Abstract
The correct navigation of axons to their targets depends on guidance molecules in the extra-cellular environment. Differential responsiveness to a particular guidance cue is largely an outcome of disparity in the expression of its receptors on the reacting axons. Here, we show that the differential responsiveness of sympathetic and sensory neurons to the transmembrane Semaphorin Sema6A is mainly determined by its co-expression in the responding neurons. Both sympathetic and sensory neurons express the Sema6A receptor Plexin-A4, but only sympathetic neurons respond to it. The expression of Sema6A counteracts this responsiveness and is detected only in sensory neurons. Remarkably, sensory neurons that lack Sema6A gain sensitivity to it in a Plexin-A4-dependent manner. Using heterologus systems, we show that the co-expression of Sema6A and Plexin-A4 hinders the binding of exogenous ligand, suggesting that a Sema6A–Plexin-A4 cis interaction serves as an inhibitory mechanism. Finally, we provide evidence for differential modes of interaction in cis versus in trans. Thus, co-expression of a transmembrane cue together with its receptor can serve as a guidance response modulator.
Keywords: axon guidance, Plexin, sensory neurons, Semaphorin
Introduction
During neural development axons navigate over long distances to form precise connections with their targets. This process is achieved by complex guidance decisions that are mediated by extra-cellular cues, which are interpreted by axonal receptors to an attraction or repulsion response. Genetic and biochemical studies over the last two decades had identified several conserved families of axon guidance molecules and their receptors (Tessier-Lavigne and Goodman, 1996; Dickson, 2002; Chilton, 2006; Yaron and Zheng, 2007). In addition, morphogenes were shown to act as axonal guidance cues as well (Charron and Tessier-Lavigne, 2005).
The Semaphorins are a large family of secreted and membrane-bound guidance cues comprised of 21 members in vertebrates that are divided into five classes according to structural homology (Pasterkamp and Kolodkin, 2003; Yazdani and Terman, 2006; Tran et al, 2007; Zhou et al, 2008). Initially, the Semaphorins were characterized according to their function as repulsive axon guidance molecules, but they are now recognized to be involved in diverse developmental and biological events including axonal fasciculation, pruning, neuronal migration, dendritic guidance and modulation of the immune system (Pasterkamp and Kolodkin, 2003; Yazdani and Terman, 2006; Mann and Rougon, 2007; Tran et al, 2007, 2009; Zhou et al, 2008).
Neuropilins were the first receptors to be identified by biochemical and genetic studies for secreted class 3 Semaphorins. As Neuropilins lack signalling domains, it was clear that they must associate with additional co-receptors that serve as signal transducers (Fujisawa, 2004). The identification of Plexin-C1 as the receptor for the viral Semaphorin paved the way for the identification of Plexins as a conserved family of receptors for the Semaphorins (Puschel, 2002; Fujisawa, 2004). Plexins are a large family of transmembrane proteins, which are divided into four types (A–D) according to sequence similarity (Tamagnone and Comoglio, 2000). In vertebrates, type A Plexins serve as the co-receptors for Neuropilins to mediate the signalling of class 3 Semaphorins (Takahashi et al, 1999; Tamagnone et al, 1999; Rohm et al, 2000; Suto et al, 2005; Yaron et al, 2005). Plexins serve as direct receptors for several other members of the Semaphorin family: class 6 Semaphorins signal through type A Plexin and class 4 Semaphorins through type B Plexins (Tamagnone et al, 1999; Toyofuku et al, 2004).
The responsiveness of axons to members of the semahporin family is mainly determined by the expression pattern of their receptors. For instance, the differential sensitivity of sympathetic, hippocampal and sensory axons to distinct members of the class 3 Semaphorins is governed by the expression of the two Neuropilins (Chedotal et al, 1998; Chen et al, 1998; Giger et al, 1998).
Sema6A is a membrane-bound Semaphorin that was originally characterized as a chemorepellent for sympathetic axons. In the central nervous system (CNS), Sema6A was shown to regulate the formation of lamina-specific axon projections in the hippocampus, as well as the formation of the cortical spinal tract and the migration of granule cells (Suto et al, 2007; Faulkner et al, 2008; Renaud et al, 2008; Runker et al, 2008). Recently, Sema6A was shown to promote the dendritic growth of spinal motor neurons (Zhuang et al, 2009). Two members of the Plexin family have been implicated as Sema6A receptors, Plexin-A2 and A4 (Suto et al, 2005, 2007; Bron et al, 2007; Renaud et al, 2008). The identification of Plexin-A4 as a Sema6A receptor was surprising as dorsal root ganglia (DRG) neurons, which strongly express Plexin-A4, barely respond to Sema6A (Xu et al, 2000). This observation prompted us to examine the mechanism that governs this suppression of response. Here, we provide evidence for a cis inhibitory activity of Sema6A. Unlike sympathetic neurons, DRG neurons express both Sema6A and its receptor Plexin-A4. Strikingly DRG neurons that lack Sema6A gain responsiveness to it in a Plexin-A4-dependent manner. We further show that Sema6A and Plexin-A4 form a stable complex, and that co-expression of Sema6A, but not Neuropilin-1 (Nrp-1), with Plexin-A4 abolishes the binding of exogenous Sema6A. Our results suggest that co-expression of trasmembrane Semaphorin with its receptor serves to attenuate the axonal response to ligand in trans, thus enabling axonal growth in an otherwise repulsive environment.
Results
Plexin-A4 and Sema6A are co-expressed in DRG sensory neurons but not in sympathetic neurons
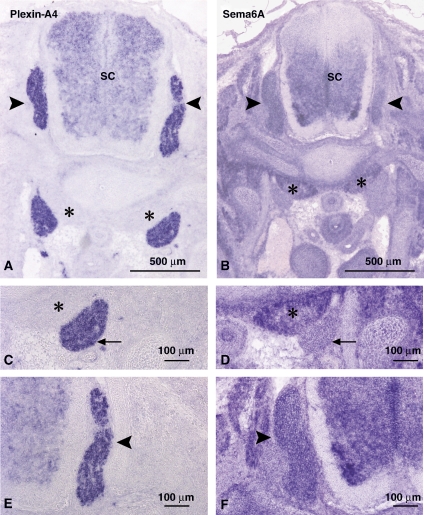
To uncover the basis for the differential responsiveness of sympathetic and DRG neurons to Sema6A, we examined the expression pattern of Sema6A and its receptor Plexin-A4. At embryonic day E13.5, an age in which sympathetic neurons are strongly repelled by Sema6A but DRG sensory neurons are not, both sympathetic and DRG neurons expressed Plexin-A4 as earlier reported (Figure 1A, C and E) (Cheng et al, 2001; Suto et al, 2005). Interestingly, we detected an inverse expression of Sema6A to the activity profile. Although sympathetic neurons did not express Sema6A, DRG neurons strongly expressed it (Figure 1B, D and F). These results are also in agreement with earlier studies (Xu et al, 2000; Suto et al, 2005).
Figure 1.
Sema6A and Plexin-A4 mRNA are co-expressed in DRG neurons, but not in sympathetic neurons. Expression patterns of Plexin-A4 and Semaphorin 6A detected by in situ hybridization on cross-sections at cervical level of E13.5 mouse embryos. Plexin-A4 mRNA is strongly detected in dorsal root ganglia (DRG) marked by black arrowheads and in sympathetic ganglia (SCG) marked by arrows (A, C, E). Sema6A mRNA is devoid from SCG, but is expressed in the anterior vertebrate muscle (marked by asterisks) and in DRG (B, D, F).
DRG from Sema6A knockout show enhanced responsiveness to Sema6A
Earlier studies on the ephrin family of repulsive cues have suggested that co-expression of ephrin and the Eph receptor can modulate the response to ephrin through several mechanisms (Hornberger et al, 1999; Sobieszczuk and Wilkinson, 1999; Yin et al, 2004; Carvalho et al, 2006). We postulated that Sema6A might function in a similar way and repress the response to Sema6A in DRG neurons. To test this idea, we compared the response of DRG neurons from wild-type (WT) embryos and Sema6A knockout (KO) littermates to Sema6A, using two different in vitro assays.
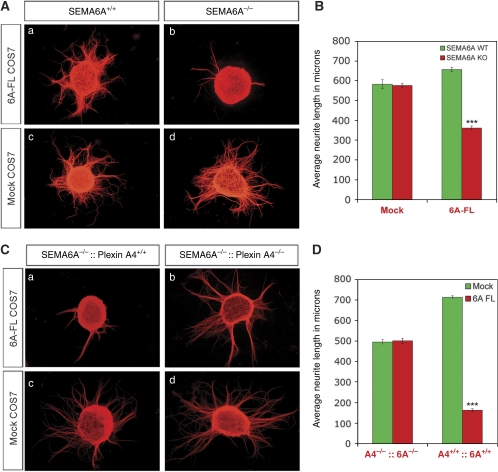
First, we examined axonal growth in response to COS7 cells that express the full-length version of Sema6A. Using this assay, Sema6A is presented to the axons in its native form as a transmembrane protein. DRG neurons from WT embryos were unresponsive to Sema6A in this assay (Figure 2A and B). In contrast, DRG neurons from the Sema6A KO mice show reduced axonal growth capacity when grown in the presence of Sema6A (Figure 2A and B). Importantly, both WT and Sema6A KO DRG neurons possess comparable axonal length and number when co-cultured on mock-transfected COS7 cells (Figure 2A and B), suggesting that the basal axonal growth capacity was not significantly different.
Figure 2.
DRG neurons from Sema6A KO mice display reduced axonal growth in response to Sema6A that is dependent on intact expression of Plexin-A4: (A) DRG explants from Sema6A WT (a, c) and Sema6A KO littermates (b, d) were cultured on top of COS7 cells transfected with Sema6A or mock plasmids. Assessment of neurite outgrowth was facilitated by Tuj-1 staining. (B) Quantification of axonal outgrowth plotted as average length in μm for each of the culturing conditions, indicating gain of responsiveness to Sema6A in KO DRG explants. (C) DRG explants from Sema6A KO (a, c) and Plexin-A4/Sema6A double-KO littermates (b, d) were co-cultured on top of Sema6A-expressing COS7 cells or control cells. Growth inhibition observed only in explants from single-KO mice, confirming the necessity of Plexin-A4 to attain responsiveness to Sema6A. (D) Quantification of the axonal outgrowth of single- and double-KO DRG in response to membrane-bound Sema6A plotted as average length in μm for each genetic background. In both quantification analyses, the average length was calculated from four independent experiments. In each experiment 10–20 explants from 3–4 littermates were analysed. Error bars correspond to standard error of the mean. Statistical analysis was preformed with GraphPad Prism using unpaired t-test (two-tailed). ***P<0.001.
This result suggests that the presence of Sema6A on the axonal membrane of DRG neurons can attenuate outgrowth inhibition by Sema6A presented in trans by cells in the surrounding environment.
Plexin-A4 serves as the receptor for Sema6A in sympathetic neurons and in the lateral motor column neurons (Suto et al, 2005; Zhuang et al, 2009). However, in cerebellar granule cells and in spinal motor neurons, Plexin-A2 can serve as a functional signalling receptor as well (Bron et al, 2007; Renaud et al, 2008). To address whether the response to Sema6A of DRG neurons is mediated by Plexin-A4, we performed the same axon outgrowth assay on Plexin-A4:Sema6A double mutant DRG explants. DRG neurons from the double KO mice were completely refractory to Sema6A, but show basal outgrowth capacity comparable to explants cultured on top of mock-transfected COS7 cells (Figure 2C and D). Thus, Plexin-A4 serves as the sole receptor that transduces the outgrowth inhibitory responses to Sema6A in DRG axons.
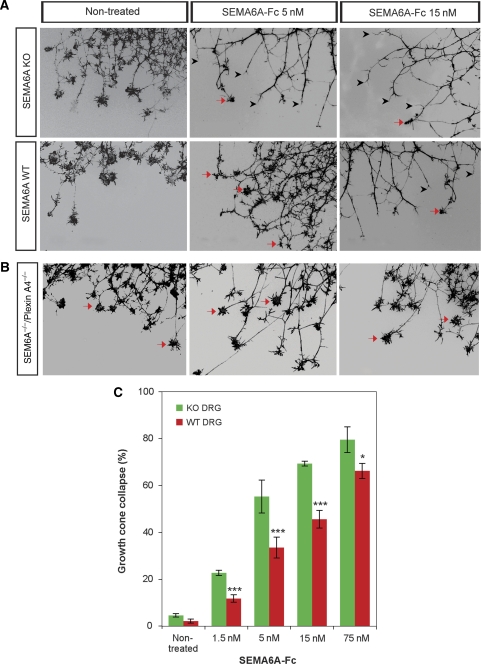
Next, we examined axonal responses to soluble Sema6A using the growth cone collapse assay. We exposed DRG explants from Sema6A KO embryos and littermate controls to a range of concentrations of soluble Sema6A-fc. A clear difference in the number of collapsed axons from Sema6A KO and WT littermates was observed, and this was directly correlated with increasing concentrations of Sema6a-fc (Figure 3A and C). To determine whether this response is also mediated by Plexin-A4, we performed the same analysis on explants from the Plexin-A4/Sema6A double KO embryos (Figure 3B). These DRG neurons were completely resistant to all dosage of Sema6A-fc applied.
Figure 3.
DRG neurons from Sema6A KO mice acquire sensitivity to Sema6A collapsing effect: cultured DRG explants from E13.5 Sema6A KO or WT littermates were treated with increasing concentrations of soluble Sema6A-fc for 45 min. Collapsed growth cones were visualized after 4% PFA fixation with Phalloidin-Rhodamine staining. (A) Black arrowheads refer to collapsed growth cones and arrows point at non-collapsed growth cones. (B) In Plexin-A4/Sema6A double KO, no collapsed growth cones were observed at any of the concentrations of Sema6A-fc that were applied. (C) Quantification of collapsed growth cones plotted against increasing concentrations of soluble Sema6A-fc. The average percentage of growth cone collapse was calculated from four independent experiments. For each concentration, four explants were analysed originating from 2 to 4 Plexin-A4/Sema6A and Sema6A littermates. Vertical bars correspond to standard error. Statistical analysis was preformed with GraphPad Prism using χ2 test. *P<0.05; ***P<0.001.
Overall, our results show that ablation of Sema6A in DRG neurons sensitize them to respond to Sema6A in a Plexin-A4-dependent manner.
Sema6A co-resides with Plexin-A4 on the cell membrane and forms a stable complex with it
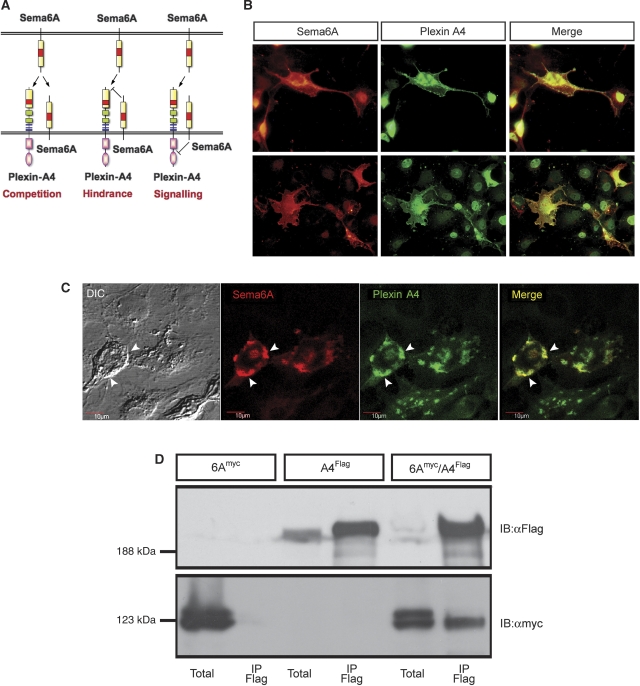
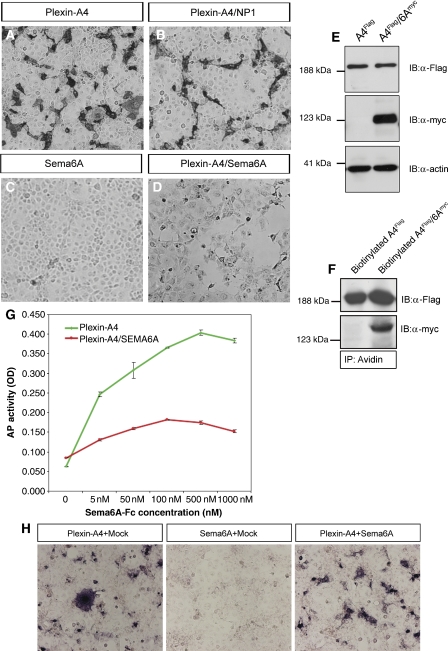
Next, we turned to heterologous cell culture systems to examine the mechanism by which co-expression of Sema6A attenuates its own receptor's response. Sema6A may inhibit Plexin-A4 signalling through inhibition of its downstream elements, preventing its cell surface expression or its ability to bind exogenous ligand. Alternatively, Sema6A may bind to itself such that the axonal Sema6A acts as a competitor to Plexin-A4 (Figure 4A). We first examined whether Plexin-A4 can be detected on the cell membrane in the presence of Sema6A, by transfecting COS7 with an N-terminus extra-cellular myc-tagged Plexin-A4 construct and a full-length Sema6A construct. Double surface staining with anti-myc and anti-Sema6A without permeabilization revealed that both Sema6A and Plexin-A4 could be detected on the cell membrane (Figure 4B). We further extended this analysis by performing confocal analysis of the stained cells, which confirmed that expression of both Plexin-A4 and Sema6A is confined to the same membrane domains (Figure 4C). Thus, the expression of Sema6A does not lead to massive intracellular sequestration of Plexin-A4. Next, we tested whether a stable complex of Sema6A and Plexin-A4 can be detected. We co-expressed Flag-tagged Plexin-A4 and myc-tagged Sema6A in HEK 293T cells and performed co-immunoprecipitation from cell lysates. Sema6A was efficiently pulled down only in the presence of Plexin-A4 by the anti-Flag sepharose beads (Figure 4D), but not HA beads (Supplementary Figure 1). Thus, a stable Sema6A–Plexin-A4 complex can be formed in transfected cells. Overall, these results argue that a complex can be formed between Sema6A and Plexin-A4; however, this does not lead to intracellular retention of either one of them.
Figure 4.
Plexin-A4 and Sema6A are co-expressed on the cell surface and form a stable complex. (A) A model representing various mechanisms by which axonal Sema6A obstruct the response to Sema6A mediated by Plexin-A4. Intracellular competition on downstream targets or intracellular sequestering of Plexin-A4 can block Plexin-A4 signalling or expression and impede the response to Sema6A (right scheme). Extra-cellular competition between Plexin-A4 and Sema6A can lead to reduce ligand availability (left Scheme). Cis interaction of axonal Sema6A with Plexin-A4 leads to hindrance in ligand binding in trans (middle Scheme). (B) COS7 expressing an extra-cellular myc tag Plexin-A4 and a Sema6A-full-length construct were co-immunostained with αmyc and αSema6A antibodies. Staining was preformed without cell permeabilization to discriminate membrane expression only. (C) Confocal microscopy analysis of Plexin-A4/Sema6A co-transfected cells, arrowheads point to membrane domains in which both can be detected. (D) HEK 293T cells were transfected with Plexin-A4-Flag, Sema6A-myc or both. Sema6A was detected by anti-myc blotting, after Flag immunoprecipitation from the double transfected cells, but not the single transfected ones.
Co-expression of Sema6A but not Nrp-1 with Plexin-A4 prevents the binding of soluble Sema6a
Next, we studied the ability of soluble Sema6A to bind to cells that express Plexin-A4 either alone or in combination with full-length Sema6A or Nrp-1, both of which can form a complex with Plexin-A4. We transfected COS7 cells with various combination of receptors and detected significant binding of Sema6A-fc to COS7 cells expressing Plexin-A4 alone or together with Nrp-1 (Figure 5A and B) in agreement with earlier data that binding of Sema6A-fc is mediated solely through Plexin-A4 and does not require Nrp-1 (Suto et al, 2005). Conversely, co-expression of Plexin-A4 with Sema6A completely blocked the binding of Sema6A-fc to Plexin-A4. Furthermore, we found that Sema6A-fc could not bind in trans to Sema6A alone (Figure 5C and D). To exclude the possibility that co-expression of Sema6A reduces the overall expression of Plexin-A4 or its cell surface presentation, we compared Plexin-A4 levels in total cell lysates and by cell surface biotinylation. Comparable levels of Plexin-A4 were detected when it was expressed alone or in combination with Sema6A (Figure 5E and F).
Figure 5.
Soluble Sema6A-fc binding to cells expressing Plexin-A4 is reduced by co-expression with Sema6A. (A–D) Binding of Sema6A-fc to COS7 cells expressing various combinations of receptor complexes. Soluble Sema6A-fc binds equally to Plexin-A4 or Plexin/NP-1-expressing cells, but could not bind in trans to Sema6A-FL-expressing cells. Moreover, the binding of Sema6A-fc to Plexin-A4/Sema6A-expressing cells is completely abolished. (E) αFlag and αmyc immunoblots of total lysates from COS7 transfected with Plexin-A4 or Plexin-A4/Sema6A, indicating equal levels of Plexin-A4 expression in single and co-transfected cells. (F) Corresponding αFlag and αmyc immunoblots of Flag-Plexin-A4 and myc-Sema6A, which were isolated by cell surface biotinylation followed by avidin immunoprecipitation, indicative of equal cell surface expression of Plexin-A4. (G) Sema6A-fc-binding curve: applying increasing concentrations of Sema6A-fc to Plexin-A4 or Plexin-A4/Sema6A-expressing COS7 cells shows that cis inhibition by Plexin-A4/Sema6A could not be prevailed at any concentration of ligand that was applied in trans. (H) Deciphering cis versus trans inhibition by Sema6A: binding assay was performed on COS7 cells that were separately microporated with Plexin-A4 or Sema6A and then mixed and cultured in a 1:1 ratio. In comparison, a mixed Plexin-A4/mock and Sema6A/mock-transfected culture was subjected to the same binding assay. Plexin-A4/mock and Plexin-A4/Sema6A cultures bind equally soluble Sema6A-fc ligand. No binding was observed in trans to Sema6A/mock cultures.
We next characterized the strength of binding inhibition derived from the Plexin-A4/Sema6A cis interaction by performing quantitative ligand-binding analysis with increasing concentrations of purified Sema6A-fc and compared the binding curve of Plexin-A4/Sema6A to that of Plexin-A4-expressing cells. Strikingly, binding was attenuated even at high concentrations of Sema6A-fc (Figure 5G). To establish that only a cis interaction between Sema6A and Plexin-A4 can specifically block trans binding of Sema6A-fc, we perform a binding experiment on a mixed culture of Sema6A-transfected and Plexin-A4-transfected COS7 cells mixed in 1:1 ratio. As shown in Figure 5H, once the two molecules are expressed in trans to each other, binding of soluble Sema6A-fc to Plexin-A4 is not blocked. These results support a mechanism whereby a cis interaction between Sema6A and Plexin-A4 specifically inhibits the binding of soluble Sema6A-fc.
Sema6B is another direct ligand of Plexin-A4 (Suto et al, 2005). Thus, we tested whether it can also inhibit the binding of Sema6A in cis. Similarly to the results we obtained with Sema6A, Sema6B did not reduce the expression of Plexin-A4, but completely abolished the binding of Sema6A-fc (Supplementary Figure 2). Therefore, both ligands of Plexin-A4 can form a complex with it and serve as cis inhibitors one of each other.
Differential interaction of Sema6A with Plexin-A4 in cis versus trans
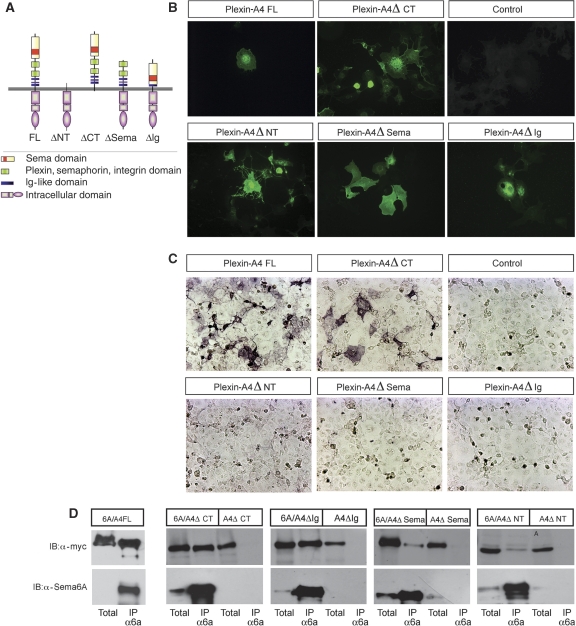
The result that even high concentrations of Sema6A-fc could not bind to cells that co-express Sema6A and Plexin-A4 might indicate that cis and trans binding involve different interactions. To test this possibility, we compared the association between Sema6A and several mutant forms of N-terminus myc-tagged Plexin-A4 (Figure 6A) in cis versus trans using cell surface binding and co-immunoprecipitation experiments. Ablation of the Sema domain eliminated the binding in trans and reduced the binding in cis, suggesting that this is the major site of interaction in both cases (Figure 6C and D).
Figure 6.
Differential modes of interaction of Sema6A and Plexin-A4 in cis versus in trans. (A) Schematic representation of the various mutant forms of N-terminus myc-tagged Plexin-A4 that were used for Co-IP and binding experiments to decipher functional domains associated with cis and trans binding to Sema6A. (B) Cell surface staining of the different Plexin-A4 variants using anti-myc on non-permeabilized cells. (C) Sema6A-fc binding in trans requires intact full-length extra-cellular domain of Plexin-A4, but is dispensable of the cytoplasmic tail. (D) Sema6A can pull down all mutated forms of myc-Plexin-A4. However, reduced interaction is seen with a mutant form lacking the Sema domain or the entire extra-cellular domain.
However, deletion of the Ig domain abolished only binding in trans without effect on the interaction in cis. It is important to note that both mutants were detected on the cell surface using anti-myc immunostaining (Figure 6B) and cell surface biotinylation (Supplementary Figure 3). Taking together, these results suggest that there are different molecular requirements for the cis versus the trans binding of Sema6A to its receptor Plexin-A4.
Interestingly, truncation of the entire extra-cellular domain but not the cytoplasmic domain reduced, but did not eliminate the formation of a Sema6A/Plexin-A4 complex in cis (Figure 6D). This type of deletion generates a constitutively active form of the Plexin receptors (Takahashi and Strittmatter, 2001; Oinuma et al, 2004; Turner and Hall, 2006). Thus, we examined whether this mutant form of Plexin-A4, which is active in a ligand-independent manner and can still interact with Sema6A, is under cis regulation.
Transfection of the Plexin-A4 Δ-NT construct into COS-7 cell induced a collapse of most of the transfected cells as earlier reported for Plexin-A1, and co-transfection of Sema6A did not inhibit this collapse (Supplementary Figure 4). Therefore, a ligand-independent Plexin-A4 signalling is not sensitive to cis inhibition by Sema6A.
Overall, our results suggest that the association of Sema6A with Plexin-A4 on the axonal membrane attenuates the activation of Plexin-A4 by Sema6A in trans through hindering its accessibility, but not by competitive binding or inhibition of signalling.
Discussion
Modulation of axonal response by ligand-receptor cis interaction
The neuronal expression patterns of axonal guidance receptors largely predict their responsiveness to specific guidance cues. However, many studies have shown that additional layers of regulation enable the neuron to modulate responses in spatial and temporal manners. Several mechanisms have been shown to govern this modulation. These include local translation within the growth cone, a switch in the expression of alternative splice isoforms, the formation of receptor complexes and the expression of signalling components. In this study, we have shown that neuronal responsiveness to transmembrane Semaphorins can be attenuated by the expression of the same transmembrane Semaphorin in the responding axon.
Earlier studies have shown that DRG neurons unlike Sympathetic neurons are insensitive to Sema6A despite their expression of its receptor Plexin-A4 (Xu et al, 2000; Suto et al, 2005). In this study, we revealed a regulatory mechanism accounting for Plexin-A4 functional attenuation. This mechanism is based on cis interaction between Sema6A and Plexin-A4 that prevents the binding of Sema6A in trans. Two independent lines of evidence strongly support this proposed mechanism. First, DRG neurons from the Sema6A KO mouse show enhanced responses to Sema6A in two independent in vitro assays. Second, in heterologous systems, Sema6A forms a complex with Plexin-A4 and prevents the binding of Sema6A in trans.
Silencing by cis interaction was earlier described in another family of axonal repellents—the ephrins. Studies on the development of the visual system have shown that retinal ganglion cells (RGCs) from the ephrin-A2/A5 double KO mice exhibit enhanced sensitivity to ephrin-A in vitro (Hornberger et al, 1999). In addition, over-expression of ephrin-A2 in chick RGCs induced overgrowth of the axons in the tectum, suggesting that it silences the response of these axons to the tectal ephrin gradient (Hornberger et al, 1999). Two mechanisms have been put forward to explain this silencing. The first, a direct competition on the ephrin-binding site in the Eph receptor, which prevents ligand binding in trans, but does not lead to the activation of the Eph receptor (Yin et al, 2004). This mechanism is also supported by transgenic over-expression of ephrin-A5 in the mouse (Sobieszczuk and Wilkinson, 1999). The second mechanism is based on interaction through a domain that is distinct from the ephrin-binding site and abolishment of the Eph receptor signalling (Carvalho et al, 2006). Interestingly, neurons have mechanisms to regulate the formation of these complexes, as it was shown that ephrin-As and Eph receptors could be targeted to discrete domains within the growth cone, thus generating two signalling cascades that work independently without cis inhibition (Marquardt et al, 2005). Our findings suggest that cis inhibition of Plexin-A4 by Sema6A also functions by prevention of trans binding, but it engages the Plexin-A4 extra-cellular domain in a different manner. Two lines of evidence support this. First, trans binding is abolished by deletion of either the Sema domain or the Ig domain in Plexin-A4, and the cis interaction is mainly affected by the deletion of the Sema domain. Second, we could not compete for the cis interaction with high concentrations of soluble ligand provided in trans. Therefore, this different mode of binding of Sema6A to Plexin-A4 prevents trans binding on one hand, but it is not functional on the other hand, ensuring that it will not result in constitutive Plexin-A4 signalling. Interestingly, based on NMR studies, differential interaction with the same domain, in cis versus trans, has been recently suggested for Notch and Delta (Cordle et al, 2008).
Co-expression of the soluble class 3 Semaphorin Sema3A with its receptor Nrp-1 in motor neurons also acts as a response modulator (Moret et al, 2007). However, in this case, it seems that this is achieved by down-regulation of Nrp-1 at the axon growth cone.
Notably, Plexin-A4 also functions as a co-receptor of Nrp-1, transmitting the signalling of Sema3A in both DRG and sympathetic neurons (Suto et al, 2005; Yaron et al, 2005). We have found that Sema6A does not attenuate ligand-independent Plexin-A4 signalling. Moreover, earlier studies have not revealed any differential response to Sema3A between DRG neurons (that express both Sema6A and Sema6B) and sympathetic neurons (that do not express Sema6A and sema6B) (Xu et al, 2000; Suto et al, 2005). Thus, one can assume the existence of different pools of complexes, with Nrp-1 or other trasmembrane proteins, which contain Plexin-A4. Which of them is subjected to cis inhibition by Sema6A is yet not clear. However, our data from the Sema6A KO DRG clearly show that there is a pool of Plexin-A4 that is under this type of regulation.
Biological function of Sema6A cis inhibition
The physiological contribution of the mechanism that we have identified to proper guidance of DRG sensory axons remains an open question. Nonetheless, an earlier study in the CNS provides an indication for the use and function of cis inhibition by Sema6A in vivo. As noted above, Plexin-A2 can also serves as functional Sema6A receptor in the CNS (Bron et al, 2007; Renaud et al, 2008). During laminar termination in the hippocampus, mossy fibres expressing Plexin-A4 invade the stratum lucidum in which Sema6A is expressed (Suto et al, 2007). This invasion depends on the co-expression of Plexin-A2 with Sema6A in the stratum lucidum. Thus, in the Plexin-A2 KO mouse, the mossy fibres are repelled and this repulsion is eliminated in the Plexin-A2/Sema6A double KO (Suto et al, 2007). These results in combination with our studies suggest that cis inhibition can go in both ways. It can serve to prevent trans binding of the ligand, as we have discovered, and to make the ligand unavailable to trans receptors. In this regard, there is another parallel to the Notch Delta system, in which earlier studies in Drosophila and a recent high throughput analysis have suggested that cis interaction makes both the ligand and the receptor involved unavailable for trans binding (Jacobsen et al, 1998; Sprinzak et al). Interestingly, in the hippocampus, the only function that was shown so far for Plexin-A2 is its function as Sema6A cis inhibitor, whether it can also act as a signalling receptor in these neurons remains to be discovered.
Earlier study in cerebellar granule cells has also speculated that Sema6A may act as cis modulator of Plexin-A2, and that ablation of Sema6A may lead to hyper-activation of the co-expressed receptor in these cells (Renaud et al, 2008). Our study offers strong biochemical support for this model as we provide indication for differential modes of interaction in cis versus in trans. Suggesting that cis interaction may lock the co-expressed receptor in a non-functional conformation.
Our results that Sema6B can also act as cis inhibitor for Sema6A suggest a complex interaction between these members of the Semaphorin family with their receptor, Plexin-A4, and may help to elucidate the phenotypes that were observed of various single and double mutants in different parts of the brain.
The members of the class 6 Semaphorins were also shown to transmit signals into the cell in a mechanism termed reverse signalling (Tran et al, 2007). Although this was not addressed in this study, this type of signalling might also be blocked by cis interaction with the Plexin.
Finally, it will be interesting to find out if other transmembrane Semaphorins, of classes 4 and 5, can attenuate their responses through cis inhibition, adding another layer of regulation to axonal responsiveness to guidance cues.
Materials and methods
ISH-procedure probe sequences
In situ hybridization using digoxigenin-labelled probes on sections from E13.5 embryos as described (Cheng et al, 2001).
KO mice strains
The Plexin-A4 KO mice and the Sema6A KO mice were earlier described (Leighton et al, 2001; Yaron et al, 2005).
Production, purification, concentration and quantification of recombinant Semaphorin 6A-fc fusion protein
A plasmid containing the extra-cellular region of Sema6a fused to the human Fc was gratefully received from J Pasterkamp (Rudolf Magnus Institute of Neuroscience, Utrech The Netherlands). Human embryonic kidney (HEK293T) cells were transfected with this plasmid using standard protocol and cultured with serum-free DMEM/F12 media. Conditioned media (CM) was collected 72 h later and used in growth cone collapse and binding assays. To concentrate Sema6a-Fc CM, 45 ml of crude CM were loaded on 15 ml Centricon 50 000 MW cutoff filter device (Milipore, Bedford, MA) After centrifugations retained samples yielded 800 μl of concentrated media. The concentration of fusion protein was determined by proteinA affinity chromatography followed by BCA protein quantification assay (Pierce) of eluted fractions and by western blot using ProteinA–HRP.
Neurite growth assay Explants of DRG from embryonic day 13.5 (E13.5) Sema6A WT and KO littermates were cultured on top of a confluent COS7 monolayer that were transfected for 48 h with Sema6a full-length construct. Explants were grown in DRG/SG media containing OptiMem/F-12 (Gibco) supplemented with glutamine, 25 mM dextrose and 12.5 ng/ml mNerve growth Factor 2.5S (Alomone Labs) for 24 h at 37°C. Cultured neurons were fixed with 4% PFA/15% sucrose for several hours and then washed with phosphate-buffered saline (PBS) and stained with 1:2000 anti-Neuronal Class IIIβ-tubulin antibody (TUJ1, COVANCE), followed by fluorescent-conjugated Goat anti-mouse cy3 antibody (Jackson ImmunoResearch, West Grove, PA laboratory). Neurite lengths were documented with Nikon's 90I microscope equipped with DXM1200C NIKON digital camera and measured with Nikon's NIS-Elements imaging software acquisition and analysis package.
Growth cone collapse assay Collapse assays of DRG were performed as earlier described (He and Tessier-Lavigne, 1997). Briefly, DRG of E13.5 were cultured on CC2 Labtek 8 well chambers (Nunc) pre-coated with 10 mg/ml laminin (Sigma-Aldrich L2020) for 3 h at 37°C. Explants were grown in DRG media supplemented with 50 ng/ml Nerve growth Factor (Alomone Labs).
After 30 h (for DRG) and 36 h, recombinant Sema6a-fc fusion proteins was added at a concentrations ranging from 1.5 to 75 nM for 45 min at 37°C. Thereafter, cultures were fixed with 4% PFA/15% sucrose for 1 h at room temperature followed by staining with Rhodamin-Phalloidin. Slides were mounted and scored for percentage of collapsed axons on 90i upright fluorescent microscope (Nikon).
Binding of Sema6A-fc to cultured COS7 cells
COS7 cells were co-transfected with Plexin-A4 and Sema6a constructs or Plexin-A4 and Nrp-1 constructs and cultured for 48 h. Transfected cells were washed with binding buffer (Hank's-Buffered Salt Solution with 0.2% BSA, 0.1% NaN3, 5 mM CaCl2, and 1 mM MgCl2 and 20 mM HEPES, pH=7.0) for 10 min and incubated for 90 min at room temperature with various concentrations of Sema6A-Fc CM containing 1:12 000 goat anti-human IgG alkaline phosphates (AP)-conjugated antibody (Jackson ImmunoResearch Laboratories). After the removal of unbound ligand, cells were fixed with 4% PFA and rinsed with 20 mM HEPES pH=7.0, 150 mM NaCl. To destroy intrinsic AP activity, cells were heat inactivate in 65°C for 30 min. Finally, cells were rinsed with AP buffer (100 Mm TRIS PH=9.5, 100 mM NaCl and to 50 mM MgCl2) and the AP activity was evaluated by the formation of precipitants after an overnight incubation with nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate substrate. For the quantification of ligand-binding, cells were lysed with 10 mM Tris/1% Triton and the AP activity was measured at 405 nm after the addition of p-nitrophenylphosphate liquid substrate.
Co-immunoprecipitation
COS7 or HEK293 cells were co-transected with Plexin-A4-FLAG and Semaphorin6A-myc constructs using standard protocols; 48 h post-transfection cells were washed with cold PBS and lysed on ice with 1 ml solubilizaion buffer (50 mM HEPES pH 7, 150 mM NaCl, 10% glycerol, 1% Triton, 5 mM EGTA supplemented with Complete® protease inhibitors cocktail (Roche) and 200 mM phenylmethylsulfonylfluoride). Lysates were pre-cleared with 50 μl agarose beads for 4 h, followed by an overnight incubation at 4°C with immunoprecipitating anti-FLAG agarose beads (Sigma-Aldrich). Subsequently, beads were washed with cold lysis buffer and boiled in Laemmili SDS protein sample buffer. Standard protocols were used for western blot analysis with mouse anti-c-myc (9E10 Sigma-Aldrich) and mouse anti-FLAG antibody followed by goat anti-mouse HRP (Jackson ImmunoResearch, West Grove, PA) diluted 1:10 000 in TBST+3% low-fat milk powder.
Cell surface biotinylation
Cell surface biotinylation was preformed on transfected COS7 cells using the cell surface protein biotinylation and isolation kit (Pierce) according to the manufacture's instructions. Isolated proteins were analysed using western blot as described above.
Co-immunocytochemistry
COS7 cells were cultured on 13 mm cover glass and co-transfected with N-terminus Myc-Plexin-A4 expression vector and the Sema6a-full-length construct; 48 h later cells were fixed with 4% PFA and stained without pre-permeablilizing the membrane with monoclonal anti-mouse c-myc antibody (9E10 Sigma-Aldrich) detected by donkey anti-mouse cy2-conjugated antibody (1:500 dilution; Jackson ImmunoResearch, West Grove, PA) and with goat anti-mouse Sema6A antibody (R&D systems, 1:100) detected with bovine anti-goat cy3-conjugated antibody (1;300 dilution, Jackson ImmunoResearch, West Grove, PA). Staining was analysed and photographed by the 90i upright fluorescent microscope (Nikon) or the FV 1000 confocal microscope (Olympus).
Microporation
COS7 cells were transfected separately with Plexin-A4, Sema6a or empty vector using a pippett MicroPorator (MP-100) electroporation device according to manufactures' protocol (NanoEnTek Inc. Seoul, Korea). Briefly, 100 000 cells were micoporated each time using 10 μl pipette containing 0.5 μg plasmid DNA. Microporation was carried out using two pulses of 1050 V for duration of 30ms. Thereafter an equal number of Plexin-A4 cells were co-cultured with Sema6a cells or with Mock-transfected cells; 48 h later, co-cultures were used in standard ligand-binding assay.
Supplementary Material
Acknowledgments
We thank the Yaron laboratory members for advice and criticism on this paper, J Pasterkamp for the Sema6A-Fc plasmid and the anonymous reviewers for their comments that greatly improved this study. An HFSP Career Development Award (CDA0024/2006-C) and a research grant from Marla Schaefer supported research in the laboratory of AY.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J (2007) Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev 2: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U (2006) Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci 9: 322–330 [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M (2005) Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development 132: 2251–2262 [DOI] [PubMed] [Google Scholar]
- Chedotal A, Del Rio JA, Ruiz M, He Z, Borrell V, de Castro F, Ezan F, Goodman CS, Tessier-Lavigne M, Sotelo C, Soriano E (1998) Semaphorins III and IV repel hippocampal axons via two distinct receptors. Development 125: 4313–4323 [DOI] [PubMed] [Google Scholar]
- Chen H, He Z, Bagri A, Tessier-Lavigne M (1998) Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21: 1283–1290 [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M (2001) Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron 32: 249–263 [DOI] [PubMed] [Google Scholar]
- Chilton JK (2006) Molecular mechanisms of axon guidance. Dev Biol 292: 13–24 [DOI] [PubMed] [Google Scholar]
- Cordle J, Johnson S, Tay JZ, Roversi P, Wilkin MB, de Madrid BH, Shimizu H, Jensen S, Whiteman P, Jin B, Redfield C, Baron M, Lea SM, Handford PA (2008) A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nat Struct Mol Biol 15: 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ (2002) Molecular mechanisms of axon guidance. Science 298: 1959–1964 [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Low LK, Liu XB, Coble J, Jones EG, Cheng HJ (2008) Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural Dev 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H (2004) Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol 59: 24–33 [DOI] [PubMed] [Google Scholar]
- Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL (1998) Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron 21: 1079–1092 [DOI] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90: 739–751 [DOI] [PubMed] [Google Scholar]
- Hornberger MR, Dutting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U (1999) Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22: 731–742 [DOI] [PubMed] [Google Scholar]
- Jacobsen TL, Brennan K, Arias AM, Muskavitch MA (1998) Cis-interactions between Delta and Notch modulate neurogenic signalling in Drosophila. Development 125: 4531–4540 [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M (2001) Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature 410: 174–179 [DOI] [PubMed] [Google Scholar]
- Mann F, Rougon G (2007) Mechanisms of axon guidance: membrane dynamics and axonal transport in semaphorin signalling. J Neurochem 102: 316–323 [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL (2005) Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 121: 127–139 [DOI] [PubMed] [Google Scholar]
- Moret F, Renaudot C, Bozon M, Castellani V (2007) Semaphorin and neuropilin co-expression in motoneurons sets axon sensitivity to environmental semaphorin sources during motor axon pathfinding. Development 134: 4491–4501 [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Negishi M (2004) Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J Neurosci 24: 11473–11480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Kolodkin AL (2003) Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol 13: 79–89 [DOI] [PubMed] [Google Scholar]
- Puschel AW (2002) The function of neuropilin/plexin complexes. Adv Exp Med Biol 515: 71–80 [DOI] [PubMed] [Google Scholar]
- Renaud J, Kerjan G, Sumita I, Zagar Y, Georget V, Kim D, Fouquet C, Suda K, Sanbo M, Suto F, Ackerman SL, Mitchell KJ, Fujisawa H, Chedotal A (2008) Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat Neurosci 11: 440–449 [DOI] [PubMed] [Google Scholar]
- Rohm B, Ottemeyer A, Lohrum M, Puschel AW (2000) Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev 93: 95–104 [DOI] [PubMed] [Google Scholar]
- Runker AE, Little GE, Suto F, Fujisawa H, Mitchell KJ (2008) Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev 3: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszczuk DF, Wilkinson DG (1999) Masking of Eph receptors and ephrins. Curr Biol 9: R469–R470 [DOI] [PubMed] [Google Scholar]
- Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, Garcia-Ojalvo J, Elowitz MB (2010) Cis-interactions between Notch Delta generate mutually exclusive signalling states. Nature 465: 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H (2005) Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci 25: 3628–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, Chedotal A, Mitchell KJ, Manabe T, Fujisawa H (2007) Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53: 535–547 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99: 59–69 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Strittmatter SM (2001) Plexina1 autoinhibition by the plexin sema domain. Neuron 29: 429–439 [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99: 71–80 [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM (2000) Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol 10: 377–383 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274: 1123–1133 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R (2007) Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol 23: 263–292 [DOI] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL (2009) Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462: 1065–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LJ, Hall A (2006) Plexin-induced collapse assay in COS cells. Methods Enzymol 406: 665–676 [DOI] [PubMed] [Google Scholar]
- Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y (2000) The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci 20: 2638–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M (2005) Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 45: 513–523 [DOI] [PubMed] [Google Scholar]
- Yaron A, Zheng B (2007) Navigating their way to the clinic: emerging roles for axon guidance molecules in neurological disorders and injury. Dev Neurobiol 67: 1216–1231 [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR (2006) The semaphorins. Genome Biol 7: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H (2004) EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res 48: 285–296 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ (2008) Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci 33: 161–170 [DOI] [PubMed] [Google Scholar]
- Zhuang B, Su YS, Sockanathan S (2009) FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 61: 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.