Abstract
Acute inflammation is a frequent, essential and beneficial response to maintain normal tissue function. PMN are the primary effector cells of acute inflammatory responses and their timely resolution by macrophages from an injured, stressed or infected tissues are required for the successful execution of this routine tissue response. Dysregulation of this fundamental program is a major factor in the global disease burden and contributes to many ocular diseases. Counter-regulatory signals are critical to the controlled activation of innate and adaptive immune responses in the eye and recent studies have identified two circuits in the cornea, uvea and/or retina, namely 15-lipoxygenase and heme-oxygenase, which control inflammation, promote resolution of PMN and afford neuroprotection. The role of these counter-regulator and pro-resolution circuits may provide insight into ocular inflammatory diseases and opportunities to restore stressed ocular tissue to a pre-inflammatory state, namely homeostasis, rather than limiting therapeutic options to palliative inhibition of pro-inflammatory circuits.
Keywords: Neutrophil, Macrophage, Acute Inflammation, Resolution, Heme-oxygenase, 15-Lipoxygenase, Retina, Cornea, ω-3 PUFA, Neuroprotection
1. Acute and Self-Resolving Inflammation: A Beneficial and Routine Tissue Response
Acute inflammation is an essential response to tissue injury, stress and infection. By design inflammation is a self-limiting and self-resolving response that evolved to provide constant protection and to maintain tissue integrity and homeostasis. Inflammation is a reoccurring and frequent response, especially for tissues that are in intimate and continuous contact with the external environment such as the gastrointestinal and pulmonary tract, skin, cornea and conjunctiva. Indeed in view of the constant exposure of these tissues to pathogens, irritants, toxins and allergens mucosal tissues are likely in a continual state of mild subclinical inflammation. Hence, it is essential that this routine tissue response is tightly regulated and controlled by highly evolved and overlapping circuits. This review will focus on emerging evidence for protective pathways in the eye that have been identified as central mediators of inflammatory resolution.
A hallmark of most inflammatory responses is early PMN recruitment from the vasculature via rolling and adhesion to the activated vascular endothelium. A notable exception is lymphocyte driven auto-immune or allergic responses, however even lymphocyte or macrophage initiated inflammatory responses (e.g., asthma, rheumatoid arthritis) often trigger pronounced recruitment of vascular PMN or eosinophils to the injured tissue. Even though many tissues in the eye exhibit privileged or highly evolved immune responses to protect the delicate visual axis the successful and essential execution of acute inflammation is governed by the same general pathways, mediators and effector cells that direct inflammatory responses in other organs (Streilein, 2003; Kumar, 2004; McDermott et al., 2005; Niederkorn & Stein-Streilein, 2010).
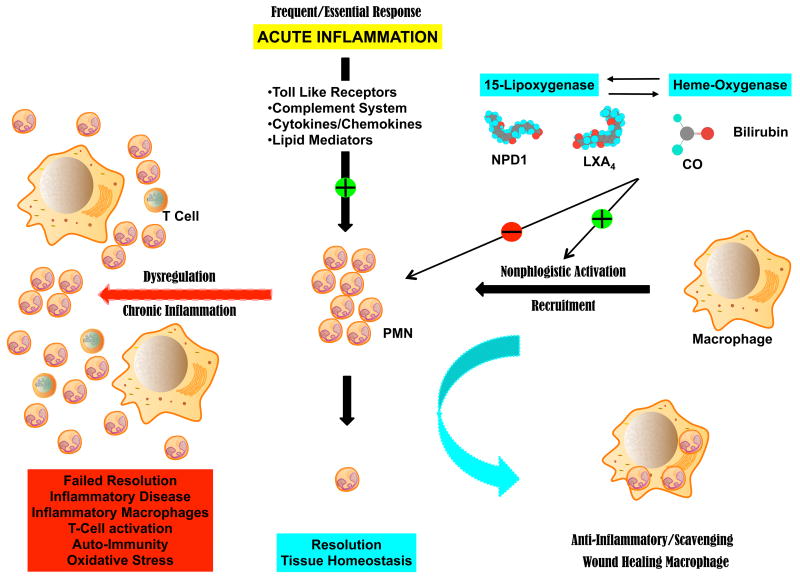
PMN are the primary line of defense against potential infection and are the most abundant leukocyte with a relative short half live and a remarkable turn over on the order of 1011 cells per day in humans (Kennedy & DeLeo, 2009). PMN tissue levels in humans can range from a few thousand that accumulate in healthy human tears overnight to over a billion per day in the synovial fluid of patients with rheumatoid arthritis (Sack et al., 1992; Nathan & Ding, 2010). These primary effector cells of the innate immune response contain a potent arsenal of bactericidal agents, reactive oxygen species (ROS), proteolytic enzymes and chemical mediators whose deployment has to be carefully controlled to minimize collateral PMN mediated tissue injury (Kumar, 2004). Indeed exuberant or dysregulated activation of PMN can cause significant tissue and organ damage that has been linked to numerous inflammatory diseases such gout, ischemia-reperfusion injury, fibrosis, corneal melts and keratitis. More importantly, this early and transient event in the inflammatory response (i.e., PMN infiltration) triggers macrophage recruitment for the efficient removal of dead and spent PMN, a critical step in resolution of acute inflammation (Figure 1). Macrophages are important immune effector cells, master regulators and scavenger cells with a remarkable plasticity to differentiate into at least three distinct macrophage populations that drive host defense, wound healing and immune regulation (Mosser & Edwards, 2008). Nonphlogistic activation, controlled differentiation and recruitment of scavenging macrophages is especially critical for maintenance of the retina where scavenging macrophages play a critical role in the non-inflammatory removal of cell waste beneath the retinal pigment epithelial cell to prevent drusen deposition (Mosser & Edwards, 2008; Raisler et al., 2008). Differentiation of macrophages is controlled by transient signals from innate immune cells and longer acting signals from antigen-specific immune cells. Hence, prolonged recruitment and activation of PMN and macrophages is precarious as it may stall inflammatory resolution and lead to PMN mediated tissue injury and protein modifications, which may promote antigen-antibody reactions and transition to chronic inflammation (Nathan & Ding, 2010).
Figure 1. Beneficial inflammation requires active resolution.
Tissues experience frequent acute inflammation in response to injury, stress or infection. Recruitment of vascular PMN is initiated and amplified by carefully regulated pro-inflammatory circuits. Successful execution of acute inflammation requires phagocytosis of spent PMN by macrophages, a non-inflammatory response. Phagocytosis of PMN induces formation of anti-inflammatory mediators and differentiation to wound healing macrophages, which are essential to restore normal tissue function. Resident circuits in the cornea, uvea and retina, namely 15-LOX and/or HO produce autacoids that counter-regulate pro-inflammatory circuits, reduce PMN infiltration and activate macrophages to remove PMN. Dysregulation of this fundamental process leads to failed PMN resolution, activation of inflammatory macrophages and T-cells, setting the stage for an inflammatory disease.
Throughout our life the vast majority of healthy inflammatory responses, especially triggered by injury, result in the rapid recruitment of PMN to ingest and kill potential microbes and a subsequent wave of macrophages that removes apoptotic PMN and promotes wound healing (Figure 1). The resolution of the inflammatory response marks the successful protection of the compromised tissue, removal of dead cells and debris, and the return of the injured tissue to homeostasis and normal function. Unfortunately, the inflammatory program is regulated by a complex array of effector cells as well as inflammatory circuits and mediators in the epithelium, vasculature and mesenchymal cells, all of which are affected by environmental and genetic factors.
It is now recognized that non-resolving or dysregulated inflammation is a major factor in the global disease burden. Chronic inflammation and prolonged inflammation is a major feature of diseases such as atherosclerosis, obesity, diabetes, rheumatoid arthritis, asthma and some cancers. Many of these prominent diseases have well-established ocular manifestation such diabetic retinopathy, and scleritis, uveitis and dry eye syndrome in patients with rheumatoid arthritis. More importantly, it is now appreciated that inflammation also contributes significantly to most ocular diseases including the leading cause of blindness, age-related macular degeneration (AMD). In human's polymorphism in the complement system, namely complement factor H, CFH), a major and obligatory circuit in the innate immune system of all organism has been identified as a risk factor for AMD (Edwards et al., 2005; Edwards & Malek, 2007; Raisler et al., 2008). Moreover, animal studies using knockout mice that are deficient in macrophage recruitment (CC-cytokine ligand 2 (Ccl2)-/- and CC-cytokine receptor 2 (Ccr2)-/-) recapitulate key pathologies of human AMD, namely bilateral drusen-like accumulation and progression to spontaneous chorodial neovascularization (CNV) (Ambati et al., 2003; Nozaki et al., 2006; Raisler et al., 2008), which underscores the essential role of controlled leukocyte recruitment and activation for normal tissue function. In addition, recent reports have demonstrated that oxidative damage to the outer retina (photoreceptors), which is highly enriched in DHA, leads to the formation of a unique reactive oxidation fragment of DHA, carboxyethylpyrrole (CEP) (Hollyfield et al., 2008). CEP modified proteins are more abundant in the drusen of eyes from patients with AMD and auto-antibodies against the CEP protein adducts are elevated in the blood of AMD patients. Mice immunized with CEP adducts generate CEP antibodies and develop AMD-like lesion as well as deposition of complement in the Bruch's membrane. These clinical and experimental data provide an elegant model that links the slow accumulation of oxidative damage in the retina and dysregulation of the complement system, which may initiate the pathogenesis or the pathological progression of AMD (Hollyfield, 2010; Hollyfield et al., 2010).
Unraveling the cause of misdirected and dysregulated inflammation is the focus of long-standing and intense global research efforts that encompass virtually every organ system in the body. Traditionally, it was assumed that resolution of inflammation is a consequence of the winding down of pro-inflammatory pathways and the eventual removal of apoptotic PMN by macrophages. However, it is now clear that resolution of inflammation requires a delicate balance of counter-regulatory signals. These protective signals are resident in the tissue to maintain an anti-inflammatory tone and by activating pathways, which raise the threshold for activation of signal transduction and expression of pro-inflammatory circuits. Moreover, they regulate macrophage and lymphocyte differentiation and directly stimulate macrophages to phagocytose apoptotic PMN; a new paradigm that has been the subjective of recent reviews (Lawrence et al., 2002; Gilroy et al., 2004; Serhan & Savill, 2005; Serhan et al., 2007; Gronert, 2008; Kennedy & DeLeo, 2009; Nathan & Ding, 2010). It is also clear that current treatment options for inflammatory diseases, which are primarily aimed at suppressing or inhibiting pro-inflammatory pathways, do not treat the underlying cause of the disease but merely provide palliative and temporary treatment. Specifically, if the pre-inflammatory state of the epithelium, parenchyma, extracellular matrix and vasculature are not restored as well as a resident anti-inflammatory tone, then stimulation of inflammation persists leading to continual reinitiating of the inflammatory response (Nathan & Ding, 2010) and potential autoimmune reactions.
2. Keeping Ocular Immune and Inflammatory Responses in Check: Counter-Regulatory Signals
The notion that endogenous suppression of immune response is an active process was recognized early in the field of ocular research. Spurred by observations that date back 130 years, which described the immune privilege of the anterior chamber of the eye, the anterior segment has been a pioneering model to study immune regulation (Niederkorn & Stein-Streilein, 2010). It was initially thought that immune privilege was a feature of the anatomical isolation of the cornea and hence antigen ignorance endowed the tissue with a privileged immune response. Seminal research by Streilein and Kaplan discovered that immune privileged in the anterior segment of the eye is an active process (Kaplan et al., 1975; Kaplan & Streilein, 1977; Streilein & Niederkorn, 1981; Niederkorn & Stein-Streilein, 2010). The dynamic immuno-regulation and active suppression of antigen-specific inflammation has been termed anterior chamber-associated immune deviation (ACAID) and extensively studied (Streilein, 1995, 2003; Niederkorn, 2007; Niederkorn & Kaplan, 2007; Stein-Streilein & Taylor, 2007; Niederkorn, 2009; Niederkorn & Stein-Streilein, 2010), it involves a complex regulatory circuits that connects the eye, thymus, spleen and sympathetic nervous system and depends on both T regulatory lymphocytes (Treg) and natural killer cells (NKT). This impressive body of work also uncovered what has been called a “pot-pourri” of soluble anti-inflammatory and immunosuppressive factors (Niederkorn, 2009) that endow the anterior chamber with an unusual tone of suppressive signal to inhibit initiation of immune-mediated inflammation such as antigen induced delayed-type hypersensitivity. This large array of diverse factors include: TGF-β, α-melanocyte stimulating hormone (αMSH), soluble Fas ligand (sFasL), FasL and TRAIL many of which inhibit activation or induce apoptosis of leukocytes and/or lymphocytes (Niederkorn, 2009).
However, our understanding of how acute inflammation, infection and recruited PMN resolve from an injured or stressed ocular tissue, a frequent and essential innate response, is still evolving. More importantly, only limited studies have investigated if a deficiency in counter-regulatory signals rather than hyperactivity of pro-inflammatory circuits (the current target for anti-inflammatory therapy) leads to the vicious cycle of inflammatory disease. The relevance of counter-regulator signals is underscored by the fact that highly evolved, conserved and essential components of the innate immune system, namely the complement and Toll-like receptor system, require redundant regulatory proteins as checkpoints to control activation (Liew et al., 2005; Serhan et al., 2007; Bora et al., 2008). Soluble decoy receptors, soluble regulatory proteins, negative intracellular regulator and/or transmembrane protein regulators are critical to restrain the cytotoxic innate immune response and control regulatory T cells activation; thus they are an indispensable component of healthy self-resolving inflammation. Loss of function mutations in humans or mice have identified at least 81 genes that are required to maintain a basal anti-inflammatory tone in tissues and loss of their activity leads to spontaneous inflammation without infection, injury or antigen stimulation (Nathan & Ding, 2010), which underscores that active and resident anti-inflammatory circuits are critical checkpoints for the initiation and amplification of inflammation.
3. Pathways for Inflammatory Resolution in the Eye
Several recent studies using gene knockout mice as loss of function models have established an essential role of two prominent early response circuits in the resolution of acute inflammation in the eye. These circuits are 15-lipoxygenase (Humans: ALOX15, 15-LOX; mouse: Alox15, 12/15-LOX), which generates protective and pro-resolving lipid signals (Bazan, 2006; Kuhn & O'Donnell, 2006; Gronert, 2008; Serhan et al., 2008a; Calandria et al., 2009) and heme-oxygenase an essential enzyme that generates potent antioxidants and the bioactive gas carbon monoxide (Otterbein et al., 2003; Bach, 2005; Ryter et al., 2006; Abraham & Kappas, 2008; Gronert, 2008).
3.1. Resident Protective Lipid Mediator Circuits
Lipid autacoids are of particular interest as virtually every cell can generate specific and highly conserved bioactive lipid signals such as arachidonic acid (AA, ω-6 C20:4, eicosatetraenoic acid) derived eicosanoids. More importantly, these short-lived lipid signals are some of the earliest cellular responses to injury, infection or stress. It is thus not surprising that primary treatment options for inflammation, such as nonsteroidal anti-inflammatory drugs (NSAIDS) and corticosteroids, target biosynthetic enzymes (cyclooxygenase and phospholipase A2) for the formation of eicosanoids. However, it is now recognized that several members in the eicosanoid family do not amplify or initiate inflammation but are key counter-regulatory signals that inhibit inflammatory circuits and promote inflammatory resolution (Lawrence et al., 2002; Gilroy et al., 2004; Serhan et al., 2007; Gronert, 2008; Serhan et al., 2008a). In this regard a key enzyme in the formation of protective lipid mediators 15-LOX is the focus of ongoing research efforts. 15-LOX is one of the most inducible genes in macrophages and its expression is induced by interleukin-13 and interleukin-4 (Kuhn & O'Donnell, 2006), cytokines that induce the wound healing macrophages (M2) phenotype (Mosser & Edwards, 2008). Its essential role in maintaining tissue function is emphasized by the fact that 15-LOX is highly expressed in mucosal epithelial cells, corneal epithelium and retinal pigment epithelium. Moreover, it is intriguing that virtually all human epithelial cancers lose expression of 15-LOX indicating that the enzyme has an essential role in regulating epithelial proliferation and apoptosis (Shureiqi et al., 2005; Wu et al., 2008).
Even though, 15-LOX is a resident enzyme the cornea and retina its tissue levels and activity is highly upregulated by recruited 15-LOX expressing macrophages during acute inflammation or normal housekeeping activity of scavenging macrophages. 15-LOX is a key enzyme for the formation of the anti-inflammatory and pro-resolving eicosanoid, lipoxin A4 (LXA4), and the recently identified family of ω-3 PUFA derived lipid signals, docosahexaenoic acid (ω-3 C22:6, DHA) derived resolvins (RvD1-4) and protectins (NPD1). 15-LOX can either directly generate protective autacoids like NPD1 or it generates pivotal metabolic intermediates that are further transformed by 5-LOX (Figure 2), a prominent enzyme in macrophages and PMN, to LXA4 or RvD1-4 (Serhan et al., 2008b). These prominent endogenous lipid autacoids restrain PMN and lymphocyte activation, dendritic cell function, formation of pro-inflammatory chemokines by epithelial cells, promote clearance of apoptotic PMN by macrophages and regulate epithelial wound healing; hence, this lipid circuit is considered critical to the resolution of acute inflammation (Lawrence et al., 2002; Gilroy et al., 2004; Serhan et al., 2007; Gronert, 2008; Serhan et al., 2008b; Kenchgowda & Bazan, 2009). Enzymatic formation and structures of these oxygenated PUFA are highly conserved in most vertebrates including fish and frogs (Gronert et al., 1995; Hong et al., 2005; Serhan et al., 2008a; Serhan et al., 2008b), and it is remarkable that human pathogens such as Pseudomonas areuginosa and Toxoplasma gondii exploit this anti-inflammatory lipid circuit to redirect host immune responses (Aliberti et al., 2002a; Aliberti et al., 2002b; Bannenberg et al., 2004; Vance et al., 2004).
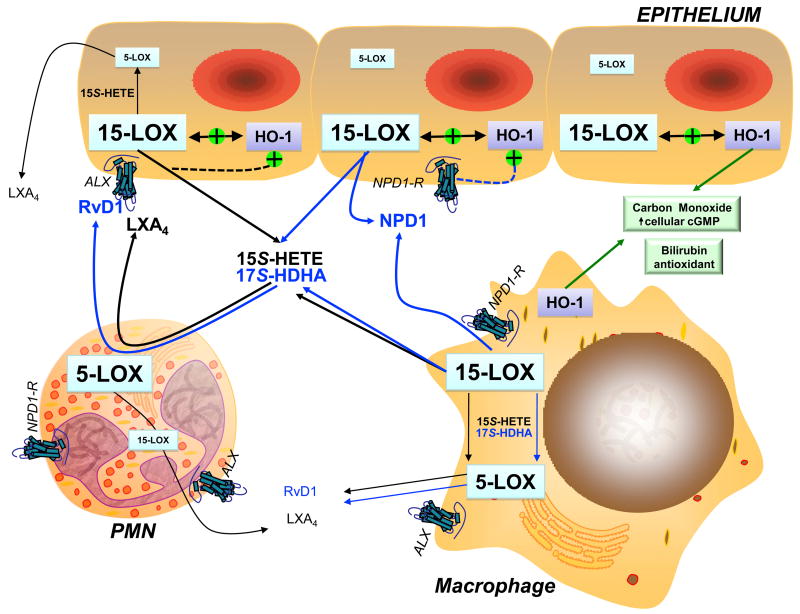
Figure 2. Biosynthetic pathways for the formation of 15-LOX-derived protective eicosanoids and docosanoids in the eye.
Scheme illustrating the potential cellular and multi-cellular biosynthetic routes for NPD1 (10R,17S-dihydroxy-DHA), RvD1 (7S,8R, 17S-trihydroxy-DHA) and LXA4 (5S,6R,15S-trihydroxy-LXA4) during an acute inflammatory response in the human eye. Interaction of the cytoprotective hemoxygenase system (HO-1) with 15-lipoxygenase (15-LOX) and regulation by the LXA4 receptor (ALX), which is also a receptor for RvD1, are indicated. Signaling by a potential NPD-1 G-protein coupled receptor is also shown. Docosahexaenoic acid (DHA, ω-3 PUFA) metabolic pathways are shown in blue and arachidonic acid (ω-6 PUFA) pathways are shown in black. Relative expression levels of 15-LOX and 5-LOX in epithelial cells, macrophages and PMN are indicated by font size.
3.2. The Lipoxin A4 Circuit
A rapidly evolving field of research now established the endogenous formation and/or protective bioactions of a 15-LOX-initiated circuit in the cornea, retina and uvea (Gronert et al., 2005; Bazan, 2006; Biteman et al., 2007; Qin et al., 2008; Bazan, 2009; Calandria et al., 2009; Kenchgowda & Bazan, 2009; Cortina et al., 2010; Leedom et al., 2010). Direct evidence for a central role of 15-LOX in corneal inflammatory response was obtained with 12/15-LOX knockout mice, which exhibit a phenotype of impaired wound healing and amplified inflammatory responses and inflammatory neovascularization, which correlate with impaired endogenous LXA4 formation (Gronert et al., 2005; Biteman et al., 2007; Leedom et al., 2010). A loss of function phenotype that is rescued by topical treatment with LXA4. In addition to LXA4, other mouse 12/15-LOX-derived metabolites, namely, 12(S)-HETE and 15(S)-HETE, can exert beneficial actions in the cornea. 12(S)-HETE and 15(S)-HETE can function as intracellular second messengers for growth factors and modulate the proliferation of corneal epithelial cells, thereby promoting wound closure (Kenchgowda & Bazan, 2009). 15-LOX in the healthy cornea is predominantly expressed in epithelial cells (Chang et al., 2005; Gronert et al., 2005) as expression and activity of 15-LOX is abrogated by de-epithelialization and restored during epithelial wound healing in mice (Gronert et al., 2005). More importantly, G-protein coupled receptors for LXA4 (ALX) are expressed in both human and mouse corneas (Gronert et al., 1998; Gronert et al., 2005; Jin et al., 2009; Leedom et al., 2010). ALX has been established as an anti-inflammatory receptor that transduces the counter-regulatory and pro-resolving bioactions of LXA4 and DHA-derived RvD1 and the receptor is expressed in epithelial cells, PMN, macrophages and peripheral blood T lymphocytes (Chiang et al., 2006; Krishnamoorthy et al., 2010). Its role in inflammatory resolution was established in a gain of function model employing mice that were engineered to express the human ALX receptor in leukocytes (Devchand et al., 2003). These transgenic mice in response to yeast antigen-induced peritonitis demonstrate reduced amplitude of acute inflammation and accelerated PMN resolution. It is thus of interest that 15-LOX and ALX receptor expression and activity are dynamically regulated during inflammatory responses in the cornea, which points towards a fundamental role of the LXA4 circuit in corneal inflammatory responses (Gronert et al., 2005; Jin et al., 2009; Leedom et al., 2010). In addition to corneal inflammatory responses the LXA4 circuit may have a role in intraocular inflammatory diseases as recent reports demonstrate that LXA4 treatment attenuates endotoxin-induced uveitis (EIU) (Medeiros et al., 2008; Karim et al., 2009). EIU is a common animal model that exhibits many of the key features of human uveitis, including dilation of vessels, macromolecule leakage, edema, and infiltration of leukocytes and lymphocytes. Topical treatment with LXA4 greatly attenuated EIU reducing protein leakage, PMN infiltration and pro-inflammatory circuits (i.e. TNF-α, COX-2, VEGF and NF-κB activation). The role of the 15-LOX/LXA4 circuit in retina or conjunctiva remains to be explored.
3.3. DHA an Essential ω-3 PUFA: Precursor for Protective Mediators
In addition to AA, cells release other essential PUFAs that are substrates for 15-LOX, especially DHA. Like AA, DHA is found in all human tissues, plasma and milk at concentrations 1-20% of total fatty acids (Arterburn et al., 2006). Moreover, in the cerebral cortex, sperm and retina, DHA is present at higher concentrations than AA. In particular, retinal photoreceptor outer segments have the highest DHA content of any cell and an unusual DHA-retention ability and numerous lines of evidence establish a critical role for DHA in human physiology, namely, neuronal and retinal photoreceptor functions (Bazan, 2006). Indeed, experimental evidence demonstrates decreased blood levels of DHA in various forms of retinitis pigmentosa and in animal models of inherited retinal degeneration (Bazan, 2006). Moreover, current clinical trials (i.e. NEI Age-Related Eye Disease Study) have demonstrated that dietary ω-3 PUFA intake is inversely associated with neovascular age-related macular degeneration (AMD) (SanGiovanni et al., 2000; SanGiovanni & Chew, 2005; SanGiovanni et al., 2007; SanGiovanni et al., 2008; SanGiovanni et al., 2009; Sangiovanni et al., 2009), namely consumption of fish oils (eicosapentaenoic acid (EPA) and DHA) lowers progression of bilateral drusen to central geographic atrophy and neovascularization. Increased dietary intake of ω-3 PUFAs also correlated with a decreased incidence of dry eye syndrome in a well-characterized population of women participating in the Women's Health Study (Miljanovic et al., 2005). These clinical findings are supported by animal studies, which show that topical treatment with α-linolenic acid (ω-3, C18:3), a precursor for essential ω-3 PUFA (EPA and DHA), reversed leukocyte infiltration and expression of TNF-α and IL-1α in a murine model of dry eye (Rashid et al., 2008). Moreover, in animal models of macular degeneration and retinopathy of prematurity diets enriched in DHA and EPA reduced the development of retinal lesions, inflammation and pathological neovascularization (Connor et al., 2007; Tuo et al., 2009). These and numerous other reports have identified dietary ω-3 PUFA, especially DHA, as an important factor for ocular health and inflammatory diseases (Bazan, 2006).
The anti-inflammatory, cardioprotective and immunosuppressive actions of dietary ω-3 PUFA are well established based on long standing research efforts; but the molecular mechanism for this striking actions was only recently uncovered (Bazan, 2006; Gronert & Hassan, 2007; Gronert, 2008; Serhan et al., 2008a; Serhan et al., 2008b). Specifically, EPA and DHA are transformed in a fashion analogous to eicosanoid biosynthesis to novel classes of ω-3 PUFA-derived mediators. These mediators have been named resolvins and protectins as they are formed during the resolution phase of inflammation and exhibit potent anti-inflammatory, pro-resolving and neuroprotective properties. Reports from several laboratories provide compelling evidence that these protective ω-3 PUFA-derived mediators provide the elusive molecular mechanism for the remarkable beneficial and essential effects of dietary ω-3 PUFA(Lukiw et al., 2005; Gonzalez-Periz et al., 2006; Connor et al., 2007; Gronert & Hassan, 2007; Levy et al., 2007; Schwab et al., 2007; Serhan et al., 2008a; Bazan, 2009; Gonzalez-Periz et al., 2009; Hassan & Gronert, 2009; Kenchgowda & Bazan, 2009).
In view of the abundance of DHA, especially in the retina and brain, considerable research interest has focused on novel protective DHA-derived autacoids, in particular NPD1, which regulates critical events in the acute inflammatory/reparative response, its resolution and protects neurons and retinal pigment epithelial cells against oxidative stress induced apoptosis (Gronert, 2008; Bazan, 2009; Kenchgowda & Bazan, 2009; Zhang & Bazan, 2010). It is of particular interest that this protective mediator is generated by a single enzyme, namely 15-LOX, which is highly expressed in retinal pigmented and corneal epithelial cells and wound healing macrophages. 15-LOX directly generates NPD1 (10,17-dihydroxy-DHA) and 17-hydroxy-DHA (17-HDHA) (Figure 2); the complete structure of NPD1 has been assigned (Serhan et al., 2006). 17-HDHA is further metabolized by leukocyte/epithelial 5-LOX (Figure 2), in mechanism analogous to lipoxin biosynthesis, to several distinct 17S-resolvins (RvD1-4), which like NPD1 exhibit anti-inflammatory actions (Serhan et al., 2008b). Hence, during acute inflammation interactions of leukocytes with epithelial cells leads to the formation of a temporally defined arrays of protective DHA-derived mediators, which are an integral component of the resolution pathway. Significant endogenous formation of these protective DHA-derived mediators (NPD1, RvD1, 17-HDHA) has been reported by several laboratories, including our group, in both human and mouse tissues (Lukiw et al., 2005; Gonzalez-Periz et al., 2006; Levy et al., 2007; Serhan et al., 2008b; Gonzalez-Periz et al., 2009; Hassan & Gronert, 2009), which includes reports in the retina and cornea (Gronert et al., 2005; Bazan, 2006; Connor et al., 2007; Qin et al., 2008). The protective DHA-derived signals have distinct but overlapping bioactions and both in vitro and in vivo experiments have demonstrated that similar to LXA4, resolvins and protectins reduce leukocyte infiltration, regulate cytokines/chemokines and reactive oxygen species, and more importantly increase macrophage phagocytosis of apoptotic PMN (Bazan, 2006; Gronert & Hassan, 2007; Schwab et al., 2007; Gronert, 2008; Zhang & Bazan, 2010). Hence, 15-LOX sets in place a resident lipid circuit that counter-regulates inflammatory circuits, contributes to the anti-inflammatory tone of the tissue and during inflammation is amplified by recruited effector cells to promote resolution of PMN.
The important role of DHA derived signals and the resident epithelial 15-LOX circuit is underscored in the retina where retinal pigment epithelial cells are essential to maintain and phagocytose photoreceptors, recycle DHA and form the critical retinal-blood barrier. Failure of RPE cells to function properly leads to photoreceptor damage or death, and consequently, impaired vision or blindness (Bazan, 2006; Zhang & Bazan, 2010). The retina is particularly prone to oxidative stress due to the high oxygen demand and blood flow in the tissue. Oxidative stress, trauma and vascular inflammation induce RPE dysfunction, which contributes to retinopathies, including pathological neovascularization and AMD. Hence, it is striking that the 15-LOX circuit is emerging as an RPE resident pathway that counteract these challenges (Bazan, 2006, 2009; Zhang & Bazan, 2010). Oxidative stress increases production of DHA-derived NPD1 in RPE cells, which inhibits oxidative stress-mediated pro-inflammatory gene induction and apoptosis, and ultimately promotes RPE cell survival. More importantly, human RPE cells deficient in 15-LOX exhibit increased sensitivity to oxidative stress-induced apoptosis, an impaired protective function that is restored selectively by treatment with NPD1. NPD1 has also been shown to inhibit retinal ganglion cell apoptosis following optic nerve transaction, pathological retinal angiogenesis and inflammation, and promote nerve regeneration in the innervated cornea (Bazan, 2006; Connor et al., 2007; Qin et al., 2008; Bazan, 2009; Cortina et al., 2010; Zhang & Bazan, 2010). Taken together, these recent studies demonstrate a role for 15-LOX-triggered DHA signals in maintaining and protecting the neural retina.
3.4. The Cytoprotective Heme-Oxygenase System
Heme-oxygenases (HO-1 and HO-2) constitute an essential cytoprotective system that is the rate-limiting enzyme for metabolizing essential heme and recycling iron. In the process, HO-1 generates the antioxidant biliverdin, which is converted to bilirubin, and the bioactive gas carbon monoxide. The heme-oxygenase system contains two principle enzymes: 1) HO-2, which is expressed at basal level in many healthy uninjured tissues and 2) HO-1, which is a highly inducible cytoprotective and early response gene. HO-1 amplification by genetic or pharmacological approaches causes striking anti-proliferative, anti-inflammatory and anti-apoptotic actions in many disease models (Otterbein et al., 2003; Ryter et al., 2006; Abraham & Kappas, 2008). HO-1 essential role in health and inflammation are underscored by the fact that human deficiencies in HO-1 and genetic deletion of HO-1 in mice leads to spontaneous chronic and non-resolving inflammation without injury or infection. Moreover, HO-1 expression is induced in macrophages specifically during the resolution phase and HO-1 mediates the anti-inflammatory actions of the key anti-inflammatory and immune regulatory cytokine IL-10 (Willis et al., 2000; Willoughby et al., 2000; Bach, 2005).
The HO system is constitutively expressed in the healthy cornea (HO-2) and is highly upregulated during acute and self-resolving injury responses (HO-1). Deletion of HO-2 in mice results not only in a defect of basal HO activity but also failed upregulation of HO-1 in acute inflammatory/reparative response of the cornea. In these HO deficient mice normal self-resolving inflammation and epithelial injury shifts to sustained and amplified leukocyte infiltration, failed wound healing and pronounced pathological neovascularization, hallmarks of chronic inflammation (Abraham et al., 1995; Seta et al., 2006; Biteman et al., 2007; Patil et al., 2008; Bellner et al., 2009). Strikingly, the phenotype of chronic inflammation correlates with impaired formation of LXA4 and in 12/15-LOX deficient mice (Alox15 KO) induction of HO-1 is impaired and correlates with amplified PMN infiltration, chemokine formation and delayed wound healing. The 15-LOX KO phenotype of dysregulated inflammation is rescued by adding back LXA4, which restores normal HO-1 expression, wound healing and inflammation. Experiments with human corneal epithelial cells have confirmed that specific 15-LOX derived mediators, LXA4, NPD1 and 17-HDHA, amplify expression of cytoprotective HO-1 (Biteman et al., 2007). In addition, systemic pharmacological upregulation of HO-1 in endotoxin-induced uveitis affords effective protection against acute inflammation by reducing leukocytes in the aqueous humor and down-regulating pro-inflammatory cytokines and inducible nitric oxide (iNOS) (Ohta et al., 2003). The role of the HO system in the retina and conjunctiva remains to be defined.
Taken together, these findings suggest an essential role of the HO system in ocular inflammation, which is consistent with an impressive body of work that has demonstrated that HO is a fundamental protective response to inflammation and oxidative stress as well as a key feature of inflammatory resolution (Willoughby et al., 2000; Ohta et al., 2003; Otterbein et al., 2003; Bach, 2005; Abraham & Kappas, 2008; Gronert, 2008). Moreover, these studies as well as reports from other inflammatory models (Willis et al., 2000; Willoughby et al., 2000; Nascimento-Silva et al., 2005) suggest an interdependence of the HO system with lipid mediators circuits that have been identified as key component of the resolution program (Lawrence et al., 2002; Gilroy et al., 2004; Serhan et al., 2007), such as the 15-LOX/LXA4 circuit. Dysregulation of these ocular resolution circuits may provide insight and therapeutic targets for inflammatory diseases in the visual axis.
Acknowledgments
I gratefully acknowledge the National Institutes of Health for supporting research in the author's laboratory (EY16136, HL34300 and P30EY003176), which is cited in this review.
Abbreviations
- PUFA
polyunsaturated fatty acid
- DHA
Docosahexaenoic Acid, ω-3 PUFA C22:4
- AA
eicosatetraenoic acid, ω-6 PUFA C20:4
- ALX
Lipoxin A4 receptor, fPRL1
- HO
Heme-oxygenase
- COX
cyclooxygenase, prostaglandin H synthase
- LOX
lipoxygenase
- PMN
polymorphonuclear leukocytes, neutrophils
- CO
carbon monoxide
- LXA4
Lipoxin A4
- NPD1
neuroprotectin D1, protectin D1, 10R, 17S-dihydroxy-DHA
- RvD
resolvin D
- VEGF
vascular endothelial growth factor
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NG, da Silva JL, Lavrovsky Y, Stoltz RA, Kappas A, Dunn MW, Schwartzman ML. Adenovirus-mediated heme oxygenase-1 gene transfer into rabbit ocular tissues. Invest Ophthalmol Vis Sci. 1995;36:2202–2210. [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nature Immunology. 2002a;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. Journal of Experimental Medicine. 2002b;196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19:1216–1219. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, Serhan CN. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. British Journal of Pharmacology. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J Lipid Res. 2009 50:S400–405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellner L, Martinelli L, Halilovic A, Patil K, Puri N, Dunn MW, Regan RF, Schwartzman ML. Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis. J Pharmacol Exp Ther. 2009;331:925–932. doi: 10.1124/jpet.109.158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, Laniado-Schwartzman M, Gronert K. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- Bora NS, Jha P, Bora PS. The role of complement in ocular pathology. Semin Immunopathol. 2008;30:85–95. doi: 10.1007/s00281-008-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG. Selective survival rescue in 15-lipoxygenase-1 deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MS, Schneider C, Roberts RL, Shappell SB, Haselton FR, Boeglin WE, Brash AR. Detection and Subcellular Localization of Two 15S-Lipoxygenases in Human Cornea. Invest Ophthalmol Vis Sci. 2005;46:849–856. doi: 10.1167/iovs.04-1166. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Arita M, Hong S, Bannenberg G, Moussignac RL, Gronert K, Serhan CN. Human ALX receptor regulates neutrophil recruitment in transgenic mice: Roles in inflammation and host defense. FASEB Journal. 2003;17:652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Malek G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007;10:119–132. doi: 10.1007/s10456-007-9064-2. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, Titos E, Horrillo R, Ferre N, Deulofeu R, Arroyo V, Rodes J, Claria J. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- Gronert K. Lipid autacoids in inflammation and injury responses: A matter of privilege. Molecular Interventions. 2008;8:28–35. doi: 10.1124/mi.8.1.7. [DOI] [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A<sub>4</sub> receptor that is regulated by interleukin (IL)-13 and interferon Œ≥ and inhibits tumor necrosis factor Œ±-induced IL-8 release. Journal of Experimental Medicine. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K, Hassan IR. Reaping the benefits of renal protective lipid autacoids. Drug Discovery Today: Disease Mechanisms. 2007;4:3–10. [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Gronert K, Virk SM, Herman CA. Thrombocytes are the predominant source of endogenous sulfidopeptide leukotrienes in the American bullfrog (Rana catesbeiana) Biochim Biophys Acta. 1995;1259:203–210. doi: 10.1016/0005-2760(95)00160-3. [DOI] [PubMed] [Google Scholar]
- Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182:3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci. 2010;51:1275–1281. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–298. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Tjonahen E, Morgan EL, Lu Y, Serhan CN, Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins-Mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana R. Novel Anti-inflammatory and Pro-resolving Lipid Mediators Block Inflammatory Angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- Kaplan HJ, Streilein JW, Stevens TR. Transplantation immunology of the anterior chamber of the eye. II. Immune response to allogeneic cells. J Immunol. 1975;115:805–810. [PubMed] [Google Scholar]
- Karim MJ, Bhattacherjee P, Biswas S, Paterson CA. Anti-inflammatory effects of lipoxins on lipopolysaccharide-induced uveitis in rats. J Ocul Pharmacol Ther. 2009;25:483–486. doi: 10.1089/jop.2008.0134. [DOI] [PubMed] [Google Scholar]
- Kenchgowda S, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2009;5:879–891. doi: 10.1194/jlr.R001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Progress in Lipid Research. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kumar V, Fausto N, Abbas A. Robbins and Cotran Pathological Basis of Disease. W.B. Saunders Company; Philadelphia: 2004. [Google Scholar]
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nature Reviews Immunology. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, Perez V, Huang AJ, Pflugfelder SC, Stern ME, Baudouin C, Beuerman RW, Burns AR, Calder VL, Calonge M, Chodosh J, Coster DJ, Dana R, Hazlett LD, Jones DB, Kim SK, Knop E, Li DQ, Mitchell BM, Niederkorn JY, Pearlman E, Wilhelmus KR, Kurie E. Pathways of corneal and ocular surface inflammation: a perspective from the cullen symposium. Ocul Surf. 2005;3:S131–138. doi: 10.1016/s1542-0124(12)70238-0. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Rodrigues GB, Figueiredo CP, Rodrigues EB, Grumman A, Jr, Menezes-de-Lima O, Jr, Passos GF, Calixto JB. Molecular mechanisms of topical antiinflammatory effects of lipoxin A(4) in endotoxin-induced uveitis. Mol Pharmacol. 2008;74:154–161. doi: 10.1124/mol.108.046870. [DOI] [PubMed] [Google Scholar]
- Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol. 2005;289:C557–563. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving Inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Regulatory T cells and the eye. Chem Immunol Allergy. 2007;92:131–139. doi: 10.1159/000099263. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Role of NKT cells in anterior chamber-associated immune deviation. Expert Rev Clin Immunol. 2009;5:137–144. doi: 10.1586/1744666X.5.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY, Kaplan HJ. Rationale for immune response and the eye. Chem Immunol Allergy. 2007;92:1–3. doi: 10.1159/000099234. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY, Stein-Streilein J. History and physiology of immune privilege. Ocul Immunol Inflamm. 2010;18:19–23. doi: 10.3109/09273940903564766. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Kikuchi T, Arai S, Yoshida N, Sato A, Yoshimura N. Protective role of heme oxygenase-1 against endotoxin-induced uveitis in rats. Exp Eye Res. 2003;77:665–673. doi: 10.1016/j.exer.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Patil K, Bellner L, Cullaro G, Gotlinger KH, Dunn MW, Schwartzman ML. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest Ophthalmol Vis Sci. 2008;49:3379–3386. doi: 10.1167/iovs.07-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Patil KA, Gronert K, Sharma SC. Neuroprotectin D1 inhibits retinal ganglion cell death following axotomy. Prostaglandins Leukot Essent Fatty Acids. 2008;79:201–207. doi: 10.1016/j.plefa.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Raisler BJ, Nozaki M, Baffi J, Hauswirth WW, Ambati J. Toward a higher fidelity model of AMD. Adv Exp Med Biol. 2008;613:185–192. doi: 10.1007/978-0-387-74904-4_21. [DOI] [PubMed] [Google Scholar]
- Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Sack RA, Tan KO, Tan A. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Investigative Ophthalmology & Visual Science. 1992;33:626–640. [PubMed] [Google Scholar]
- SanGiovanni JP, Agron E, Clemons TE, Chew EY. Omega-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch Ophthalmol. 2009;127:110–112. doi: 10.1001/archophthalmol.2008.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY. omega-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90:1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Agron E, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R, Sperduto RD. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris FL, 3rd, Gensler GR, Kurinij N, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105:1292–1298. doi: 10.1542/peds.105.6.1292. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual antiinflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008a;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008b;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta F, Bellner L, Rezzani R, Regan RF, Dunn MW, Abraham NG, Gronert K, Laniado-Schwartzman M. Heme Oxygenase-2 Is a Critical Determinant for Execution of an Acute Inflammatory and Reparative Response. Am J Pathol. 2006;169:1612–1623. doi: 10.2353/ajpath.2006.060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shureiqi I, Wu Y, Chen D, Yang XL, Guan B, Morris JS, Yang P, Newman RA, Broaddus R, Hamilton SR, Lynch P, Levin B, Fischer SM, Lippman SM. The critical role of 15-lipoxygenase-1 in colorectal epithelial cell terminal differentiation and tumorigenesis. Cancer Res. 2005;65:11486–11492. doi: 10.1158/0008-5472.CAN-05-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J, Taylor AW. An eye's view of T regulatory cells. J Leukoc Biol. 2007;81:593–598. doi: 10.1189/jlb.0606383. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Unraveling immune privilege. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nature Reviews Immunology. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Ross RJ, Herzlich AA, Shen D, Ding X, Zhou M, Coon SL, Hussein N, Salem N, Jr, Chan CC. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2135–2139. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, Moore AR, Willoughby DA. Heme oxygenase isoform expression in cellular and antibody-mediated models of acute inflammation in the rat. J Pathol. 2000;190:627–634. doi: 10.1002/(SICI)1096-9896(200004)190:5<627::AID-PATH556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Willoughby DA, Moore AR, Colville-Nash PR, Gilroy D. Resolution of inflammation. Int J Immunopharmacol. 2000;22:1131–1135. doi: 10.1016/s0192-0561(00)00064-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Fang B, Yang XQ, Wang L, Chen D, Krasnykh V, Carter BZ, Morris JS, Shureiqi I. Therapeutic molecular targeting of 15-lipoxygenase-1 in colon cancer. Mol Ther. 2008;16:886–892. doi: 10.1038/mt.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bazan NG. Lipid-Mediated Cell Signaling Protects against Injury and Neurodegeneration. J Nutr. 2010;140:858–863. doi: 10.3945/jn.109.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]