Abstract
Despite significant advances in antiviral treatment, solid organ transplant (SOT) recipients remain at heightened risk for developing late cytomegalovirus (CMV) disease. Elevated inhibitory immune signaling suggestsa state of immune impairment in SOT recipients, who do not control CMV infection and develop severe clinical symptoms after discontinuation of antiviral prophylaxis. We longitudinally monitored the negative immune modulator programmed death (PD)-1 receptor on both CD4 and CD8 T cells, co-expressing the CD137 surface marker of recent activation, in a liver transplant cohort. Liver recipients who progressed to CMV disease expressed elevated levels of PD-1 on CD137+ CD4 and CD8 T cells, following stimulation with either full-length peptide libraries or CMV lysate. This novel approach, applicable to a multitude of human leukocyte antigen types, enhances the usefulness of the PD-1 measurements, as a clinical strategy to predict late CMV disease. In parallel, we detected an increased level of the immunosuppressive cytokine interleukin (IL)-10, in plasma of liver recipients diagnosed with CMV disease. CMV-specific T cells were still functional when both PD-1 and IL-10 were up-regulated; however they showed a marked proliferation deficit, which may limit their ability to contain viremia and lead to CMV disease. Our preliminary observations support further investigation of dual monitoring of PD-1 and IL-10, as potential immune markers of CMV disease.
Keywords: programmed death-1 receptor, interleukin-10, liver transplant, late cytomegalovirus disease, solid organ transplant, CMV, PD-1, IL-10, CD137
Cytomegalovirus (CMV) is the single most important viral pathogen that affects recovery of immunosuppressed transplant recipients (1). Antiviral prophylaxis for 3 months post transplant, used by ~90% of American solid organ transplant (SOT) centers, reduces the incidence of CMV disease to <5% in CMV-seropositive SOT recipients (R+). In contrast, unacceptably high rates of CMV disease (~30%) affect CMV-seronegative recipients (R−) of transplant from CMV \-seropositive donors (D+), presumably as a result of inadequate CMV-specific cellular immunity.
Defining the qualitative and quantitative aspects of CMV-specific immune responses that protect against clinical CMV symptoms is critical to identify patients requiring enhanced prevention strategies, and devise novel treatments to reduce the incidence and/or the impact of this life-threatening transplant complication. CMV-specific T-cell levels after SOT can be indicative of long-term susceptibility to CMV infection (2, 3). A number of functional bio-markers have been associated with episodes of reactivated CMV infection, correlate with high CMV viremia risk profiling, and can be clinically useful to identify SOT patients at higher risk of CMV replication (4–6). T-cell lytic function, the ability to produce interferon-gamma (IFN-γ) in response to rising CMV viremia, maturation, and effector function surface markers, was an insufficient indicator of virus-associated disease activity in D+/R− liver recipients (7, 8). In contrast, elevated PD-1 levels expressed on CMV-specific tetramer+ CD8+ T cells were associated with risk of disease in the same SOT cohort; this finding suggested a promising predictive tool for PD-1 as an immune parameter (9).
Based on our findings and those of others (9, 10), we hypothesized that up-regulation of inhibitory immune signaling indicates a state of immune impairment, which may lead to CMV disease. Characterizing the post-transplant time course of immune-suppressive factors can lay the ground work for a strategy to block negative immune modulators, restoring protective CMV immunity in SOT recipients.
In the present work we expanded the clinical applicability of PD-1 immune-monitoring. We used a non-human leukocyte antigen (HLA) restricted approach, which tracks the CD137 surface-activation marker for measuring PD-1 levels, on both CMV-specific CD4 and CD8 T cells (11). Since increased production of the immunosuppressive cytokine interleukin (IL)-10 has been associated with persistent viral infections (12), we longitudinally investigated post-transplant levels of IL-10 in plasma of SOT patients symptomatic for CMV, as a possible further marker of immune dysregulation during CMV disease. Finally, we measured the proliferative capacity of CMV-specific T cells in transplant recipients developing CMV disease, to assess whether upregulation of inhibitory immune signaling impacts the expansion of the anti-CMV T-cell population (10, 12).
Materials and methods
Subjects
A total of 17 D+/R− liver transplant patients were enrolled at University of Washington Medical Center, after signing informed consent. Peripheral blood mononuclear cells (PBMC) from all blood draws were stored at the City of Hope, Division of Translational Vaccine Research. Patients received 3-month prophylaxis with valganciclovir (Valcyte™, Roche, Nutley, New Jersey USA) and belong to the same SOT cohort analyzed in previously published reports by our group, using different strategies (7–9). Based on timing and frequency of CMV disease in D+/R− liver transplant cohorts (13), blood specimens were longitudinally collected 3–12 months post transplant (twice a month between 3 and 6 months, once afterwards).
Cell stimulation and surface marker staining
PBMC were stimulated for 1½ h with 1 µg/mL of pp65 peptide library (JPT, Germany) at a concentration of 106 cells/100 µL, in serum-free Royal Park Memorial Institute (RPMI) medium (8). Cells were then brought to a concentration of 106/mL in 5% fetal bovine serum (FBS) RPMI medium and incubated for additional 23 h. When CMV lysate (Microbix Biosystems Inc., Toronto, Ontario, Canada) was used for stimulation, 106 PBMC/mL were incubated at 37°C for 24 h with 10 µg/mL lysate, in RPMI containing 5% FBS serum. Following either antigen stimulation (no co-stimulatory antibodies were added), cells were stained with antibodies anti-CD8, CD4, CD137, and PD-1 and analyzed by FACSCanto flow cytometry system using FACSDiva software (all from BD Biosciences, San Jose, Calfornia USA) (8, 9). PD-1 values >105.5 mean fluorescence intensity (MFI) were considered elevated, based on an estimated upper 99th percentile for the PD-1 levels of healthy volunteers and liver recipients, at asymptomatic time points (9).
Cytometric bead array (CBA)
CBA was performed using the Human Th1/Th2 Cytokine Kit II (BD Biosciences, San Jose, California USA) to quantitatively measure IL-2, IL-4, IL-6, IL-10, tumor necroisis factor (TNF), IFN-γ protein levels in serum samples. The anti-IL-10 antibody used in this report recognizes endogenous as well as viral IL-10 (14).
Proliferation assay
Proliferation was analyzed using the carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution method (Molecular Probes, Carlsbad, California USA). To evaluate CD4 T-cell proliferation, PBMC were CFSE labeled and incubated for 5 days with CMV lysate (32 µL/mL) (10) or uninfected MRC-5 fibroblasts (Microbix Biosystems, Toronto, Canada) as control. Cells were then washed and co-stained with anti-CD4 for tracking cell generation, by FACS analysis of CFSE fluorescence dilution. To assess CD8 T-cell proliferation, PBMC were labeled with CFSE and incubated for 6 days with 1 µg/mL pp65 library or dimethylsulfoxide (DMSO) diluent as control, in the presence of anti-CD49b and CD28 (1 µg/mL) (10). Cells were then washed and co-stained with anti-CD8 (all antibodies were from BD Biosciences) and FACS analyzed for CFSE fluorescence levels.
Statistical methods
Non-parametric (two-tailed) Mann-Whitney test was calculated using GraphPadPrism software (GraphPad, San Diego, California, USA). Association analyses and Student T test were performed using SigmaPlot 10 (Systat Software Inc., Chicago, Illinois USA). The p values are indicated for each statistical analysis.
Results
CD137 levels in T cells activated by CMV peptide library and lysate
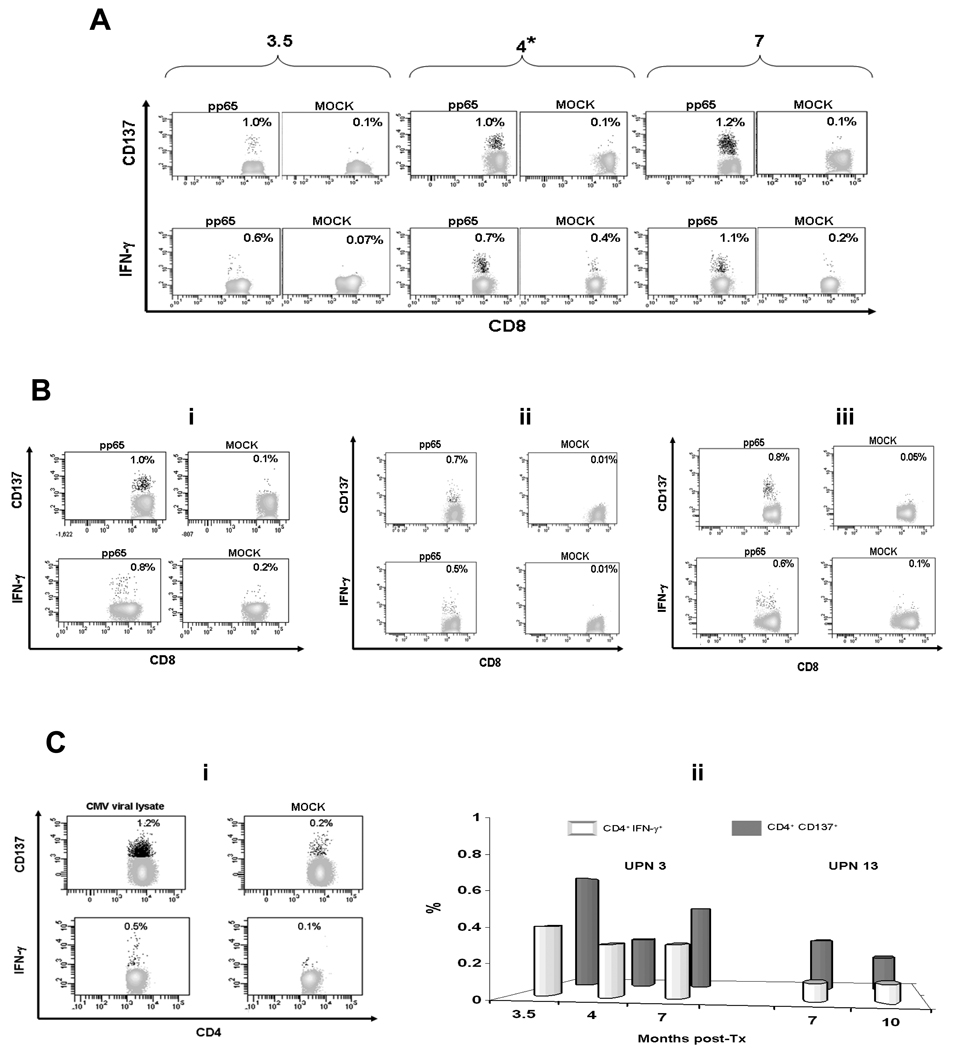
CD137 is a surface marker of recently activated T cells, whose expression correlates with functional activation of CD8 T cells (11). We performed a pilot study to compare IFN-γ production and CD137 expression levels in T cells stimulated either with a CMV-specific library or lysate from a SOT liver cohort. For each patient, selected time points were evaluated, in which measurable levels of IFN-γ were previously detected (8). A CMV-specific peptide library and a CMV viral lysate were used to activate T cells in the liver recipients tested. From our results, CD137 surface marker is consistently activated on both CD8 and CD4 T cells after 24 h stimulation with the pp65 library (Fig. 1 A and B upper panels, and C ii grey bars), and on CD4 T cells, following 24 h incubation with the CMV lysate (Fig. 1 Ci upper panel). ICS assays performed in parallel using the same CMV antigens (8) showed levels of T cell IFN-γ production (Fig. 1 A, B, Ci lower panels, and Cii white bars) that trended significantly lower (Mann-Whitney test p=0.0024) than those detected by monitoring CD137 surface marker.
Fig. 1. CD137 expression and interferon-gamma (IFN-γ) production.
CD137 surface staining analyses and the IFN-γ production detected by ICS assays (on fixed cells) were performed separately for each patient/time point. (A) 3.5, 4 (*symbol indicates cytomegalovirus [CMV] disease diagnosis), and 7 months post transplant (as shown on the plot tops) peripheral blood mononuclear cells (PBMC) from CMV symptomatic UPN 3 were stimulated with 1 µg/mL of pp65 library to measure CD137 cell surface expression (upper panel) and intracellular IFN-γ production (detected by ICS, lower panel) on CD8 T-cells. In (B), asymptomatic patients UPN 4, tested at 6 months post-transplant (i); UPN 6, tested at 5.5 months post-transplant; (ii) UPN 12, tested at 5 months post-transplant (iii) are shown. PBMC were stimulated and analyzed as described in A. (Ci) 8 months post transplant (asymptomatic time point) PBMC from CMV symptomatic UPN 8 are stimulated using 10 µg/mL of CMV lysate to detect CD137 expression and IFN-γ production (by the method used in A) on CD4 T-cells. (Cii) PBMC of disease patients UPN 3 and 13 (as reported on the bar tops) were stimulated at the post-transplant time points indicated on the x axes, using the pp65 library to measure the percentages of CD4+ T-cells expressing the CD137 surface marker (grey bars) or producing IFN-γ (detected by ICS, white bars). FITC conjugated antibodies were used for CD8 and CD4 T-cells, and APC conjugates for CD137 and IFN-γ. In MOCK plots, peptide library diluent (DMSO) was added in A, and uninfected MRC-5 lysate, in Ci. Numbers inside the plots indicate percentages of CD8 (A and B) or CD4 (Ci) T-cells expressing CD137 (upper panels) or producing IFN-γ (lower panels). In Cii, background activities to dimethylsulfoxide (DMSO), used as control, were subtracted. Events acquired for UPN 3 at 3.5 months were 100,000 (due to shortage of cells at this time point), while ranged 300 to 400,000 in the other cases.
Measurements of PD-1 levels using peptide libraries
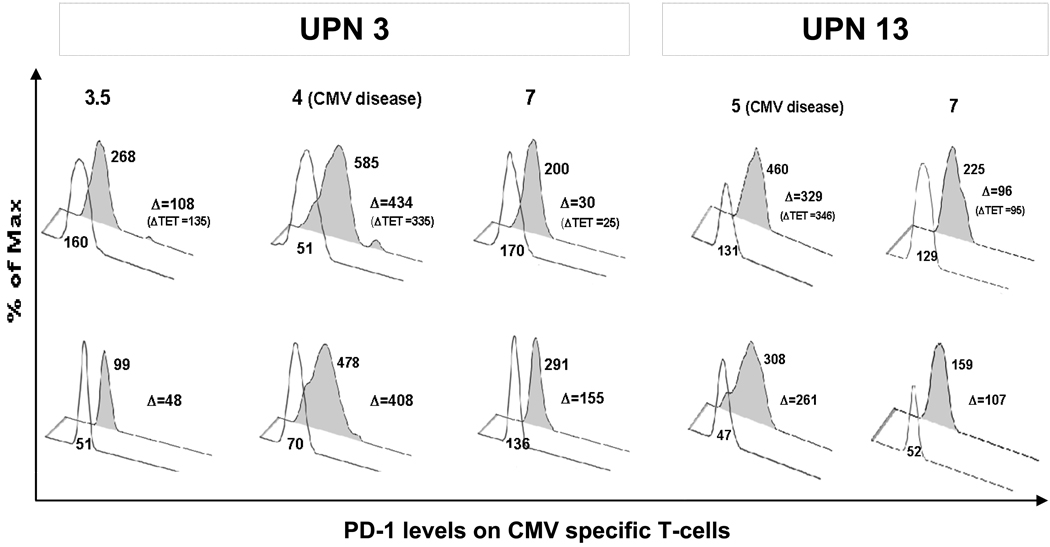
Based on the CD137 monitoring results, we assessed whether PD-1 levels could be simultaneously measured on CD137+ CD8+ and CD4+ CMV-specific T cells. As shown in Figure 2, coupling CD137 and PD-1 specific measurements was successfully tested in liver transplant CMV disease patients (8), evaluated at asymptomatic and CMV disease time points. The offset histogram plots show that PD-1 expression on pp65 library specific CD137+ CD8+ T cells (Fig. 2, upper panel) was elevated before disease (UPN 3) and at time of disease (UPN 3 and 13). Importantly, the MFI values we got measuring PD-1 either on CMV-specific class I tetramer cells or on CD137 positive T cells stimulated with a CMV specific library are similar to what found using CMV-specific major histocompatibility (MHC) Class I tetramers (9). Additionally, PD-1 measured on CD137+ CD4+ T cells specific for the pp65 library (Fig. 2, lower panel) showed that the PD-1 upregulation involves also the CD4 compartment, and that levels of CD4 PD-1 levels measured at disease time points are significantly higher than those measured at asymptomatic time points (Student T test p=0.04) (9, 10). Our preliminary data point to the feasibility of combined measurements of CD137 and PD-1 on CMV specific T cells.
Fig. 2. Expression of programmed death (PD)-1 on CD137+ T cells.
Peripheral blood mononuclear cells (PBMC) from representative cytmegalovirus (CMV) disease patients UPN 3 and UPN 13 were stimulated for 24 h with 1 µg/mL of pp65 library and then stained for CD8, CD4, CD137 (as in 1A) and PD-1 (PE-conjugated). Grey-filled histograms represent mean fluorescence intensity (MFI) of PD-1 expressed on CD137+ CD8+ T cells (upper panel) or on CD137+ CD4+ T cells (lower panel). A PD-1 matching isotype control was used in the transparent offset histograms. Δ indicates the MFI difference after subtracting the isotype control MFI. In parenthesis, the Δ MFI values of PD-1 measured on CMV-specific major histocompatibility Class I tetramers are reported (9). On the histogram tops are indicated the post-transplant month tested. Time points of CMV disease diagnosis are 4 months post-transplant for UPN 3, and 5 months post-transplant for UPN 13.
Analyses of soluble IL-10 in CMV disease patients
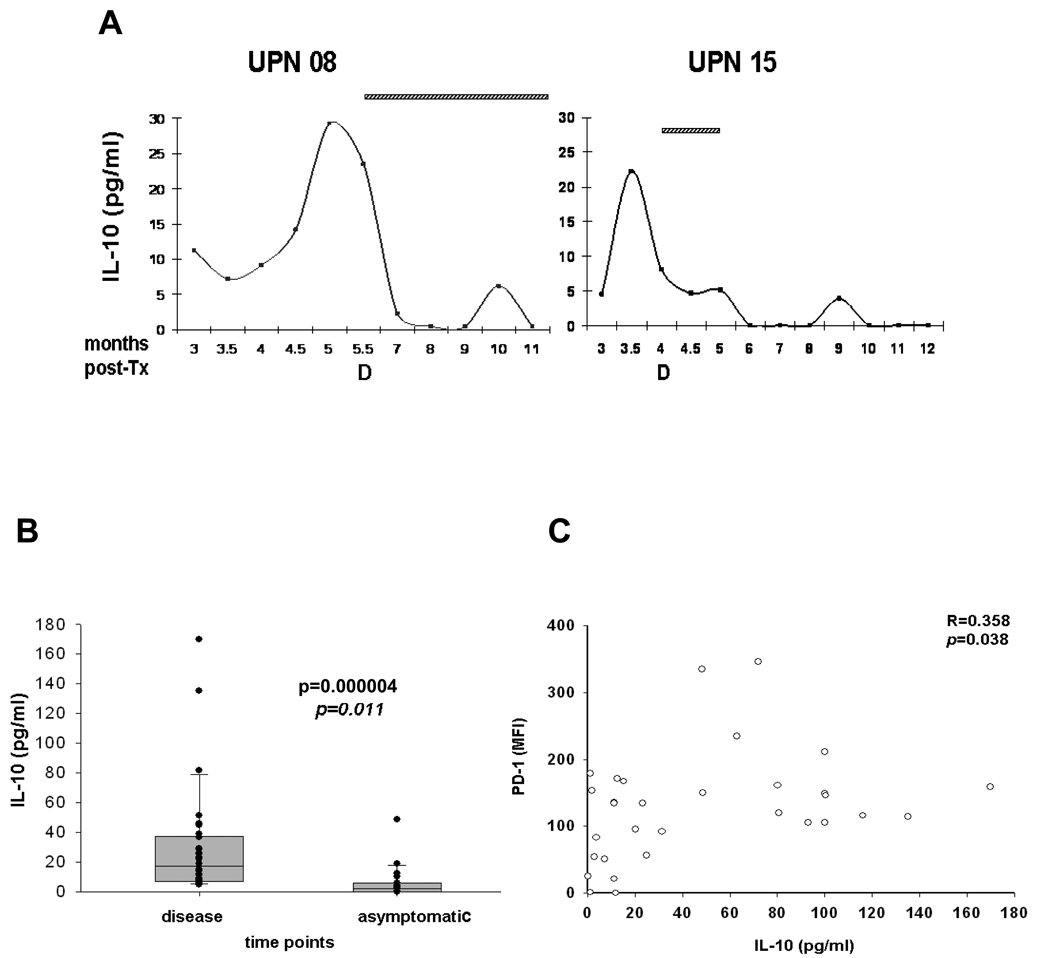
To address whether at time of CMV disease SOT recipients had an altered profile of plasma cytokines, we longitudinally tested the levels of soluble cytokines in the recipients who progressed to CMV disease in our liver transplant cohort (8, 9). By using a CBA kit, Th1/Th2 cytokines were quantitatively measured. IL-2, IL-4, IL-6, TNF, IFN-γ cytokine patterns varied greatly among SOT subjects, and their levels were neither associated with viremia nor with CMV disease (data not shown). In contrast, pronounced peaks of IL-10 were consistently found in all recipients who developed CMV disease (Fig. 3A). High levels of IL-10 were present before CMV disease, when CMV was diagnosed and also during intravenous ganciclovir administration (Fig. 3A). IL-10 was significantly lower (Mann-Whitney test p=0.000004) at asymptomatic time points (Fig. 3B), mirroring our PD-1 findings (9). Moreover, there was a moderate association (regression analysis: R=0.358 and p=0.038) between PD-1 expression and IL-10 plasma production (Fig. 3C). Our results indicate that dysregulated levels of both IL-10 and PD-1 may play a dual role in the immune impairment of SOT recipients, who fail to contain a primary CMV infection and progress to CMV disease (12).
Fig. 3. Soluble levels of plasma interleukin (IL)-10 at asymptomatic vs. cytomegalovirus (CMV) disease time points.
Levels of IL-10 were longitudinally monitored in plasma of CMV symptomatic liver transplant UPN 3, 8, 9, 13, 15 and 18 by CBA. (A) IL-10 levels (pg/mL, y axes) measured in representative UPN 8 and 15 are reported. The x-axes of plots show the post-transplant month in which the measurement was performed. Horizontal pattern bars indicate ganciclovir (GCV) treatment at time of disease (as D, on x axes). (B) Vertical box plots show median, 25th and 75th percentiles, and all values (filled circles) measured for the two groups indicated. The p value indicates the significance of the difference between groups. Observations listed in disease time points comprise IL-10 levels measured immediately preceding CMV disease, during CMV symptomatology and GCV treatment; in the asymptomatic time points are listed IL-10 levels measured at CMV disease-free time points (excluding the last measurement before CMV disease), and when anti-viral therapy was not administered. (C) The dot plot shows the association between PD-1 mean fluorescence intensity (MFI) levels on CMV-specific CD8+ T-cells (y axes) and IL-10 (pg/mL) plasma levels (x axes). The coefficient of correlation (R) and significance (p) of the association are reported on the upper right side.
Quantitation of CMV-specific proliferation
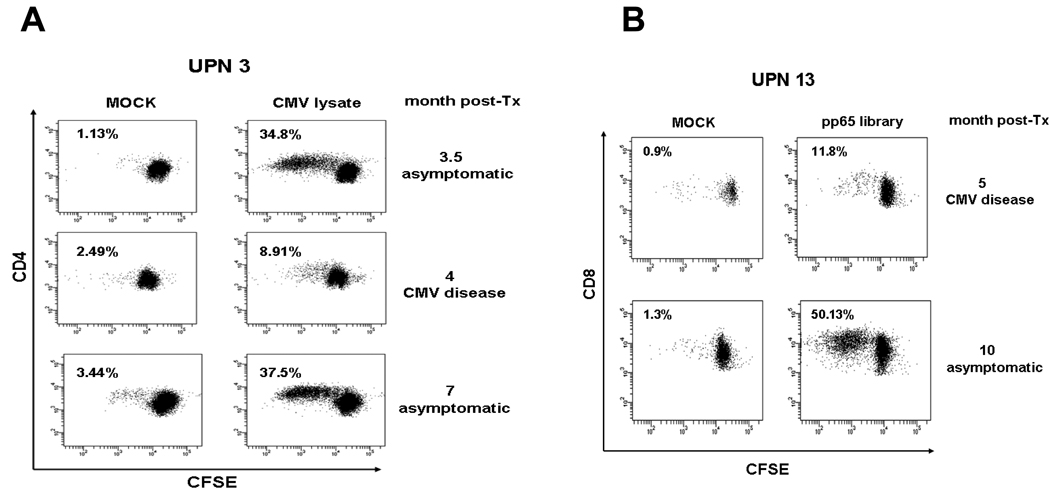
We assessed the capability of their CD4 and CD8 T cells to proliferate, upon stimulation with CMV lysate or pp65 peptide library. The analysis of cell growth kinetics was performed using the CFSE dilution method for cell division tracking (8, 12). We found that at time of CMV disease diagnosis (Fig. 4), liver recipients showed a marked proliferation deficit for both CD4 and CD8 T cells specific for CMV lysate and pp65 library respectively. In contrast, pre- and post-disease time points (Fig. 4) showed higher lymphoproliferative profiles (Student T test p=0.01). Importantly, parallel PD-1 and IL-10 up-regulation was detected at time of CMV disease (Figs. 2 and 3). Our preliminary data from CMV disease liver recipients suggest that high levels of IL-10 and PD-1 may contribute to diminish cell proliferation in both CMV-specific CD8 and CD4 T-cell compartments.
Fig. 4. Proliferation of cytomegalovirus (CMV)-specific CD8 and CD4 T cells.
(A) Peripheral blood mononuclear cells (PBMC) from CMV disease UPN 3 were labeled with carboxyfluorescein diacetate succinimidyl (CFSE) and incubated for 5 days with CMV lysate (32 µL/mL) or uninfected MRC-5 fibroblasts (MOCK), as indicated on the top plots. Cells were washed and co-stained with CD4 APC for tracking cell generation, by FACS analysis of CFSE fluorescence dilution, at the time points shown on the right margins (month post-transplant: 4, CMV disease; 3.5 and 7, asymptomatic). The number inside the plots indicates the percentage of divided CD4 T-cells (%Divided, is the percentage of the CD4 T-cells which proliferated). (B) PBMC from UPN 13 CMV-disease patient were stimulated with 1 µg/mL pp65 library (as shown on top plots) or dimethylsulfoxide (DMSO) peptide library diluent as control (MOCK), in the presence of co-stimulatory CD49b and CD28 molecules (1 µg/mL) for 6 days. Cells were then washed and co-stained with CD8 APC, and %Divided CD8 T-cells were calculated as in A. CMV disease and asymptomatic time points (respectively 5 and 10 months post-transplant) tested are shown on the plot right margin.
Discussion
Elevated levels of the negative immune-modulator PD-1, measured on CMV-specific and MHC Class I tetramer+ CD8 T cells, have been shown to be significantly associated with CMV disease and viremia in solid organ liver recipients (9). Because CMV epitopes have not been identified for all HLA alleles, usage of this tetramer-based protocol has limitations for clinical application (15). Importantly, PD-1 measurements conducted on both CD4 and CD8 T cells need to be included, since elevated levels have been detected on CMV-specific CD4 T cells from a cohort of kidney transplant patients (10).
Towards these aims, we explored whether PD-1 upregulation could be measured on CMV-specifically stimulated T cells, by monitoring CD137. CD137 (4–1BB) molecule, expressed on activated T cells, has been used to detect antigen-specific T cells (11). The published data from Greenberg only apply to antigen-specific T cells against a single epitope (11). In our results (Fig. 1) we have shown a significant advance for a broader use of this technique: the CD137 assay works consistently also with a CMV peptide library and the CMV lysate, extending its usefulness to a multitude of HLA types. Interestingly, monitoring IFN-γ and CD137 in parallel showed that levels of CMV-specific T cells producing IFN-γ were lower than those expressing the CD137 activation marker (Fig. 1). Our novel, but limited data support the concept that T-cell responses against CMV may not be fully characterized using a single cytokine functional assay, while an antigen specific activation marker, such as CD137, which is associated with multiple T-cell functions, may provide an enhanced tool for CMV immune-monitoring in the SOT setting (11, 16). Further studies are warranted to establish whether CD137 levels correlate with declining viremia or protection from CMV disease.
The CD137 results obtained in liver recipients laid the ground work for the development of a novel strategy, in which PD-1 levels were measured on CD137+ CD8+ and CD4+ CMV-specific T cells, upon stimulation with the pp65 peptide library and the CMV lysate (Fig. 2). This convenient technique provides an enhancement for clinical application of PD-1, as a tool for early detection of CMV disease: by using peptide libraries instead of epitopes (9), the PD-1 monitoring can be broadly performed, regardless of the recipient’s HLA type. Investigations using a panel of multiple immune-dominant CMV antigens during stimulation and surface memory markers are critical to determine the extent of the PD-1 up-regulation and the maturation state of CMV-specific T cells in SOT recipients who develop CMV disease (9).
IL-10 is an immune-modulatory cytokine that suppresses cellular immune responses by altering the function of T cells and antigen-presenting cells. Elevated levels of IL-10 production have been associated with persistent infection by hepatitis B virus, hepatitis C virus, human immunodeficiency virus, and Epstein-Barr virus (reviewed in [17]). In the case of CMV, the virus encodes an host cytokine homolog IL-10, which has been found to suppress cytokine production, inhibit in vitro PBMC proliferation, and likely contribute to immune evasion during viral infection (14). Recently, a dysregulated cytokine response has been reported during CMV infection in renal transplant recipients. Levels of endogenous IL-10 were found to be significantly enhanced during CMV viremia (18). In our study, in which both human and viral IL-10 were measured, IL-10 levels were significantly elevated at time of CMV disease in liver recipients, markedly declined at asymptomatic time points and were associated with PD-1 levels (Fig. 3). In agreement with previous findings, our results support the hypothesis the IL-10 might be involved in CMV activation and uncontrolled replication in SOT patients (18).
To evaluate the impact of elevated PD-1 and IL-10 on the proliferation of CMV-specific T cells, we assessed CD4 and CD8 T-cell expansion levels in SOT patients with CMV disease (8, 12). A significantly diminished proliferation for both CMV-specific and CD4 and CD8 T cells characterized CMV disease time points, while vigorous expansions were detected at asymptomatic time points (Fig. 4). Importantly, at time of reduced CMV specific proliferation, substantial PD-1 upregulation was expressed on CMV-specific T cells, and high levels of IL-10 were detected in plasma (Figs. 2 and 3). In contrast, T-cell function, measured as IFN-γ production, did not seem impaired (compare Figs. 1, 2, and 3) (12). Our results suggest that a dual strategy to block both PD-1 and/or IL-10 pathways may restore the proliferative capacity of anti-CMV T cells, enhancing their ability to contain CMV viremia and clinical complications (12, 19, 20). In vitro blocking studies are indicated to lay the ground work for an innovative therapeutic strategy, focused on reducing the incidence of life-threatening CMV disease, in high-risk SOT recipients.
Acknowledgments
We are grateful to Prof. Jeff Longmate (Department of Biostatistics, City of Hope) for advices with the statistical analysis. We acknowledge the administrative assistance of Denise Marsano and secretarial support of Donna Packer.
These studies were partially supported by grants from the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III) to D.J.D.; from the National Institute of Health to SFL (R21 DK077374), C.L.R. and A.P.L. (R21 AI084019) from NCI (CA33572) to The City of Hope Comprehensive Cancer Center, and by The Edwin and Bea Wolfe Charitable Foundation to the Division of Translational Vaccine Research.
Footnotes
The authors declare no competing financial interests.
References
- 1.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006. 2006;81(12):1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 2.Sester U, Gartner BC, Wilkens H, et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am J Transplant. 2005;5(6):1483–1489. doi: 10.1111/j.1600-6143.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 3.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201(7):1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerna G, Lilleri D, Fornara C, et al. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am J Transplant. 2006;6(10):2356–2364. doi: 10.1111/j.1600-6143.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 5.Lilleri D, Zelini P, Fornara C, Comolli G, Gerna G. Inconsistent responses of cytomegalovirus-specific T cells to pp65 and IE-1 versus infected dendritic cells in organ transplant recipients. Am J Transplant. 2007;7(8):1997–2005. doi: 10.1111/j.1600-6143.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 6.Egli A, Binet I, Binggeli S, et al. Cytomegalovirus-specific T-cell responses and viral replication in kidney transplant recipients. J Transl Med. 2008;6:29. doi: 10.1186/1479-5876-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacey SF, La Rosa C, Zhou W, et al. Functional comparison of T cells recognizing cytomegalovirus pp65 and intermediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J Infect Dis. 2006;194(10):1410–1421. doi: 10.1086/508495. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195(5):633–644. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 9.La Rosa C, Krishnan A, Longmate J, et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197(1):25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 10.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8(7):1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 11.Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limaye AP, Bakthavatsalam R, Kim HW, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation. 2004;78(9):1390–1396. doi: 10.1097/01.tp.0000145989.22373.03. [DOI] [PubMed] [Google Scholar]
- 14.Spencer JV, Lockridge KM, Barry PA, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76(3):1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longmate J, York J, La Rosa C, et al. Population coverage by HLA class-I restricted cytotoxic T-lymphocyte epitopes. Immunogenetics. 2001;52(3–4):165–173. doi: 10.1007/s002510000271. [DOI] [PubMed] [Google Scholar]
- 16.Boeckh M, Riddell SR. Immunologic predictors of late cytomegalovirus disease after solid organ transplantation--an elusive goal? J Infect Dis. 2007;195(5):615–617. doi: 10.1086/511311. [DOI] [PubMed] [Google Scholar]
- 17.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15(4):143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi M, Daniel V, Naujokat C, et al. Dysregulated cytokine responses during cytomegalovirus infection in renal transplant recipients. Transplantation. 2008;86(2):275–285. doi: 10.1097/TP.0b013e31817b063d. [DOI] [PubMed] [Google Scholar]
- 19.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205(3):533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]