Abstract
Inhibitory receptors play a crucial role in regulating CD8 T-cell function during chronic viral infection. T-cell Ig- and mucin-domain–containing molecule–3 (Tim-3) is well known to negatively regulate T-cell responses, but its role in CD8 T-cell exhaustion during chronic infection in vivo remains unclear. In this study, we document coregulation of CD8 T cell exhaustion by Tim-3 and PD-1 during chronic lymphocytic choriomeningitis virus infection. Whereas Tim-3 was only transiently expressed by CD8 T cells after acute infection, virus-specific CD8 T cells retained high Tim-3 expression throughout chronic infection. The majority (approximately 65% to 80%) of lymphocytic choriomeningitis virus–specific CD8 T cells in lymphoid and nonlymphoid organs coexpressed Tim-3 and PD-1. This coexpression of Tim-3 and PD-1 was associated with more severe CD8 T-cell exhaustion in terms of proliferation and secretion of effector cytokines such as IFN-γ, TNF-α, and IL-2. Interestingly, CD8 T cells expressing both inhibitory receptors also produced the suppressive cytokine IL-10. Most importantly, combined blockade of Tim-3 and PD-1 pathways in vivo synergistically improved CD8 T cell responses and viral control in chronically infected mice. Taken together, our study defines a parameter for determining the severity of CD8 T cell dysfunction and for identifying virus-specific CD8 T cells that produce IL-10, and shows that targeting both PD-1 and Tim-3 is an effective immune strategy for treating chronic viral infections.
During chronic viral infection, virus-specific CD8 T cells become unresponsive to viral antigens and persist in a nonfunctional exhausted state (1). These exhausted CD8 T cells are characterized by the inability to produce immune-stimulatory cytokines, lyse virally infected cells, and proliferate (1). After CD8 T-cell exhaustion was initially characterized in the murine lymphocytic choriomeningitis virus (LCMV), such a functional impairment has been a common feature in human chronic viral infections such as, HIV, hepatitis B virus, and hepatitis C virus (HCV) (2). These functional defects in responding T cells are probably a primary reason for failure of immunological control of these persisting pathogens.
Recent studies have focused on the crucial role of inhibitory receptors in regulating T-cell exhaustion during chronic viral infections. Programmed death 1 (PD-1), an inhibitory receptor of the CD28 superfamily, was shown to be highly expressed on exhausted CD8 T cells compared with functional memory T cells in the LCMV system, and in vivo blockade of this pathway restored the function of virus-specific CD8 T cells, resulting in enhanced viral control (3). Involvement of the PD-1 pathway has also been shown in various chronic viral infections including HIV, hepatitis B virus, and HCV in humans (4, 5), and during simian immunodeficiency virus infection in nonhuman primates (6). These studies have suggested that PD-1 could be a major inhibitory pathway during chronic infection and manipulation of this pathway may have therapeutic potential. However, blockade of PD-1 pathway does not completely restore T-cell function (4, 5, 7), indicating the involvement of other negative regulatory pathways in CD8 T-cell exhaustion. Gene expression profiling studies have identified the presence of a number of other potential inhibitory receptors on exhausted CD8 T cells such as 2B4, LAG-3, CTLA-4, PirB, GP49, and CD160 (8). Moreover, considerable evidence indicates that the expression of these receptors is important for regulating multiple functional aspects of CD8 T-cell exhaustion (7, 9). Therefore, a more thorough understanding of the importance of inhibitory receptors in CD8 T-cell exhaustion may reveal potential therapeutic targets leading to the restoration of CD8 T-cell function and better viral control.
T-cell Ig- and mucin-domain–containing molecule-3 (Tim-3) was initially identified as a molecule expressed on T helper (Th) 1, but not Th2 (10). Interaction of Tim-3 with its ligand, galectin-9, regulates Th1 responses by promoting the death of Th1 cells and induces peripheral tolerance (11). Recently, it was reported that Tim-3 was expressed by virus-specific T cells during HIV-1 and HCV infections, and the expression levels correlated with the state of CD8 T-cell exhaustion (12, 13). In addition, blockade of Tim-3 improved the responsiveness of the exhausted T cells in vitro (12, 13), suggesting Tim-3 as another inhibitory marker of exhausted T cells during chronic viral infection. However, it is currently unclear whether Tim-3 regulates CD8 T cell exhaustion in cooperation with PD-1 during chronic viral infection. Furthermore, it will be important to explore the possibility of a synergistic effect of blocking both the Tim-3 and PD-1 pathways for providing new opportunities in antiviral therapy.
In this study, we longitudinally investigated the expression of Tim-3 on virus-specific CD8 T cells during acute and chronic LCMV infection. We were especially interested in determining the coexpression of Tim-3 and PD-1 on CD8 T cells to identify populations with differential dysfunction during chronic viral infection. In addition, we examined the effect of in vivo blockade of Tim-3 and PD-1 regulatory pathways on the reversal of exhausted CD8 T cells and viral control.
Results
Tim-3 Expression Defines a Subpopulation of PD-1+ Exhausted CD8 T Cells During Chronic LCMV Infection.
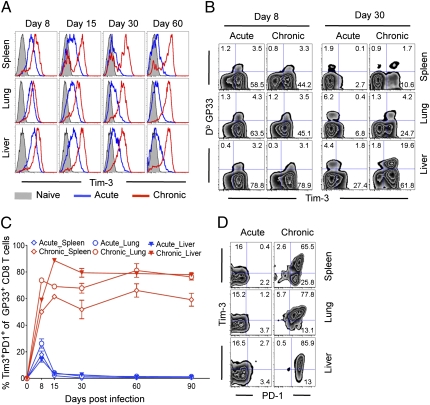
We examined expression of Tim-3 on LCMV-specific effector, memory, and exhausted CD8 T cells during acute or chronic infection using the Armstrong (Arm) or clone-13 (CL-13) strains of LCMV (14). First, we analyzed Tim-3 expression on LCMV-specific CD8 (GP33+CD8) T cells in lymphoid and nonlymphoid organs at various time points after infection. During acute infection, the percentage of GP33+CD8 T cells expressing Tim-3 (Tim3+GP33+) was significantly increased in spleen, lung, and liver at d 8 after infection (Fig. 1A and Fig. S1A). However, this frequency rapidly decreased to approximately 10% in all tissues at day 30 after Arm infection (Fig. 1B and Fig. S1A). On the contrary, during chronic infection, the frequency of Tim3+GP33+ T cells was markedly elevated at day 8 in all tissues, which was sustained at approximately 70% to 80% for at least 90 d (Fig. 1A and Fig. S1A). Although the frequency of Tim3+GP33+ T cells was similarly increased at day 8 in acute and chronic infection, we observed significantly stronger mean fluorescence intensity (MFI) of Tim-3 expression during chronic versus acute viral infections at day 8 (Fig. S1B). Moreover, this high intensity of Tim-3 expression on virus-specific CD8 T cells was maintained during chronic viral infection. These results indicate that Tim-3 is transiently up-regulated at intermediate levels on activated T cells, and is quickly down-regulated in acute infection, but continues to be expressed at much higher levels during chronic infection.
Fig. 1.
Sustained coexpression of Tim-3 and PD-1 on antigen-specific CD8 T cells during chronic LCMV infection. Expression of Tim-3 and PD-1 on GP33-specific CD8 T cells during LCMV Arm (acute) or Clone-13 (chronic) infections was analyzed by multiparameter flow cytometry. (A) Intensity of Tim-3 expression on GP33-specific CD8 T cells during infection. (B) Representative data show the frequency of GP33-specific CD8 T cells expressing Tim-3 on day 8 and 30 after infection. (C) The frequency of GP33-specific CD8 T cell coexpressing Tim-3 and PD-1 is shown over time. (D) Representative data showing coexpression of PD-1 and Tim-3 by exhausted LCMV-specific CD8 T cells on day 30 after infection. Numbers in quadrants indicate percent of each subset. Error bars represent SEM. Data are representative of three independent experiments with three mice per experiment.
Because it was not yet clear whether Tim-3 and PD-1 are expressed by distinct or overlapping populations of CD8 T cells during chronic infection (12, 13), we next analyzed the coexpression of Tim-3 and PD-1. During acute viral infection, only at day 8 after infection was a small portion of GP33-specific CD8 T cells coexpressing Tim-3 and PD-1 (Tim3+PD1+) detectable (Fig. 1C). In contrast, chronic viral infection resulted in much higher frequency of Tim3+PD1+ cells, which persisted even at day 90 (Fig. 1C). We could also detect another dominant population expressing only PD-1 but not Tim-3 (Tim3−PD+) during chronic infection (Fig. 1D). Thus, exhausted CD8 T cells found during chronic LCMV infection could be divided into Tim3+PD1+ and Tim3−PD1+ subsets. Notably, the distribution of Tim3+PD1+ or Tim3−PD+ was slightly different depending on the tissues analyzed, and a small percentage of CD8 T cells detectable in the lung expressed only Tim-3 but not PD-1 (Tim3+PD1−; Fig. 1D), suggesting differential phenotypic characteristics of exhausted CD8 T cells in varying anatomical sites. The presence of this Tim3+PD1+ cell could also be defined by looking at total CD8 T cells (Fig. S1C).
Coexpression of Tim-3 and PD-1 Correlates with More Severe Exhaustion of Virus-Specific CD8 T Cells During Chronic Virus Infection.
We identified two dominant populations of virus-specific CD8 T cells expressing PD-1 alone (Tim3−PD1+) or coexpressing Tim-3 (Tim3+PD1+) during chronic LCMV infection. We further assessed the phenotypic profile of virus-specific Tim3−PD1+ and Tim3+PD1+ CD8 T cells by using a panel of markers, including other inhibitory and differentiation markers. The Tim3+PD1+ virus-specific CD8 T cells displayed higher levels of other inhibitory receptors such as LAG3 and 2B4, along with lower levels of CD62L and CD127, than the Tim3−PD1+ population (Fig. S2). Considering the phenotypic signature of CD8 T-cell exhaustion (8), this suggests that the virus-specific Tim3+PD1+ T cells may represent a more severely exhausted subpopulation.
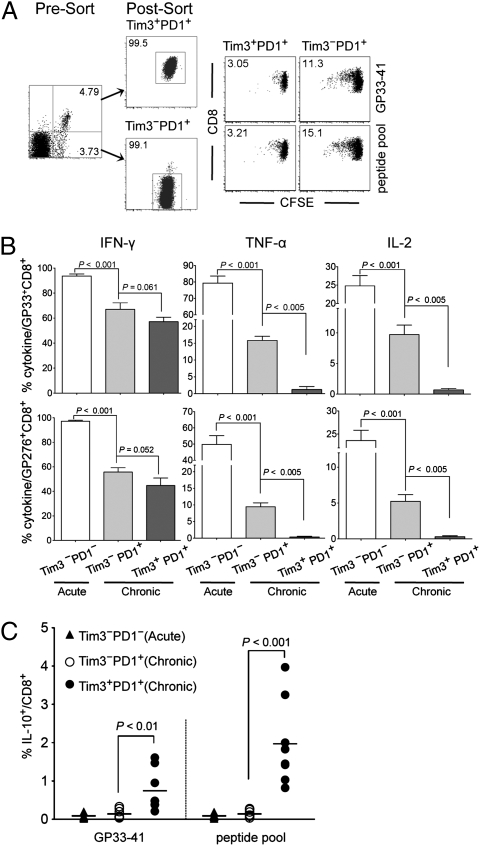
We next directly compared the functional properties of purified population of Tim3−PD1+ and Tim3+PD1+ CD8 T cells. As the majority of antigen-specific CD8 T cells in the spleen are Tim3−PD1+ or Tim3+PD1+ during chronic infection (Fig. 1 and Fig. S1), we focused on investigating the functionality of these two subsets. To investigate the proliferation capacity, equal numbers of GP33-specific Tim3−PD1+ or Tim3+PD1+ cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with GP33-41 peptide or LCMV peptide pool. When cell proliferation was assessed by CFSE dilution, some division of Tim3−PD1+ cells was observed, whereas Tim3+PD1+ cells hardly showed any proliferation after peptide stimulation (Fig. 2A). These results show that the proliferative dysfunction is greater in the Tim3+PD1+ T cell population.
Fig. 2.
Coexpression of Tim-3 and PD-1 correlates with more severe exhaustion of LCMV-specific CD8 T cells during chronic infection. Functions of Tim3+PD1+ or Tim3−PD1+ CD8 T cells were analyzed using splenocytes at d 50 after infection. (A) The isolated Tim3+PD1+ or Tim3−PD1+ CD8 T cells were labeled with CFSE and stimulated with GP33 peptide or a pool of LCMV peptides for 3 d. Proliferation was determined by dilution of CFSE, and the number in flow cytometry plot indicates the frequency of proliferating cells. (B) Frequency of GP33- or GP276-specific CD8 T cells producing cytokine after stimulation for 5 h with GP33-41 or GP276-286 peptides. (C) Frequency of Tim3+PD1+, Tim3−PD1+, or Tim3−PD1− CD8 T cells producing IL-10 was analyzed after stimulation for 5 h with the LCMV peptide. Data are representative of three independent experiments. Error bars represent SEM. LCMV peptide pool consists of GP33-41, GP276-286, GP70-77, GP92-101, NP166-175, NP205-212, NP235-249, and NP396-404.
We then examined cytokine secretion by Tim3−PD1+ or Tim3+PD1+ cells in response to LCMV peptide stimulation. Consistent with our previous report (14), we confirmed the functional exhaustion of CD8 T cells from chronically infected mice characterized by a highly reduced ability to secret IFN-γ, TNF-α, and IL-2 compared with CD8 T cells from acutely infected mice (Fig. S3A). When we further accessed the dysfunction of Tim3−PD1+ or Tim3+PD1+ CD8 T cells in chronic infection, the frequency of IFN-γ–secreting cells was similar between the two populations, but the intensity of IFN-γ secretion (i.e., MFI) was approximately 2-fold higher in the Tim3−PD1+ subset compared with the Tim3+PD1+ subset (Fig. S3B). Furthermore, TNF-α and IL-2 secretion from CD8 T cells in response to stimulation was observed predominantly from the Tim3−PD1+ population, with minimal cytokine production observed in the Tim3+PD1+ population (Fig. S3B). To more quantitatively determine the functionality of these subsets, we examined cytokine secretion on a “per-cell” basis by calculating the frequency of epitope-specific CD8 T cells in each subset. The Tim3−PD1+ population showed a much higher percentage of both GP33- and GP276-specific CD8 T cell producing TNF-α or IL-2 than the Tim3+PD1+ population (Fig. 2B). These results therefore indicate that functional exhaustion of CD8 T cells during chronic infection is associated with coexpression of Tim-3 and PD-1, and that Tim3+PD1+ cells exhibit a deeper exhaustion.
Tim3+PD1+ Exhausted CD8 T Cell Produces the Inhibitory Cytokine IL-10.
A novel population of HIV-specific CD8 T cells has been shown to express IL-10 during HIV-1 infection (15, 16), suggesting that this specific population may have an important regulatory role in immune dysfunction in chronic infections. However, no phenotypic marker has been defined to identify the IL-10–positive CD8 T cells. In this regard, we examined whether Tim3−PD1+ or Tim3+PD1+ cells secrete IL-10 in response to stimulation with LCMV peptides. In contrast to effector cytokines (IFN-γ, TNF-α, and IL-2), IL-10 production was significantly observed only by the Tim3+PD1+ but not the Tim3−PD1+ populations (Fig. 2C and Fig. S3C). These results indicate that Tim-3 coexpression with PD-1 may provide an immunophenotypic marker for determining the IL-10–producing CD8 T cells as well as severely exhausted CD8 T cells.
Simultaneous in Vivo Blockade of Tim-3 and PD-1 Pathways Synergistically Restores CD8 T Cell Function and Enhances Viral Control.
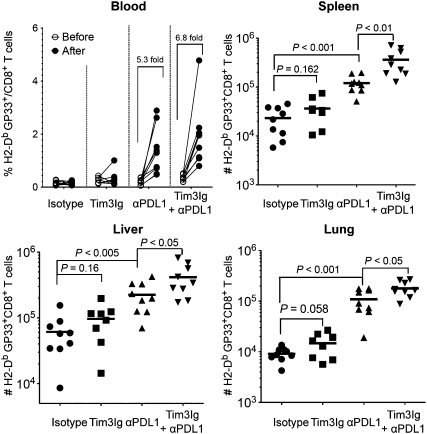
Our results show a correlation between coexpression of Tim-3 with PD-1 and deeper CD8 T cell exhaustion. However, it is not clear whether the Tim-3 and PD-1 pathways have overlapping functions or contribute independently to T cell dysfunction during chronic viral infection. To address this issue, we blocked the Tim-3 and PD-1 pathways with Tim-3-Ig fusion protein (Tim3Ig) and blocking antibody to PD-L1 (αPDL1), either alone or in combination during chronic LCMV infection. We first measured the frequency of GP33-specific CD8 T cells in the blood before and after treatment (Fig. 3). Tim-3 blockade alone (Tim3Ig) induced almost no change in GP33-specific CD8 (GP33+CD8) T-cell population. PD-1 blockade alone (i.e., αPDL1) resulted in a 5.3-fold increase of GP33+CD8 T-cell population (P = 0.0013), and dual blockade of Tim-3 and PD-1 (Tim3Ig + αPDL1) led to the 6.8-fold increase of GP33+CD8 T cell pool (P = 0.001). We then analyzed virus-specific CD8 T cells responses in lymphoid and nonlymphoid tissues. Treatment with Tim3Ig alone showed only marginal effects on the absolute number of GP33+CD8 T cells, whereas blocking the PD-1 pathway significantly augmented the GP33+CD8 T cell response compared with isotype control (Fig. 3). More interestingly, the simultaneous administration of Tim3Ig and αPDL1 further increased the number of GP33+CD8 T cells even when compared with αPDL1 alone (Fig. 3). It is worth noting that, when treatments started at 200 d after infection, dual blockade still increased the quantity of virus-specific CD8 T cells, but αPDL1 alone was only minimally effective (Fig. S4).
Fig. 3.
In vivo blockade of Tim-3 and PD-1 pathways enhances virus-specific CD8 T-cell responses during chronic viral infection. Chronically infected C57BL/6 mice (80 d after infection) were treated every third day or every other day for 2 wk with αPDL1 or Tim3Ig, respectively. Frequency of GP33-specific CD8 T cells before and after treatment of individual mouse is shown in the blood. Total number of GP33-specific CD8 T cells in the indicated tissues at 2 wk after treatment. Data are representative of three independent experiments with five to six mice per group in each experiment.
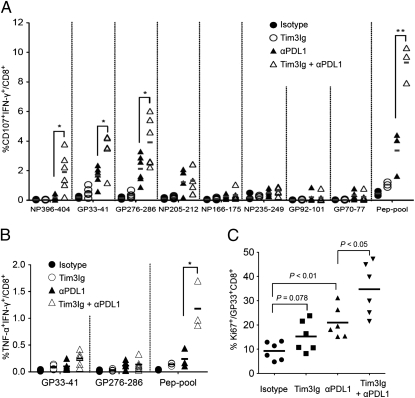
To determine the effect of dual blockade of Tim-3 and PD-1 pathways on the function of virus-specific CD8 T cells during chronic viral infection, we measured the ability of LCMV epitope-specific CD8 T cells to produce the cytokine IFN-γ and to degranulate by monitoring CD107a/b expression. The treatment with Tim3Ig alone showed only a modest increase of CD107a/b expression and IFN-γ production, whereas treatment with αPDL1 significantly increased the percentage of functional CD8 T cells responding to multiple LCMV epitopes (Fig. 4A and Fig. S5A). Importantly, dual blockade with Tim3Ig and αPDL1 showed a much greater increase in the degranulation and IFN-γ–producing CD8 T cell population, particularly to GP33-41, GP276-286, NP396-404 peptides, and a pooled peptide mixture, compared with either treatment alone or isotype control (Fig. 4A and Fig. S5A). Furthermore, only dual blockade elevated the percentage of polyfunctional CD8 T cells coproducing IFN-γ and TNF-α (Fig. 4B). Next, to test the effect of the blockade treatments on the proliferation of virus-specific CD8 T cells, we analyzed the frequency of Ki67-positive GP33+CD8 T cells. Tim-3 blockade alone slightly increased the frequency of Ki67-positive GP33+CD8 T cells compared with isotype control (Fig. 4C and Fig. S5B). PD-1 blockade alone showed a significant increase in the Ki67-positive population, and dual blockade further increased the frequency of Ki67-positive GP33+CD8 T cells compared with αPDL1 alone (Fig. 4C and Fig. S5B). Taken together, these results indicate that dual blockade of Tim-3 and PD-1 pathways substantially enhanced virus-specific CD8 T cell responses in quality as well as quantity during chronic viral infection.
Fig. 4.
Dual blockade of Tim-3 and PD-1 pathways enhances function in exhausted virus-specific CD8 T cells. (A) IFN-γ production and degranulation by CD8 T cells in treated mice at 2 wk after therapy. The percentage of IFN-γ+CD107+ CD8 T cells specific for each of the LCMV peptides are summarized. (B) Polyfunctional (TNF-α+IFN-γ+) CD8 T cells in treated mice at 2 wk after therapy. (C) The proliferation of antigen-specific CD8 T cell after dual blockade is shown as the percentage of Ki67+ on LCMV GP33-specific CD8 T cells. Data are representative of three independent experiments with five to six mice per group in each experiment. *P < 0.05; **P < 0.01.
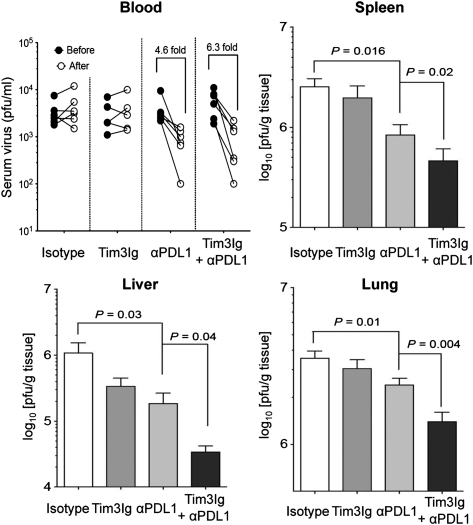
We next assessed if the heightened CD8 T-cell response induced by dual blockade of these inhibitory receptors also enhanced viral control. The viral load of mice treated with isotype control or Tim3Ig alone in the serum was not significantly altered after treatment (Fig. 5). In contrast, αPDL1 alone or dual blockade (Tim3Ig + αPDL1) treatments led to 4.2-fold and a 6.3-fold reductions, respectively, in virus titer of serum (Fig. 5). Similarly, treatment with Tim3Ig alone resulted in slightly lower viral loads in the spleen, liver, and lung, whereas treatment with αPDL1 alone showed significant reduction of viral load in all tissues compared with the isotype control group (Fig. 5). More impressively, dual blockade with Tim3Ig and αPDL1 led to further reduction in viral loads in all tissues compared with blockade with αPDL1 alone (Fig. 5).
Fig. 5.
Dual blockade of Tim-3 and PD-1 pathways enhances viral control. Viral titer was determined by plaque assay in the blood before and after treatment. Viral load in spleen, liver, and lung at 2 wk after treatment is shown. Data are representative of three independent experiments with five to six mice per group in each experiment. Error bars represent SEM.
Discussion
In this study, we found that, although Tim-3 was transiently expressed by CD8 T cells after acute LCMV infection, it was rapidly down-regulated, whereas CD8 T cells retained high Tim-3 expression throughout chronic LCMV infection. Moreover, Tim-3 was mainly coexpressed with PD-1 on virus-specific CD8 T cells during chronic infections. Importantly, this subset of CD8 T cells coexpressing Tim-3 and PD-1 (Tim3+PD1+) showed the phenotypic and functional characteristics of more severely exhausted CD8 T cells than did those expressing only PD-1 (Tim3−PD1+). Finally, simultaneous in vivo blockade of Tim-3 and PD-1 pathways had synergistic effects in restoring antiviral immunity and viral control compared with blockade of either pathway alone. Collectively, these results indicate that Tim-3 and PD-1 pathways may cooperate and independently contribute to negatively regulate CD8 T cell responses during chronic viral infections.
The subset of CD8 T cells coexpressing Tim-3 and PD-1 (Tim3+PD1+) also showed high expression of other inhibitory receptors, such as 2B4 and Lag3 (Fig. S2). 2B4 and Lag3 are still not clearly characterized for their inhibitory function on CD8 T cells. 2B4 expression is up-regulated and remains high on virus-specific exhausted CD8 T cells (8), and Lag3 is known to play a negative role in T cell homeostasis and expansion (17, 18). In addition, a recent study found that coexpression of multiple these receptors was associated with greater T-cell exhaustion (9), which is consistent with our result showing that Tim3+PD1+ subset exhibits a deeper exhaustion than the Tim3−PD1+ subset.
We identified subsets of exhausted CD8 T cells based on Tim-3 and PD-1 expression during chronic LCMV infection. A majority of virus-specific CD8 T cells expressed both Tim-3 and PD-1 (Tim3+PD1+), indicating that Tim-3 and PD-1 mark overlapping subsets of exhausted CD8 T cells. In our study, the frequency of the CD8 T cell subset expressing only Tim-3 (Tim3+PD1−) was too low to analyze the phenotype and functionality of this subset. However, given that there is a hierarchical process of T cell exhaustion—thus, diverse inhibitory receptors may regulate distinct aspects of T-cell exhaustion—this Tim3+PD1− subset could also be one of the stages in differentiation of exhausted T cells during chronic infection. Contrary to our study, the Tim3+PD1− subset was shown in another study to be one of the major populations of CD8 T cells in peripheral blood of HIV-1 infection (12). This difference may be explained by the different type and/or severity of infection. Many studies have demonstrated the relationship between severity of infection and the extent of CD8 T-cell exhaustion (14, 19, 20). Moreover, these exhausted CD8 T cells segregated into a series of discrete subsets that expressed different numbers and combinations of inhibitory receptors (9). Alternatively, the precise phenotypic characteristic of exhausted CD8 T-cell subsets may vary in different anatomical sites. During chronic infection, there was substantial redistribution of virus-specific CD8 T cells to nonlymphoid tissues (14). Following this redistribution, expression of inhibitory receptors on virus-specific CD8 T cells might be differentially regulated by different tissue microenvironments. Indeed, during chronic LCMV infection, virus-specific CD8 T cells in the liver, brain, and bone marrow expressed higher PD-1 than those in the blood, which was dependent on the differential levels of virus antigen load and PD-1 ligand and PD-L1 expression in the respective tissues (21). Herein, we also observed compartmental differences in Tim-3 and PD-1 expression on CD8 T cells during chronic LCMV infection (Fig. 1D and Fig. S1C). Thus, together with previous reports (14, 21), our results suggest that analysis of one compartment during chronic viral infection may not predict the phenotypic characteristics as well as the number of antigen-specific CD8 T cells present in other tissues.
Recently, a novel population of HIV-specific suppressor CD8 T cells has been shown to express IL-10 during HIV infection (15, 16). However, as a surface marker has not yet been identified, the use of functional characterization by IL-10 production is required to discriminate suppressor from effector CD8 T cells during chronic infections. In the present study, we demonstrated that the subset of CD8 T cells producing IL-10 was phenotypically identical to the severely exhausted CD8 T cells coexpressing Tim-3 and PD-1. These results suggest that coexpression of Tim-3 and PD-1 may be used as a phenotypic marker for defining antigen-specific suppressor CD8 T cells. In addition, it is possible that therapeutic vaccine-derived antigenic stimulation may drive these suppressor CD8 T cells to produce IL-10 during chronic infection, further limiting the magnitude of CD8 T-cell responses. However, considering that many therapeutic vaccines occur in the setting when viral load is substantially reduced by antiviral drug treatment, it should be first addressed whether this IL-10–producing CD8 subset would exist when antigen load is decreased. Interestingly, it was recently found that triggering of PD-1 by PD-L1 induced IL-10 production from monocytes and led to reversible CD4 T cell dysfunction (22). Thus, it would be important to further investigate how PD-1 or Tim-3 pathways are involved in IL-10 production on Tim3+PD1+ CD8 T cells, which might provide new insights in developing immune therapies against chronic infections.
Tim-3 was initially identified as a molecule expressed on Th1 but not on Th2 cells and plays an important role in inducing peripheral tolerance (11). This suppressive effect of Tim-3 was mediated by interaction with the Tim-3 ligand, galectin-9, followed by promoting the apoptosis of Th1 cells (23). However, in the present study, exhausted CD8 T cells expressing Tim-3 were sustained without elimination during chronic viral infection. Although our results indicate that Tim-3 pathway is a central negative regulator of the T cell response, it is not clear why these exhausted CD8 T cells expressing Tim-3 were not killed by apoptosis. One possible explanation is that the expression of galectin-9 may be limited in concentration in the chronically infected hosts. Another possibility is that Tim-3 engagement may induce distinct signaling in exhausted CD8 T and Th1 cells, resulting in differential susceptibility to galectin-9–mediated cell death. Differential responsiveness to Tim-3 pathway is supported by a recent report showing that regulatory T cells expressing Tim-3 were more resistant to galectin-9–induced apoptosis than CD4+ effector T cells (24).
Although blockade of the Tim-3 pathway alone had only minimal effect on T-cell function and viral control, combined blockade of Tim-3 and PD-1 pathways resulted in substantially better reversal of exhaustion and control of virus than did blockade of PD-1 pathway alone. Similarly, in another study (9), blockade of Lag3 alone had minimal to no effect on the severity of exhaustion but combined blockade of Lag3 and PD-1 synergistically improved T-cell responses and diminished viral load in vivo. Conversely, in vitro blockade of Tim-3 alone restored the function of HIV-1–specific T cells in which the Tim3+PD1− subset was one of the major populations of CD8 T cells (12). Considering that Tim3−PD1+ and Tim3+PD1+ subsets were major populations of exhausted CD8 T cells during chronic LCMV infection, it appears that the PD-1 pathway is the major regulator in promoting and maintaining exhausted CD8 T cells expressing PD-1. However, during late stage of chronic LCMV infection (>200 d), single blockade of PD-1 pathway also showed only modest effect on antiviral immunity, whereas dual blockade still expanded the virus-specific CD8 T cells responses (Fig. S4). Although the precise role of Tim-3 and PD-1 pathways in CD8 T cell exhaustion should be further investigated, these results indicate that the Tim-3 and PD-1 pathways may play independent roles in CD8 T-cell exhaustion during chronic viral infection. This can be further supported by our data showing that the population of CD8 T cell coexpressing Tim-3 and PD-1 exhibited a more severe defect in proliferation and production of cytokines, such as IFN-γ, TNF-α, and IL-2, than did those cells expressing only PD-1. Thus, dual blockade of Tim-3 and PD-1 pathways may allow for a more comprehensive reversal of T-cell exhaustion, potentially leading to potent combination therapies.
Materials and Methods
Mice and Infections.
Six-week-old female C57BL/6 mice were purchased from Jackson Laboratory. LCMV strains were propagated, titered, and used as previously described (14). For acute infection, mice were intraperitoneally infected with 2 × 105 pfu of LCMV Armstrong strain. For chronic infection, mice were infected i.v. with 2 × 106 pfu of LCMV clone-13 strain, and were intraperitoneally injected with 500 μg anti-CD4 antibody (GK 1.5; BioExpress) on day −1 and day +1 relative to infection with LCMV clone-13 on d 0. All mice were used in accordance with National Institutes of Health and the Emory University Institutional Animal Care and use Committee guidelines.
Flow Cytometry and Intracellular Cytokine Staining.
Lymphocytes were isolated from tissue including spleen, liver, lung, and blood as previously described (3). The antibodies to CD8 (53-6.7), Ki67 (B56), IFN-γ (XMG 1.2), TNF-α (MP6-XT22), IL-2 (JES6-5H4), IL-10 (JES5-16E3), CD107a (1D4B), and CD107b (ABL093) were purchased from BD Bioscience. Anti-CD44 (IM7), anti–Tim-3 (215008), and anti–PD-1 (RMP 1–30) were obtained from eBioscience, R&D Systems, and BioLegend, respectively. MHC class I tetramers or multimers conjugated with APC and Qdot565 (Invitrogen) were generated and used as previously described (14, 25). Surface and intracellular cytokine staining was performed as described (14). To detect degranulation, splenocytes were stimulated with individual LCMV peptides or a pool of eight LCMV epitopes for 5 h in the presence of brefeldin, monensin, anti–CD107a-FITC, and anti–CD107b-FITC. Cells were then analyzed on an LSR II flow cytometer (BD Immunocytometry Systems). Data were analyzed with FlowJo v.8.8 (TreeStar). Dead cells were removed by gating on Live/Dead NEAR IR (Invitrogen).
In Vitro Proliferation Assay.
CD8 T cells were purified to more than 90% purity by using magnetic beads (Miltenyi Biotec). Tim3+PD1+ and Tim3−PD1+ CD8 T cells were isolated by FACS sorting. CFSE-labeled Tim3+PD1+ and Tim3−PD1+ CD8 T cells were cocultured with splenocytes from Thy1.1+ C57BL/6 mice in the presence of LCMV peptides for 3 d. To analyze proliferation, loss of CFSE in Thy1.2+-gated cells was determined by flow cytometry.
In Vivo Blockade and Virus Titration.
For blockade of PD-1 pathway, 200 μg of rat antimouse PD-L1 antibody (10F.9G2; prepared in house) or rat IgG2b isotype control were administered intraperitoneally every 3 d for 2 wk. For blockade of Tim-3 pathway, 100 μg of Tim-3-Ig fusion protein (prepared in house) were injected intraperitoneally every 2 d for 2 wk. The ability of Tim-3-Ig and anti–PD-L1 to block the Tim-3 and PD-1 pathways, respectively, has been previously demonstrated (3, 26). Titers of virus from serum or homogenized tissue sample were determined by plaque assay on Vero cells as previously described (27).
Statistical Analysis.
Data were analyzed using Prism 5.0 software (GraphPad). Experiments were repeated two or three times. The data presenting the differences between the groups were assessed using two-tailed unpaired Student t tests. P < 0.05 indicated that the value of the test sample was significantly different from that of the relevant controls.
Supplementary Material
Acknowledgments
We thank B. T. Konieczny and H. Wu for technical assistance and members of the R.A. laboratory for helpful discussion. This work was supported by grants from the Gates Foundation Grand Challenge in Global Health (R.A.); National Institutes of Health Grants AI054456, AI08080192, AI056299 (to G.J.F.), AI73748, NS038037, NS045937, NS030843 (to V.K.K.), and NS054096 (to A.C.A.); Korean Health Technology Research and Development Project A091204 from the Ministry for Health, Welfare and Family Affairs, Republic of Korea (to S.-J.H.); National Multiple Sclerosis Society (V.K.K.); Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard University (V.K.K.); and an Innovation Award from the Ragon Institute of Massachusetts Institute of Technology, Massachusetts General Hospital, and Harvard University (V.K.K.). V.K.K. is a recipient for the Javits Neuroscience Investigator Award from the National Institutes of Health.
Footnotes
Conflict of interest statement: R.A. received licensing fees from Genentech on using antibodies to block the PD-1 inhibitory pathway. G.J.F. received patent royalties on the PD-1 and TIM-3 pathways. No other authors have any financial conflicts.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009731107/-/DCSupplemental.
References
- 1.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 6.Velu V, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RB, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden-Mason L, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elrefaei M, Barugahare B, Ssali F, Mugyenyi P, Cao H. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol. 2006;176:1274–1280. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 16.Elrefaei M, et al. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178:3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 17.Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 18.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 19.Streeck H, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackburn SD, et al. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: Implications for CD8 T-cell exhaustion. J Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Said EA, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 24.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: Shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabatos CA, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.