Summary
Costimulation through Junctional Adhesion Molecule-Like protein interaction with Coxsackie and Adenovirus Receptor mediates epithelial γδ T cell-specific activation and effector function during tissue repair.
γδ T cells present in epithelial tissues provide a crucial first line of defence against environmental insults, including infection, trauma and malignancy, yet the molecular events surrounding their activation remain poorly defined. Here we identify an epithelial γδ T cell-specific costimulatory molecule, Junctional Adhesion Molecule-Like protein (JAML). Binding of JAML to its ligand Coxsackie and Adenovirus receptor (CAR) provides costimulation leading to cellular proliferation and cytokine and growth factor production. Inhibition of JAML costimulation leads to diminished γδ T cell activation and delayed wound closure akin to that seen in the absence of γδ T cells. Our results identify JAML as a crucial component of epithelial γδ T cell biology and have broader implications for CAR and JAML in tissue homeostasis and repair.
αβ and γδ T cells, key players in cellular immunity, fulfill distinct, yet equally important, functions. αβ T cells are primarily involved in foreign antigen recognition, whereas γδ T cells function in tissue homeostasis and recognition of damaged self (1–3). Epithelial γδ T cells reside at the interface between organism and environment. They provide a rapid response to environmental insults and are crucial to the maintenance of epithelial integrity (2, 4, 5). γδ T cells in the skin, also known as Dendritic Epidermal T Cells (DETC), are a prototypic epithelial γδ T cell population. DETC have cellular projections that are in constant contact with multiple neighboring keratinocytes and Langerhans cells (6) and are activated following interaction with damaged or malignant keratinocytes (2, 4). DETC activation likely relies on the interplay of numerous cell surface molecules, as for αβ T cell activation, but the underlying mechanisms of DETC activation are unknown. Furthermore, many of the rules that apply to αβ T cell activation do not hold for γδ T cells. Coreceptor engagement and costimulation are integral to effective αβ T cell activation, but DETC do not express the CD4 or CD8 coreceptors or the costimulatory molecule CD28 (1, 7). Moreover, expression of MHC molecules or the costimulatory ligand B7 on keratinocytes is not required for DETC recognition of antigen (1, 7). Identification of equivalent activation molecules for γδ T cells has remained elusive. Although DETC do express some costimulatory molecules, such as NKG2D (4, 8), these are not unique to γδ T cells and their role in γδ T cell activation is not clearly defined.
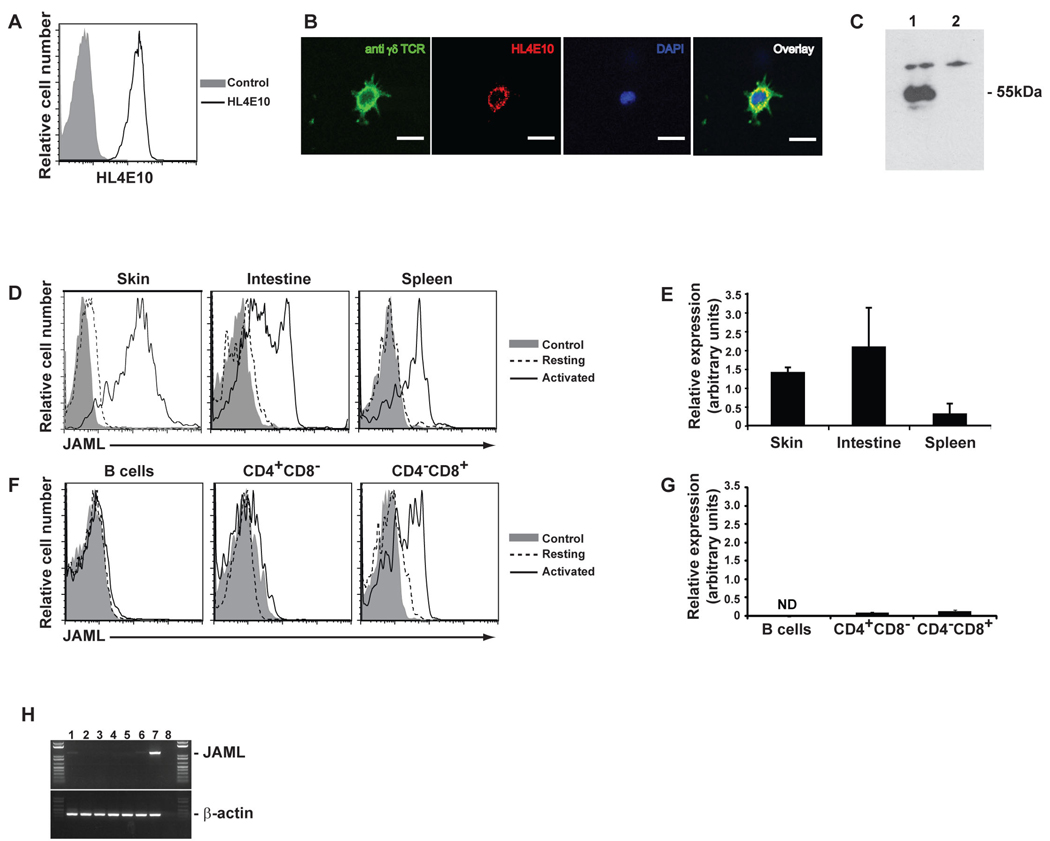
To shed light on the activation requirements of γδ T cells, we generated monoclonal antibodies (mAbs) to cell surface molecules expressed by DETC (9). Initial screens identified one mAb, HL4E10, as inducing potent proliferation of DETC. The ligand for HL4E10 was expressed on the cell surface (Fig. 1A), showing a somewhat punctate expression pattern, colocalizing with much of the T cell receptor (TCR) (Fig. 1B) and had a molecular weight of approximately 55kDa (Fig. 1C). Mass spectrometry identified the ligand as mouse Junctional Adhesion Molecule-Like protein (JAML) and the specificity of HL4E10 for mouse JAML was confirmed by flow cytometry and immunoprecipitation (fig. S1 and S2).
Fig. 1.
Murine JAML, a 55kDa protein expressed on γδ T cells, is recognized by mAb HL4E10. (A) 7–17 cells were analyzed by flow cytometry for HL4E10 or control mAb staining. (B) γδ TCR and HL4E10 fluorescent staining (magnification ×600) of 7–17 cells grown on coverslips. The nucleus is stained with DAPI. Scale bar represents 50µm. (C) Western blot analysis of 7–17 lysates immunoprecipitated with mAb HL4E10 (lane 1) or a control hamster mAb, 1F4, (lane 2). Protein identification by LC-ESI-MS gave a high scoring match with mouse JAML (Mascot score 151). (D) Flow cytometric analysis of JAML expression by resting and ConA activated epithelial and peripheral γδ T cells. Cells were gated on γδ TCR+. (E) Quantitative RT-PCR analysis of JAML expression in γδ T cells from epidermis, intestine and spleen. Relative amounts of JAML mRNA were normalized to β-actin and are shown as arbitrary units. (F) Flow cytometric analysis of JAML expression by splenic B cells, CD4+ T cells and CD8+ T cells. Both resting and activated B cells (LPS-activated) and αβ T cells (PMA and ionomycin) were analyzed. (G) Quantitative RT-PCR analysis of JAML expression in CD4+CD8− and CD4−CD8+ splenocytes. Relative amounts of JAML mRNA were normalized to β-actin and are shown as arbitrary units. ND=not determined. (H) JAML mRNA isolated from representative epithelial and lymphocyte cell lines: PAM keratinocytes (lane 1), 3T3 fibroblasts (lane 2), DP thymocytes (DPK; lane 3), CD4+ T cells (D10; lane 4), CD8+ T cells (CTLL; lane 5), B cells (A20; lane 6), γδ T cells (7–17; lane 7) and RT control (lane 8). Data in (A) through (H) are representative of at least three experiments.
JAML is a member of the Junctional Adhesion Molecule (JAM) family (10) and in humans is expressed on neutrophils and, to a lesser extent, on memory T cells and monocytes (10, 11). JAM family members are thought to regulate leukocyte-endothelial interactions, facilitate tight junction assembly and help orchestrate recruitment of leukocytes to sites of inflammation and wound repair (12, 13). As DETC are primary responders to epidermal insult, JAML may play a crucial role in epidermal wound repair.
Low level expression of JAML was found on resting γδ T cells isolated from mouse epidermis and intestine, but not spleen (Fig. 1, D and E). 70–95% of γδ T cells from each of these tissues upregulated JAML upon mitogenic stimulation (Fig. 1D). Increased expression was evident on DETC within 4hrs levels and continued to increase for 24–48 h (fig. S3). JAML was not found on B cells, CD4+αβ T cells or resting CD8+αβ T cells (Fig. 1, F and G). However, approximately 50% of Concanavalin A (Con A) activated, splenic CD8+αβ T cells did express JAML (Fig. 1F), as did activated CD8αα+ and CD8αβ+ αβ T cells in the intestine (fig. S4). In the spleen, the activated JAML+CD8+ population was indistinguishable from the JAML−CD8+ T cells on the basis of expression of several activation markers (fig. S5). Analysis of a number of cell lines further supported a restricted expression of mouse JAML (Fig. 1H) and we found no evidence of JAML expression on adult αβ or γδ thymocytes (fig. S6). Thus, the expression pattern, and the proliferation results from our initial screen, suggested that JAML plays an important role in γδ T cell activation.
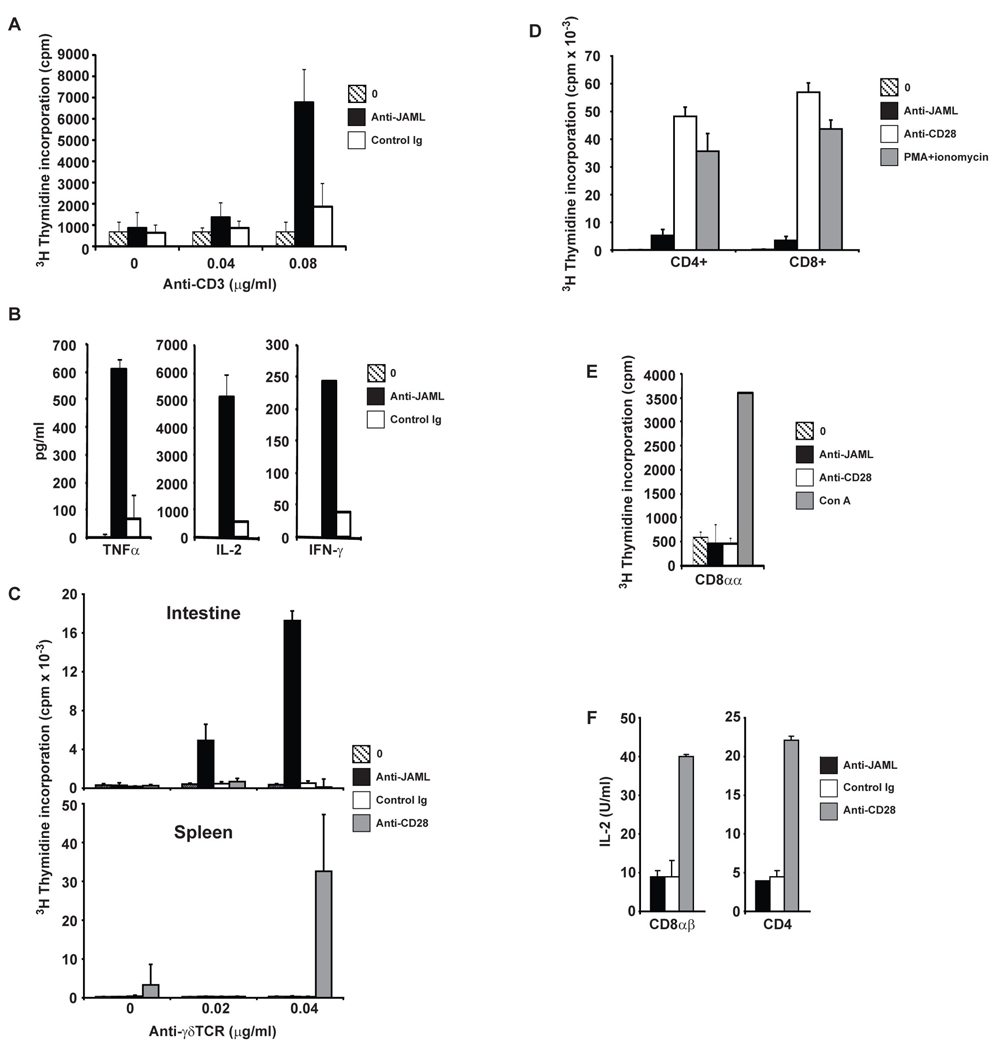
Ligation of JAML with HL4E10 induced costimulation of both short-term cell lines from freshly isolated DETC (Fig. 2A) and the established 7–17 DETC cell line (fig. S7). Costimulation through JAML induced proliferation (Fig. 2A and fig. S7) and production of the cytokines interleukin-2 (IL-2), tumor necrosis factor-α (TNFα) and interferon-γ (IFNγ) (Fig. 2B and fig. S7 and S8), supporting a role for JAML in the activation and effector function of epidermal γδ T cells. HL4E10 also induced potent costimulation of intestinal γδ T cells, but not splenic γδ T cells (Fig. 2C), while CD28, expressed by γδ T cells in the spleen but not intestine, only costimulated splenic γδ T cells (Fig. 2C). This dichotomy of JAML function between epithelial and lymphoid γδ T cells is perhaps surprising due to the expression of JAML on both γδ T cell populations upon stimulation; however, tissue-resident γδ T cells constitutively express low levels of JAML, whereas lymphoid γδ T cells require activation to produce detectable JAML (Fig. 1, D and E). Indeed, unlike splenic γδ T cells, epithelial-resident γδ T cells were responsive to JAML costimulation directly ex vivo (Fig. 2C and fig. S9). JAML may thus contribute to latter stages of lymphoid γδ T cell activation, as suggested for ICOS-mediated costimulation of αβ T cells (14).
Fig. 2.
JAML functions as a costimulatory molecule on epithelial γδ T cells, but not lymphoid γδ T cells or αβ T cells. (A) Proliferation of short-term DETC cell lines to immobilized anti-CD3 either alone or in combination with anti-JAML mAb, HL4E10, or a control mAb, 1F4. (B) JAML costimulation induces cytokine production. ELISA analysis for TNFα, IL-2 and IFNγ from culture supernatants of DETC stimulated with anti-CD3 alone, anti-CD3 and anti-JAML, or anti-CD3 and control mAb, 1F4. (C) Proliferation of intestinal or splenic γδ T cells to immobilized anti-γδ TCR mAb (GL3) either alone or in combination with anti-JAML, control mAb 1F4, or anti-CD28. (D) Proliferation of splenic CD4+ and CD8+ T cells in response to immobilized anti-CD3 (0.4µg/ml) alone or in combination with anti-JAML or anti-CD28. PMA and ionomycin was used as a positive control. (E) Proliferation of sorted CD8αα+ αβ IEL to immobilized anti-CD3 (0.4µg/ml) alone or in combination with anti-JAML or anti-CD28. Con A was used as a positive control. (F) IL-2 production by CD8αβ+ and CD4+ αβ IEL in response to anti-CD3 (0.4µg/ml) in combination with anti-JAML, control mAb 1F4, or anti-CD28. IL-2 was measured by MTT assay. Data in (A) through (F) are representative of at least three experiments.
While the necessity of costimulation in γδ T cell activation remains an open question, αβ T cells rely on efficient costimulation, most notably through CD28, for their activation and effector function (15, 16). Despite expression of JAML on activated CD8+ αβ T cells (Fig. 1F), it was unable to costimulate either CD4+ or CD8+ αβ T cells (Fig. 2D). Furthermore, intestinal CD8αα+, CD8αβ+ and CD4+ αβ T cells did not respond to JAML costimulation (Fig. 2, E and F). Thus, the costimulatory function of JAML appears to be specific for epithelial γδ T cells, in contrast to other putative γδ T cell costimulatory molecules (4).
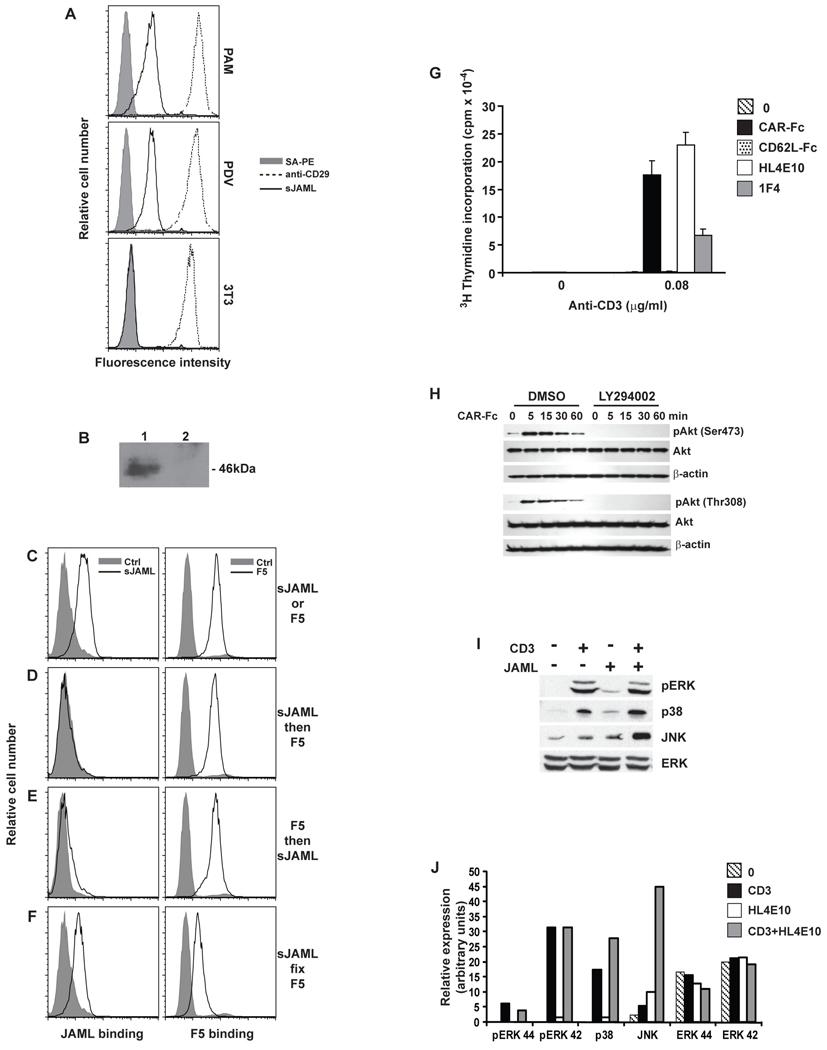
Recently, epithelial-expressed Coxsackie and Adenovirus Receptor (CAR) has been shown to be a ligand for JAML on neutrophils (17). We, therefore, reasoned that the skin JAML ligand might also be CAR. CAR migrates at 46kDa (18) and is not expressed by 3T3 fibroblasts (19). As expected, soluble JAML bound only to keratinocyte cell lines and not fibroblasts (Fig. 3A), and precipitated a 46kDa protein from keratinocytes (Fig. 3B).
Fig. 3.
CAR is a functional ligand for JAML in the epidermis. (A) PAM 2–12 and PDV keratinocyte and 3T3 fibroblast cell lines were analyzed by flow cytometry for binding of soluble JAML (sJAML), anti-CD29 (positive control) or streptavidin PE (SA-PE; negative control). (B) Western blot analysis of PDV lysates immunoprecipitated with sJAML (lane 1) or empty beads (lane 2). (C–F) PDV keratinocytes were analyzed by flow cytometry for binding of sJAML and soluble adenovirus fiber protein (F5). Keratinocytes were stained with sJAML or F5 alone (C), sJAML followed by F5 (D), F5 followed by sJAML (E), or stained with sJAML, fixed and then stained with F5 (F). (G) Proliferation of the 7–17 γδ T cell line to immobilized anti-CD3 either alone or in combination with CAR-Fc, a negative control Fc fusion protein, CD62L-Fc, HL4E10, or control mAb 1F4. (H) Western blot analysis of phosphorylation of Akt Ser473 and Thr308 and total Akt in 7–17 DETC following JAML ligation with CAR-Fc, in the presence or absence (DMSO vehicle control) of the PI3K inhibitor LY924002. β-actin was used as a loading control. (I,J) Western blot analysis of ERK 1,2 phosphorylation and p38 and JNK kinase activity in 7–17 lysates following 5 min stimulation with anti-CD3, anti-JAML or both. Total ERK is shown as a control. Quantification of data in (I) is shown in (J). Data in (A) through (J) are representative of at least three experiments.
CAR is best characterized as the primary receptor for Coxsackie B virus and Adenovirus (18, 20, 21). To confirm CAR as the ligand for JAML in the epidermis, we therefore performed competitive binding analysis using a soluble adenovirus fiber protein (Ad5 fiber knob; F5) and JAML (Fig. 3, C to F). FACS analysis confirmed that the JAML ligand and CAR are expressed on keratinocytes (Fig. 3C). Addition of F5 to keratinocytes, displaced (Fig. 3D) or blocked (Fig. 3E) binding of JAML, whereas fixation of keratinocyte bound JAML prior to F5 addition allowed simultaneous binding of some JAML and F5 (Fig. 3F). These data confirmed that CAR is the keratinocyte ligand for JAML and, based on displacement of JAML by F5, appears to represent a lower affinity interaction (Kd~5µM) (22) compared to a Kd~8nM for adenovirus serotype 5 (23), but comparable to other lymphocyte cell-cell molecular interactions (24).
Binding of soluble CAR and HL4E10 to JAML single-domain constructs demonstrated that CAR and HL4E10 recognize different epitopes of JAML. HL4E10 bound the membrane proximal JAML D2 Ig domain, whereas CAR-Fc bound the membrane distal JAML D1 Ig domain (fig. S10). This binding mode was confirmed in the crystal structure of JAML in complex with CAR (22), in contrast to previous suggestions (17).
In vitro costimulation assays demonstrated that CAR is a functional ligand for JAML. CAR ligation of JAML induced proliferation (Fig. 3G) and IL-2 production (fig. S11) by DETC that was comparable to mAb HL4E10. Although CAR has been extensively studied as a virus receptor (18, 20, 21) and, more recently, as an adhesion molecule (11, 17), these data demonstrate its importance as a signaling molecule in the immune system.
CAR ligation of JAML leads to rapid and sustained phosphatidylinosytol 3-kinase (PI3K) association with a YMxM motif in the JAML intracellular domain (22). In CD28 and ICOS, phosphorylated YMxM is also required for PI3K binding (25). Strikingly, CD28 and JAML are the only two molecules that contain a YMxMxPxxP motif in their intracellular domains (22), suggesting that they may also share other downstream signals. We, therefore, examined the effect of JAML ligation on Akt, the key downstream target of PI3K and one of the central molecules for CD28-mediated costimulation. Rapid phosphorylation of Akt at both Ser473 and Thr308 was induced in DETC in response to CAR-JAML ligation and was entirely abrogated in the presence of a PI3K inhibitor (Fig. 3H). Thus, CAR-induced PI3K recruitment to JAML is tightly linked to activation of Akt and initiation of downstream signaling events.
Activation of MAP kinase pathways downstream of PI3K association with CD28 is a hallmark feature of αβ T cell activation (25). In DETC, costimulation through JAML led to JNK kinase activity at 5 min of TCR and JAML coligation (Fig. 3I) compared with 20 min for TCR ligation alone (fig. S12), inducing a 9-fold increase in JNK activity over TCR stimulation alone and a 5-fold increase over HL4E10 alone (Fig. 3J). In contrast, ERK phosphorylation and p38 kinase activity were very similar to TCR ligation alone (Fig. 3, I and J and fig. S12). The early JNK activation suggests that JAML can enhance signals through the γδ TCR and it remains to be seen if alternate pathways can also be activated.
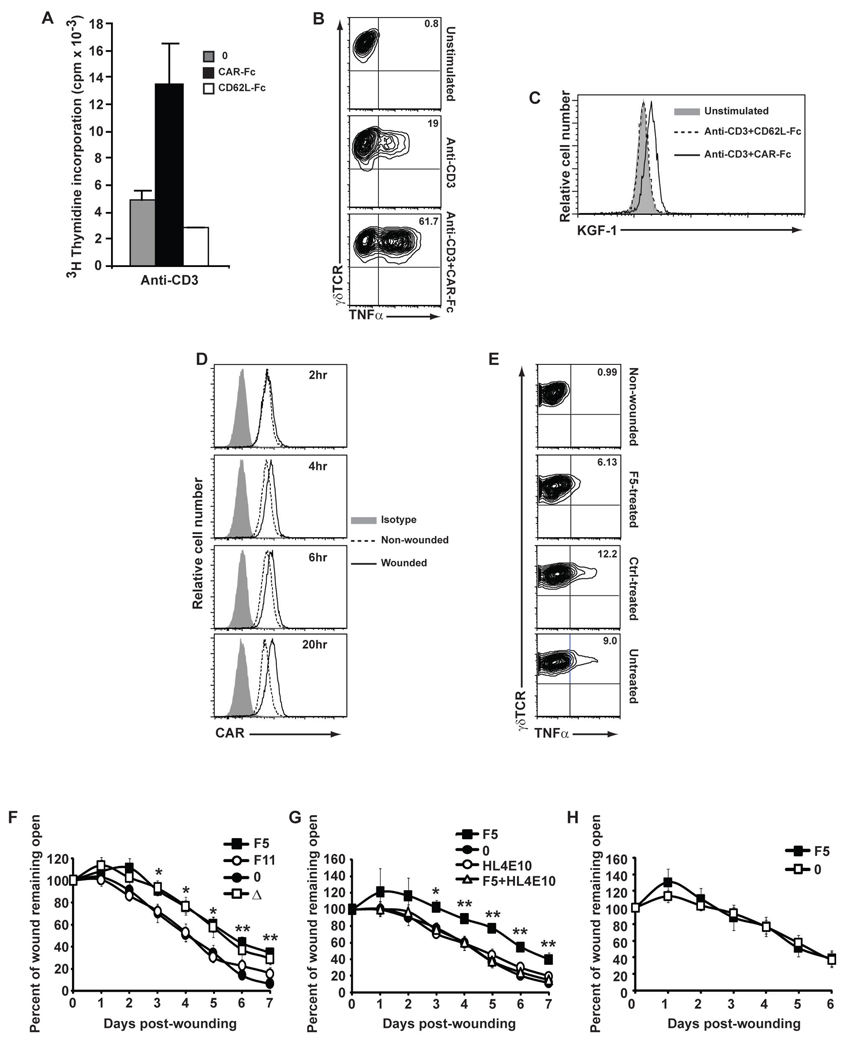
To address the role of JAML-CAR interactions in vivo, we first analyzed DETC directly isolated from the epidermis, where JAML costimulation resulted in proliferation (Fig. 4A) and cytokine production (fig. S13) comparable to that seen with DETC lines (Fig. 2, A and B and Fig. 3G). Strikingly, costimulation of DETC was required for effective cytokine production (Fig. 4B). Proliferation of DETC in vitro can be achieved with high dose TCR stimulation (fig. S14), as for αβ T cells (26). Using CD3 alone, at a concentration effective for maximal proliferation of DETC (fig. S14), only minimal TNFα (Fig. 4B) production was apparent. In contrast, costimulation through JAML resulted in substantially more production of TNFα (Fig. 4B), suggesting that JAML costimulation is necessary for rapid and robust production of the cytokines required for DETC effector function. Consistent with its role as a mediator of γδ T cell function, costimulation through JAML also upregulated expression of keratinocyte growth factor 1 (KGF-1) in primary DETC (Fig. 4C), a vital component of the wound repair process (2).
Fig. 4.
Costimulation through JAML-CAR interactions mediates epithelial γδ T cell activation and effector function. (A) Proliferation of freshly isolated epidermal γδ T cells to immobilized anti-CD3 (1µg/ml) either alone (0) or in combination with CAR-Fc or a negative control Fc fusion protein, CD62L-Fc. (B) Intracellular flow cytometric analysis of TNFα production by unstimulated, anti-CD3 stimulated (0.5µg/ml) or anti-CD3 (0.05µg/ml) and CAR-Fc (10µg/ml) stimulated 7–17 DETC. (C) Flow cytometric analysis of KGF expression by unstimulated primary DETC or after stimulation with immobilized anti-CD3 (1µg/ml) and CAR-Fc or anti-CD3 and control Fc fusion protein, CD62L-Fc. (D) Flow cytometric analysis of CAR expression by keratinocytes isolated from epidermis of C57Bl/6 mice. Skin was either non-wounded or wounded and keratinocytes were isolated at the indicated times. (E) Flow cytometric analysis of TNFα production by DETC isolated from epidermis of C57Bl/6 mice. Skin was either non-wounded, wounded and treated with soluble F5 protein (F5-treated), wounded and treated with control soluble F11 protein (Ctrl-treated) or wounded and not treated. (F) Wound closure in vivo in the presence of soluble F5 or a control protein, F11. Untreated C57Bl/6 mice (0) and TCRδ−/− mice (Δ) were used as controls. (G) Wound closure in the presence of F5, HL4E10 or F5 and HL4E10. Untreated C57Bl/6 mice (0) were used as a control for rate of wound closure. (H) Wound closure in F5-treated (F5) and untreated (0) TCRδ−/− mice. Data represent mean ± SEM of 4–8 wounds per condition. Student’s t test p values (**p<0.005 and *p<0.05). Data in (A) through (H) are representative of more than three experiments.
A requirement for JAML-CAR costimulation in vivo is further supported by the response of keratinocytes and DETC to epidermal wounding. Keratinocytes immediately adjacent to the wound site upregulate CAR expression beginning 4 h post-wounding (Fig. 4D). The neighboring DETC become activated as evident by an increased production of cytokines and growth factors (2, 27). Blockade of JAML-CAR interactions, immediately after wounding, reduced activation of DETC isolated from the wound edge (Fig. 4E) and impaired the healing response (Fig. 4F). A significant delay in wound closure followed treatment of wounds with a single dose of soluble F5 protein (Fig. 4F) (28). Notably, the kinetics of wound closure followed very closely the repair seen in the skin in the absence of γδ T cells (Fig. 4F). Although no effect was seen following addition of the HL4E10 mAb, presumably due to high levels of constitutive expression of CAR in the epidermis available for JAML binding, strikingly, addition of the HL4E10 to F5-treated wounds restored wound closure kinetics to untreated levels (Fig. 4G). HL4E10 thus appears to be able to substitute for CAR and restore JAML-mediated costimulation to γδ T cells at the wound site. The specificity of F5-treatment for JAML-CAR blockade on γδ T cells was confirmed by the observation that no further delay in wound closure was found upon addition of F5 to TCRδ−/− wounds (Fig. 4H). These data strongly suggest that the JAML-CAR interaction contributes to natural γδ T cell activation and effector function.
Epithelial γδ T cells provide a crucial first line defense for epithelial barrier tissues. The complete activation of these cells is vital to their responses to epithelial insults, such as infection, trauma and malignancy. Assessment of the full extent to which epithelial γδ T cells require JAML-CAR interactions in concert with TCR signals cannot be fully addressed until the cognate ligand for these cells is identified. Nevertheless, manipulation of the interaction between JAML and CAR may well increase the effectiveness of the epithelial γδ T cell response in cases of chronic disease, such as non-healing wounds and epithelial malignancies.
Supplementary Material
References and Notes
- 1.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 2.Jameson J, et al. A role for skin γδ T cells in wound repair. Science. 2002;296:747. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 3.Strid J, Tigelaar RE, Hayday AC. Skin immune surveillance by T cells-a new order? Semin. Immunol. 2009;21:110. doi: 10.1016/j.smim.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Girardi M, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605. [PubMed] [Google Scholar]
- 5.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 2005;6:73. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 6.Jameson J, Havran WL. Skin γδ T-cell functions in homeostasis and wound healing. Immunol. Rev. 2007;215:114. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000;18:975. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 8.Whang MI, Guerra N, Raulet DH. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J. Immunol. 2009;182:4557. doi: 10.4049/jimmunol.0802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Materials and Methods are available as supporting material on Science Online.
- 10.Moog-Lutz C, et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood. 2003;102:3371. doi: 10.1182/blood-2002-11-3462. [DOI] [PubMed] [Google Scholar]
- 11.Luissint AC, Lutz PG, Calderwood DA, Couraud PO, Bourdoulous S. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by α4β1 integrin activation. J. Cell Biol. 2008;183:1159. doi: 10.1083/jcb.200805061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazzoni G. The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 2003;15:525. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- 13.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell. Sci. 2004;117:19. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 14.Coyle AJ, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 15.Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit. Rev. Immunol. 1997;17:89. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 16.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J. Clin. Invest. 2002;109:295. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zen K, et al. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol. Biol. Cell. 2005;16:2694. doi: 10.1091/mbc.E05-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv. Drug. Deliv. Rev. 2005;57:869. doi: 10.1016/j.addr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3352. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergelson JM, et al. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 1998;72:415. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howitt J, Anderson CW, Freimuth P. Adenovirus interaction with its cellular receptor CAR. Curr. Top. Microbiol. Immunol. 2003;272:331. doi: 10.1007/978-3-662-05597-7_11. [DOI] [PubMed] [Google Scholar]
- 22.Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010 doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby I, et al. Adenovirus type 9 fiber knob binds to the coxsackie B virus-adenovirus receptor (CAR) with lower affinity than fiber knobs of other CAR-binding adenovirus serotypes. J. Virol. 2001;75:7210. doi: 10.1128/JVI.75.15.7210-7214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MM, et al. Dynamics of cell surface molecules during T cell recognition. Annu. Rev. Biochem. 2003;72:717. doi: 10.1146/annurev.biochem.72.121801.161625. [DOI] [PubMed] [Google Scholar]
- 25.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat. Rev. Immunol. 2003;3:544. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 27.Havran WL, Jameson JM. Epidermal T cells and wound healing. J. Immunol. 2010;184:5423. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.F5 and JAML recognize overlapping binding sites on CAR (22). As such, F5 is able to block the natural JAML-CAR interaction (Fig 3, D and E).
- 29.We thank M. Park and W. Low for MS analysis, G. Nemerow for Fiber 5 and Fiber 11 reagents, J. Kaye for DPK cell line, and M. Haynes, M. Svoboda, J. Barcas, K. Sendaydiego, D. Yeh and B. Atteberry for technical assistance. J. Lewis provided advice on culture of short-term DETC lines. M. Kronenberg, R. Boismenu, J. Jameson, K. Mowen, T. Meehan, and K. Komori provided advice and critical reading of the manuscript. This work was supported by NIH grants to W.L.H. (AI52257, AI064811) and I.A.W. (AI42266, CA58896) as well as an Erwin-Schroedinger Fellowship of the Austrian Science Fund (P.V.) and The Leukemia and Lymphoma Society (S.E.R.). MS instrumentation was acquired with an NSF shared equipment grant. The MS Laboratory at the Salk Institute is supported by the Vincent J. Coates Foundation and NIH Blueprint NS057096. This is manuscript number 17711-IMM from The Scripps Research Institute.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.