Abstract
Background: Bevacizumab, the anti-vascular endothelial growth factor agent, provides clinical benefit when combined with platinum-based chemotherapy in first-line advanced non-small-cell lung cancer. We report the final overall survival (OS) analysis from the phase III AVAiL trial.
Patients and methods: Patients (n = 1043) received cisplatin 80 mg/m2 and gemcitabine 1250 mg/m2 for up to six cycles plus bevacizumab 7.5 mg/kg (n = 345), bevacizumab 15 mg/kg (n = 351) or placebo (n = 347) every 3 weeks until progression. Primary end point was progression-free survival (PFS); OS was a secondary end point.
Results: Significant PFS prolongation with bevacizumab compared with placebo was maintained with longer follow-up {hazard ratio (HR) [95% confidence interval (CI)] 0.75 (0.64–0.87), P = 0.0003 and 0.85 (0.73–1.00), P = 0.0456} for the 7.5 and 15 mg/kg groups, respectively. Median OS was >13 months in all treatment groups; nevertheless, OS was not significantly increased with bevacizumab [HR (95% CI) 0.93 (0.78–1.11), P = 0.420 and 1.03 (0.86–1.23), P = 0.761] for the 7.5 and 15 mg/kg groups, respectively, versus placebo. Most patients (∼62%) received multiple lines of poststudy treatment. Updated safety results are consistent with those previously reported.
Conclusions: Final analysis of AVAiL confirms the efficacy of bevacizumab when combined with cisplatin–gemcitabine. The PFS benefit did not translate into a significant OS benefit, possibly due to high use of efficacious second-line therapies.
Keywords: bevacizumab, chemotherapy, NSCLC, overall survival, vascular endothelial growth factor
introduction
Advanced non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related death. Although there is currently no universally accepted standard regimen for the first-line treatment of advanced NSCLC, cisplatin–gemcitabine (CG) is widely used in Europe on the basis of its favourable efficacy and tolerability profile [1, 2]. Bevacizumab is an anti-vascular endothelial growth factor mAb developed from the murine antibody A4.6.1 [3, 4]. The clinical benefit of bevacizumab is well established across a range of tumour types, including colorectal cancer [5, 6], metastatic breast [7] and renal cancers [8]. In nonsquamous NSCLC, the proven efficacy and well-established safety profile of bevacizumab as a first-line treatment have been demonstrated in two randomised phase III trials [9, 10].
The Eastern Cooperative Oncology Group (ECOG) phase III trial E4599 reported significantly improved overall survival (OS; study primary end point) with bevacizumab plus carboplatin–paclitaxel versus carboplatin–paclitaxel alone [hazard ratio (HR) 0.79; median 12.3 versus 10.3 months; P = 0.003] [9]. Bevacizumab plus carboplatin–paclitaxel also significantly improved progression-free survival (PFS) and response rates versus carboplatin–paclitaxel alone and had an acceptable safety profile. The results of E4599 showed that bevacizumab is the first agent to increase OS beyond the historical 1-year benchmark.
AVAiL (BO17704), a randomised placebo-controlled phase III trial, evaluated bevacizumab (7.5 and 15 mg/kg every 3 weeks) in combination with CG. In AVAiL, the primary end point of PFS was met, further proving the efficacy of bevacizumab in NSCLC. The results of the PFS analysis, other secondary efficacy end points and the safety analysis have already been published [10]. Here, we report results of the OS analysis from AVAiL, on the basis of a follow-up period of up to 32 months (median ≥12.5 months). Some of these results have been presented in abstract form [11].
patients and methods
patients
Eligible patients had histologically or cytologically documented, advanced (stage IIIb with supraclavicular lymph node metastasis, malignant pleural or pericardial effusion or stage IV) or recurrent nonsquamous NSCLC. Patients with mixed non-small-cell tumour and small-cell tumour or mixed adenosquamous carcinomas with a predominant squamous component were excluded. Full details of patient inclusion and exclusion criteria have been published previously [10]. Patients were recruited from February 2005 to August 2006 by 150 centres in 20 countries. The protocol was approved by local independent ethics committees.
study design and treatments
Eligible patients were randomised to receive bevacizumab 7.5 mg/kg plus CG, bevacizumab 15 mg/kg plus CG or high- or low-dose placebo plus CG. Cisplatin was administered i.v. at 80 mg/m2 on day 1 and gemcitabine was administered i.v. at 1250 mg/m2 on days 1 and 8. Chemotherapy was repeated every 3 weeks for up to six cycles unless there was evidence of disease progression or unacceptable toxicity. Blinded placebo or bevacizumab was administered i.v. and given concurrently with chemotherapy every 3 weeks on day 1. Further information on randomisation, stratification and strategies to maintain blinding is detailed elsewhere [10]. Crossover from placebo to the bevacizumab groups was not permitted. Bevacizumab and placebo were supplied by F. Hoffmann-La Roche Ltd (Basel, Switzerland).
statistical analysis
The primary analysis population was the intent-to-treat (ITT) population, which included all randomised patients. The per protocol (PP) population included all patients with no major protocol violations who received at least four cycles of study treatment and had at least one tumour assessment during treatment or who terminated treatment before four cycles because of disease progression or death. The PP analysis was a protocol-specified efficacy analysis.
The study was originally initiated with a two-stage adaptive design and a primary end point of OS (defined as the time from randomisation to death from any cause). One of the bevacizumab groups was to be stopped on the basis of an interim analysis of efficacy (PFS) and safety at the end of the first stage, resulting in a two-arm study. Following the positive OS results from E4599 [9], the study protocol was amended to facilitate trial completion and interpretation, to accelerate reporting of the efficacy data and thus expedite the availability of a potentially active treatment option for patients and to minimise the risk of the OS end point being confounded by the increasing use of second-line therapies. The primary end point was changed to PFS, and it was decided that both bevacizumab doses would be explored. The study was not powered to detect an OS benefit retaining the two doses of bevacizumab but was fully powered for the primary end point of PFS. No analysis comparing the pooled bevacizumab groups versus placebo was carried out, as the criteria required to pool these data were not met. Exploratory OS analyses for patients who did not receive postprotocol therapies were carried out for the two bevacizumab arms (pooled data from both groups) versus the placebo arm. Pre-planned exploratory subgroup analyses of OS data were carried out on the basis of a variety of important baseline factors: ECOG performance status, stage, gender and age (and also albumin, lactate dehydrogenase and alkaline phosphatase but results are not presented for these factors). Event-time distributions were estimated by the Kaplan–Meier method. All analyses reported here were carried out from a data cut-off on 30 November 2007.
results
Randomised patients (n = 1043) were assigned to bevacizumab 7.5 mg/kg plus CG (n = 345), bevacizumab 15 mg/kg plus CG (n = 351) or placebo plus CG (n = 347). Of these, 57 patients did not receive study therapy because of eligibility violations (n = 27), withdrawal of consent (n = 16), adverse events (n = 8) and other reasons (n = 6). Demographic and baseline characteristics of all randomised patients, which have been previously reported [10], were well balanced across treatment groups.
treatment duration
The median number of cycles of chemotherapy and bevacizumab–placebo was five for the placebo and 15 mg/kg bevacizumab groups and six for the 7.5 mg/kg bevacizumab group [10]. Most patients (94%) who were eligible to receive single-agent bevacizumab maintenance therapy at cycle 7 did so.
progression-free survival
The trial met its primary end point at the time of the first analysis, showing that PFS was significantly prolonged with bevacizumab compared with placebo (clinical cut-off 7 October 2006) [10].
The observed PFS benefit was maintained with longer follow-up (median 12.5–12.9 months across groups). At the time of the OS analysis, PFS was significantly longer in both bevacizumab groups compared with placebo. Compared with the placebo group, the risk of progression or death at any time was reduced by 25% in the bevacizumab 7.5 mg/kg group [HR 0.75, 95% confidence interval (CI): 0.64–0.87; P = 0.0003] and by 15% in the bevacizumab 15 mg/kg group (HR 0.85, 95% CI 0.73–1.00; P = 0.0456).
response rate
At the time of the follow-up analysis, objective response rates were 37.8% (P < 0.0001) and 34.6% (P = 0.0002) for bevacizumab 7.5 mg/kg and 15 mg/kg, respectively, compared with 21.6% for placebo.
overall survival
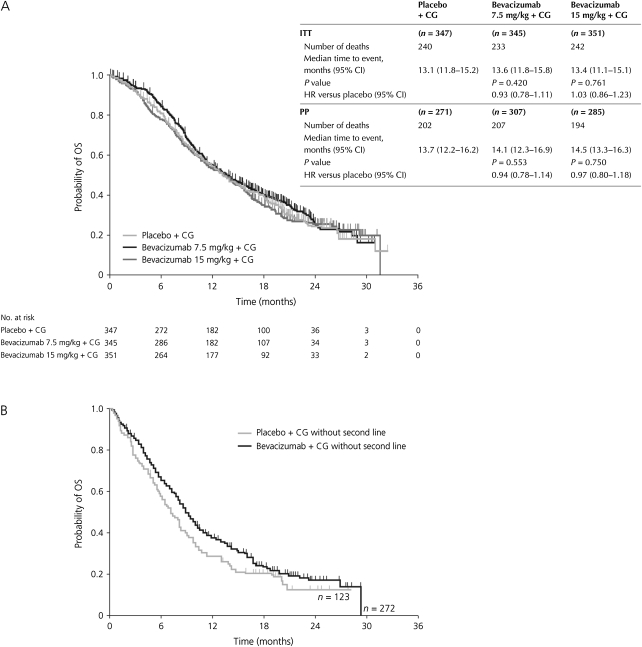
The total number of reported deaths was 715. Despite the trend seen in OS at the time of the initial analysis favouring the bevacizumab 7.5 mg/kg arm and the confirmed reduction in the risk of progression or death seen with longer follow-up, final analysis shows that the PFS benefit did not translate into a significant OS benefit. In the ITT population, the median OS was nevertheless the longest ever reported in NSCLC trials, increasing from 13.1 months for the placebo group to 13.6 and 13.4 months in the bevacizumab 7.5 mg/kg and bevacizumab 15 mg/kg groups, respectively (HR 0.93, 95% CI 0.78–1.11; P = 0.420; HR 1.03, 95% CI 0.86–1.23; P = 0.761, respectively) (Figure 1A). The results were similar in the PP population.
Figure 1.
Plots of Kaplan–Meier estimates for OS (ITT population) for the bevacizumab 7.5 mg/kg group and the bevacizumab 15 mg/kg group relative to placebo, together with time to event data for the OS analysis in the ITT and PP populations (A) and for the subgroup of patients who did not receive poststudy therapy (B). In panel b, data from the two bevacizumab groups have been pooled. CG, cisplatin–gemcitabine; CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; OS, overall survival; PP, per protocol.
A large proportion of patients in AVAiL (61%–65%) received poststudy therapy in each of the treatment arms (Table 1). In order to estimate the impact of postprotocol therapies on the results of the OS analysis, a hypothesis-generating exploratory analysis examining the duration of OS in patients who did not receive poststudy therapy was conducted (Figure 1B). Without the potential influence of poststudy therapies, the median OS (95% CI) was 8.7 (7.8–9.9) months for the pooled bevacizumab groups versus 7.3 (5.9–8.9) months for the placebo group (HR 0.84; P = 0.20). A clear separation of the Kaplan–Meier curves for OS was observed between the bevacizumab and the placebo groups; however, owing to the small sample size, the results did not reach statistical significance. A similar analysis conducted for patients who did receive poststudy therapy showed no difference between treatment groups.
Table 1.
Poststudy therapy (ITT population)
| Placebo + CG (n = 347), n (%) | Bevacizumab 7.5 mg/kg + CG (n = 345), n (%) | Bevacizumab 15 mg/kg + CG (n = 351), n (%) | |
| Patients with at least one treatment | 224 (65) | 210 (61) | 214 (61) |
| Targeted therapy | |||
| Erlotinib | 66 (19) | 82 (24) | 69 (20) |
| Gefitinib | 20 (6) | 18 (5) | 15 (4) |
| Bevacizumab | 2 (<1) | 1 (<1) | 2 (<1) |
| Chemotherapy | |||
| Taxane | 93 (27) | 76 (22) | 84 (24) |
| Pemetrexed | 52 (15) | 47 (14) | 46 (13) |
| Gemcitabine | 20 (6) | 18 (5) | 21 (6) |
| Radiotherapya | 66 (19) | 76 (22) | 60 (17) |
CG, cisplatin–gemcitabine.
Included palliative irradiation of bone metastases.
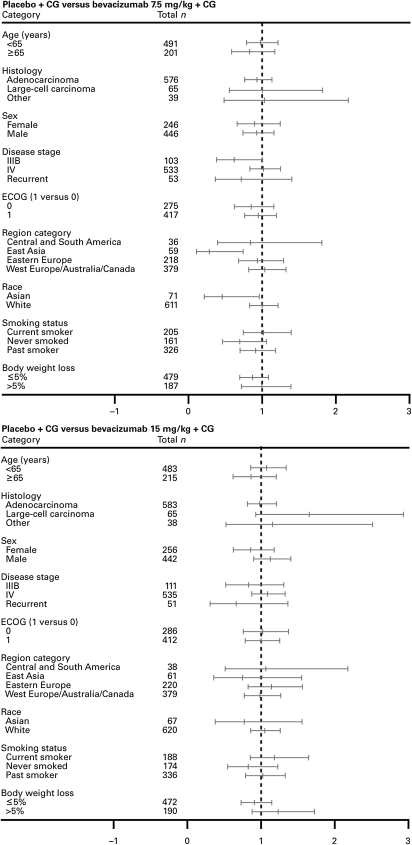
The analysis of OS for subgroups in AVAiL produced results that were largely consistent with those for the overall ITT population, except for the small subgroup of Asian patients for whom a significant benefit of bevacizumab 7.5 mg/kg was observed (Figure 2).
Figure 2.
Forest plots of HRs for OS by subgroup for each bevacizumab group relative to placebo (ITT population). CG, cisplatin–gemcitabine; ECOG, Eastern Cooperative Oncology Group.
safety
The adverse event profile, including overall incidence of adverse events, severe (grade ≥3) adverse events and events of special interest, has been previously reported [10]. Updated safety results at the time of the follow-up OS analysis are consistent with those previously reported and no new safety signals were detected.
discussion
AVAiL is the second randomised phase III trial to show a clinically significant benefit from bevacizumab-based therapy in patients with advanced NSCLC. Efficacy analyses showed that the primary end point of prolonged PFS was met; the response rate and response duration were also significantly improved [10]. However, the significant prolongation of PFS observed with bevacizumab did not translate into significantly longer OS. This is likely due to a number of confounding factors. It is notable that all three treatment groups achieved a median OS of >13 months, the longest reported to date in this nonsquamous NSCLC patient population and well beyond the historical survival benchmark of 1 year.
To establish the possible reasons for the extended OS seen across all treatment groups and to ascertain why the significant OS benefit of bevacizumab in E4599 was not confirmed in AVAiL, it is instructive to examine the impact of variables such as baseline prognostic factors and postprotocol therapies. Patients in the overall AVAiL population generally had slightly more favourable prognostic features when compared with those in the E4599 trial [9]: they were younger (median age 57–59 versus 63 years), 8% had dry stage IIIb (0% in E4599, which only enrolled wet stage IIIb) and a high proportion had adenocarcinoma histology (82%–85%) and were never smokers (22%–26%) [10, 11]. Together, these factors are not only likely to have contributed to the longest survival duration reported for CG (13.1 months) in a predominantly non-Asian population but may also explain the unusually high use of second and subsequent lines of therapy. Indeed, it appears that the long duration of survival in AVAiL may also reflect the growing influence of further lines of therapy on patient outcomes.
AVAiL was conducted at a time when several efficacious second-line therapies, such as the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib and pemetrexed, became widely used in routine clinical practice and their use may have introduced a confounding factor in the OS end point analysis. Our data show that the proportion of patients who received poststudy therapies in AVAiL (62%) was one of the highest ever reported in NSCLC trials (e.g. 54% in the JMDB trial [12], 57% in the FLEX trial [13]). Additionally, the percentage of patients receiving second-line therapy was slightly higher for placebo (65%) versus either bevacizumab group (61%); this may also have led to a more favourable than expected outcome for the placebo group. Although the various types of agents used in the poststudy setting appeared to be balanced across study arms, the real impact of these therapies is difficult to assess, as specific information on the combinations, dosing, compliance, duration or sequencing of the therapies is not available. However, the heterogeneity in poststudy therapies was high (with >66 different regimens identified). The exploratory OS analysis for patients who did not receive additional therapies indicates that when the influence of second-line therapies is removed, bevacizumab may have a favourable impact on OS over and above that of chemotherapy alone. Clearly, the therapeutic options now available for the treatment of NSCLC have become more complex and need to be individually tailored to achieve the most favourable patient outcomes. In a recent investigation [14], the effect of bevacizumab on levels of circulating endothelial progenitor cells differed depending on whether it was combined with paclitaxel or gemcitabine, indicating that bevacizumab may interact with different cytotoxic chemotherapy agents in a variable manner. However, these findings were generated in preclinical tumour models and have not been confirmed in clinical trials.
As an increasing number of effective options for second- and third-line therapies in advanced NSCLC become available, the sensitivity of OS as a primary end point in NSCLC trials is likely to be increasingly challenged. The OS findings from the AVAiL and BETA Lung trials [15] are consistent with this notion and tend to validate the adoption of PFS as the primary end point in AVAiL. Both the Food and Drug Administration [16] and the European Medicines Agency [17] accept PFS as a valid measure of clinical benefit, particularly in situations where it is expected that further lines of treatment may hamper the detection of a relevant OS benefit. Recent regulatory approval of a number of cancer therapies on the basis of a PFS advantage underscores the increasing importance and validity of PFS as an end point in phase III clinical trials of oncologic agents where multiple efficacious lines of therapy are accepted clinical standards or where there is unmet clinical need.
The safety profile for bevacizumab plus CG in AVAiL was previously reported [10] and no new safety signals were observed in the current analysis, supporting the well-established and manageable adverse event profile for bevacizumab.
In summary, the primary end point of AVAiL (PFS) was met and the magnitude of bevacizumab benefit was maintained with longer follow-up. This magnitude (HR 0.75–0.85) is clinically significant, allowing patients to live longer without their disease progressing. The final analysis of the AVAiL data confirms the significant prolongation of PFS when bevacizumab is combined with CG, but the PFS finding did not translate into a significant OS benefit. The positive results from AVAiL, together with those from the E4599 study, demonstrate that bevacizumab combined with standard platinum-based chemotherapy doublets in the first-line setting leads to significantly improved outcomes for patients with advanced nonsquamous NSCLC.
funding
Hoffmann-La Roche Ltd (study number BO17704; registered at clinicaltrials.gov, NCT00806923).
disclosure
MR has received consulting fees and speaking fees from AstraZeneca, Eli Lilly, F. Hoffmann-La Roche and Merck. VA and NM are employed by and hold stock in F. Hoffmann-La Roche. All other authors report no involvements that might raise the question of bias.
Acknowledgments
The authors would like to acknowledge medical writing support by David Cutler of Gardiner-Caldwell Communications.
References
- 1.Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group—EORTC 08975. J Clin Oncol. 2003;21:3909–3917. doi: 10.1200/JCO.2003.03.195. [DOI] [PubMed] [Google Scholar]
- 2.Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 4.Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 7.Miller KD, Wang M, Gralow J, et al. A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer: a trial coordinated by the Eastern Cooperative Oncology Group (E2100) Breast Cancer Res Treat. 2005;94:S6. (Abstr 3) [Google Scholar]
- 8.Hainsworth JD, Sosman JA, Spigel DR, et al. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889–7896. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 11.Manegold C, von Pawel J, Zatloukal P, et al. BO17704 (AVAiL): a phase III randomised study of first-line bevacizumab combined with cisplatin/gemcitabine (CG) in patients (pts) with advanced or recurrent non-squamous, non-small cell lung cancer (NSCLC) Ann Oncol. 2008;19(Suppl 8):viii1. (Abstr LBA1) [Google Scholar]
- 12.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt W, von Pawel J, Vynnychenko I, et al. FLEX: cetuximab in combination with platinum-based chemotherapy (CT) vs CT alone improves survival in 1st-line treatment of patients with advanced non-small cell lung cancer (NSCLC) Ann Oncol. 2008;19(Suppl 8):viii45. (Abstr 73O) [Google Scholar]
- 14.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hainsworth J, Lin M, O'Connor P, et al. A phase III, multicenter, placebo-controlled, double-blind, randomized, clinical trial to evaluate the efficacy of bevacizumab (Avastin) in combination with erlotinib (Tarceva) compared with erlotinib alone for treatment of advanced non-small cell lung cancer (NSCLC) after failure of standard first line chemotherapy (BETA) J Thorac Oncol. 2008;3(Suppl 4):S302. [Google Scholar]
- 16.US Department of Health and Human Services. Food and Drug Administration. Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071590.pdf (1 February 2010, date last accessed) [Google Scholar]
- 17.European Agency for the Evaluation of Medicinal Products. Guideline on the Evaluation of Anticancer Medicinal Products in Man. http://www.tga.gov.au/docs/pdf/euguide/ewp/020595enrev3.pdf (1 February 2010, date last accessed) [Google Scholar]