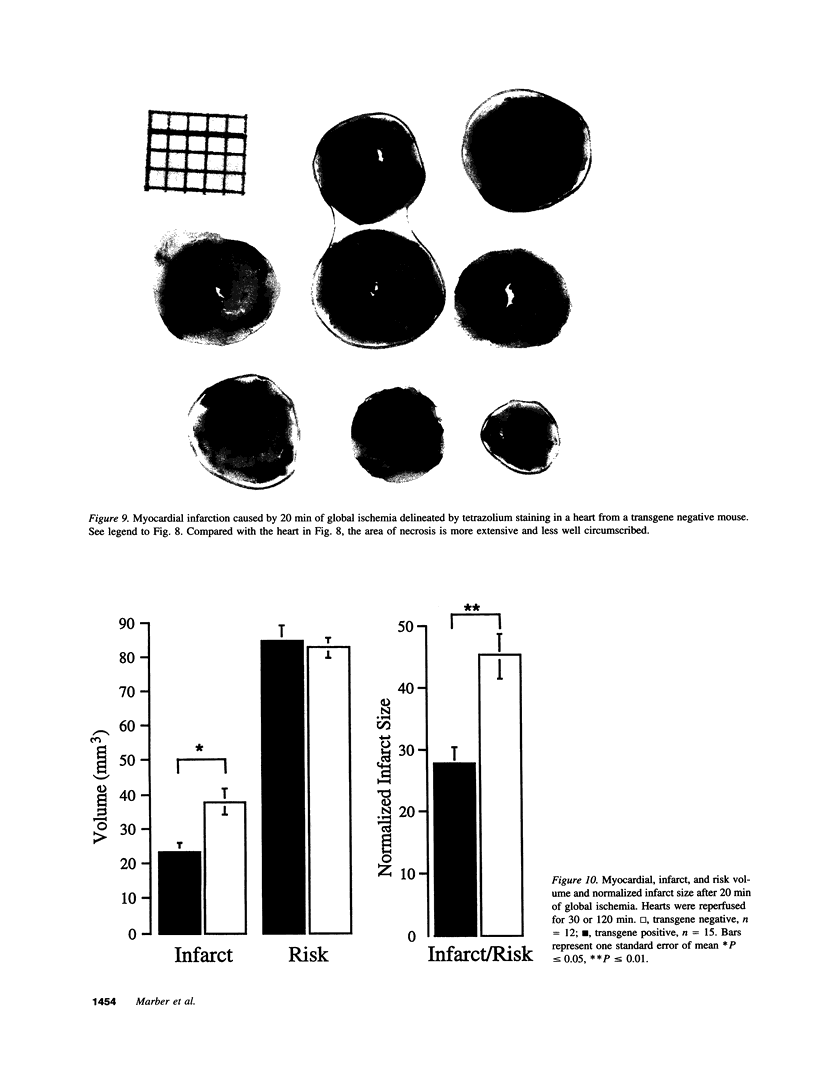
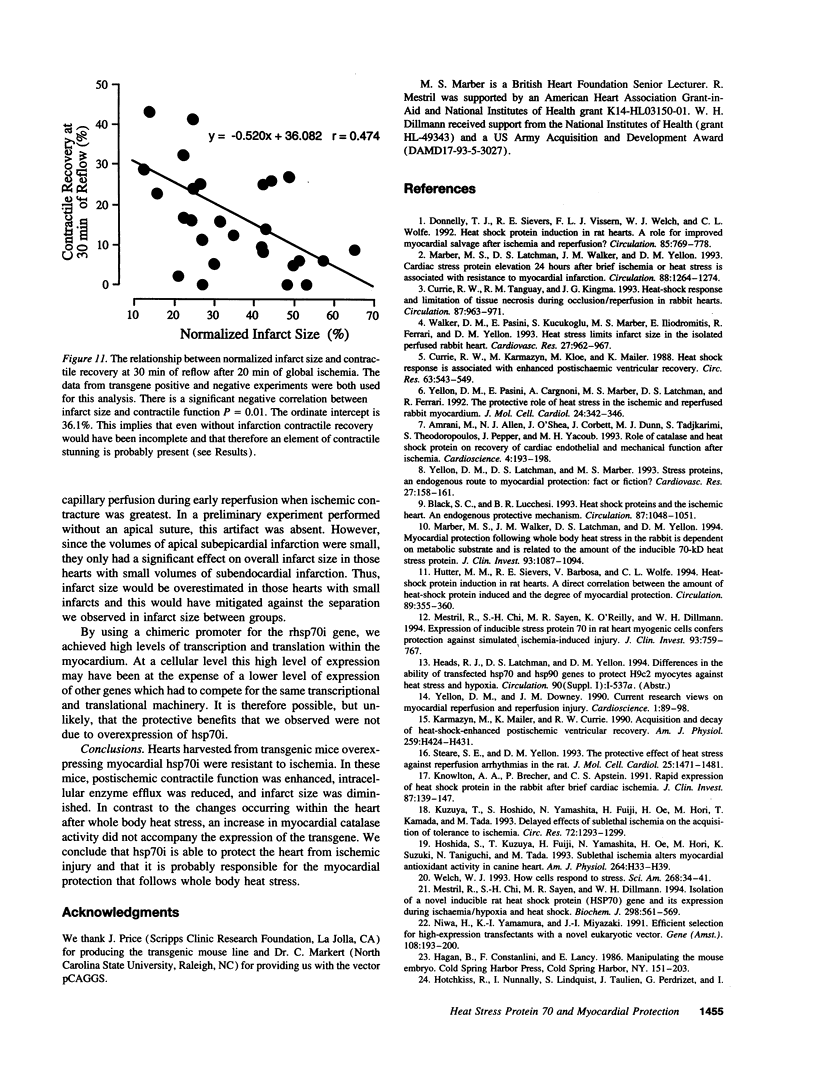
Myocardial protection and changes in gene expression follow whole body heat stress. Circumstantial evidence suggests that an inducible 70-kD heat shock protein (hsp70i), increased markedly by whole body heat stress, contributes to the protection. Transgenic mouse lines were constructed with a cytomegalovirus enhancer and beta-actin promoter driving rat hsp70i expression in heterozygote animals. Unstressed, transgene positive mice expressed higher levels of myocardial hsp70i than transgene negative mice after whole body heat stress. This high level of expression occurred without apparent detrimental effect. The hearts harvested from transgene positive mice and transgene negative littermates were Langendorff perfused and subjected to 20 min of warm (37 degrees C) zero-flow ischemia and up to 120 min of reflow while contractile recovery and creatine kinase efflux were measured. Myocardial infarction was demarcated by triphenyltetrazolium. In transgene positive compared with transgene negative hearts, the zone of infarction was reduced by 40%, contractile function at 30 min of reflow was doubled, and efflux of creatine kinase was reduced by approximately 50%. Our findings suggest for the first time that increased myocardial hsp70i expression results in protection of the heart against ischemic injury and that the antiischemic properties of hsp70i have possible therapeutic relevance.
Full text
PDF

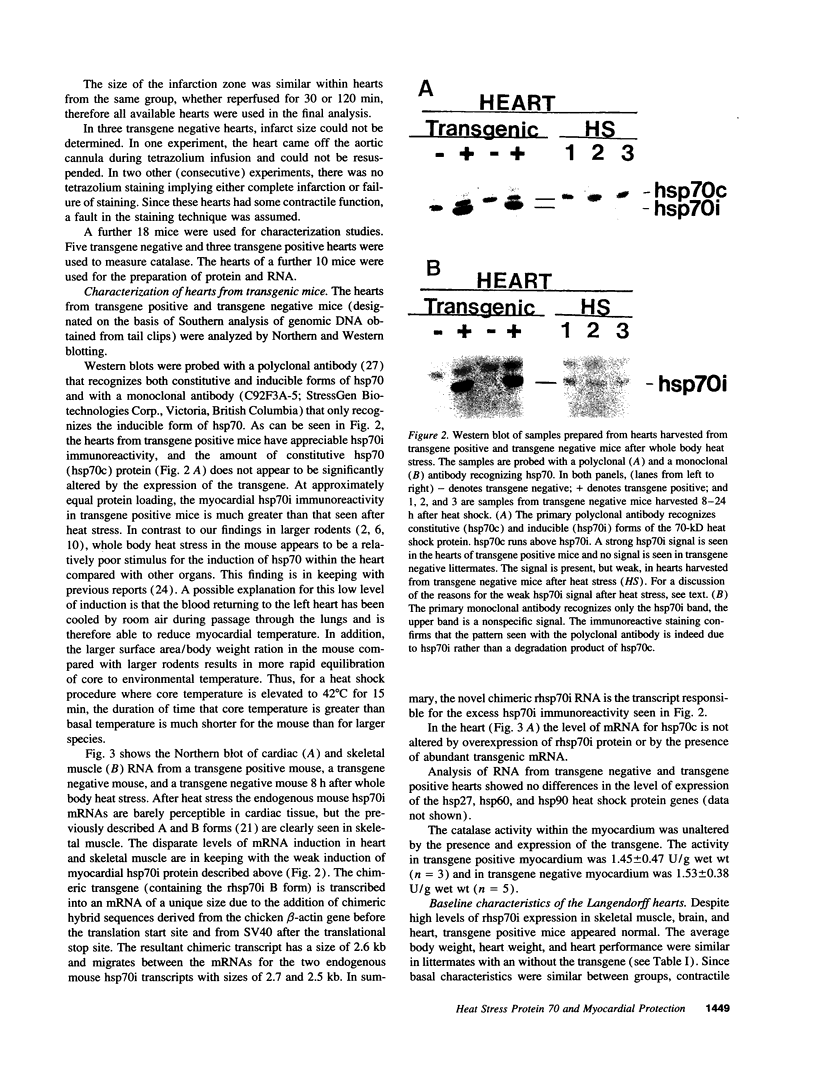
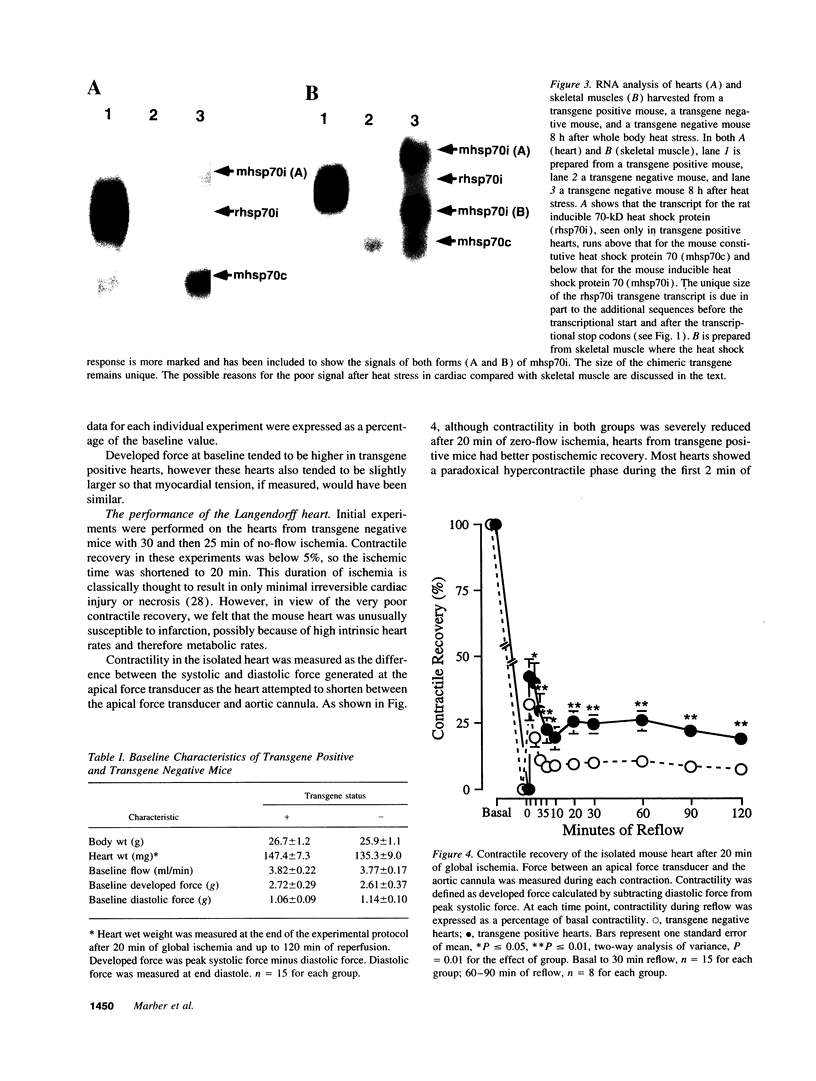
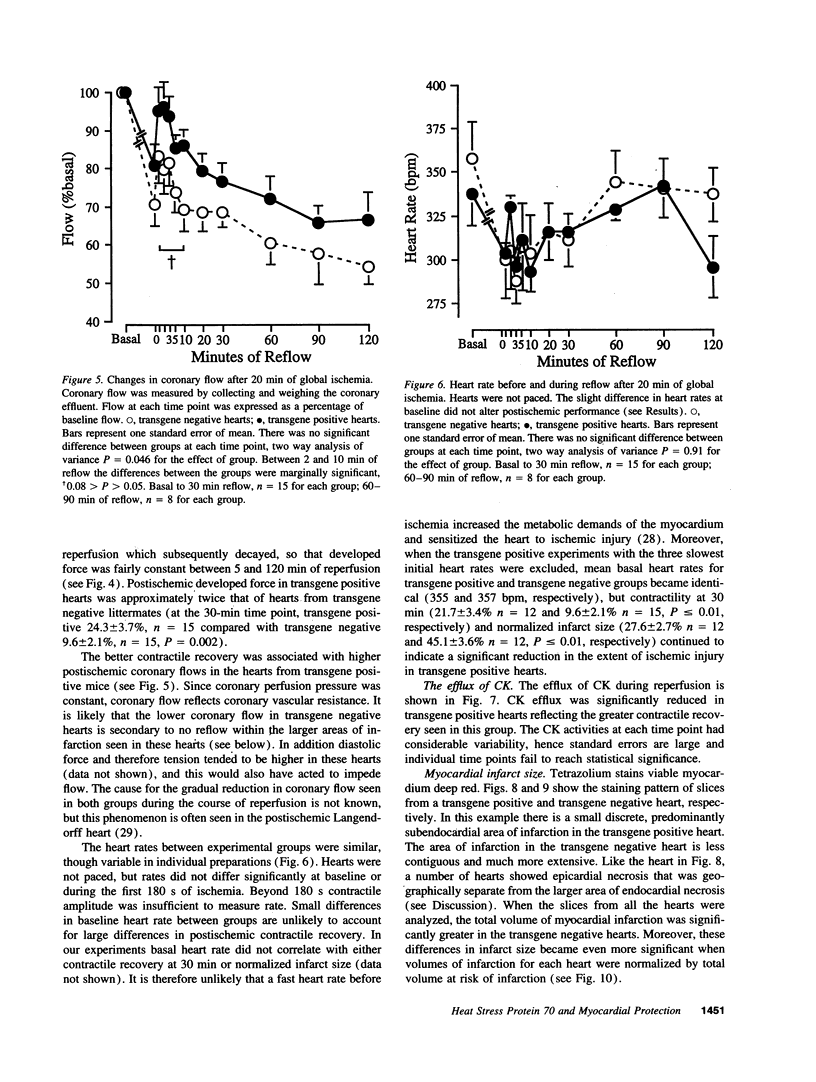
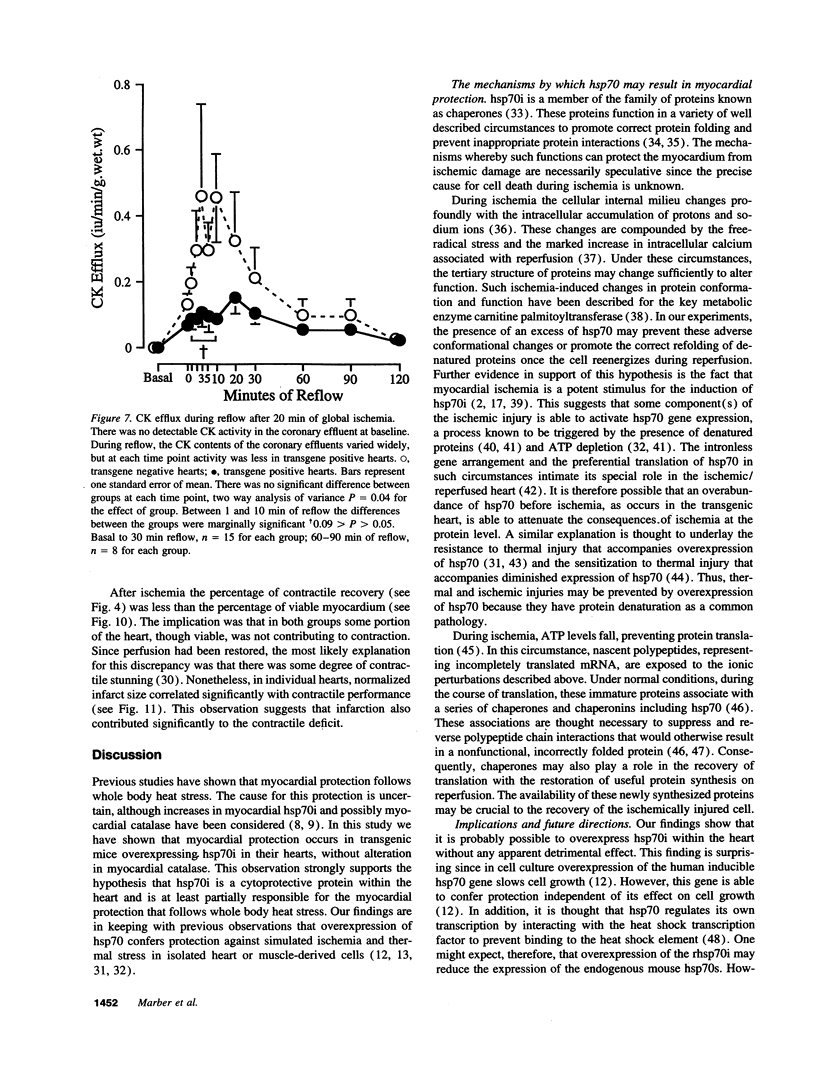

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Amrani M., Allen N. J., O'Shea J., Corbett J., Dunn M. J., Tadjkarimi S., Theodoropoulos S., Pepper J., Yacoub M. H. Role of catalase and heat shock protein on recovery of cardiac endothelial and mechanical function after ischemia. Cardioscience. 1993 Sep;4(3):193–198. [PubMed] [Google Scholar]
- Baler R., Welch W. J., Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J Cell Biol. 1992 Jun;117(6):1151–1159. doi: 10.1083/jcb.117.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Locke-Winter C., Rogers K. B., Mitchell M. B., Brew E. C., Cairns C. B., Bensard D. D., Harken A. H. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ Res. 1993 Oct;73(4):656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- Beckmann R. P., Lovett M., Welch W. J. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992 Jun;117(6):1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Black S. C., Lucchesi B. R. Heat shock proteins and the ischemic heart. An endogenous protective mechanism. Circulation. 1993 Mar;87(3):1048–1051. doi: 10.1161/01.cir.87.3.1048. [DOI] [PubMed] [Google Scholar]
- Bolli R. Mechanism of myocardial "stunning". Circulation. 1990 Sep;82(3):723–738. doi: 10.1161/01.cir.82.3.723. [DOI] [PubMed] [Google Scholar]
- Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970 Mar;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Karmazyn M., Kloc M., Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988 Sep;63(3):543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Tanguay R. M., Kingma J. G., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993 Mar;87(3):963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Mehta H. B., Barrieux A., Guth B. D., Neeley W. E., Ross J., Jr Ischemia of the dog heart induces the appearance of a cardiac mRNA coding for a protein with migration characteristics similar to heat-shock/stress protein 71. Circ Res. 1986 Jul;59(1):110–114. doi: 10.1161/01.res.59.1.110. [DOI] [PubMed] [Google Scholar]
- Donnelly T. J., Sievers R. E., Vissern F. L., Welch W. J., Wolfe C. L. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 1992 Feb;85(2):769–778. doi: 10.1161/01.cir.85.2.769. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. Chaperoning nascent proteins. Nature. 1994 Jul 14;370(6485):96–97. doi: 10.1038/370096a0. [DOI] [PubMed] [Google Scholar]
- Feder J. H., Rossi J. M., Solomon J., Solomon N., Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992 Aug;6(8):1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Meerbaum S., Rit J., Lando U., Kanmatsuse K., Mercier J. C., Corday E., Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981 May;101(5):593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Ohtsuka K., Hartl F. U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994 Jul 14;370(6485):111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Heads R. J., Latchman D. S., Yellon D. M. Stable high level expression of a transfected human HSP70 gene protects a heart-derived muscle cell line against thermal stress. J Mol Cell Cardiol. 1994 Jun;26(6):695–699. doi: 10.1006/jmcc.1994.1084. [DOI] [PubMed] [Google Scholar]
- Hoshida S., Kuzuya T., Fuji H., Yamashita N., Oe H., Hori M., Suzuki K., Taniguchi N., Tada M. Sublethal ischemia alters myocardial antioxidant activity in canine heart. Am J Physiol. 1993 Jan;264(1 Pt 2):H33–H39. doi: 10.1152/ajpheart.1993.264.1.H33. [DOI] [PubMed] [Google Scholar]
- Hutter M. M., Sievers R. E., Barbosa V., Wolfe C. L. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994 Jan;89(1):355–360. doi: 10.1161/01.cir.89.1.355. [DOI] [PubMed] [Google Scholar]
- Iwaki K., Chi S. H., Dillmann W. H., Mestril R. Induction of HSP70 in cultured rat neonatal cardiomyocytes by hypoxia and metabolic stress. Circulation. 1993 Jun;87(6):2023–2032. doi: 10.1161/01.cir.87.6.2023. [DOI] [PubMed] [Google Scholar]
- Karmazyn M., Mailer K., Currie R. W. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990 Aug;259(2 Pt 2):H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Brecher P., Apstein C. S. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991 Jan;87(1):139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya T., Hoshida S., Yamashita N., Fuji H., Oe H., Hori M., Kamada T., Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993 Jun;72(6):1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L. G., Liu Y. K., Mak J. Y., Chen L. L., Lee W. M. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marber M. S., Latchman D. S., Walker J. M., Yellon D. M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993 Sep;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Walker J. M., Latchman D. S., Yellon D. M. Myocardial protection after whole body heat stress in the rabbit is dependent on metabolic substrate and is related to the amount of the inducible 70-kD heat stress protein. J Clin Invest. 1994 Mar;93(3):1087–1094. doi: 10.1172/JCI117059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta H. B., Popovich B. K., Dillmann W. H. Ischemia induces changes in the level of mRNAs coding for stress protein 71 and creatine kinase M. Circ Res. 1988 Sep;63(3):512–517. doi: 10.1161/01.res.63.3.512. [DOI] [PubMed] [Google Scholar]
- Mestril R., Chi S. H., Sayen M. R., Dillmann W. H. Isolation of a novel inducible rat heat-shock protein (HSP70) gene and its expression during ischaemia/hypoxia and heat shock. Biochem J. 1994 Mar 15;298(Pt 3):561–569. doi: 10.1042/bj2980561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R., Chi S. H., Sayen M. R., O'Reilly K., Dillmann W. H. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Invest. 1994 Feb;93(2):759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991 Dec 15;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Reperfusion injury and its pharmacologic modification. Circulation. 1989 Oct;80(4):1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- Pauly D. F., Kirk K. A., McMillin J. B. Carnitine palmitoyltransferase in cardiac ischemia. A potential site for altered fatty acid metabolism. Circ Res. 1991 Apr;68(4):1085–1094. doi: 10.1161/01.res.68.4.1085. [DOI] [PubMed] [Google Scholar]
- Rabindran S. K., Wisniewski J., Li L., Li G. C., Wu C. Interaction between heat shock factor and hsp70 is insufficient to suppress induction of DNA-binding activity in vivo. Mol Cell Biol. 1994 Oct;14(10):6552–6560. doi: 10.1128/mcb.14.10.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Sandhu R., Diaz R. J., Wilson G. J. Comparison of ischaemic preconditioning in blood perfused and buffer perfused isolated heart models. Cardiovasc Res. 1993 Apr;27(4):602–607. doi: 10.1093/cvr/27.4.602. [DOI] [PubMed] [Google Scholar]
- Schaper J., Schaper W. Time course of myocardial necrosis. Cardiovasc Drugs Ther. 1988 May;2(1):17–25. doi: 10.1007/BF00054248. [DOI] [PubMed] [Google Scholar]
- Steare S. E., Yellon D. M. The protective effect of heat stress against reperfusion arrhythmias in the rat. J Mol Cell Cardiol. 1993 Dec;25(12):1471–1481. doi: 10.1006/jmcc.1993.1163. [DOI] [PubMed] [Google Scholar]
- Walker D. M., Pasini E., Kucukoglu S., Marber M. S., Iliodromitis E., Ferrari R., Yellon D. M. Heat stress limits infarct size in the isolated perfused rabbit heart. Cardiovasc Res. 1993 Jun;27(6):962–967. doi: 10.1093/cvr/27.6.962. [DOI] [PubMed] [Google Scholar]
- Williams E. H., Kao R. L., Morgan H. E. Protein degradation and synthesis during recovery from myocardial ischemia. Am J Physiol. 1981 Mar;240(3):E268–E273. doi: 10.1152/ajpendo.1981.240.3.E268. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Thomas J. A., Fina M., German Z., Benjamin I. J. Human heat shock protein 70 (hsp70) protects murine cells from injury during metabolic stress. J Clin Invest. 1993 Jul;92(1):503–508. doi: 10.1172/JCI116594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon D. M., Downey J. M. Current research views on myocardial reperfusion and reperfusion injury. Cardioscience. 1990 Jun;1(2):89–98. [PubMed] [Google Scholar]
- Yellon D. M., Latchman D. S., Marber M. S. Stress proteins--an endogenous route to myocardial protection: fact or fiction? Cardiovasc Res. 1993 Feb;27(2):158–161. doi: 10.1093/cvr/27.2.158. [DOI] [PubMed] [Google Scholar]