Abstract
The anti-CCR5 antibody PRO 140 has shown potent and prolonged antiretroviral activity in subjects infected with CCR5-tropic (R5) HIV-1. Prior studies have examined single intravenous doses ranging up to 5 mg/kg of body weight or up to three subcutaneous doses ranging up to 324 mg. Here we report the results of a randomized, double-blind, placebo-controlled trial that examined the antiviral activity, tolerability, and pharmacokinetics of single 5-mg/kg and 10-mg/kg intravenous infusions of PRO 140 in 31 treated subjects. Eligibility criteria included HIV-1 RNA levels of >5,000 copies/ml, CD4+ cell counts of >300/μl, no antiretroviral therapy for ≥12 weeks, and detection of only R5 HIV-1 in the original Trofile assay. Following poststudy testing with an enhanced-sensitivity Trofile assay, one subject treated with 10 mg/kg was reclassified as having dual/mixed-tropic virus at screening, and the data for that subject were censored from efficacy analyses. The mean maximum reduction of the HIV-1 RNA level from the baseline level was 1.8 log10 units for both the 5-mg/kg and 10-mg/kg doses (P < 0.0001 relative to placebo). Viral loads reached their nadir at day 12 posttreatment and remained significantly (P < 0.01) reduced through day 29 for both PRO 140 dose groups. Treatment was generally well tolerated, with no dose-limiting toxicity being observed. Peak serum concentrations and overall exposures increased proportionally with dose. In summary, single 5-mg/kg and 10-mg/kg doses of PRO 140 exhibited potent, long-lived antiviral activity and were generally well tolerated. The findings further delineate the safety and antiviral properties of this novel, long-acting antiretroviral agent.
The chemokine receptor CCR5 plays a physiological role in the activation and migration of T cells and other leukocytes. CCR5 also binds to the HIV-1 envelope glycoprotein gp120 and serves as a coreceptor for HIV-1 entry into CD4+ cells (11). Certain strains of HIV-1 can use the chemokine receptor CXCR4 either exclusively (X4 viruses) or in addition to CCR5 (R5X4 or dual-tropic viruses). Viruses that use CCR5 exclusively (R5 viruses) are the only strains detected in most individuals during the initial to middle stages of disease. CXCR4-using virus can be detected in an increasing percentage of individuals as disease progresses (1, 2, 14, 24). A small-molecule CCR5 antagonist (maraviroc; Pfizer/ViiV Healthcare) has been approved by the U.S. Food and Drug Administration (FDA) for use in patients with only R5 virus detectable (5) and serves to validate CCR5 as a target for new anti-HIV-1 therapies.
PRO 140 is a humanized monoclonal antibody that binds to CCR5 and potently inhibits R5 but not CXCR4-using viruses in laboratory studies (15, 22). PRO 140 or its murine counterpart shows synergy and limited cross-resistance with small-molecule CCR5 antagonists in vitro (9, 13, 15). Both intravenous (i.v.) and subcutaneous (s.c.) forms of PRO 140 have previously been evaluated in short-term monotherapy studies with HIV-1-infected subjects with only R5 virus detectable. Both dosage forms of PRO 140 were generally well tolerated relative to placebo and demonstrated potent, prolonged, and dose-dependent antiretroviral activity (6, 7).
In a prior study, intravenous PRO 140 was evaluated as single doses of 0.5 mg/kg of body weight, 2 mg/kg, or 5 mg/kg. Antiviral effects increased in a dose-dependent manner, with a 1.83-log10-unit mean reduction in the HIV-1 RNA level being observed with the 5-mg/kg dose. On the basis of these findings, the present study was conducted to evaluate single 5-mg/kg and 10-mg/kg intravenous doses for their antiviral effects, tolerability, and pharmacokinetics (PK) in individuals infected with R5 HIV.
MATERIALS AND METHODS
Study design.
A randomized, double-blind, placebo-controlled, parallel-group study was conducted with HIV-infected adults. Subjects (approximately 30 planned) were randomized 1:1:1 to receive a single intravenous infusion of placebo, 5 mg/kg PRO 140, or 10 mg/kg PRO 140. The protocol was approved by the institutional review board at each site. All subjects provided written informed consent. Eligibility criteria included age of ≥18 years, plasma HIV-1 RNA level of ≥5,000 copies/ml, CD4+ lymphocyte counts of ≥300/μl and no documented count being ≤250/μl, no antiretroviral therapy for ≥12 weeks, no history of an AIDS-defining illness, and only R5 HIV-1 detectable in the original Trofile assay (Monogram Biosciences, Inc.) (23). PRO 140 was provided at a concentration of 10 mg/ml in a sterile phosphate-buffered solution. Placebo was a matched, sterile buffer solution without PRO 140. The study drug was administered over 30 min. Subjects were followed for 58 days posttreatment.
Virological evaluations.
The Amplicor HIV-1 Monitor test (version 1.5; Roche Diagnostics) was used to measure plasma levels of HIV-1 RNA at screening, the baseline (predosing on day 1), and days 3, 5, 8, 10, 12, 15, 22, 29, 43, and 59. Samples with <400 copies/ml were reanalyzed with the ultrasensitive specimen processing procedure. Coreceptor tropism was determined at screening for all subjects and after viral rebound for PRO 140-treated subjects using the original Trofile assay (23), given that the enhanced assay was not yet available. Tropism data were reported as R5 if only CCR5 use was observed, X4 if only CXCR4 use was observed, or dual/mixed if use of both CCR5 and CXCR4 was observed in the assay. When it became available poststudy, a version of Trofile with enhanced sensitivity in detecting CXCR4-using virus (17) was used in poststudy analyses. Blood samples for viral susceptibility analyses were collected on days 1 (predosing), 15, 29, and 59. Viral susceptibility to PRO 140 was determined for all subjects on day 1 and after viral rebound for PRO 140-treated subjects using the Phenosense Entry assay (Monogram Biosciences, Inc.) (6). Susceptibility data were reported as fold change values, defined as (the concentration required for 50% inhibition [EC50] for test isolate/EC50 for reference isolate).
Safety assessments.
Vital signs, concomitant medications, and adverse events were recorded during screening and on days 1, 2, 3, 5, 8, 10, 12, 15, 22, 29, 43, and 59. Physical examinations and laboratory safety tests (serum chemistries, hematology, and urinalysis) were performed during screening and on days 1, 8, 15, 29, and 59. Twelve-lead electrocardiograms were obtained during screening and on days 1, 5, 15, and 59.
Bioanalytical methods.
Serum concentrations of PRO 140 were determined by enzyme-linked immunosorbent assay (ELISA), as described previously (6). The assay detection range was 80 to 5,000 ng/ml. The coefficients of variation were 13% and 19% at the low and high concentrations, respectively. Antibodies to PRO 140 were measured by ELISA, as described elsewhere (6). Sera with detectable levels of anti-PRO 140 antibodies were tested for neutralizing activity according to a published method (7). Serum for PK analysis was obtained at 0 h (predosing), 0.5 h, 1 h, 3 h, 6 h, 24 h, 32 h, 48 h, 56 h, and 96 h posttreatment during the first week and then on days 8, 10, 12, 15, and 22. Serum for detecting anti-PRO 140 antibodies was obtained on days 1 (predosing), 8, 15, 29, and 59. CD4+ lymphocytes and CCR5 receptor occupancy were measured as described previously (7) in samples collected on days 1 (predosing), 3, 8, 12, 15, 22, 29, 43, and 59.
PK and pharmacodynamic analyses.
PK metrics were estimated after noncompartmental analysis using WinNonlin software (version 5.2; Pharsight). PK metrics included the maximum observed serum concentration (Cmax), area under the concentration-time curve (AUC) from time zero to infinity (AUC0-∞), clearance, mean residence time (MRT), volume of distribution following a single dose (V), and terminal serum half-life (t1/2). t1/2 was estimated by linear regression of the log-transformed concentration data as a function of time during the terminal phase of the decay curve. PK metrics were calculated for individual subjects and then summarized by dose cohort. Antiviral and PK data were fit to a maximum-effect equation using the WinNonlin program: E = Emax × AUC/(AUC + AUC50), where E is the log10 change in the HIV-1 RNA level, Emax is the maximum predicted change in the HIV-1 RNA level, and AUC50 is the AUC required to achieve 50% of Emax. AUC0-∞ values and nadir log10 HIV-1 RNA level changes for individual subjects were used in the model. Modeling was performed using data from subjects treated in the present study and in a prior study of single intravenous doses of PRO 140 (6).
Statistical methods.
All subjects who received study drug were included in the safety evaluations. The primary efficacy variable was the maximum change in viral load at any time following treatment. Efficacy analyses were performed on log10-transformed HIV-1 RNA level data, and changes were calculated relative to the baseline load (day 1, predosing). The results for treatment and placebo groups were compared using an analysis of variance model and using pairwise t tests, as described previously (6). Fisher's exact tests were used to compare treatment groups with the placebo group for the percentage of subjects with a ≥1-log10- or ≥2-log10-unit reduction in HIV-1 RNA levels from the baseline at any time posttreatment. Results are reported for two-sided tests.
RESULTS
Subject characteristics and disposition.
A total of 115 subjects were screened, of whom 35 were randomized and 31 were treated with study drug. All 31 treated subjects completed the study. Table 1 summarizes the demographic and other characteristics of the treated subjects, which comprised 29 males and 10 nonwhite individuals. At screening, treated subjects had a median age of 42.7 years, CD4+ cell count of 382 cells/μl, and plasma HIV-1 RNA level of 33,100 copies/ml. These characteristics were similar for the different treatment groups. Twelve subjects reported at least one historical antiretroviral therapy. Genotypic resistance to existing antiretroviral drugs was limited to single-class resistance in six subjects and two-class resistance in one subject. Viruses from all subjects were genotyped as subtype B.
TABLE 1.
Demographic and baseline characteristics
| Characteristic | Value for groupa |
|||
|---|---|---|---|---|
| Placebo (n = 11) | 5 mg/kg PRO 140 (n = 10) | 10 mg/kg PRO 140 (n = 10) | All subjects (n = 31) | |
| Age (yr) | 40.2 (22.3-56.6) | 44.7 (28.0-55.9) | 45.3 (25.9-57.2) | 42.7 (22.3-57.2) |
| Sex (no. male/no. female) | 9/2 | 10/0 | 10/0 | 29/2 |
| Race (no. black/no. white/no. other) | 4/7/0 | 2/8/0 | 3/6/1 | 9/21/1 |
| Wt (kg) | 82.4 (65.7-101.5) | 79.1 (62.2-126.0) | 82.3 (67.5-95.0) | 81.4 (62.2-126.0) |
| CD4+ cell count (no. of cells/μl) | 414.5 (316-738) | 389 (321-519) | 368 (264-595) | 382 (264-738) |
| HIV-1 RNA level (no. of log10 copies/ml) | 4.52 (3.76-5.12) | 4.58 (3.88-4.75) | 4.63 (3.79-5.53) | 4.52 (3.76-5.53) |
Data are median (range) values, unless otherwise indicated. Data were collected during screening.
Antiviral effects.
Both dose levels (5 mg/kg and 10 mg/kg) of PRO 140 demonstrated potent, rapid, and prolonged antiviral effects that were highly statistically significant relative to the effects of placebo (Table 2). All PRO 140-treated subjects except one subject treated with 10 mg/kg experienced a ≥1-log10-unit reduction in HIV-1 RNA level; the subject who was the exception experienced a minimal (<0.5-log10-unit) decrease in viral load. This subject had dual/mixed virus detected in the original Trofile assay at day 15. A poststudy analysis using the enhanced-sensitivity Trofile assay determined that this subject had dual/mixed virus at screening. The data for this subject were therefore censored from the efficacy analyses described in this report.
TABLE 2.
Change in HIV-1 RNA levels
| Effect | Value (P value) for groupa |
||
|---|---|---|---|
| Placebo | 5 mg/kg PRO 140 | 10 mg/kg PRO 140 | |
| Maximum log10 change in HIV-1 RNA load | −0.32 ± 0.24 | −1.83 ± 0.23 (<0.0001) | −1.83 ± 0.41 (<0.0001) |
| Day 12 log10 change in HIV-1 RNA load | 0.02 ± 0.18 | −1.69 ± 0.36 (<0.0001) | −1.73 ± 0.37 (<0.0001) |
| No. of subjects with a ≥1-log10-unit decrease in HIV-1 RNA level/total no. in group (%) | 0/11 (0) | 10/10 (100) (<0.0001) | 9/9 (100) (<0.0001) |
| No. of subjects with a ≥2-log10-unit decrease in HIV-1 RNA level/total no. in group (%) | 0/11 (0) | 2/10 (20) | 5/9 (56) (<0.01) |
Data are mean ± SD values, unless otherwise indicated. The analysis excludes data for one subject in the 10-mg/kg PRO 140 group who was reclassified as having dual/mixed virus at screening.
The mean maximum reduction from the baseline viral load was 1.83 log10 units for each of the PRO 140 dose groups. The reductions are statistically significant (P < 0.0001) relative to the 0.32-log10-unit mean reduction observed for the placebo group. The corresponding median reductions are 0.23, 1.84, and 2.09 log10 units for the placebo, 5-mg/kg dose, and 10-mg/kg dose groups, respectively. The mean and median maximum reductions for the 10-mg/kg group are 1.67 log10 and 1.82 log10 units, respectively, if the data for the censored subject are included (P < 0.0001 relative to placebo). Individual viral nadirs were observed on day 10 (five subjects) or day 12 (five subjects) for subjects treated with 5 mg/kg PRO 140 and on day 12 (five subjects), day 15 (three subjects), or day 22 (one subject) for subjects in the 10-mg/kg group.
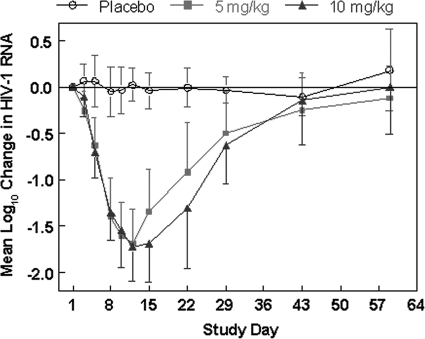
Similar mean log10 decreases in viral load were observed for the 5-mg/kg and 10-mg/kg dose groups through day 12, when the nadir reduction was observed in each group (Fig. 1). Thereafter, mean viral loads rebounded somewhat more slowly for the 10-mg/kg dose group; however, the differences between the 5-mg/kg and 10-mg/kg dose groups were not statistically significant at any time point (P > 0.1).
FIG. 1.
Mean log10 change in plasma levels of HIV-1 RNA over time by treatment group. P values were <0.0001 for each PRO 140 group relative to the placebo group at all time points from day 5 through day 15. P values were <0.001 and <0.01 for each PRO 140 group relative to the placebo group at day 22 and day 29, respectively. The analysis excludes data for one subject in the 10-mg/kg group who was reclassified as having dual/mixed virus at screening. Subjects received a single infusion of study drug on day 1. Data reflect mean values and 1 standard deviation.
As noted above, all PRO 140-treated subjects with the exception of the individual who was reclassified as having dual/mixed virus prior to treatment experienced a ≥1-log10-unit decrease in HIV-1 RNA posttreatment. No subject in the placebo group experienced a ≥1-log10- unit decline in viral load during the study (Table 2). Two subjects in the 5-mg/kg group and five subjects in the 10-mg/kg group (P < 0.01 relative to placebo) experienced ≥2-log10-unit decreases in viral loads. Five subjects treated with 5 mg/kg PRO 140 (P = 0.012 relative to placebo) and two treated with 10 mg/kg had viral loads reduced to <400 copies/ml, but no subject in the placebo group did. One subject in the 10-mg/kg group had a viral load of 50 copies/ml on days 10 and 12.
Coreceptor tropism and viral susceptibility to PRO 140 in vitro.
Tropism was assessed at screening and at the time of viral rebound in PRO 140-treated subjects. As noted above, one subject in the 10-mg/kg group was observed to have dual/mixed virus at day 15. This subject was later reclassified as having dual/mixed virus at screening, based on data generated poststudy using the enhanced-sensitivity Trofile assay. All other PRO 140-treated subjects maintained R5 coreceptor tropism following treatment.
Viral susceptibility to PRO 140 was measured in the PhenoSense Entry assay prior to treatment in all subjects and at the time of viral rebound in PRO 140-treated subjects. In the R5 Phenosense Entry assay, which examined CCR5-mediated viral entry into U87-CD4-CCR5 cells, PRO 140 inhibited all study viruses tested. Prior to treatment, the mean fold change was 1.7 (range, 0.77 to 3.1). On the basis of the ratio of the fold change value at the time of viral rebound to the value prior to treatment, no appreciable change in R5 virus susceptibility was observed in PRO 140-treated subjects (median ratio, 0.83; range, 0.49 to 1.71). Threefold or lower differences in fold change are considered to be within the normal range of interassay variation (3, 8). The maximum inhibition was ≥98% in all cases prior to treatment and was ≥99% in all cases following treatment. CXCR4-mediated entry of dual/mixed viruses into U87-CD4-CXCR4 cells was not inhibited by PRO 140, as expected.
Safety.
No serious adverse events (AEs) or dose-limiting toxicities were reported. All 11 subjects in the placebo group and 26 of 31 subjects overall reported at least one AE. AEs reported in more than two subjects were headache in one subject in the 5-mg/kg group and two subjects in the 10-mg/kg group, nasal congestion in two subjects in the placebo group and one subject in the 10-mg/kg group, and pruritus in three subjects in the placebo group. No obvious dose-related trend in the incidence of AEs was observed. There was no clinically relevant change in any electrocardiogram parameter, including QTc intervals, associated with administration of PRO 140 or placebo. There were no notable findings in clinical laboratory hematology or chemistry assessments or in vital sign measurements.
Pharmacokinetics and pharmacodynamics.
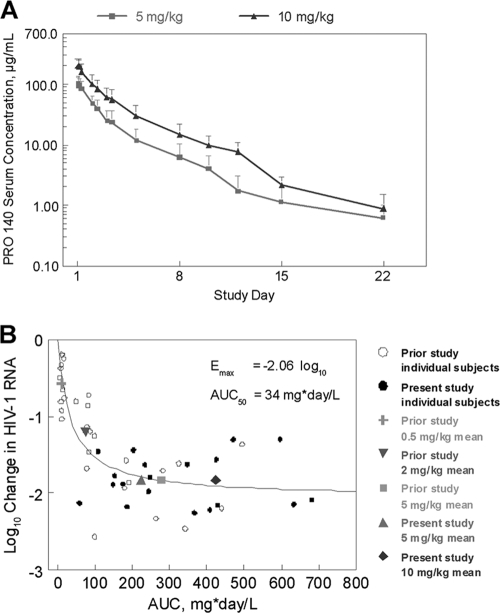
Mean serum concentrations of PRO 140 over time are illustrated by dose group in Fig. 2A, and PK metrics are listed in Table 3. Cmax was reached within 2 h for both PRO 140 dose groups. The mean Cmaxs were 109 ± 31 μg/ml and 211 ± 57 μg/ml for the 5- and 10-mg/kg dose groups, respectively. The AUC0-∞ also increased in approximate proportion with the dose, from 224 ± 60 μg·day/ml at the 5-mg/kg dose to 423 ± 150 μg·day/ml at the 10-mg/kg dose. The corresponding mean terminal half-lives were 3.13 ± 1.30 days and 3.33 ± 0.70 days. Clearance (1.97 ± 0.61 and 2.36 ± 1.85 liters/day), mean residence time (2.76 ± 0.84 and 3.15 ± 0.39 days), and volume of distribution (9.17 ± 5.52 and 10.8 ± 6.5 liters) were similar for the 5- and 10-mg/kg dose groups, respectively.
FIG. 2.
Pharmacokinetics and Emax analysis. (A) Arithmetic mean serum concentrations of PRO 140 over time are shown by treatment group. Error bars depict standard deviations. (B) Emax analysis. The maximum log10 changes in HIV-1 RNA levels are plotted against AUC0-∞ for subjects treated with single intravenous infusions of PRO 140 in the present study and a prior study (6). Data were fit to an Emax equation: E = Emax × AUC/(AUC + AUC50). The best-fit parameters (± standard errors) are an Emax value of −2.06 ± 0.12 log10 units and an AUC50 value of 34.1 ± 9.7 mg·day/liter (R = 0.80). Mean data for the different treatment groups in each study are plotted for illustration purposes but were not used for curve fitting.
TABLE 3.
Pharmacokinetic parametersa
| Dose (mg/kg) | Cmax (μg/ml) | AUC0-∞ (μg·day/ml) | t1/2 (days) | CL (ml/day/kg) | MRT (days) | V (liters) |
|---|---|---|---|---|---|---|
| 5 | 109 ± 31 | 224 ± 60 | 3.13 ± 1.30 | 1.97 ± 0.61 | 2.76 ± 0.84 | 9.17 ± 5.52 |
| 10 | 211 ± 57 | 423 ± 150 | 3.33 ± 0.70 | 2.36 ± 1.85 | 3.15 ± 0.39 | 10.8 ± 6.5 |
Data represent arithmetic means ± standard deviations.
The relationship between viral load reductions and PRO 140 exposure was modeled using a hyperbolic Emax equation. Combined data from the present study and a prior study of single-dose i.v. PRO 140 (6) were used in the analysis (Fig. 2B). The best-fit parameters for the combined data (Emax = −2.06 ± 0.12 log10 units, AUC50 = 34.1 ± 9.7 mg·day/liter) are similar to those reported previously for data from the prior study only (Emax = −2.14 ± 0.22 log10 units, AUC50 = 43.6 ± 15.6 mg· day/liter) (6).
Antibodies to PRO 140 were detected in two subjects in each of the PRO 140 dose groups. Antibodies were first detected on day 15 (n = 1), day 29 (n = 2), or day 59 (n = 1). In all cases, the anti-PRO 140 antibodies were of low titer (1:32 or less) and did not neutralize binding of PRO 140 to CCR5-positive (CCR5+) cells in vitro. The anti-PRO 140 antibodies did not have any apparent effect on the PKs or viral load reductions.
Lymphocyte and receptor occupancy analyses.
The changes in CD4+ lymphocyte counts following treatment with PRO 140 were not statistically significant. For the combined PRO 140 dose groups, the median (range) changes in CD4+ lymphocyte counts were +1 (−225 to +407), +111 (−204 to +286), +57 (−198 to +386), and +18 (−362 to +370) cells/μl at days 8, 12, 15, and 22, respectively. The corresponding values for the placebo group were +24 (−250 to +279), +38.5 (−399 to +145), +45 (−117 to +339), and +82 (−173 to +291) cells/μl at these time points.
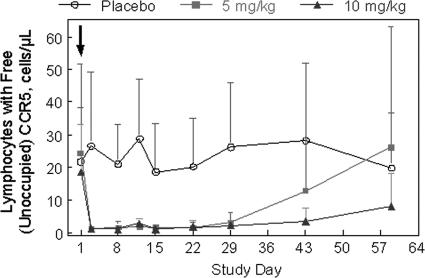
Receptor occupancy was assessed by flow cytometry using fluorescently labeled PRO 140. Occupancy of CCR5 by study drug results in a reduction in the number of lymphocytes with detectable levels of free CCR5. High levels of receptor occupancy (>85% reduction in the number of cells detected) were observed from day 3 through day 29 for both PRO 140 dose groups (Fig. 3). The results were statistically significant (P < 0.01 relative to placebo) throughout this time period. Significant receptor occupancy (81%, P < 0.01) was also observed at day 43 for the 10-mg/kg group. At day 59, receptor occupancy levels were not statistically significant for either PRO 140 dose group relative to that for the placebo group (P > 0.05). Lymphocytes were analyzed in parallel with a noncompeting fluorescently labeled CCR5 antibody, as described previously (6), and this analysis demonstrated that CCR5+ lymphocytes were not depleted from the circulation following treatment (data not shown).
FIG. 3.
Receptory occupancy. Receptor occupancy was determined by flow cytometry using fluorescently labeled PRO 140. In this assay, occupancy of CCR5 by study drug is reflected as a reduction in the number of cells that have detectable levels of free CCR5. Mean cell counts are shown over time by treatment group. Error bars depict standard deviations. P was <0.01 relative to placebo for 5 mg/kg PRO 140 at all time points from day 3 through day 29 and for 10 mg/kg PRO 140 at all time points from day 3 through day 43. P was >0.05 relative to placebo at all other time points. CCR5+ cells were not depleted from the circulation (data not shown). Subjects received a single infusion of study drug on day 1, as indicated by the arrow.
DISCUSSION
In this study, PRO 140 demonstrated potent, rapid, and prolonged antiretroviral activity when it was administered as single 5-mg/kg or 10-mg/kg intravenous infusions to individuals infected with CCR5-tropic HIV-1. The mean maximum decrease in viral load was 1.8 log10 units at each dose level, and this value compares favorably to the reductions observed in prior studies of PRO 140 (6, 7) and with small-molecule CCR5 antagonists (4, 10, 16, 19). Overall, single doses of 5 mg/kg or 10 mg/kg were generally well tolerated when they were administered as short-term monotherapy. Notably, we observed that 10 mg/kg, the highest dose tested to date, did not demonstrate any dose-dependent pattern of adverse events relative to placebo or to the 5-mg/kg dose. The present study adds to our understanding of the pharmacologic, pharmacokinetic, and safety profiles of this agent.
There was a striking consistency in the antiviral effects observed in the present study and a prior study of intravenous PRO 140 (6). Remarkably, the mean maximum reduction in the HIV-1 RNA level was 1.8 log10 units for doses of 5 mg/kg or higher in each study. The consistency of outcomes underscores the robustness of the single-dose activity observed for intravenous PRO 140. The median reduction in viral load was slightly higher in the 10-mg/kg group, and more subjects in the 10-mg/kg group achieved a ≥2-log10-unit reduction in HIV-1 RNA levels. In addition, there was a trend toward more prolonged antiviral effects with the 10-mg/kg dose. Overall, the findings indicate that doubling the dose from 5 mg/kg to 10 mg/kg resulted in modestly greater single-dose antiviral effects.
The original Trofile assay was used to determine coreceptor tropism for enrollment into the study. The original assay was validated to have 100% sensitivity in detecting CXCR4-using viruses when they were present at 10% or more of a virus population (23). The original Trofile assay has since been replaced with an enhanced-sensitivity assay that was validated to have 100% sensitivity in detecting 0.3% CXCR4-using viruses in a virus population (17). The enhanced-sensitivity assay is the method currently used in clinical practice. One subject enrolled into the 10-mg/kg group based on R5 tropism in the original Trofile assay was found to have dual/mixed virus 2 weeks posttreatment. This subject was later reclassified using the enhanced-sensitivity assay as having dual/mixed virus at screening, and the data for that subject were censored from the efficacy analysis. Similar approaches have been adopted in efficacy analyses of other studies of CCR5 coreceptor antagonists (18, 21), consistent with the view that the enhanced-sensitivity Trofile assay provides an improved method of identifying candidates for therapy with CCR5 coreceptor antagonists. Similar censoring of subjects treated in the MERIT study of maraviroc (18) was performed for efficacy analyses that supported FDA approval of this agent's use in antiretroviral treatment-naïve patients.
Compared with the 5-mg/kg dose, the 10-mg/kg dose of PRO 140 resulted in proportionally higher peak (Cmax) and overall (AUC0-∞) exposures to PRO 140. The higher drug exposures attained with 10 mg/kg were not associated with any obvious toxicity or pattern of toxicity. The maximum tolerated dose of i.v. PRO 140 has not been determined. The 10-mg/kg i.v. dose resulted in peak serum concentrations that are 15-fold higher, on average, than those observed following s.c. dosing (7), suggesting a sizeable margin of safety for s.c. PRO 140.
All pretreatment viruses were susceptible to inhibition by PRO 140 in vitro. The concentrations required for 50% inhibition varied by <5-fold across the panel of 31 viruses, and all viruses were efficiently inhibited (98 to 100%) at higher concentrations. With the exception of the one subject in the 10-mg/kg group who was reclassified as having dual/mixed virus at screening, there was no change in coreceptor tropism or emergence of PRO 140-resistant virus during the course of this study. The results support the view that PRO 140 broadly inhibits R5 HIV-1 with a high barrier to resistance.
High levels of receptor occupancy were observed following treatment with either 5 mg/kg or 10 mg/kg PRO 140. Statistically significant levels of receptor occupancy preceded significant reductions in viral load by at least 2 days. This result is concordant with the dynamics of inhibiting HIV-1 entry and with the half-life of virus-producing T cells (12). Receptor occupancy values also appeared to rebound later than viral loads. This apparent discordance could reflect issues related to assay sensitivity and sampling. Given the modest numbers of CCR5+ lymphocytes at the baseline (∼20 cells/μl, on average), the assay had a limited ability to determine mean receptor occupancy levels above 90%. Therefore, the times of maximum receptor occupancy and of initial rebound in receptor occupancy levels could not be determined precisely. In addition, receptor occupancy is measured on cells in the periphery, whereas HIV replication occurs primarily within tissues (20). PRO 140 concentrations and levels of receptor occupancy may differ at local sites of HIV-1 replication. As with viral load reductions, the duration of receptor occupancy was modestly greater with the 10-mg/kg dose relative to that with the 5-mg/kg dose, consistent with the higher serum concentrations of drug achieved at the higher dose.
To date, 84 HIV-infected individuals have been treated with i.v. or s.c. forms of PRO 140 in three short-term monotherapy studies (6, 7). In each study, 1.5- to 2.0-log10-unit mean reductions in HIV-1 RNA levels were observed with the higher dose levels. The viral load reductions were long-lived and highly statistically significant. No dose-limiting toxicity or pattern of toxicity was identified in these studies. In addition, no emergence of R5 viral resistance was observed, even though >1-log10-unit reductions in viral loads were observed for up to 6 weeks in some subjects.
In the present study, the duration of antiviral activity increased somewhat as the i.v. dose was increased from 5 mg/kg to 10 mg/kg. However, neither i.v. dose would appear to support highly infrequent (e.g., monthly) administration, and Emax analysis indicated that further increases in i.v. dose would result in incremental increases in antiviral effects. In a study of s.c. PRO 140, significant antiviral effects were observed when the drug was administered weekly or every other week, and virologic suppression was maintained between successive doses (7). While both i.v. and s.c. dosage forms have demonstrated favorable antiviral and tolerability profiles, s.c. PRO 140 was selected for further development on the basis of its potential to be self-administered by patients. Self-administration may offer greater convenience for many patients. Nevertheless, the s.c. dosage form is undergoing clinical study, and i.v. administration may be preferred in certain treatment settings.
In summary, single intravenous infusions of 5 mg/kg and 10 mg/kg PRO 140 demonstrated potent, long-lived antiretroviral activity and a favorable tolerability profile in this study. The findings provide new insights into the safety and virological properties of this agent, which represents a novel and long-acting approach to treating R5 HIV-1 infection.
Acknowledgments
We thank the subjects for their participation in the study. We gratefully acknowledge the assistance of all site personnel and study investigators, including Nicholaos Bellos, Daniel Berger, Margaret Fischl, Ralph Liporace, Amneris Luque, Mahesh Patel, Robert Redfield, Richard Reichman, Jihad Slim, and Michael Wohlfeiler.
The study was supported by Public Health Service grant AI066329 from the National Institutes of Health.
Progenics has a proprietary interest in PRO 140. Authors include employees who have equity interests in Progenics.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Brumme, Z. L., J. Goodrich, H. B. Mayer, C. J. Brumme, B. M. Henrick, B. Wynhoven, J. J. Asselin, P. K. Cheung, R. S. Hogg, J. S. Montaner, and P. R. Harrigan. 2005. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. J. Infect. Dis. 192:466-474. [DOI] [PubMed] [Google Scholar]
- 2.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 3.Demarest, J. F., H. Amrine-Madsen, D. M. Irlbeck, and K. M. Kitrinos. 2009. Virologic failure in first-line human immunodeficiency virus therapy with a CCR5 entry inhibitor, aplaviroc, plus a fixed-dose combination of lamivudine-zidovudine: nucleoside reverse transcriptase inhibitor resistance regardless of envelope tropism. Antimicrob. Agents Chemother. 53:1116-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatkenheuer, G., A. L. Pozniak, M. A. Johnson, A. Plettenberg, S. Staszewski, A. I. M. Hoepelman, M. S. Saag, F. D. Goebel, J. K. Rockstroh, B. J. Dezube, T. M. Jenkins, C. Medhurst, J. F. Sullivan, C. Ridgway, S. Abel, I. T. James, M. Youle, and E. van der Ryst. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 11:1170-1172. [DOI] [PubMed] [Google Scholar]
- 5.Gulick, R. M., J. Lalezari, J. Goodrich, N. Clumeck, E. DeJesus, A. Horban, J. Nadler, B. Clotet, A. Karlsson, M. Wohlfeiler, J. B. Montana, M. McHale, J. Sullivan, C. Ridgway, S. Felstead, M. W. Dunne, E. van der Ryst, and H. Mayer. 2008. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 359:1429-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson, J. M., M. S. Saag, M. A. Thompson, M. A. Fischl, R. Liporace, R. C. Reichman, R. R. Redfield, C. J. Fichtenbaum, B. S. Zingman, M. C. Patel, J. D. Murga, S. M. Pemrick, P. D'Ambrosio, M. Michael, H. Kroger, H. Ly, Y. Rotshteyn, R. Buice, S. A. Morris, J. J. Stavola, P. J. Maddon, A. B. Kremer, and W. C. Olson. 2008. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J. Infect. Dis. 198:1345-1352. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson, J. M., M. A. Thompson, J. P. Lalezari, M. S. Saag, B. S. Zingman, P. D'Ambrosio, N. Stambler, Y. Rotshteyn, A. J. Marozsan, P. J. Maddon, S. A. Morris, and W. C. Olson. 2010. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J. Infect. Dis. 201:1481-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitrinos, K. M., H. Amrine-Madsen, D. M. Irlbeck, J. M. Word, and J. F. Demarest. 2009. Virologic failure in therapy-naive subjects on aplaviroc plus lopinavir-ritonavir: detection of aplaviroc resistance requires clonal analysis of envelope. Antimicrob. Agents Chemother. 53:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhmann, S. E., P. Pugach, K. J. Kunstman, J. Taylor, R. L. Stanfield, A. Snyder, J. M. Strizki, J. Riley, B. M. Baroudy, I. A. Wilson, B. T. Korber, S. M. Wolinsky, and J. P. Moore. 2004. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J. Virol. 78:2790-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalezari, J., M. Thompson, P. Kumar, P. Piliero, R. Davey, K. Patterson, A. Shachoy-Clark, K. Adkison, J. Demarest, Y. Lou, M. Berrey, and S. Piscitelli. 2005. Antiviral activity and safety of 873140, a novel CCR5 antagonist, during short-term monotherapy in HIV-infected adults. AIDS 19:1443-1448. [DOI] [PubMed] [Google Scholar]
- 11.Lederman, M. M., A. Penn-Nicholson, M. Cho, and D. Mosier. 2006. Biology of CCR5 and its role in HIV infection and treatment. JAMA 296:815-826. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz, M., M. Louie, A. Hurley, E. Sun, M. Di Mascio, A. S. Perelson, and D. D. Ho. 2003. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J. Virol. 77:5037-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marozsan, A. J., S. E. Kuhmann, T. Morgan, C. Herrera, E. Rivera-Troche, S. Xu, B. M. Baroudy, J. Strizki, and J. P. Moore. 2005. Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D). Virology 338:182-199. [DOI] [PubMed] [Google Scholar]
- 14.Moyle, G. J., A. Wildfire, S. Mandalia, H. Mayer, J. Goodrich, J. Whitcomb, and B. G. Gazzard. 2005. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J. Infect. Dis. 191:866-872. [DOI] [PubMed] [Google Scholar]
- 15.Murga, J., M. Franti, D. C. Pevear, P. J. Maddon, and W. C. Olson. 2006. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 50:3289-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pett, S. L., M. C. McCarthy, D. A. Cooper, K. MacRae, A. Tendolkar, R. Norris, J. M. Strizki, K. M. Williams, and S. Emery. 2009. A phase I study to explore the activity and safety of SCH532706, a small molecule chemokine receptor-5 antagonist in HIV type-1-infected patients. Antivir. Ther. 14:111-115. [PubMed] [Google Scholar]
- 17.Reeves, J. D., E. Coakley, C. J. Petropoulos, and J. M. Whitcomb. 2009. An enhanced-sensitivity Trofile™ HIV coreceptor tropism assay for selecting patients for therapy with entry inhibitors targeting CCR5: a review of analytical and statistical studies. J. Viral Entry 3:94-102. [Google Scholar]
- 18.Saag, M., J. Heera, J. Goodrich, E. DeJesus, N. Clumeck, D. Cooper, S. Walmsley, N. Ting, E. Coakley, J. Reeves, M. Westby, E. van der Ryst, and H. Mayer. 2008. Reanalysis of the MERIT Study with the Enhanced Trofile Assay (MERIT-ES), abstr. H-1232A. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 19.Schurmann, D., G. Fatkenheuer, J. Reynes, C. Michelet, F. Raffi, J. van Lier, M. Caceres, A. Keung, A. Sansone-Parsons, L. M. Dunkle, and C. Hoffmann. 2007. Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS 21:1293-1299. [DOI] [PubMed] [Google Scholar]
- 20.Stebbing, J., B. Gazzard, and D. C. Douek. 2004. Where does HIV live? N. Engl. J. Med. 350:1872-1880. [DOI] [PubMed] [Google Scholar]
- 21.Su, Z., R. M. Gulick, A. Krambrink, E. Coakley, M. D. Hughes, H. Dong, C. Flexner, T. J. Wilkin, P. R. Skolnik, W. L. Greaves, D. Kuritzkes, and J. D. Reeves. 2009. Response to vicriviroc in treatment-experienced subjects, as determined by an enhanced-sensitivity coreceptor tropism assay: reanalysis of AIDS Clinical Trials Group A5211. J. Infect. Dis. 200:1724-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitcomb, J. M., W. Huang, S. Fransen, K. Limoli, J. Toma, T. Wrin, C. Chappey, L. D. Kiss, E. E. Paxinos, and C. J. Petropoulos. 2007. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob. Agents Chemother. 51:566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkin, T. J., Z. Su, D. R. Kuritzkes, M. Hughes, C. Flexner, R. Gross, E. Coakley, W. Greaves, C. Godfrey, P. R. Skolnik, J. Timpone, B. Rodriguez, and R. M. Gulick. 2007. HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211. Clin. Infect. Dis. 44:591-595. [DOI] [PubMed] [Google Scholar]