Abstract
Regulatory T cells (T reg cells) play a major role in controlling the pathogenic autoimmune process in type 1 diabetes (T1D). Interleukin 2 (IL-2), a cytokine which promotes T reg cell survival and function, may thus have therapeutic efficacy in T1D. We show that 5 d of low-dose IL-2 administration starting at the time of T1D onset can reverse established disease in NOD (nonobese diabetic) mice, with long-lasting effects. Low-dose IL-2 increases the number of T reg cells in the pancreas and induces expression of T reg cell–associated proteins including Foxp3, CD25, CTLA-4, ICOS (inducible T cell costimulator), and GITR (glucocorticoid-induced TNF receptor) in these cells. Treatment also suppresses interferon γ production by pancreas-infiltrating T cells. Transcriptome analyses show that low-dose IL-2 exerts much greater influence on gene expression of T reg cells than effector T cells (T eff cells), suggesting that nonspecific activation of pathogenic T eff cells is less likely. We provide the first preclinical data showing that low-dose IL-2 can reverse established T1D, suggesting that this treatment merits evaluation in patients with T1D.
In type 1 diabetes (T1D), the immune system destroys the insulin-producing β cells of the pancreas. The conventional treatment, consisting of life-long daily and multiple insulin injection only imperfectly prevents severe hypoglycemia and vascular complications. Thus, there is a clear need for improved treatments of T1D. At clinical diabetes onset, residual β cells still produce insulin, offering a window for therapeutic intervention to stop the autoimmune destruction and rescue β cell function. Extensive research and clinical studies are being developed in this direction (Chatenoud and Bluestone, 2007).
Even though the etiology and pathogenesis of human T1D are still poorly known, major paradigms of its physiopathology have been established from studies in the nonobese diabetic (NOD) mice. We and others have shown that the CD4+CD25+Foxp3+ regulatory T cells (T reg cells) play a major role in the control of T1D (Salomon et al., 2000; Sakaguchi et al., 2006). Moreover, injecting islet-specific T reg cells can reverse established diabetes in NOD mice (Tang et al., 2004). However, at present the lack of good manufacture practice procedures to obtain antigen-specific T reg cells precludes the translation of such approach to the clinic. Stimulating the patient’s own T reg cell compartment to down-regulate the autoimmune process represents a more accessible alternative.
IL-2 was identified 30 yr ago for its strong capacity to stimulate T cells in vitro. Therefore, it has been used in the clinic for boosting the immune response in certain cancers and infectious diseases (Zhang et al., 2005; Ahmadzadeh and Rosenberg, 2006). However, the results were often disappointing (Zhang et al., 2005). The unexpected and severe T cell–mediated autoimmune syndrome of IL-2–deficient mice showed the complex role of this cytokine in the immune system (Sadlack et al., 1993). These findings were then explained by the critical role of IL-2 on the peripheral survival and suppressive function of T reg cells (Papiernik et al., 1998). Consistent with this, IL-2 administration has been shown to expand and activate T reg cells in humans and mice (Zhang et al., 2005; Ahmadzadeh and Rosenberg, 2006). Thus, although IL-2 has pleiotropic functions, its major impact is to favor T reg cell activity (Malek, 2008). Besides, NOD mice present a qualitative diminution of IL-2 production (Yamanouchi et al., 2007), and a genetic predisposing factor to T1D development in humans and NOD mice is linked to IL-2/IL-2R gene polymorphisms (Vella et al., 2005).
We have recently reported that insufficient IL-2 amounts in the pancreas are responsible for poor T reg cell survival in this tissue, which could lead to progressive breakdown of self-tolerance and development of diabetes in NOD mice (Tang et al., 2008). We and others also showed that young prediabetic NOD mice treated with low-dose IL-2 alone, or together with rapamycin, can be protected from the development of disease (Serreze et al., 1989; Rabinovitch et al., 2002; Tang, et al., 2008). However, although >200 different treatments can prevent T1D in NOD mice, only very few are effective to cure established disease (Shoda et al., 2005). In this paper, we show that only 5-d administration of low-dose IL-2 at diabetes onset can induce long-lasting remission in the treated animals. IL-2 did not stimulate the diabetogenic effector T cells (T eff cells) but rather specifically stimulated CD4+Foxp3+ T reg cells in the pancreas to dampen the inflammatory milieu. Thus, in the presence of pathogenic T cells, IL-2 at a low dose is a selective T reg cell stimulator endowed with a great therapeutic potential.
RESULTS AND DISCUSSION
Short-term administration of low-dose IL-2 induces long-lasting diabetes remission in NOD mice but is inefficient in T reg cell–deficient mice
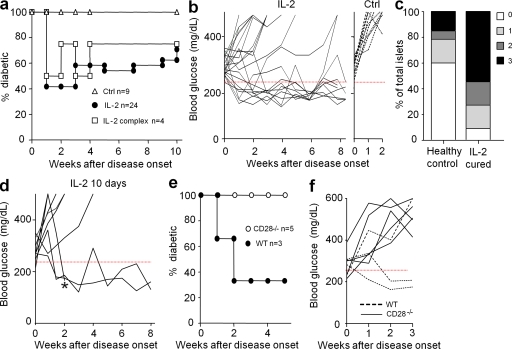
We assessed if 5 d of low-dose IL-2 administration could be effective to cure clinical diabetes in NOD mice. Remarkably, this treatment induced diabetes remission in 60% of the mice within 1 wk, and most of them remained normoglycemic over the 10-wk duration of the experiment (Fig. 1, a and b). Five mice were followed up for 30–50 wk after treatment and were still diabetes free (unpublished data). Administration of an IL-2/anti–IL-2 mAb complex, which induces a more dramatic T reg cell expansion than IL-2 alone (Tang et al., 2008), provided a similar but not better curative effect. Histological examination of mice that remained diabetes free for 12–35 wk after IL-2 treatment showed that pancreatic islets were more infiltrated than age-matched nondiabetic controls, indicating that the treatment did not induce a long-lasting clearance of insulitis (Fig. 1 c). We tried to optimize treatment efficacy by prolonging IL-2 administration or by combining IL-2 with drugs that are favorable for T reg cells, such as rapamycin, glucocorticoids, and α-CD3 (Battaglia et al., 2005; Chen et al., 2006; Chatenoud and Bluestone, 2007). Prolonging IL-2 administration from 5 to 10 d induced disease remission in three out of eight mice that had not reverted after 5 d (Fig. 1 d). The other treatments did no show obvious improved outcome (unpublished data).
Figure 1.
Diabetes remission by IL-2 therapy. (a and b) Spontaneous new-onset diabetic NOD mice (two consecutive blood glucose concentrations between 250 and 350 mg/dl) were treated for 5 d with PBS or denatured IL-2 (Ctrl), 25,000 IU IL-2 (IL-2), or 0.5 µg IL-2 + 5 µg anti–IL-2 (IL-2 complex). Blood glucose concentrations were monitored. (a) Percentage of diabetic mice. (b) Blood glucose concentrations in IL-2–treated (left) or control (right) mice. The 250 mg/dl blood glucose value is indicated by a dashed red line. Age at treatment onset was 26 ± 9.4 wk in mice that did not revert and 24 ± 7.6 wk in the cured ones. (c) Intraislet infiltration in IL-2–treated mice that remain free of overt diabetes 12–35 wk after treatment (IL-2 cured) and diabetes-free age-matched controls (healthy controls) was evaluated histologically. The graph shows the percentage of islets with no infiltration (0), peri-insulitis (1), moderate insulitis with <50% islet area infiltrated (2), and severe insulitis with >50% islet area infiltrated (3). Data for the control group represent 60 islets from four mice, and for the IL-2–treated group represent 22 islets from three mice. (d) Blood glucose concentrations in new onset diabetic mice that had not reverted after 5 d of IL-2 treatment and were further treated for 5 more days. Age at treatment onset was 22 ± 4.5 wk in mice that did not revert and 22 ± 9.2 in the cured ones. * indicates a mouse that was sacrificed for analysis. (e and f) New onset diabetic CD28−/− or WT littermate NOD mice were treated for 5 d with 25,000 IU IL-2 and blood glucose concentrations were monitored. (e) Percentage of diabetic mice. (f) Blood glucose concentrations.
Our previous results indicated that disease prevention by the IL-2/anti–IL-2 mAb complex was associated with increased T reg cell survival in the pancreas (Tang et al., 2008). To address the role of T reg cells in IL-2 remission of overt diabetes, we repeated the treatment in CD28 KO NOD mice, which have a profound deficit in T reg cells (Salomon et al., 2000). IL-2 administration did not reverse hyperglycemia in CD28 KO mice, whereas diabetes remission was observed in two out of three WT littermates (Fig. 1, e and f), suggesting that low-dose IL-2 therapy is inefficient in the absence of natural T reg cells. Overall, our results show, for the first time, that low-dose IL-2 administration is one of the few treatments that can cure spontaneous overt diabetes in NOD mice (Shoda et al., 2005).
Short-term administration of low-dose IL-2 does not modify the peripheral homeostasis of hematopoietic cells
Given that T reg cells are not the only cells sensitive to IL-2 and that a systemic IL-2–mediated activation of T reg cells could lead to detrimental global immunosuppression, we performed a comprehensive analysis of the effect of IL-2 administration on the different hematopoietic subpopulations potentially sensitive to IL-2 in the spleen and pancreatic LN (PLN). Prediabetic (8–14 wk old) NOD mice were treated for 5 d with 25,000 IU (the low dose mentioned in the Introduction) or 250,000 IU IL-2. No significant change was observed at low-dose IL-2 on the total cell number, the proportion of CD4+, CD8+, CD4+Foxp3+, CD8+Foxp3+, NK, B, and CD11b+ cells (Table S1), or their activation status (unchanged CD44 expression; unpublished data). Also, the proportions of the different dendritic cell subpopulations remained unchanged (unpublished data). Only when high-dose IL-2 was used was cell homeostasis modified, with a significant increase of the cell numbers in PLN and the proportion of CD4+Foxp3+, CD4+CD25+Foxp3−, and CD8+Foxp3+ populations in the spleen. These data indicated that 5 d of low-dose IL-2 administration did not modify steady-state homeostasis of hematopoietic cells in lymphoid organs. In contrast, we have previously reported that low-dose administration of the IL-2/anti–IL-2 complex favored the expansion of T reg, T eff, NK, and CD8+ T cells in lymphoid tissues leading to a possible undesired systemic response (Tang et al., 2008). We thus focused on the mechanism of action of low-dose plain IL-2 for the rest of our study.
Low-dose IL-2 shows a profound and specific effect on pancreatic T reg cells
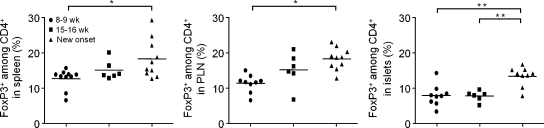
Data suggest that T reg cells present in the pancreas control the progression to destructive insulitis in prediabetic mice (Feuerer et al., 2009). However, under highly inflammatory conditions, reduced survival or insufficient function of T reg cells may unleash T eff cell autoimmune attack (Tang et al., 2008). To establish whether the IL-2 curative effect was related to a modification of T reg cell numbers and/or characteristics in the pancreas, we quantified their proportion in the spleen, PLN, and pancreas of 8–9- and 15–16-wk-old prediabetic and new onset diabetic NOD mice. The proportion of T reg cells gradually increased with age and disease evolution in the spleen, PLN, and islets (Fig. 2). Unexpectedly, in prediabetic mice (8–16 wk old), significantly lower proportions of T reg cells (8% of CD4+ T cells) were found in the pancreas compared with normal ratios (10–15% of CD4+ T cells) in the spleen and PLN (Fig. 2 legend, p-values). At disease onset, the percentage of pancreatic T reg cell was significantly increased to reach 14% of CD4+ T cells. A similar increase in the proportion of T reg cell in PLN with aging has been reported (Mellanby et al., 2007). However, in this same study, T reg cell proportions were unchanged between nondiabetic and recent-onset diabetic mice, which is at odds with our results. This could be a result of variations between different NOD mice colonies or of the technique used to purify islet-infiltrating T cells. Our results confirm and complete our previous and paradoxical observation that T reg cells increase with age and disease development but not sufficiently to control autoimmunity (Tang et al., 2008). Also, we show for the first time that over time, consistently lower proportions of T reg cell are found in the pancreas compared with lymphoid tissues.
Figure 2.
The proportion of T reg cells increases with age and disease in lymphoid tissues and in the pancreas of NOD mice. The percentage of Foxp3+ cells among CD4+ cells in the spleen, PLN, and pancreas of untreated nondiabetic NOD mice of different ages and recent onset diabetic NOD mice (new onset) was quantified by FACS. Graphs show cumulative individual and mean (horizontal bars) data from two to five independent experiments. Each symbol represents an individual mouse. *, P < 0.05; **, P < 0.001. The percentage of T reg cells in the pancreas at 8–9 wk is significantly different from the percentage of T reg cells in the PLN (P < 0.05) and spleen (P < 0.05) of mice of the same age. The percentage of T reg cells in the pancreas at 15–16 wk is significantly different from the percentage of T reg cells in the PLN (P < 0.01) and spleen (P < 0.001) of mice of the same age.
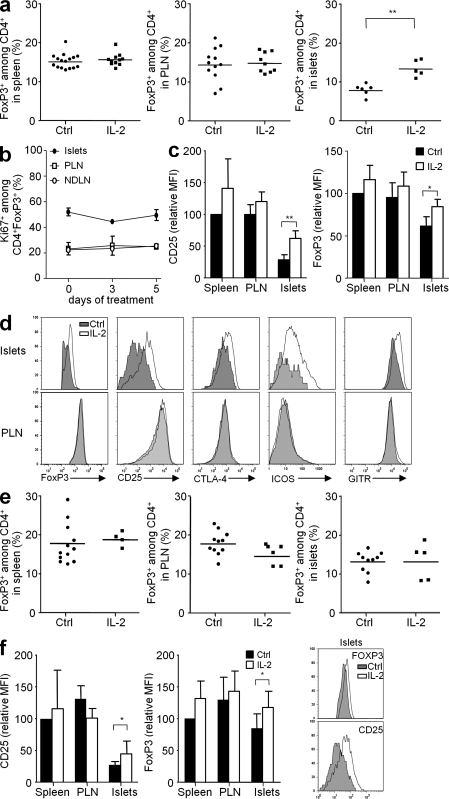
We then studied the IL-2 effect on the inflammatory pancreatic infiltrate of late prediabetic mice (14–19 wk old). After 5 d of low-dose IL-2 administration, the proportion of T reg cells increased 1.7-fold in the pancreas of prediabetic mice, reaching similar proportions to those in the spleen and PLN, where T reg cell proportions were unchanged (Fig. 3 a). This increase was not associated with augmented T reg cell division, as the percentage of cycling cells, determined by the expression of Ki67 (Fig. 3 b) and EdU incorporation (not depicted), was unaffected during IL-2 treatment. T reg cell division in LN was also unaffected by IL-2 treatment (Fig. 3 b). We also verified that T eff cell cycling was not modified under IL-2 treatment (Fig. S1 a). The increase in the numbers of pancreatic T reg cells induced by IL-2 is most probably the result of an improvement of T reg cell survival (Tang et al., 2008) or, alternatively, of the conversion of T eff cells into induced T reg cells (Zheng et al., 2007), the reexpression of Foxp3 in ex–T reg cells (Zhou et al., 2009), or the increased recruitment of T reg cells in the pancreas.
Figure 3.
IL-2 treatment increases T reg cell proportions and reinforces the T reg cell phenotype specifically in inflamed pancreas. Prediabetic (a, c, and d) or new onset diabetic (e and f) NOD mice received five daily injections of PBS (Ctrl) or 25,000 IU IL-2 and were sacrificed 2 h after the last injection. (a and e) The percentage of Foxp3+ cells among CD4+ cells was determined by FACS in the spleen, PLN, and pancreas. Symbols represent individual mice and horizontal bars are the means. (b) Percentage of Ki67+ cells among CD4+Foxp3+ cells from islets, PLN, and nondraining LN (NDLN) of prediabetic mice untreated (day 0) or treated with daily injections of 25,000 IU IL-2 for 3 or 5 d (days 3 and 5). n = 2–5 mice per group. The graph is representative of three independent experiments. (c and f) Mean fluorescence intensity (MFI) of CD25 and Foxp3 expression among CD4+Foxp3+ cells infiltrating the spleen, PLN, and pancreas, expressed as relative percentage from the mean fluorescence intensity value in the spleen, which has an arbitrary value of 100%. Graphs show cumulative mean data ± SEM from control mice (black) and mice treated with IL-2 for 5 d (white), from four to six independent experiments. *, P < 0.05; **, P < 0.001. A representative histogram of CD25 and Foxp3 intensities on CD4+ gated islet cells is shown for new onset diabetic animals (f, right). (d) Expression of the indicated molecules by CD4+Foxp3+ cells from mice treated with PBS (gray) or IL-2 (white) for 5 d. Graphs are representative of two to six independent experiments.
We also observed that IL-2 treatment was associated with a rise in the expression of molecules associated with T reg cell function such as CD25, Foxp3, CTLA-4 (CTL antigen 4), inducible T cell costimulator (ICOS), and glucocorticoid-induced TNF receptor (GITR; Fig. 3, c and d). Again, this effect was only noticed in pancreas-infiltrating T reg cells and not in T eff cells of the islets or PLN (Fig. S1 b).
Similar analyses were repeated in new-onset diabetic mice. In this case, in which high percentages of pancreatic T reg cells are already present before treatment, we did not observe a further increase in their numbers (Fig. 3 e). However, and as in prediabetic mice, IL-2 treatment induced a significant rise in the expression of CD25 and Foxp3 in pancreatic T reg cells but not in lymphoid tissues (Fig. 3 f).
To evaluate if the specific effect of IL-2 on pancreatic T reg cells was unique to this nonlymphoid tissue, we studied the effect of IL-2 on T reg cells residing in other nonlymphoid sites, such as salivary glands, skin, colon, and liver. The percentage of T reg cells only significantly augmented after IL-2 administration in the salivary glands (Fig. S2 a). However, IL-2 treatment induced the up-regulation of CD25 expression in all the assessed tissues and a minimal increase of Foxp3 expression in salivary glands and liver (Fig. S2 b).
The dual specificity achieved by the low-dose IL-2 treatment is noteworthy. IL-2 effects are more marked in tissues with ongoing autoimmunity (pancreas and salivary glands) at the spatial level and to the T reg cells at the cellular level. This could be explained by the fact that there is a local deficit of IL-2, at least in the pancreas of NOD mice (Tang et al. 2008), and that exogenous low-dose IL-2 administration corrects this particular deficiency.
IL-2 treatment decreases IFN-γ production in the pancreas
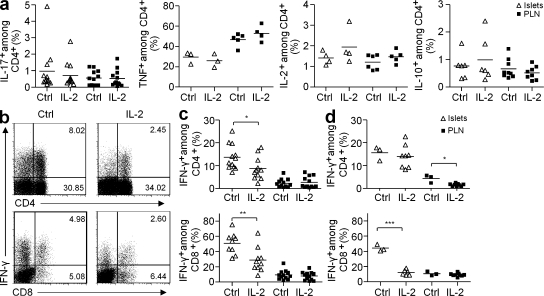
IL-10, IL-2, and TGF-β can contribute to the control of pancreatic inflammation (Rabinovitch et al., 2002; You et al., 2007; Tang et al., 2008). Thus, we assessed the production of these cytokines by pancreas-infiltrating T cells after 5 d of IL-2 or PBS treatment in prediabetic mice. The proportions of CD4+ and CD8+ T cell subsets among total CD45+ cells were similar in PBS- versus IL-2–treated animals (33 ± 13 vs. 31 ± 15, n = 11, respectively for CD4+ T cells and 10 ± 5 vs. 9 ± 7, n = 10, respectively for CD8+ T cells). IL-10 and IL-2 production were not significantly increased in the CD4+ T cells of IL-2–treated animals and we could not detect TGF-β under the same stimulation conditions (Fig. 4 a).
Figure 4.
IL-2 treatment induces a decrease in IFN-γ production by pancreas-infiltrating T cells. Cytokine production by CD4+ and CD8+ T cells from pancreas and PLN of NOD mice, treated as in Fig. 3, was quantified after ex vivo restimulation with PMA-ionomycin. (a) Percentage of CD4+ producing IL-10, IL-2, TNF, or IL-17 in control or IL-2–treated prediabetic NOD mice. Data cumulate two to three independent experiments. (b) Representative dot plot of IFN-γ production in CD4+ (top) and CD8+ (bottom) T cells in the pancreas of prediabetic mice treated or not with IL-2. Numbers in quadrants indicate percentage of cells. (c and d) Percentage of IFN-γ+ CD4+ (top) and CD8+ (bottom) T cells in islets and PLN of prediabetic (c) and new-onset diabetic (d) NOD mice. Data cumulate five and three experiments, respectively. Symbols represent individual mice and horizontal bars are the means. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
We then studied the production of TNF, IL-17, and IFN-γ, which are known to be toxic for β cells. TNF production by T cells, macrophages, and dendritic cells remained unchanged under IL-2 treatment (Fig. 4 a and not depicted) and similar small amounts of IL-17 were detected in control and IL-2–treated mice (Fig. 4 a). Remarkably, IL-2 treatment induced an important reduction of IFN-γ production by both CD4+ and CD8+ pancreas-infiltrating T cells but no change in the PLN of prediabetic treated animals (Fig. 4, b and c).
Interestingly, when we repeated the experiment in new onset diabetic mice, IFN-γ production in IL-2–treated mice was also massively suppressed, specifically in pancreatic CD8+ T cells and to a lesser extent in PLN CD4+ T cells (Fig. 4 d). Consistent with this, it has been shown that T reg cells limit IFN-γ production specifically in the pancreas, precluding the progression from nondestructive insulitis to diabetes (Feuerer et al., 2009). Also, a similar reduction of intraislet IFN-γ production is associated with other treatments shown to prevent T1D development in NOD mice, such as CFA, BCG, all-trans retinoic acid, and p277 peptide treatments (Ablamunits et al., 1998; Serreze et al., 2001; Van et al., 2009). These data suggest that one of the mechanisms of action of low-dose IL-2 is the limitation of IFN-γ production by the pancreas-infiltrating T cells.
In vivo low-dose IL-2 treatment selectively modifies the T reg cell transcriptome, leaving the T eff cell gene profile practically unaltered
To have a global understanding of the mode of action of low-dose IL-2 on T cells in vivo, we performed gene array analysis. We compared the transcriptome of highly purified T reg cells and T eff cells obtained from spleen and LNs of Foxp3-GFP NOD mice that received the same low-dose IL-2 treatment that cured new onset diabetic mice (T reg cell–IL-2 and T eff cell–IL-2) or PBS (T reg cell–PBS and T eff cell–PBS).
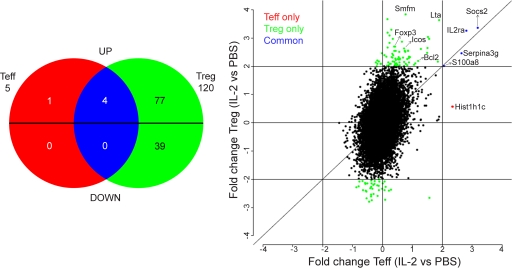
Analysis of genes with a fold change equal to or greater than two revealed that in T reg cells, IL-2 induced the overexpression of 81 genes and down-regulation of 39 genes (Fig. 5, green). Surprisingly, only the expression of five genes (out of the ∼31,500 present in the gene chip) was modified in T eff cells. Four were also up-regulated in T reg cells (Socs2, IL2Ra, Serpina3g, and S100a8; Fig. 5, blue) and one was up-regulated only in T eff cells (Hist1h1c; Fig. 5, red). This result puts forward that nonspecific activation of pathogenic T eff cells as a result of low-dose IL-2 administration is less likely.
Figure 5.
In vivo IL-2 treatment selectively modifies the T reg cell transcriptome. Gene array analysis was performed on FACS-sorted T eff cells and T reg cells from spleen and LN of Foxp3-GFP NOD mice treated with PBS or IL-2, as in Fig. 3. (Left) Venn diagram comparing the two gene lists (T eff cell–IL-2 vs. T eff cell–PBS and T reg cell–IL-2 vs. T reg cell–PBS) filtered at twofold changes and with overlapping probes discarded. Probes are separated in up- or down-regulation by IL-2 from reference PBS. Genes modified by IL-2 only in T eff cells are in red, only in T reg cells are in green, and in both subsets are in blue. Each number represents the number of genes in each subgroup. (Right) Fc/Fc plot comparison of fold change expression values of IL-2 effect on T reg cells versus T eff cells for probes, with detection p-values <0.05. Only significant probes with fold change ≥2 are colored. Data are from one experiment with biological duplicates for each condition.
The complete list of genes affected by IL-2 treatment in T reg cells is shown in Fig. S3. Globally, most of these 120 genes do not have a direct relationship with the biology and suppressive function of T reg cells, which is in accordance with the absence of effect of IL-2 on peripheral T reg cells that we observed in our FACS analysis (Table S1 and Fig. 3). Only a few genes involved in T reg cell function and survival, such as IL-2Ra, Foxp3, Icos, and Bcl-2, were up-regulated by IL-2. This increased mRNA expression was only reflected by a minor, but nonstatistically significant, augmentation of the protein expression for CD25 and Foxp3 (Fig. 3 c) and no modification for ICOS protein expression (Fig. 3 d) in T reg cells of lymphoid tissues. Interestingly, the important rise in the expression level of these three proteins in pancreatic T reg cells (Fig. 3, c and d) could reflect an enhanced sensibility to IL-2 caused by a local deficit of this cytokine in this tissue (Tang et al., 2008).
In conclusion, we show that a short course of low-dose IL-2 administration at diabetes onset can reverse established disease in NOD mice. This effect is long lasting and inefficient in the absence of naturally occurring T reg cells. The main mechanism seems to be an increased T reg cell activity in the pancreas. Drugs that boost the patients’ T reg cell compartment are under extensive investigation. This approach has been tested in a clinical trial using agonistic α-CD28 (Suntharalingam et al., 2006) and in preclinical models with high doses of IL-2, IL-2/α–IL-2 complex, or Flt3-L (Swee et al., 2009). However, secondary effects caused by an action of these drugs on other cells, such as NK and T eff cells, have been observed and these drugs can lead to a systemic increase of T reg cells. As opposed to these treatments, low-dose IL-2 appears to act specifically on T reg cells in inflamed nonlymphoid tissues, with no detectable effect on other cells, minimizing the risk of generalized immune suppression. There is one ongoing clinical trial testing the use of IL-2 combined with rapamycin to treat T1D patients (NCT00525889), a combination which has been shown to prevent disease in mice (Rabinovitch et al., 2002), but neither IL-2 alone nor its association with rapamycin have ever been shown to cure established disease. Our findings, together with the fact that IL-2 has been used in the clinic for >25 yr, constitute the first preclinical data indicating that low-dose IL-2 can control established autoimmunity and may place IL-2 as a safe therapeutic option for the treatment of pathologies with local and chronic inflammation.
MATERIALS AND METHODS
Mice.
NOD mice were obtained from Charles River Laboratories or from our own colony. NOD Cd28−/− (Lenschow et al., 1996) and NOD mice expressing GFP under the control Foxp3 gene promoter (generated by V. Kuchroo, Brigham and Women’s Hospital, Boston, MA) were maintained in our animal facility under specific pathogen-free conditions in agreement with current European legislation on animal care, housing, and scientific experimentation. All protocols were approved by The Regional Ethics Committee in Animal Experiment N°3 of Ile-de-France region.
IL-2 treatment.
Mice were treated with daily i.p. injections of 25,000 IU of recombinant human IL-2 (Proleukin; Novartis), 250,000 IU IL-2, or 0.5 µg of recombinant mouse IL-2 and 5 µg of anti–IL-2 mAb complex for 5 consecutive days. For Cd28−/− mice, treatment was done during 10 d. Denatured IL-2 was used as control. Glycosuria was measured using colorimetric strips (Multistix; Bayer) and glycemia was quantified by a glucometer (Optium Xceed; Abbott Diabetes Care).
Spleen, LN, and tissue-infiltrating lymphocyte preparation.
Spleen and LN were isolated and dissociated in PBS 3% FCS. For the other tissues and organs, mice were perfused with 0.9% NaCl, digested with collagenase/DNase solution, and filtered, as previously described (Cassan et al., 2006). For pancreas and liver, a percoll density gradient step was performed as previously described (Baekkeskov et al., 1981).
Antibodies and flow cytometry analysis.
Anti-CD4, anti-CD25, anti-CD314 (NKG2D), anti-ICOS, anti-B220, anti-GITR, anti-CD44, anti–CTLA-4, anti-CD11b, anti-CD11c, anti–IL-2, anti–IL-10, anti-TNF, anti–IL-17, anti–IFN-γ, and anti-Ki67 labeled with PE, allophycocyanin, PerCP, or Alexa Fluor 700, were purchased from BD. The PE- or Pacific blue–labeled anti-Foxp3 staining was performed using a kit (eBioscience). In some cases, anti-Ki67 antibody (Ab) was added together with the anti-Foxp3 Ab. For intracellular cytokine staining, cells were restimulated with 1 µg/ml PMA (Sigma-Aldrich) and 0.5 µg/ml ionomycin (Sigma-Aldrich) for 4 h in the presence of 1 µl/ml GolgiPlug (BD). After cell surface staining, intracellular staining was performed using the Cytofix/Cytoperm kit (BD). Cells were acquired on an LSR II (BD) and analyzed with FlowJo (Tree Star, Inc.) software.
Histology.
Pancreas were fixed in formol solution and processed for paraffin embedding. 5-µm-thick sections were stained with hematoxylin/eosin and the degree of insulitis was evaluated microscopically.
Sample generation and DNA microarray hybridization and analysis.
GFP-NOD mice were treated for 5 d with PBS or 25,000 IU IL-2 and sacrificed 2 h after the last injection. Untouched CD4+ T cells were enriched by magnetic cell separation (CD4+ T Cell Isolation kit; Miltenyi Biotec), labeled with CD4-PE Ab, and FACS sorted as CD4+GFP+ (T reg cells) and CD4+GFP− (T eff cells). RNA was generated (RNeasy Mini kit; QIAGEN) from the sorted cells. RNA quality was verified in a Bioanalyzer (Agilent Technologies) and measured with a NanoDrop 1000 (Thermo Fisher Scientific).
Microarray experiments were performed on a MouseWG-6 BeadChip (Illumina), a genome-wide array with 45,282 probes. Data were extracted and Quantile normalized using BeadStudio software (Illumina). The working lists were created, filtering probes with detection p-values <0.05 for at least half of the chips involved in the comparison and discarding overlapping probes. Each dataset was derived from two biologically independent replicate samples. Independent samples were compared by computing fold ratios and filtered at twofold. The Microarray data accession no. is E-MEXP-2689.
Statistical analyses.
Statistical significances were calculated using the two-tailed unpaired Student t test with 95% confidence intervals.
Online supplemental material.
Fig. S1 shows that T eff cell division and phenotype are not modified by low-dose IL-2 treatment. Fig. S2 provides information on IL-2 effect on T reg cells in different nonlymphoid tissues. Fig. S3 shows a heat map of IL-2 effect on T reg cell gene expression. Table S1 shows the systemic effects of low and high doses of IL-2. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100209/DC1.
Acknowledgments
We thank Professors Vijay Kuchroo and Anne Cooke for providing us with the Foxp3-GFP NOD mice, Professor Jose Cohen and Alexandre Boissonnas for critical reading of the manuscript, and Gaëlle Martin for technical help.
This work was supported by the Juvenile Diabetes Research Foundation (1-2005-1056) and Agence Nationale de la Recherche (ANR-05-MIIM-003-01). Y. Grinberg-Bleyer, A. Baeyens, and G. Fourcade were supported by Ministère de la Recherche.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- GITR
- glucocorticoid-induced TNF receptor
- ICOS
- inducible T cell costimulator
- NOD
- nonobese diabetic
- PLN
- pancreatic LN
- T1D
- type 1 diabetes
- T eff cell
- effector T cell
- T reg cell
- regulatory T cell
References
- Ablamunits V., Elias D., Reshef T., Cohen I.R. 1998. Islet T cells secreting IFN-gamma in NOD mouse diabetes: arrest by p277 peptide treatment. J. Autoimmun. 11:73–81 10.1006/jaut.1997.0177 [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M., Rosenberg S.A. 2006. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 107:2409–2414 10.1182/blood-2005-06-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Kanatsuna T., Klareskog L., Nielsen D.A., Peterson P.A., Rubenstein A.H., Steiner D.F., Lernmark A. 1981. Expression of major histocompatibility antigens on pancreatic islet cells. Proc. Natl. Acad. Sci. USA. 78:6456–6460 10.1073/pnas.78.10.6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Stabilini A., Roncarolo M.G. 2005. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 105:4743–4748 10.1182/blood-2004-10-3932 [DOI] [PubMed] [Google Scholar]
- Cassan C., Piaggio E., Zappulla J.P., Mars L.T., Couturier N., Bucciarelli F., Desbois S., Bauer J., Gonzalez-Dunia D., Liblau R.S. 2006. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J. Immunol. 177:1552–1560 [DOI] [PubMed] [Google Scholar]
- Chatenoud L., Bluestone J.A. 2007. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 7:622–632 10.1038/nri2134 [DOI] [PubMed] [Google Scholar]
- Chen X., Oppenheim J.J., Winkler-Pickett R.T., Ortaldo J.R., Howard O.M. 2006. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 36:2139–2149 10.1002/eji.200635873 [DOI] [PubMed] [Google Scholar]
- Feuerer M., Shen Y., Littman D.R., Benoist C., Mathis D. 2009. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 31:654–664 10.1016/j.immuni.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D.J., Herold K.C., Rhee L., Patel B., Koons A., Qin H.Y., Fuchs E., Singh B., Thompson C.B., Bluestone J.A. 1996. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 5:285–293 10.1016/S1074-7613(00)80323-4 [DOI] [PubMed] [Google Scholar]
- Malek T.R. 2008. The biology of interleukin-2. Annu. Rev. Immunol. 26:453–479 10.1146/annurev.immunol.26.021607.090357 [DOI] [PubMed] [Google Scholar]
- Mellanby R.J., Thomas D., Phillips J.M., Cooke A. 2007. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 121:15–28 10.1111/j.1365-2567.2007.02546.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiernik M., de Moraes M.L., Pontoux C., Vasseur F., Pénit C. 1998. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371–378 10.1093/intimm/10.4.371 [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Suarez-Pinzon W.L., Shapiro A.M., Rajotte R.V., Power R. 2002. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes. 51:638–645 10.2337/diabetes.51.3.638 [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A.C., Horak I. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 75:253–261 10.1016/0092-8674(93)80067-O [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8–27 10.1111/j.0105-2896.2006.00427.x [DOI] [PubMed] [Google Scholar]
- Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440 10.1016/S1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- Serreze D.V., Hamaguchi K., Leiter E.H. 1989. Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2:759–776 10.1016/0896-8411(89)90003-6 [DOI] [PubMed] [Google Scholar]
- Serreze D.V., Chapman H.D., Post C.M., Johnson E.A., Suarez-Pinzon W.L., Rabinovitch A. 2001. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 166:1352–1359 [DOI] [PubMed] [Google Scholar]
- Shoda L.K., Young D.L., Ramanujan S., Whiting C.C., Atkinson M.A., Bluestone J.A., Eisenbarth G.S., Mathis D., Rossini A.A., Campbell S.E., et al. 2005. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 23:115–126 10.1016/j.immuni.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., Panoskaltsis N. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355:1018–1028 10.1056/NEJMoa063842 [DOI] [PubMed] [Google Scholar]
- Swee L.K., Bosco N., Malissen B., Ceredig R., Rolink A. 2009. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 113:6277–6287 10.1182/blood-2008-06-161026 [DOI] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Bi M., Finger E.B., Szot G., Ye J., Masteller E.L., McDevitt H., Bonyhadi M., Bluestone J.A. 2004. In vitro–expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199:1455–1465 10.1084/jem.20040139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Adams J.Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E., Piccirillo C.A., Salomon B.L., Bluestone J.A. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 28:687–697 10.1016/j.immuni.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Y.H., Lee W.H., Ortiz S., Lee M.H., Qin H.J., Liu C.P. 2009. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 58:146–155 10.2337/db08-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A., Cooper J.D., Lowe C.E., Walker N., Nutland S., Widmer B., Jones R., Ring S.M., McArdle W., Pembrey M.E., et al. 2005. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am. J. Hum. Genet. 76:773–779 10.1086/429843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi J., Rainbow D., Serra P., Howlett S., Hunter K., Garner V.E., Gonzalez-Munoz A., Clark J., Veijola R., Cubbon R., et al. 2007. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat. Genet. 39:329–337 10.1038/ng1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S., Leforban B., Garcia C., Bach J.F., Bluestone J.A., Chatenoud L. 2007. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc. Natl. Acad. Sci. USA. 104:6335–6340 10.1073/pnas.0701171104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chua K.S., Guimond M., Kapoor V., Brown M.V., Fleisher T.A., Long L.M., Bernstein D., Hill B.J., Douek D.C., et al. 2005. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat. Med. 11:1238–1243 10.1038/nm1312 [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Wang P., Gray J.D., Horwitz D.A. 2007. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 178:2018–2027 [DOI] [PubMed] [Google Scholar]
- Zhou X., Bailey-Bucktrout S.L., Jeker L.T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J.A. 2009. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10:1000–1007 10.1038/ni.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]