Abstract
SitC is one of the predominant lipoproteins in Staphylococcus aureus. Recently, SitC was shown to be capable of stimulating Toll-like receptor 2 (TLR2), but the mechanism of TLR2 activation by SitC has not been analyzed in detail so far. In this study, we purified C-terminally His-tagged SitC (SitC-His) from Staphylococcus aureus. SitC-His induced interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) release in human monocytes and also NF-κB activation in TLR2-transfected HEK293 cells, indicating TLR2-specific activation. SitC not only induced a TLR2-dependent release of IL-6 in primary murine keratinocytes (MKs) but also induced intracellular accumulation of TLR2, which was time and concentration dependent. Cy2-labeled SitC-His colocalized specifically with TLR2 in MKs and was also internalized in TLR2 knockout MKs, suggesting a TLR2-independent uptake. Neither activation nor colocalization of SitC-His was observed with TLR4 or Nod2. The results show that the native lipoprotein SitC-His specifically colocalizes with TLR2, is internalized by host cells, induces proinflammatory cytokines, and triggers intracellular accumulation of TLR2.
Recognition of intruding pathogens is the first step of host defense. The innate immune response is capable of recognizing microbes and provides a first line of defense to the host. It is mediated in part by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), that specifically recognize microbe-associated molecular patterns (MAMPs) derived from microorganisms (2). TLR2 has been shown to play a crucial role in the host response to Staphylococcus aureus (47). It appears to be a receptor for various structurally unrelated MAMPs, e.g., lipoproteins (27, 39), glycolipids from spirochetes, lipoarabinomannan from mycobacteria, porins from Neisseria (45), lipoteichoic acid (LTA) (32, 40), and peptidoglycan (PGN) (1, 4, 12, 20, 33). However, an increasing number of studies suggest that bacterial lipoproteins are the major, if not sole, TLR2-activating molecules of Gram-positive bacteria (18, 27, 43, 48, 52).
The N termini of bacterial lipoproteins contain a unique S-diacylglyceryl cysteine moiety. In Escherichia coli and other Gram-negative bacteria, as well as in mycobacterial and spirochetal species, an additional acyl group is linked to the amino group of this cysteine (16), resulting in the formation of triacylated membrane anchor structures. Lipoproteins are synthesized as preproteins with a distinct type II signal sequence containing a conserved lipobox consensus motif (28). In the S. aureus genome, more than 50 genes contain the type II signal sequence typical for lipoproteins. Some of them have been annotated as substrate binding components of ABC transporters that are involved in nutrient and iron acquisition, and one of the predominant lipoproteins is SitC (43). SitC is part of the iron transporter SitABC (10).
Until now, only synthetic lipoprotein analogs, such as N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cyste- inyl-(lysyl)3-lysine (Pam3Cys; tripalmitoyl cysteinyl) lipopeptide, Pam3CSK4 (adapted from the Escherichia coli Braun's lipoprotein), and dipalmitoyl MALP-2 (macrophage-activating lipopeptide 2 kDa) from Mycoplasma fermentans (31), have been shown to mimic the proinflammatory properties of bacterial lipoproteins (46). This led to a model in which triacylated lipopeptides signal through TLR2/TLR1 heterodimers, whereas diacylated lipopeptides signal through TLR2/TLR6 heterodimers. Indeed, the structure of TLR1/TLR2 heterodimers with Pam3CSK4 suggests that both receptors are involved in binding the lipopeptide (24). On the other hand, stimulation of TLR1- and TLR6-deficient mice with di- and triacylated lipopeptides (e.g., Pam2CSK4 and Pam3CGNNDESNISFKEK) revealed that neither TLR1 nor TLR6 was necessary for stimulation (8).
Whether lipoproteins of S. aureus are di- or triacylated is still not clear. Gram-negative bacteria possess an N-acyltransferase (Lnt) that transfers an acyl group to the amino group of the S-diacylated cysteine residue, yielding a triacylated (N-acylated, S-diacylated) lipoprotein. However, an Lnt homolog has not been found in Gram-positive bacteria such as staphylococci. Therefore, it was assumed that the lipoproteins in S. aureus were diacylated. Indeed, N-terminal analysis of an S. aureus lipoprotein (SAOUHSC_02699) revealed that it is diacylated (48). However, it has also been reported that purified SitC (another lipoprotein) is triacylated (27). Although the major role of lipoproteins in TLR2 activation seems to be established, detailed information on molecular lipoproteins interacting with TLR2 in host cells is still unclear.
In this study, the interaction of the staphylococcal lipoprotein SitC with TLR2 of primary murine keratinocytes (MKs) was examined. We show that SitC colocalizes specifically with TLR2 and stimulates proinflammatory cytokines and intracellular TLR2 expression.
MATERIALS AND METHODS
Bacterial strains, growth media, and plasmids.
S. aureus SA113, S. aureus(pTX30SitC-his), and Staphylococcus carnosus ΔRKET(pPSHG5ΩHis6mSHL) were used in this study. Strains were grown aerobically at 37°C in basal medium (BM) with the supplements indicated in Table 1.
TABLE 1.
Strains used in this study
| Strain | Relevant property | Supplementa | Reference |
|---|---|---|---|
| S. aureus SA113 | Laboratory strain | 22 | |
| S. aureus(pTX30SitC-his) | Overproduction of SitC-His | Tc | This work |
| S. carnosus ΔRKET(pPSHG5ΩHis6mSHL) | galRKET::aadA overproduction of shl (cytosolic) | Cm | Q. Gao, unpublished data |
Tc, tetracycline; Cm, chloramphenicol.
Mouse strains.
C57BL/6 wild-type mice were purchased from Charles River (Sulzfield, Germany), and Nod2-deficient mice were purchased from Jackson Laboratory, ME. TLR2-deficient mice were obtained from C. Kirschning (Technical University, Munich, Germany). All deficient strains were in the C57BL/6 background. The animals were bred under specific-pathogen-free conditions at the animal facility of the University of Tübingen according to European guidelines and German law, and the preparation of oral mucosa was approved by the Regierungspräsidium Tübingen (Az 25.04.07 and Az 05.06.09).
Cells and cell lines.
Primary cultures of murine epithelial cells (keratinocytes) were obtained from adult oral mucosa. After overnight treatment with the epidermis upside down in trypsin solution at 4°C, the epidermis was separated from the dermis, and epidermal cells were collected by centrifugation. Murine epithelial cells were cultured in defined medium (64.5% Dulbecco's modified Eagle's medium [DMEM], 21.5% Ham's medium, 10% fetal calf serum, 2% penicillin-streptomycin) for 2 days at 37°C and 5% CO2. Subsequently, cells were transferred to a second medium (94% MCDB [basal nutrient medium], 2% fetal calf serum, 2% penicillin-streptomycin) and grown for 3 days before experiments were started. Human Mono Mac 6 (MM6) cells were cultured in RPMI 1640 medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal calf serum (Biochrom AG, Berlin, Germany), 2× nonessential amino acids, and OPI supplement (Sigma-Aldrich, Taufkirchen, Germany). Cells were grown for 3 days before experiments were started.
Construction of SitC-His-expressing plasmid pTX30SitC-his.
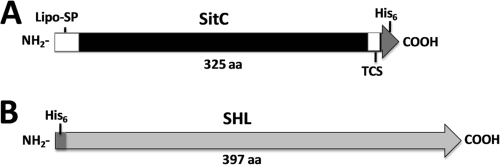
The sitC gene was cloned into a derivative of the xylose-inducible expression vector pTX15 (37) and expressed with a C-terminal extension of 12 amino acids (thrombin cleavage site and histidine tag [KVPRGSHHHHHH]), and the product was named SitC-His (Fig. 1 A). sitC was amplified from S. aureus SA113 genomic DNA by using the primer pair BamHI-fwd (TATTTAGGATCCGAAACGAGGAAGTTTAACATGAAAAAATTAG) and SacI-rev (ATAATTGAGCTCTTAATGATGATGATGATGATGTGAACCACGTGGAACTAATTTCAG CTTCCGTGTAC) (underlining indicates restriction enzyme sites) and High Fidelity PCR enzyme mix (Fermentas, St. Leon-Rot, Germany). The sequence of the PCR product (999 bp) contained the sitC gene with a C-terminal thrombin-cleavable His6 tag. This PCR product was cloned in frame into the polylinker upstream of the xylA promoter region of the pTX15 derivative, resulting in pTX30sitC-His. This plasmid was transformed into S. aureus SA113 by electroporation (5), and the insert was sequenced using a Li-Cor Long Reader DNA sequencer (Lincoln Corporation, Inc., Lincoln, NE). Computer sequence analysis was performed with the Vector NTI program.
FIG. 1.
Protein constructs. (A) C-terminally histidine-tagged preprotein of S. aureus SitC. (B) Cytoplasmic histidine-tagged S. hyicus lipase (SHL) from pPSHG5His6mSHL. TCS, thrombin cleavage site; Lipo-SP, signal peptide; His6, histidine tag; aa, amino acids.
Purification of SitC-His.
S. aureus(pTX30SitC-his) cells were grown at 37°C in the presence of xylose for 13 h prior to growth at 37°C in the absence of xylose for 2 h. Cells were harvested and broken via FastPrep. Cell membranes were obtained by ultracentrifugation. SitC-His was solubilized in extraction buffer (20 mM Tris-HCl, 50 mM NaCl, 2% Triton X-100, pH 8.0) for 18 h at 6°C. After another ultracentrifugation step, the supernatant was taken and incubated with Ni-nitrilotriacetic acid (Ni-NTA) superflow beads (Qiagen, Hilden, Germany) that had been equilibrated with extraction buffer for 18 h at 6°C. The beads were then used to fill a column and washed four times with washing buffer (20 mM Tris-HCl, 50 mM NaCl, 0.25% Triton X-100, 40 mM imidazole) prior to elution with 20 mM Tris-HCl, 50 mM NaCl, 0.25% Triton X-100, and 400 mM imidazole, pH 8.0. The elution was performed in two steps. The two fractions were combined and concentrated 10-fold via a Vivaspin centrifugal ultrafilter unit with a molecular mass cutoff of 10 kDa (Sartorius, Göttingen, Germany). The concentrated eluate was analyzed by SDS-PAGE (Fig. 2 A). The purified protein was concentrated, and the total amount was estimated by Bradford assay and stored at −80°C.
FIG. 2.
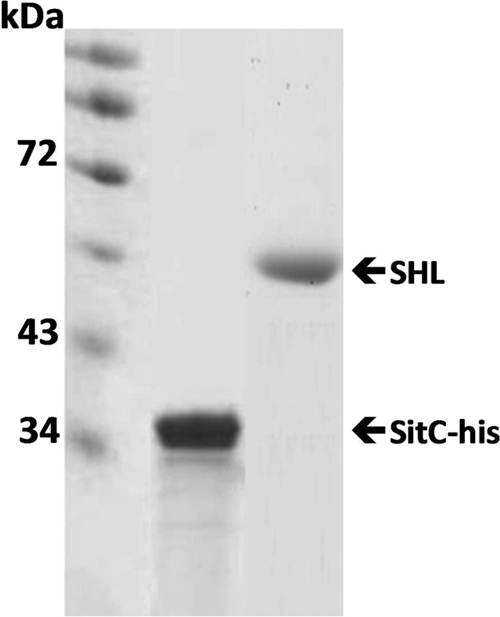
Verification of purified SitC-His and SHL. SitC-His and SHL in the elution fractions after purification by use of Ni-NTA beads were verified by SDS-PAGE (with Coomassie blue staining).
Purification of SHL from S. carnosus ΔRKET(pPSHG5ΩHis6mSHL).
Mature lipase (without PP) with an N-terminal His tag (SHL) was expressed intracellularly in S. carnosus ΔRKET(pPSHG5ΩHis-SHL) (26). SHL was isolated under denaturing conditions with 8 M urea and subsequently purified by Äkta fast-performance liquid chromatography (FPLC) with a His-trap column.
Determination of LPS contamination.
Lipopolysaccharide (LPS) concentrations in the stock solutions of the stimulants used for this study were determined by QCL-1000 LAL assay (Cambrex, Walkersville, MD). The LPS concentrations in 10-μg preparations of SitC-His, Pam3Cys, and SHL were 0.005 endotoxin unit (EU), 0.02 EU, and 0.01 EU, respectively (10 EU = 1 ng LPS).
Labeling of purified proteins.
Protein buffers were changed to LPS-free 0.1 M sodium carbonate buffer, pH 10 to 11. Cy2 or Cy5 labeling was performed as recommended by the manufacturer (GE Healthcare, Freiburg, Germany). The samples were spun at 5,000 × g for 90 min at room temperature (RT) in a centrifugal ultrafilter unit (cutoff, 10 kDa) to remove unbound dye and to change the buffer conditions to neutral pH (LPS-free phosphate-buffered saline [PBS]). Fluorescently labeled proteins were finally diluted in 1 ml LPS-free PBS and stored at −80°C.
Reagents used in cell culture.
Penicillin-streptomycin, Accutase, nonessential amino acids, and PBS were obtained from PAA (Pasching, Austria). LPS from E. coli was purchased from Sigma-Aldrich (Taufkirchen, Germany). The synthetic lipopeptide Pam3Cys (EMC Microcollections, Tübingen, Germany), mirroring the diacylated lipoproteins of bacteria, is also known to activate TLR2 (7). The antibodies used were rabbit anti-TLR2, rabbit anti-TLR4, rabbit anti-Nod2 (all from Santa Cruz Biotechnology, Santa Cruz, CA), and a Cy3-conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch Laboratory, West Grove, PA).
Sample preparation for confocal microscopy.
A total of 3 × 105 freshly isolated, live keratinocytes were seeded in chamber slides for 3 to 5 days at 37°C, 5% CO2, and 95% relative humidity. Afterwards, cells were incubated with different stimuli for 15 to 180 min. Cells were fixed with PLP (0.1 M l-lysin-HCl, 2% paraformaldehyde, 0.01 M sodium meta-periodate, pH 7.4) for 10 min at RT and permeabilized with 0.5% Triton X-100 for 10 min at RT. Cells were subsequently stained with antibodies against TLR2, Nod2, and TLR4 (all from rabbits). A Cy3-conjugated anti-rabbit antibody was used as a secondary antibody. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen, Karlsruhe, Germany) or Yo-Pro-1 iodide (Invitrogen, Karlsruhe, Germany). Cells were observed by confocal laser scanning microscopy (CLSM), using a Leica TCS SP 2 spectral confocal and multiphoton inverted microscope (Leica, Heidelberg, Germany). Images were taken using a Plan-Apochromat 63×/1.32-numerical-aperture oil immersion objective, with fluorescence excitation at 488 nm (Ar laser), 543 nm (HeNe laser), and 633 nm (HeNe laser) and with an Enterprise II 351/364-nm DAPI laser (Coherent, Dieburg, Germany). Appropriate filters were selected for individual staining. Fluorescence analysis was carried out by Leica confocal software.
Cytokine enzyme-linked immunosorbent assay (ELISA).
For stimulation experiments, Mono Mac 6 cells were washed two times with PBS and seeded at 500 μl/well in 24-well plates at a density of 106 cells/ml. Mono Mac 6 cells were pulsed with each SitC-His protein as well as with Pam3Cys for 24 h at 37°C in a humidified 5% CO2 environment. MKs were seeded at 500 μl/well in 24-well plates at a density of 105 cells/ml. Cells were cultured in defined medium for 2 days at 37°C and 5% CO2. Subsequently, cells were transferred to a second medium and grown for 3 days before experiments were started. MKs were stimulated with different amounts of SitC-His, Pam3Cys, and LPS for 48 h.
The cell supernatants were collected and stored at −20°C. Human interleukin-6 (hIL-6), human tumor necrosis factor alpha (hTNF-α), and murine IL-6 (mIL-6) levels were determined by use of ELISA kits according to the manufacturer's instructions (eBioscience, San Diego, CA). The optical density was measured at 450 nm and 570 nm by a Tecan Infinite 200 reader (Tecan, Crailsheim, Germany).
Reporter assay of NF-κB activation.
Human embryonic kidney (HEK293) cells (ATCC, Manassas, VA) were cultured in DMEM supplemented with 2 mM l-glutamine and 10% fetal calf serum (all from Biochrom, Berlin, Germany). HEK293 and stably transfected HEK293-hTLR2 cells (Invivogen, San Diego, CA) were plated at 2 × 105 cells/well in 24-well plates 1 day before transfection. Cells were then transiently transfected by use of Lipofectamine 2000 transfection reagent (Invivogen, San Diego, CA) with 100 ng of an NF-κB reporter plasmid (pNFkB-TA-Luc; Clontech, BD Biosciences, Heidelberg, Germany) and 10 ng of a plasmid that directed expression of Renilla luciferase under the control of the constitutively active thymidine kinase promoter (pRL-TK; Promega). The pcDNA3.1 vector (Promega) was used to balance the transfected DNA concentration. Twenty-four hours after transfection, cells were stimulated with cell wall components in serum-free medium for 24 h, and luciferase activity was measured using a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions.
RESULTS
For the analysis of staphylococcal lipoprotein interactions with host receptors, C-terminally His-tagged SitC (Fig. 1A) was isolated from the membrane fraction of S. aureus clone SA113(pTX30SitC-his) and purified via Ni-NTA beads almost to homogeneity (Fig. 2). For fluorescence monitoring, SitC-His was labeled with Cy2 and then designated Cy2-SitC-His. As a nonlipidated control protein, we also isolated the cytoplasmic 46-kDa SHL lipase (Fig. 1B) from S. carnosus ΔRKET(pPSHG5ΩHis6mSHL), which was also purified by use of Ni-NTA beads (Fig. 2) and subsequently labeled with Cy5. The LPS contamination of both preparations was very low (0.005 EU), and none of the preparations caused a TLR4-dependent activation.
Cy2-SitC-His incorporates into MKs and elicits TLR2 accumulation.
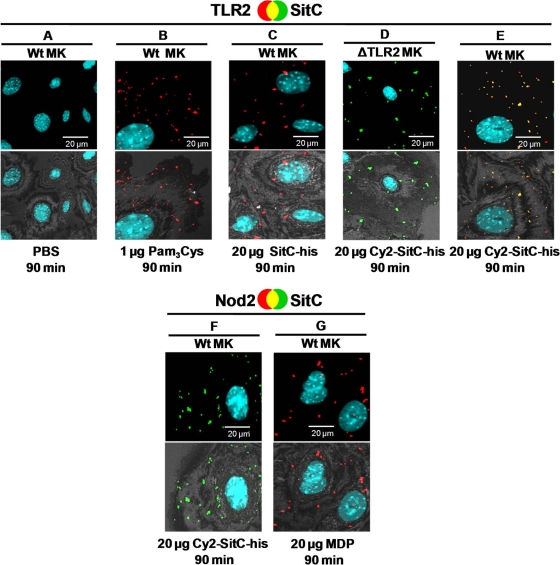
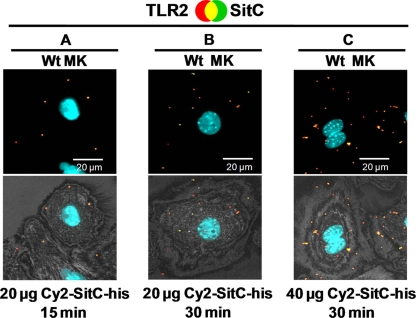
Although it was recently described that the S. aureus lipoprotein SitC has an immune-stimulatory activity toward TLR2 (27), internalization of SitC into host cells and colocalization with TLR2 have not yet been studied. To monitor the effect of SitC on TLR2 synthesis in MKs, these cells were stimulated with different amounts of Cy2-SitC-His (15, 20, and 40 μg, corresponding to 0.45, 0.6, and 1.2 nmol, respectively) over different periods (15, 30, 90, and 180 min) and analyzed by CLSM. As controls, the synthetic lipopeptide Pam3Cys and purified Cy5-SHL lipase were used. Cy2-SitC-His stimulated intracellular TLR2 production which was time (15, 30, and 90 min) and concentration (15 and 40 μg of Cy2-SitC-His) dependent (Fig. 3 and 4).
FIG. 3.
Cy2-SitC-His is incorporated into MKs and colocalizes with TLR2. Confocal images show primary MKs stained intracellularly with a TLR2 or Nod2 antibody from rabbits (detected by a Cy3-conjugated anti-rabbit antibody [red]). Nuclei were stained with DAPI (blue). SitC-His was labeled with Cy2 (Cy2-SitC-his; green). The upper panels show merged images; colocalization events are visualized in yellow. The lower images show an overlay of the fluorescence merge and the host cell, acquired in reflection mode of the confocal microscope. (A) PBS control. (B and C) TLR2 accumulated after stimulation with Pam3Cys (B) and with nonlabeled SitC-His (C). (D) Cy2-SitC-His was incorporated into TLR2-deficient MKs. No TLR2 was detectable in those cells. (E) TLR2 accumulated and colocalized with Cy2-SitC-His in wild-type MKs. (F) Internalized Cy2-SitC-His did not lead to accumulation of Nod2. (G) Stimulation with muramyl dipeptide (MDP) led to an accumulation of Nod2. Images of cells shown are representative of the cells observed in each dish and are representative of three experiments.
FIG. 4.
Cy2-SitC-His elicits TLR2 accumulation time and concentration dependently. For descriptions of the images, see the legend to Fig. 3. (A to C) Wild-type MKs were stimulated with different amounts of Cy2-SitC-His for 15 or 30 min. Cy2-SitC-His and TLR2 displayed strong colocalization in all tested experimental setups. Images of cells shown are representative of the cells observed in each dish and are representative of three experiments.
In nonstimulated MKs (PBS controls), no TLR2 was detectable (Fig. 3A). Stimulation of MKs with the synthetic lipopeptide Pam3Cys (Fig. 3B), which mimics triacylated lipoproteins, and with nonlabeled SitC-His (Fig. 3C) augmented intracellular TLR2 accumulation. Cy2-SitC-His was internalized into MKs (Fig. 3D and E) and elicited intracellular production and accumulation of TLR2 (Fig. 3F). After 90 min of stimulation, the TLR2 accumulation was significantly increased in comparison to that at 15 and 30 min of stimulation (Fig. 4A and B). Stimulation for over 90 min did not lead to further increases of detectable intracellular Cy2-SitC-His and TLR2 (data not shown). Furthermore, stimulation with 40 μg of Cy2-SitC-His induced a significantly stronger accumulation of TLR2 than did stimulation with 20 μg for the same amount of time (Fig. 4B and C).
Cy2-SitC-His was taken up by TLR2-deficient MKs, but TLR2 was not detected (Fig. 3D). Thus, the cell uptake of Cy2-SitC-His appeared not to be TLR2 dependent. Uptake of Cy2-SitC-His did not lead to Nod2 augmentation in MKs (Fig. 3F). Nod2 was detectable after stimulation with muramyl dipeptides (Fig. 3G).
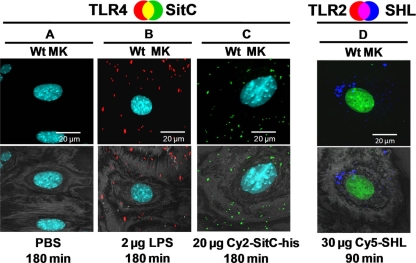
Cy2-SitC-His did not affect TLR4 (Fig. 5 A to C), a known LPS receptor (9, 17). These data demonstrate the specificity of our results. The occurrence of TLR2 was not enhanced after stimulation with Cy5-SHL, which contains no lipid modification (Fig. 5D).
FIG. 5.
Cy2-SitC-His does not elicit TLR4 accumulation and Cy5-SHL does not elicit TLR2 accumulation in MKs. Confocal images of MKs stained with a TLR4 antibody (A to C) or a TLR2 antibody (D) (detected by a Cy3-conjugated anti-rabbit antibody [red]) are shown. Nuclei were stained with DAPI (blue; A to C) or with Yo-Pro-1 (green; D). SitC-His was labeled with Cy2 (Cy2-SitC-his; green), and SHL was labeled with Cy5 (Cy5-SHL; blue). The upper panels show merged images. The lower images show an overlay of the fluorescence merge and the host cell, acquired in reflection mode of the confocal microscope at 488 nm. (A) PBS control. (B) Stimulation with LPS led to an accumulation of TLR4. (C) TLR4 was not detectable after stimulation with Cy2-SitC-His. (D) Cy5-SHL was taken up by MKs, but no TLR2 was detectable. Images of cells shown are representative of the cells observed in each dish and are representative of three experiments.
SitC-His colocalizes with TLR2 and mediates TLR2-dependent signaling.
Cy2-SitC-His not only induced intracellular TLR2 accumulation but also was internalized and colocalized with TLR2 (Fig. 3). This colocalization indicates an interaction of the SitC lipoprotein with TLR2, which is thought to be essential for triggering the innate immune response.
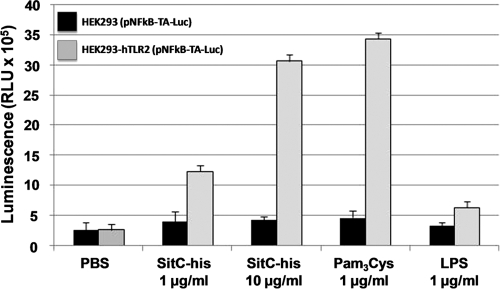
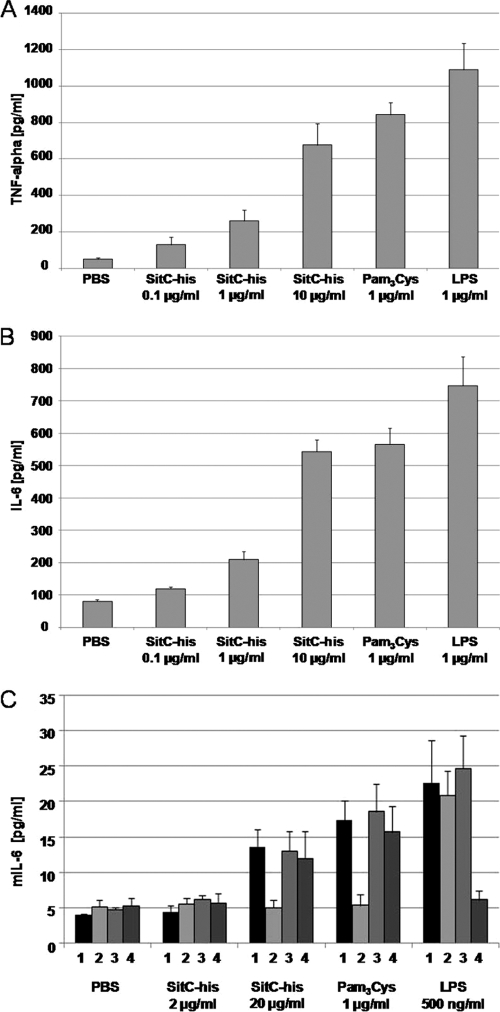
Staphylococcal SitC has been shown to induce a TLR2-dependent activation of the innate immune system (27, 43). This was confirmed here with an NF-κB luciferase reporter plasmid in TLR2- and/or Nod2-expressing HEK293 cells. Only TLR2-expressing HEK cells showed an increase in luciferase expression when stimulated with SitC-His or Pam3Cys; no stimulation was seen with LPS (Fig. 6). Stimulation with SitC-His resulted in proinflammatory cytokine production (TNF-α and IL-6), as investigated with the human monocytic cell line Mono Mac 6 (Fig. 7 A and B). SitC-His also induced the release of murine IL-6 in wild-type MKs, Nod2-deficient MKs, and TLR4-deficient MKs but not in TLR2-deficient MKs (Fig. 7C).
FIG. 6.
TLR2-dependent NF-κB activation by isolated SitC-His. A reporter assay was performed with NF-κB reporter plasmid (pNFkB-TA-Luc)-transfected HEK293 cells with hTLR2 (light gray bars) and without any receptors (black bars). Cells were stimulated with different amounts of isolated SitC-His and with Pam3Cys and LPS as controls. SitC-His showed a clearly TLR2-dependent activity. The data shown are means ± standard deviations (SD) for three independent experiments. RLU, relative light units.
FIG. 7.
SitC-His induces TLR2-dependent release of cytokines. Mono Mac 6 cells (A and B) and MKs (C) were stimulated with different amounts of SitC-His for 24 h. The concentrations of human tumor necrosis factor alpha (A), human interleukin-6 (B), and murine interleukin-6 (C) in the culture supernatants were measured using enzyme-linked immunosorbent assays. Bars: 1, wild-type MKs; 2, TLR2-deficient MKs; 3, Nod2-deficient MKs; 4, TLR4-deficient MKs. The data shown are means ± SD for three independent experiments.
Taken together, these data demonstrate that the S. aureus lipoprotein SitC-His is incorporated into MKs, provokes intracellular TLR2 accumulation, colocalizes with TLR2, and induces a TLR2-dependent immune response.
DISCUSSION
It is not trivial to unambiguously correlate pathogen-derived macromolecules with immune-stimulating activity. The problem is that macromolecule preparations such as murein, LTA, and wall teichoic acids are frequently contaminated with highly reactive pathogen-associated molecular patterns (PAMPs) such as LPS or lipopeptides. To rule out such contaminations, defined mutants are very helpful. It has been shown, for example, that cells and crude lysate of the S. aureus lgt mutant (nonlipidated prolipoprotein) induce much lower levels of proinflammatory cytokines than do those of the wild type (43), although the LTA content and structure are not altered (19). These results indicate that not LTA, as proclaimed in many publications, but lipoproteins are the major immune-stimulating players in S. aureus. Indeed, an LTA-deficient (ΔltaS) S. aureus mutant can activate TLR2 in vitro as well as the wild type does (27). It has long been known that bacterial lipoproteins lead to cell activation and apoptosis via TLR2 (3), and it is also well documented that S. aureus lipoprotein signaling is TLR2 specific (18, 27). Furthermore, lipoproteins contribute via TLR2 and MyD88 to pathogenesis in a sepsis model with C57BL/6 mice with IL-1β, chemokine-mediated inflammation, and large bacterial numbers (39). This shows that lipidation of prolipoprotein plays a crucial role in virulence, mainly because the lgt mutant, among other things, is impaired in iron acquisition and therefore attenuated in virulence.
Although it is well demonstrated that native lipoproteins such as SitC signal via TLR2, no colocalization studies of SitC with TLR2 in primary cells have been described. First, we confirmed with HEK293/hTLR2 as well as TLR2 knockout MKs that SitC-His induces TLR2-dependent activation of proinflammatory cytokines, corroborating the data reported recently for SitC in a CHO cell/hCD14/hTLR2-based assay (27). Next, we carried out colocalization studies with Cy2-SitC-His and MK cells isolated from C57BL/6 wild-type mice as well as Nod2- and TLR2-deficient mice. Four effects could be observed. First, Cy2-SitC-His colocalized only with TLR2, not with TLR4 or Nod2. Second, Cy2-SitC-His induced concentration- and time-dependent TLR2 production, but not TLR4 or Nod2 production, in wild-type MKs, supporting the notion that lipoproteins or synthetic lipopeptides are not recognized by Nod2, a peptidoglycan receptor (13, 21). Third, the majority of the SitC-His-induced TLR2 was intracellular and not at the cell surface. This finding is in agreement with recent studies of human keratinocytes investigated by flow cytometry demonstrating that TLR2 is expressed mainly intracellularly in these cells (6, 25). It has been shown that TLR2 is recruited to subcellular sites such as endolysosomal compartments (11, 23, 34, 36, 49). Fourth, Cy2-SitC-His was internalized readily in MKs or MM6 cells, in a TLR2-independent way. Our findings confirm the data of Shamsul and colleagues, who showed that the uptake of FSL-1, a known TLR2 ligand, is not dependent on the presence of TLR2 (41).
Stimulation of TLR2 in MK cells is accompanied by an increase of its transcription (25), and here we show that the increase of transcription correlated with an increase of TLR2, which reached a maximum after 90 min of stimulation; longer incubation times (180 min) (data not shown) did not lead to a further increase of mainly intracellular TLR2.
In nonstimulated MK cells, TLR2 was not detectable. In fact, low-level expression of TLR2 has been reported for nonstimulated human keratinocytes (25). Therefore, we assume that TLR2 is under the detection limit in nonstimulated MKs. The low-level occurrence of PRRs in nonstimulated MKs might serve as a regulating mechanism to avoid an overreaction of the immune system.
Where TLR2 is localized intracellularly is unknown, but most likely it is bound to the Golgi apparatus or the vesicular compartments of the endosomal pathway (11, 23, 34, 36, 41, 49). TLR4 has been described to cycle between the Golgi apparatus and the plasma membrane until engaged by LPS (29).
An interesting question is whether intracellular TLR2 can be activated by SitC or Pam3Cys. This would afford its interaction with MyD88, as the classical induction pathway is TLR2→MyD88→IRAK→TRAF6→TBK1→IKK→NF-κB (35, 38, 51), which leads to activation not only of proinflammatory genes but also of the TLR2 gene (51). Antibody binding studies revealed that TLR1, TLR2, TLR4, TLR5, and TLR6 are all localized to the plasma membrane, whereas TLR3, TLR7, TLR8, and TLR9 are preferentially expressed in intracellular compartments such as endosomes (44). Our impression is, however, that upon TLR2 stimulation, incorporation of TLR2 into the plasma membrane cannot keep up with the expression speed. The stimulation of innate immunity would be boosted enormously if in addition to the surface-bound TLR2, the intracellular TLR2 molecules also could be activated by lipoprotein. This would explain the dominant role of lipoproteins in activating the innate immune system.
On the other hand, too much TLR2 presentation on the MK cell surface might consistently lead to an excessive inflammatory response to skin-associated bacteria. In a recent study, it was suggested that intracellular TLR2 may function as a receptor for cell-invading pathogenic bacteria (34). Indeed, S. aureus is known to infiltrate various types of nonprofessional phagocytes, such as keratinocytes (30). However, what happens inside the host cells leaves many questions open. Rather before whole bacteria infiltrate the host cells, lipoproteins are internalized and can interact with intracellular TLR2. The main question, therefore, is whether intracellular TLR2 is able to induce the activation cascade.
As a positive control in our studies, we used Pam3Cys, a strong inducer of TLR2-mediated immune responses in host cells (42). But it should be mentioned here that apart from stimulation of the immune system, lipoproteins might also contribute to sepsis. Recently, it was described that Pam3CSK4 triggers eryptosis by stimulation of phosphatidylserine exposure, increased cytosolic Ca2+ activity, and moderately stimulated erythrocytic ceramide formation, which are major triggers of eryptosis (50). Thus, lipoprotein-dependent suicidal erythrocyte death may contribute to the pleiotropic effects of sepsis.
As a negative control, the well-studied Staphylococcus hyicus lipase (SHL lipase), a typical secreted and nonlipidated protein, was used (14, 15). The purified and Cy5-labeled SHL lipase was readily taken up by MK cells, indicating that lipidation is not necessary for incorporation. However, SHL lipase exerted no immune-stimulating activity. Another control was stimulation with LPS, which, as expected, stimulated only TLR4, not TLR2 (9, 47).
In conclusion, we showed here that the staphylococcal lipoprotein SitC specifically colocalizes with TLR2 and induces concentration- and time-dependent TLR2, but not TLR4 or Nod2, production in wild-type MKs, that the majority of induced TLR2 is intracellular and not membrane anchored, and finally, that SitC is readily internalized in MKs or MM6 cells, in a TLR2-independent way. Further studies are required to substantiate whether there is a cytoplasmic route of receptor activation that may boost the TLR2 response in mammalian cells.
Acknowledgments
This work was supported by the DFG (SFB 766) and the Infection Biology Graduate College (GKI 685). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 2 August 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Ajuwon, K. M., W. Banz, and T. A. Winters. 2009. Stimulation with peptidoglycan induces interleukin 6 and TLR2 expression and a concomitant downregulation of expression of adiponectin receptors 1 and 2 in 3T3-L1 adipocytes. J. Inflamm. (London) 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 4.Asong, J., M. A. Wolfert, K. K. Maiti, D. Miller, and G. J. Boons. 2009. Binding and cellular activation studies reveal that Toll-like receptor 2 can differentially recognize peptidoglycan from Gram-positive and Gram-negative bacteria. J. Biol. Chem. 284:8643-8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin, J., and F. Götz. 1990. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol. Lett. 54:203-207. [DOI] [PubMed] [Google Scholar]
- 6.Begon, É., L. Michel, B. Flageul, I. Beaudoin, F. Jean-Louis, H. Bachelez, L. Dubertret, and P. Musette. 2007. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur. J. Dermatol. 17:497-506. [DOI] [PubMed] [Google Scholar]
- 7.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 8.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2006. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J. Biol. Chem. 281:9049-9057. [DOI] [PubMed] [Google Scholar]
- 9.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 10.Cockayne, A., P. J. Hill, N. B. Powell, K. Bishop, C. Sims, and P. Williams. 1998. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect. Immun. 66:3767-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich, N., S. Lienenklaus, S. Weiss, and N. O. Gekara. 2010. Murine Toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One 5:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dziarski, R., and D. Gupta. 2005. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect. Immun. 73:5212-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 14.Götz, F., F. Popp, E. Korn, and K. H. Schleifer. 1985. Complete nucleotide sequence of the lipase gene from Staphylococcus hyicus cloned in Staphylococcus carnosus. Nucleic Acids Res. 13:5895-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Götz, F., H. M. Verheij, and R. Rosenstein. 1998. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem. Phys. Lipids 93:15-25. [DOI] [PubMed] [Google Scholar]
- 16.Hantke, K., and V. Braun. 1973. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur. J. Biochem. 34:284-296. [DOI] [PubMed] [Google Scholar]
- 17.Hao, H. N., J. D. Peduzzi-Nelson, P. J. VandeVord, K. Barami, S. P. DeSilva, D. Pelinkovic, and L. G. Morawa. 2009. Lipopolysaccharide-induced inflammatory cytokine production by Schwann's cells dependent upon TLR4 expression. J. Neuroimmunol. 212:26-34. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto, M., K. Tawaratsumida, H. Kariya, K. Aoyama, T. Tamura, and Y. Suda. 2006. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int. Immunol. 18:355-362. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto, M., K. Tawaratsumida, H. Kariya, A. Kiyohara, Y. Suda, F. Krikae, T. Kirikae, and F. Götz. 2006. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 177:3162-3169. [DOI] [PubMed] [Google Scholar]
- 20.Hussain, T., N. Nasreen, Y. Lai, B. F. Bellew, V. B. Antony, and K. A. Mohammed. 2008. Innate immune responses in murine pleural mesothelial cells: Toll-like receptor-2 dependent induction of beta-defensin-2 by staphylococcal peptidoglycan. Am. J. Physiol. Lung Cell. Mol. Physiol. 295:L461-L470. [DOI] [PubMed] [Google Scholar]
- 21.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 22.Iordanescu, S., and M. Surdeanu. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277-281. [DOI] [PubMed] [Google Scholar]
- 23.Ip, W. K., K. Takahashi, K. J. Moore, L. M. Stuart, and R. A. B. Ezekowitz. 2008. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J. Exp. Med. 205:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin, M. S., S. E. Kim, J. Y. Heo, M. E. Lee, H. M. Kim, S. G. Paik, H. Lee, and J. O. Lee. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071-1082. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi, M., R. Yoshiki, J. Sakabe, K. Kabashima, M. Nakamura, and Y. Tokura. 2009. Expression of Toll-like receptor 2, NOD2 and dectin-1 and stimulatory effects of their ligands and histamine in normal human keratinocytes. Br. J. Dermatol. 160:297-304. [DOI] [PubMed] [Google Scholar]
- 26.Krismer, B. A. 1999. Studium der Funktion der Sekretierten Proteine SceA und SceB, Analyse des Galaktoseoperons galRKET und Konstruktion von Sekretions- und Expressionsvektoren in Staphylococcus carnosus. Ph.D. thesis. University of Tübingen, Tübingen, Germany.
- 27.Kurokawa, K., H. Lee, K. B. Roh, M. Asanuma, Y. S. Kim, H. Nakayama, A. Shiratsuchi, Y. Choi, O. Takeuchi, H. J. Kang, N. Dohmae, Y. Nakanishi, S. Akira, K. Sekimizu, and B. L. Lee. 2009. The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for Toll-like receptor 2. J. Biol. Chem. 284:8406-8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madan Babu, M., and K. Sankaran. 2002. DOLOP—database of bacterial lipoproteins. Bioinformatics 18:641-643. [DOI] [PubMed] [Google Scholar]
- 29.McGettrick, A. F., and L. A. O'Neill. 2010. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 22:20-27. [DOI] [PubMed] [Google Scholar]
- 30.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Weidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943-951. [DOI] [PubMed] [Google Scholar]
- 31.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullaly, S. C., and P. Kubes. 2006. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J. Immunol. 177:8154-8163. [DOI] [PubMed] [Google Scholar]
- 33.Natsuka, M., A. Uehara, S. Yang, S. Echigo, and H. Takada. 2008. A polymer-type water-soluble peptidoglycan exhibited both Toll-like receptor 2- and NOD2-agonistic activities, resulting in synergistic activation of human monocytic cells. Innate Immun. 14:298-308. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell, C. M., I. A. Ionova, A. J. Quayle, A. Visintin, and R. R. Ingalls. 2006. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J. Biol. Chem. 281:1652-1659. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill, L. A. J. 2008. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Rev. 226:10-18. [DOI] [PubMed] [Google Scholar]
- 36.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peschel, A., B. Ottenwalder, and F. Gotz. 1996. Inducible production and cellular location of the epidermin biosynthetic enzyme EpiB using an improved staphylococcal expression system. FEMS Microbiol. Lett. 137:279-284. [DOI] [PubMed] [Google Scholar]
- 38.Sato, S., H. Sanjo, K. Takeda, J. Ninomiya-Tsuji, M. Yamamoto, T. Kawai, K. Matsumoto, O. Takeuchi, and S. Akira. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6:1087-1095. [DOI] [PubMed] [Google Scholar]
- 39.Schmaler, M., N. J. Jann, F. Ferracin, L. Z. Landolt, L. Biswas, F. Götz, and R. Landmann. 2009. Lipoproteins in Staphylococcus aureus mediate inflammation by TLR2 and iron-dependent growth in vivo. J. Immunol. 182:7110-7118. [DOI] [PubMed] [Google Scholar]
- 40.Schröder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zähringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 41.Shamsul, H. M., A. Hasebe, M. Iyori, M. Ohtani, K. Kiura, D. Zhang, Y. Totsuka, and K. Shibata. 2010. The Toll-like receptor 2 (TLR2) ligand FSL-1 is internalized via the clathrin-dependent endocytic pathway triggered by CD14 and CD36 but not by TLR2. Immunology 130:262-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spohn, R., U. Buwitt-Beckmann, R. Brock, G. Jung, A. J. Ulmer, and K. H. Wiesmuller. 2004. Synthetic lipopeptide adjuvants and Toll-like receptor 2—structure-activity relationships. Vaccine 22:2494-2499. [DOI] [PubMed] [Google Scholar]
- 43.Stoll, H., J. Dengjel, C. Nerz, and F. Götz. 2005. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 73:2411-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 45.Takeda, K., and S. Akira. 2003. Toll receptors and pathogen resistance. Cell. Microbiol. 5:143-153. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 48.Tawaratsumida, K., M. Furuyashiki, M. Katsumoto, Y. Fujimoto, K. Fukase, Y. Suda, and M. Hashimoto. 2009. Characterization of N-terminal structure of TLR2-activating lipoprotein in Staphylococcus aureus. J. Biol. Chem. 284:9147-9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 402:39-43. [DOI] [PubMed] [Google Scholar]
- 50.Wang, K., H. Mahmud, M. Foller, R. Biswas, K. S. Lang, E. Bohn, F. Gotz, and F. Lang. 2008. Lipopeptides in the triggering of erythrocyte cell membrane scrambling. Cell. Physiol. Biochem. 22:381-386. [DOI] [PubMed] [Google Scholar]
- 51.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-kappaB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zähringer, U., B. Lindner, S. Inamura, H. Heine, and C. Alexander. 2008. TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205-224. [DOI] [PubMed] [Google Scholar]