Summary
Anaplasma phagocytophilum is an obligate intracellular bacterium that infects neutrophils to reside within a host cell-derived vacuole. The A. phagocytophilum-occupied vacuole (ApV) fails to mature along the endocytic pathway and is non-fusogenic with lysosomes. Rab GTPases regulate membrane traffic. To better understand how the bacterium modulates the ApV’s selective fusogencity, we examined the intracellular localization of 20 green fluorescent protein (GFP) or red fluorescent protein (RFP)-tagged Rab GTPases in A. phagocytophilum infected HL-60 cells. GFP-Rab4A, GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, and GFP-Rab35, which regulate endocytic recycling, and GFP-Rab1, which mediates endoplasmic reticulum to Golgi apparatus trafficking, localize to the ApV. Fluorescently tagged Rabs are recruited to the ApV upon its formation and remain associated throughout infection. Endogenous Rab14 localizes to the ApV. Tetracycline treatment concomitantly promotes loss of recycling endosome-associated GFP-Rabs and acquisition of GFP-Rab5, GFP-Rab7, and the lysosomal marker, LAMP-1. Wild-type and GTPase-deficient versions, but not GDP-restricted versions of GFP-Rab1, GFP-Rab4A, and GFP-Rab11A localize to the ApV. Strikingly, GFP-Rab10 recruitment to the ApV is guanine nucleotide-independent. These data establish that A. phagocytophilum selectively recruits Rab GTPases that are primarily associated with recycling endosomes to facilitate its intracellular survival and implicate bacterial proteins in regulating Rab10 membrane cycling on the ApV.
Introduction
Diverse intravacuolar pathogens hijack membrane traffic to modify the host cell-derived vacuoles in which they reside into permissive safe havens. Anaplasma phagocytophilum is a tick-transmitted obligate intravacuolar bacterium that infects granulocytes and bone marrow progenitor cells to cause the emerging infection, human granulocytic anaplasmosis (HGA) (Chen et al., 1994, reviewed in Thomas et al., 2009). HGA manifestations range from sub-clinical infection to severe disease, including death. The ability of A. phagocytophilum to flourish within the otherwise hostile confines of the primary effector cell of microbial killing indicates that the bacterium is able to evade intracellular killing pathways. Indeed, the A. phagocytophilum-occupied vacuole (ApV) is altered in its fusogenicity. It does not resemble early endosomes as it lacks transferrin receptor (TFR), early endosomal antigen I, Rab5, and Annexins I, II, IV, and VI (Mott et al., 1999, Webster et al., 1998). It lacks endosomal markers α-adaptin and clathrin heavy chain. It does not acidify, does not acquire the late endosomal/lysosomal markers myeloperoxidase, CD63, LAMP-1, and V-type H+ ATPase and avoids lysosomal fusion. The ApV excludes fusion with secretory vesicles and specific granules harboring NADPH oxidase and proteolytic enzymes (Carlyon et al., 2004, IJdo et al., 2004, Mott et al., 2002). Furthermore, it fails to acquire Golgi vesicular markers β-COP or C6-NBD-ceramide, and bacterial growth is unhindered by the Golgi apparatus destabilizing agent, Brefeldin A (BFA) (Mott et al., 1999).
While the ApV stains negative for most endosomal, lysosomal, and Golgi markers, it is not an inert compartment that is completely sequestered from membrane traffic. Indeed, the ApV acquires endocytosed BSA-gold (Webster et al., 1998), which indicates that it intercepts some arm of endocytic traffic. Moreover, mannose-6-phosphate receptor, which is found on late endosomes, pre-lysosomes, and trans-Golgi vesicles (Geuze et al., 1988), and major histocompatibility complex (MHC) class I and class II molecules, which traffic from the Golgi to recycling endosomes and the plasma membrane (Amigorena et al., 1994, Neefjes et al., 1990), are found on the ApV (Mott et al., 1999). How the ApV interfaces with desired vesicular trafficking pathways while excluding those that facilitate microbial killing is poorly understood.
The Rab GTPase family (>60 members) is the largest member of the Ras superfamily of small guanosine triphosphatases (reviewed in Brumell et al., 2007 and Stenmark, 2009). Rab GTPases localize to distinct organelles and dictate organelle identity. Specific Rabs cycle on and off endosomes in a dynamic process to control endosomal maturation, which ultimately results in lysosomal fusion. Each of the 4 steps in membrane trafficking that Rabs regulate – vesicle budding, vesicle delivery, vesicle tethering, and fusion of the vesicle and target compartment membranes – are carried out by a diverse collection of effector molecules that bind to specific Rabs in their GTP-bound states. Rab GTPases are molecular switches and cycle between a cytoplasmic, GDP-bound, inactive state and a membrane-associated GTP-bound, active state.
The switch of a GDP-bound Rab to a GTP-bound-Rab is controlled by guanine nucleotide exchange factors (GEFs), which trigger the release of GDP, and GTPase-activating proteins (GAPs), which accelerate hydrolysis of the bound GTP to GDP (Brumell et al., 2007, Stenmark, 2009). In their GDP-bound state, Rabs are soluble and bound to guanine nucleotide dissociation inhibitor (GDI). At the acceptor membrane, Rab-GDI interacts with GDI displacement factor (GDF), which removes GDI to enable Rab membrane insertion. Next, a GEF catalyzes the conversion of the membrane-bound Rab to its GTP-bound state, which allows it to interact with its effectors to control membrane traffic and mediate membrane fusion. After inactivation by their specific GAPs, the GDP-bound Rabs are extracted from the membrane by GDI and returned to the cytosol.
Endosomal recycling pathways return much of the membrane proteins and lipids that are internalized during endocytosis to the plasma membrane (Grant et al., 2009). Endocytic mechanisms can be classified as either clathrin-dependent or clathrin-independent. TFR is an example of a clathrin-dependent endocytic cargo. In contrast to clathrin-dependent endocytosis, clathrin-independent endocytosis comes in many forms including macropinocytosis and phagocytosis. Unrelated forms of clathrin-independent endocytosis require free cholesterol. Proteins and lipids that reside in sphingolipid-rich lipid rafts are prominent clathrin-independent endocytosis cargo (Sandvig et al., 2008, Grant et al., 2009, Mayor et al., 2007). Endogenous proteins that enter cells via clathrin-independent endocytosis include MHC class I proteins and proteins involved in amino acid uptake (Radhakrishna et al., 1997, Eyster et al., 2009, Brown et al., 2001). Clathrin-independent endocytosis- and clathrin-dependent endocytosis-generated vesicles are delivered to the early endosome, which functions as a sorting station. From there, cargo can be routed for degradation to late endosomes and lysosomes or recycled along rapid or slow recycling endosome pathways. Clathrin-dependent endocytic cargo, such as TFR, can be returned to the plasma membrane via either rapid recycling endosomes or slow recycling endosomes, while clathrin-independent endocytic cargo are recycled exclusively by the slow recycling endosome pathway. In slow endocytic recycling, cargo is sorted from the early endosome to an endocytic recycling compartment from which recycling endosomes or tubular recycling endosomes emanate to return cargo to the plasma membrane (Grant et al., 2009). Several Rab GTPases regulate traffic and confer vesicle identities along RE pathways (Table 1). Rab5 is found on the early endosomes. Rab4 and Rab35 are on rapid recycling endosomes. Rab35 is also associated with tubular recycling endosomes that return cargo to the plasma membrane in clathrin-independent endocytosis. Whether Rab35 functions or is simply a passenger on tubular recycling endosomes is unknown. Rab10, Rab11, Rab14, and Rab22A are required for moving cargo from the early endosome to the endocytic recycling compartment (Brumell et al., 2007, Kelly et al., 2009). The endocytic recycling compartment is defined by the presence of Rab11 and Rab14. Rab11 is also found on recycling endosomes, mediates delivery to the plasma membrane, is essential for MHC class I cargo recycling, and is needed to transport vesicles that bud off from tubular recycling endosomes to the plasma membrane (Brumell et al., 2007). Rab14 is also found on early endosomes and the trans-Golgi network and mediates vesicular transport from the trans-Golgi network to the endocytic recycling compartment (Kelly et al., 2009). Rab8 and Rab22A are required to transport cargo from the endocytic recycling compartment to Rab10-/Rab22A-/Rab35-positive tubular recycling endosomes (Grant et al., 2009, Stenmark, 2009).
Table 1.
Rab GTPases examined in this studya
| Rab GTPaseb | Location(s)c | Transport Function(s) |
|---|---|---|
| Rab1 | ER exit sites, IC | ER to Golgi, Golgi to ER, IC to PM |
| Rab2A | IC | ER to Golgi, Golgi to ER |
| Rab3A | SV, SG | Regulated exocytosis |
| Rab4A | Rapid RE | Endocytic recycling |
| Rab5 | EE, PM, caveosomes | Endocytosis, EE formation |
| Rab6A | Golgi | Intra-Golgi, ER to Golgi, endosome to Golgi, Golgi to PM |
| Rab7 | LE | EE to LE, lysosome biogenesis, phagosome maturation |
| Rab8A | ERC to tubular RE, TGN | ERC to tubular RE, TGN to PM |
| Rab9A | LE | LE to TGN |
| Rab10 | EE to ERC, tubular RE, TGN | Endocytic recycling, TGN to PM |
| Rab11A | ERC, RE, tubular RE | Endocytic recycling |
| Rab13 | Tight junctions, EE | Biogenesis of tight junctions |
| Rab14 | EE, ERC, TGN | Endocytic recycling, TGN to EE |
| Rab18 | ER, SG | ER to Golgi |
| Rab20 | Dense apical tubules | Connexin-43 trafficking |
| Rab22A | EE to ERC, tubular RE | Endocytic recycling, EE to TGN |
| Rab23 | PM, EE | Phagosome to lysosome fusion |
| Rab27A | SG, melanosomes, T-cell granules | Regulated secretion, Myosin Va recruitment to melanosomes & T- cell granules |
| Rab33A | Medial Golgi | Golgi to ER, autophagy |
| Rab35 | Tubular RE, rapid RE | Endocytic recycling |
Data summarized from references by Grant and Donaldson, Kelly et al, and Stenmark.
ApV-associated Rab GTPases are highlighted.
EE, early endosome; ER, endoplasmic reticulum; ERC, endocytic recycling center; IC, pre-Golgi intermediate compartment; LE, late endosome; PM, plasma membrane; RE, recycling endosome; SG, secretory granule; SV, synaptic vesicle; TGN, trans-Golgi network.
Selective retention of specific Rab proteins with the concomitant exclusion of others has been shown to regulate the construction of vacuolar organelles inhabited by several intracellular bacterial pathogens including Mycobacterium tuberculosis (Kelley et al., 2003, Kyei et al., 2006, Via et al., 1997), Coxiella burnetii (Gutierrez et al., 2004, Gutierrez et al., 2005, Romano et al., 2007), Salmonella enterica serovar Typhimurium (Mukherjee et al., 2001, Smith et al., 2007, Smith et al., 2005, Steele-Mortimer et al., 1999), Chlamydia species (Cortes et al., 2007, Moorhead et al., 2007, Rzomp et al., 2006, Rzomp et al., 2003), and Legionella pneumophila (Derre et al., 2004, Kagan et al., 2004, Machner et al., 2006, Murata et al., 2006). In this study, we examined a panel of fluorescent protein-tagged Rab GTPases that function in a variety of membrane trafficking pathways and cellular processes (Table 1). We demonstrate that the ApV selectively recruits a subset of fluorescently tagged Rabs that predominantly associate with recycling endosomes in a tetracycline-sensitive manner. Endogenous Rab14 localizes to a comparable percentage of ApVs as its green fluoresecent protein (GFP)-tagged counterpart. Recruitment of GFP-Rab1, GFP-Rab4A, and GFP-Rab11A is guanine nucleotide-dependent, while recruitment of GFP-Rab10 is strikingly guanine nucleotide-independent. These data shed light on how A. phagocytophilum facilitates its intravacuolar survival and implicates the requirement of bacterial-derived proteins for this strategy.
Results
Fluorescent protein-tagged versions of Rab1, Rab4A, Rab10, Rab11A, Rab14, Rab22A, and Rab35 localize to the ApV
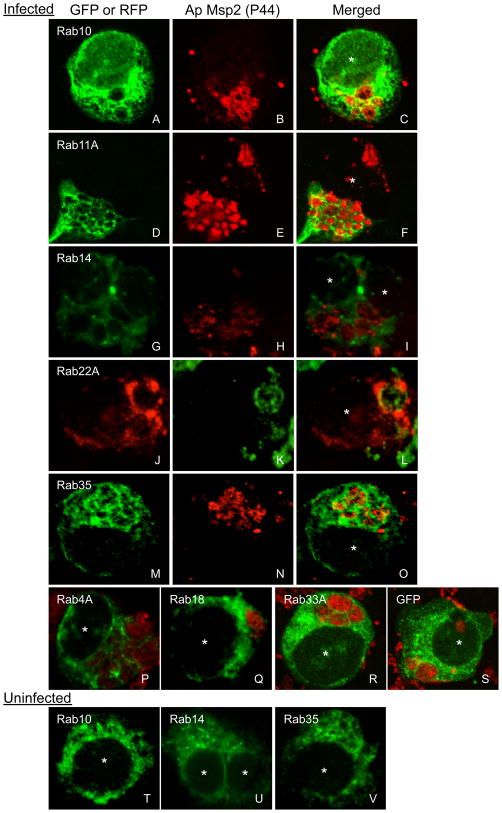
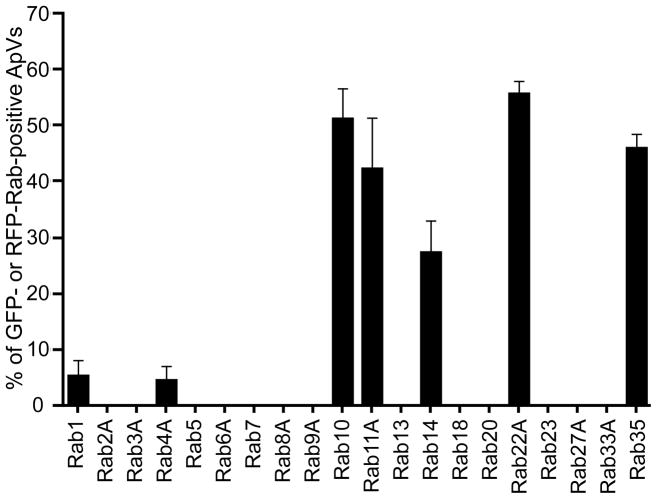
We rationalized if Rab GTPases are important for the formation, development, and/or altered fusogenicity of the ApV, then specific Rab proteins would be recruited to the ApV while others would be excluded. To investigate this, we transiently nucleofected A. phagocytophilum infected HL-60 cells with plasmids encoding a representative panel of 20 Rab GTPases N-terminally fused to GFP or red fluorescent protein (RFP). The collection of Rabs examined, their organelle locales, and functions are listed in Table 1. Fluorescently-tagged Rabs used here have been confirmed to localize to subcellular compartments similar to those of their endogenous counter parts (Rzomp et al., 2003). At 5 h post-nucleofection, we analyzed the association of GFP- or RFP-tagged Rabs, or GFP alone to the ApV by confocal microscopy. A. phagocytophilum inclusions were demarcated by labeling with a monoclonal antibody against the major surface protein Msp2 (P44), which is present on the Anaplasma surface, but not the ApV membrane (AVM) (Ijdo et al., 1998). A distinct subset of fluorescent protein tagged-Rab GTPases localizes to the ApV (Figures 1 and 2). GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, GFP-Rab35, GFP-Rab1, and GFP-Rab4A localize to 51.3 ± 9.45, 42.5 ± 15.5, 27.5 ± 7.8, 55.9 ± 2.9, 46.2 ± 3.5, 5.5 ± 3.5, and 4.7 ± 3.2% of inclusions, respectively. For most cells exhibiting Rab localization to the ApV, either all or none of the ApVs within a given HL-60 cell were fluorescent protein-tagged Rab-positive. As an example of a GFP-Rab that does not localize to all of the ApVs within a single infected cell, GFP-Rab11A associates with most but not all of the bacterial inclusions in the cell presented in Figure 1, panels D-F. The accumulations of these fluorescent protein-Rabs at the ApV produce distinct aggregate signal that can be seen as either rim-like or punctate signal at the periphery of the inclusion membrane. Rim-like staining, which is typified by GFP-Rab10 (Figure 1, panels A–C) and GFP-Rab35 (Figure 1, panels M–O) localization to the ApV, indicates that the GFP-Rab is uniformly distributed over the AVM’s entirety. Punctate staining, as typified by GFP-Rab11A (Figure 1, panels D–F), GFP-Rab14 (Figure 1, panels G–I), RFP-Rab22A (Figure 1, panels J–L), and GFP-Rab4A (Figure 1P) indicates recruitment to microdomains on the AVM. GFP alone and all other fluorescently-tagged Rabs fail to associate specifically with the ApV and exhibit fluorescent signal that is diffuse throughout the cell or that is vesicle- and/or Golgi apparatus-associated (Figure 1, panels Q–S and data not shown).
Figure 1. Selective GFP- or RFP-Rab GTPase localization to the ApV.
A. phagocytophilum infected (A to S) or uninfected control (T to V) HL-60 cells were transiently nucleofected with DNA constructs to express the indicated GFP- or RFP-tagged Rab-GTPases or GFP alone. At 5 h post-nucleofection, the cells were fixed and viewed by indirect immunofluorescence. A to S, Representative confocal micrograph images depicting localization of GFP-Rab10A (A to C), GFP-Rab11A (D to F), GFP-Rab14 (G to I), RFP-Rab22A (J to L), GFP-Rab35 (M to O), or GFP-Rab4A (P) or lack of recruitment of GFP-Rab18 (Q), GFP-Rab33A (R), or GFP alone (S) to the ApV are presented. Intravacuolar and HL-60 cell surface-associated A. phagocytophilum organisms were denoted by indirect immunofluorescence staining with mAb 20B4, which recognizes the A. phagocytophilum surface protein, Msp2 (P44), but not the AVM, followed by anti-mouse IgG conjugated to Alexa Fluor-594 (B, E, H, M, P, Q, R, S) or Alexa Fluor-488 (K). Panels A, B, D, E, G, H, J, K, M, and N present single channel GFP (A, D, G, M), RFP (J), or Msp2 (P44) (B, E, H, K, N) fluorescence. Panels C, F, I, L, O, P, Q, R, and S present merged fluorescent images. T to V, In the absence of infection, GFP-Rabs exhibit diffuse and/or organelle-like patterns of fluorescence. Asterisks demarcate HL-60 cell nuclei.
Figure 2. Percentages of ApVs exhibiting GFP- or RFP-Rab GTPase localization.
A. phagocytophilum inclusions as demarcated by anti-Msp2 (P44) staining of intravacuolar bacteria were scored for GFP- or RFP-Rab GTPase localization as in Figure 1. The number of GFP- or RFP-positive ApVs was divided by the total number of ApVs, which was multiplied by 100 to determine the percentage of ApVs to which each GFP- or RFP-Rab GTPase localized. Results are presented as the mean percentages (±SD) of ApVs exhibiting GFP- or RFP-Rab GTPase and are derived from two to four separate experiments. Up to 972 bacterial inclusions were examined for localization of each GFP-or RFP-Rab GTPase.
In the absence of infection, those fluorescent protein-tagged Rabs that otherwise localize to the ApV demonstrate organelle-like localization and/or diffuse staining patterns (Figure 1, panels T–V and data not shown). Since not all fluorescently tagged Rab proteins associate with the ApV, GFP-Rab1, GFP-Rab4A, GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, and GFP-Rab35 localization to the ApV is likely specific and not due to non-specific association with GFP or RFP. The lack of detection of GFP-Rab5 recruitment to the ApV is consistent with the inability to detect endogenous Rab5 on the AVM (Mott et al., 1999). From these data, it can be concluded that the ApV primarily hijacks endocytic recycling traffic. The fluorescent protein-tagged Rabs that exhibit the highest degrees of association with the ApV – Rab10, Rab11A, Rab14, Rab22A, and Rab35 – are specific to the endocytic recycling compartment, recycling endosomes, and tubular recycling endosomes associated with the slow clathrin-independent endocytic recycling pathway (Grant et al., 2009). GFP-Rab4A, which is rapid recycling endosome-specific, is only found on 4.7% of ApVs (Figure 2). Thus, the ApV predominantly hijacks the slow clathrin-independent endocytic recycling pathway.
The ApV recruits Rabs upon formation & continues to do so throughout infection
We previously determined that the A. phagocytophilum developmental cycle, which consists of four distinct phases – (i) entry of a surface bound infectious dense core (DC) organism into a nascent ApV; (ii) conversion of the bacterium to a metabolically active reticulate cell (RC) organism; (iii) intravacuolar bacterial replication of RC organisms; and (iv) condensation of RC bacteria back to an inclusion full of DC organisms – takes approximately 24 h (Troese et al., 2009). Since the recruitment of Rab GTPases to the ApV may be temporally regulated with respect to the A. phagocytophilum developmental cycle and this, in turn, may influence the role that each Rab plays during A. phagocytophilum infection, we examined the association of several fluorescent protein-tagged Rabs to the ApV during the first 24 h of infection. At time 0, Anaplasma organisms were added to synchronously infect HL-60 cells. Consistent with previous reports by our and other laboratories (Borjesson et al., 2005, Carlyon et al., 2004, IJdo et al., 2004), it took approximately 4 h for bound A. phagocytophilum bacteria to enter cells and form nascent vacuoles (data not shown). Beginning at 4 h, all fluorescent protein-tagged Rabs examined can be detected at the ApV (Figure 3). The percentages of ApVs positive for GFP-Rab4A, GFP-Rab10, GFP-Rab11A, RFP-Rab22A, and GFP-Rab35, each of which are involved in endocytic recycling, continually increased throughout the course of infection. GFP-Rab14 localized to a considerably higher percentage of inclusions at 4 h than any other recycling endosome-associated GFP-Rab. By 24 h, however, the number of GFP-Rab14-positive ApVs had waned. The percentages of ApVs exhibiting association of endoplasmic reticulum/Golgi-specific GFP-Rab1 remained consistently low at all time points.
Figure 3. Time course analyses of GFP- or RFP-Rab GTPase recruitment to the ApV.
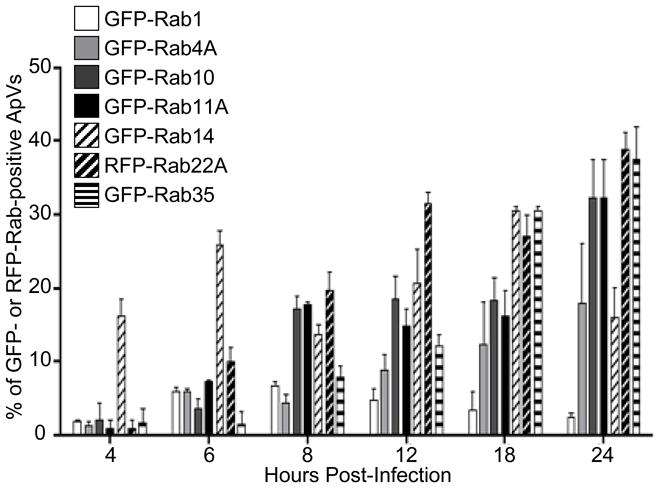
Percentages of ApVs to which GFP- or RFP-Rab GTPases associated are presented. Results are the mean (±SD) of two to five experiments. Up to 575 bacterial inclusions were examined for recruitment of each GFP- or RFP-Rab per time point. For assessment of GFP- or RFP-Rab GTPase association with the ApV at 4, 6, 8, and 12 h post-infection, HL-60 cells were transiently nucleofected with DNA constructs encoding GFP- or RFP-Rab GTPases. At 12 h post-nucleofection, the cells were synchronously infected with A. phagocytophilum for the time periods indicated and then fixed and examined by indirect immunofluorescence microscopy. To assess GFP- or RFP-Rab GTPase recruitment to the ApV at 12, 18, and 24 h, HL-60 cells were synchronously infected with A. phagocytophilum. At 7 h post-infection, the cells were nucleofected to express GFP- or RFP-Rab GTPases and fixed and examined at 12, 18, and 24 h post-infection.
Endogenous Rab14 localizes to the ApV
We next examined whether endogenous Rab14 associates with the ApV. A. phagocytophilum infected HL-60 cells were screened with antibodies against Msp2 (P44) and human Rab14 and examined by confocal microscopy. As previously reported, Rab14 localized to vesicular structures that were ubiquitously distributed throughout the host cell cytoplasm (Kelly et al., 2009). Examination of 1031 ApVs detected aggregate Rab14 staining on 37.9% of ApVs (Figure 4). Attempts at detecting endogenous Rab11 at the ApV were unsuccessful (data not shown).
Figure 4. Endogenous Rab14 localizes to the ApV.
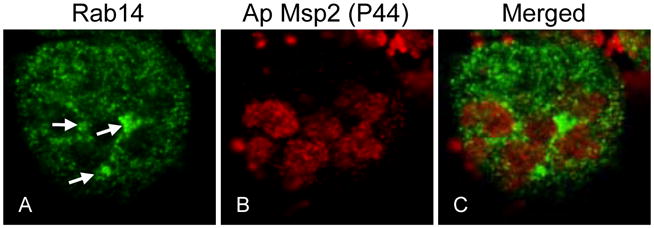
A. phagocytophilum infected HL-60 cells were fixed and viewed by indirect immunofluorescence. Rab14 was detected using rabbit polyclonal antisera against Rab14 followed by anti-rabbit IgG conjugated to Alexa Fluor-488. Intravacuolar and HL-60 cell surface-associated A. phagocytophilum organisms were denoted by indirect immunofluorescence staining with mAb 20B4, which recognizes the A. phagocytophilum surface protein, Msp2 (P44), but not the AVM, followed by anti-mouse IgG conjugated to Alexa Fluor-594. Panels A, B, and C present single channel Rab14 detection, single channel Msp2 (P44) detection, and merged fluorescent images, respectively. Arrows denote ApVs that exhibit pronounced Rab14 staining. Results are representative of a total of 1031 inclusions examined for two separate experiments.
GFP-Rab10 and GFP-Rab14 localization to the ApV is not dependent on an intact Golgi apparatus
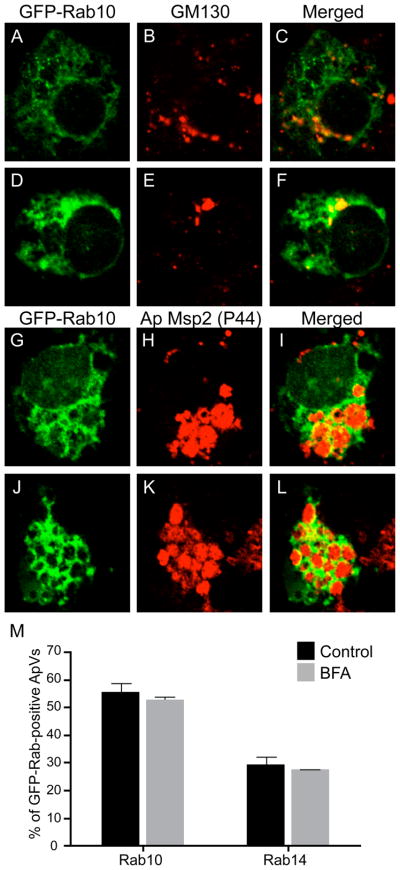
In addition to mediating cargo transfer from the early endosome to the endocytic recycling compartment, Rab10 is also found at the trans-Golgi network and directs transport of cargo from the trans-Golgi network to the plasma membrane (Table 1) (Grant et al., 2009). Likewise, Rab14 is not only found on early endosomes and the endocytic recycling compartment, but is also found on the trans-Golgi network and mediates traffic from the trans-Golgi network to the endocytic recycling compartment (Table 1) (Junutula et al., 2004, Kelly et al., 2009). Therefore, Rab10 and/or Rab14 association with the ApV could be due to A. phagocytophilum hijacking endocytic recycling- or trans-Golgi network-derived traffic. To investigate the contribution of an intact Golgi network to GFP-Rab10 and GFP-Rab14 delivery to the ApV, infected HL-60 cells expressing GFP-Rab10 or GFP-Rab14 were treated with BFA. BFA treatment resulted in Golgi fragmentation, as detected by staining for the Golgi marker, GM130 (Figure 5, A–C). Vehicle control treated cells exhibited intact Golgi apparatuses (Figure 5, D–F) and GFP-Rab10 localization to the ApV (Figure 5, J–M). GFP-Rab10 localization to the ApV in BFA-treated cells exhibited no difference from that observed for control cells whether BFA was added to cells with established A. phagocytophilum infections (Figure 5, panels G–I and M) or prior to infection (data not shown). Highly similar results were observed for GFP-Rab14 recruitment to the ApV in BFA-treated cells (Figure 5M).
Figure 5. GFP-Rab10 and GFP-Rab14 localization to the ApV is insensitive to BFA.
A. phagocytophilum infected HL-60 cells expressing GFP-Rab10 or GFP-Rab14 were treated with BFA or vehicle control (methanol) for 3 h, after which the cells were fixed, processed for immunofluorescence microscopy, and examined by LSCM. Panels A–L, confocal micrograph images depicting GFP-Rab10 and immunofluorescent staining for the Golgi marker, GM130 or A. phagocytophilum Msp2 (P44). Panels A to C and G to I, BFA-treated cells. Panels D to F and J to L, vehicle control-treated cells. Panels A, D, G, and J, GFP-Rab10. Panels B and E, GM130. Panels H and K, Msp2 (P44). Panels C, F, I, and L, merged images. Panel M, Percentages of ApVs to which GFP-Rab10 or GFP-Rab14 associates in the absence or presence of BFA. Results are the mean (± SD) of two separate experiments. At least 361 ApVs inclusions were examined for GFP-Rab GTPase localization.
GFP-Rab1, GFP-Rab4A, and GFP-Rab11A associate with the ApV in a guanine nucleotide-dependent manner, but GFP-Rab10 localizes to the ApV in a guanine nucleotide-independent manner
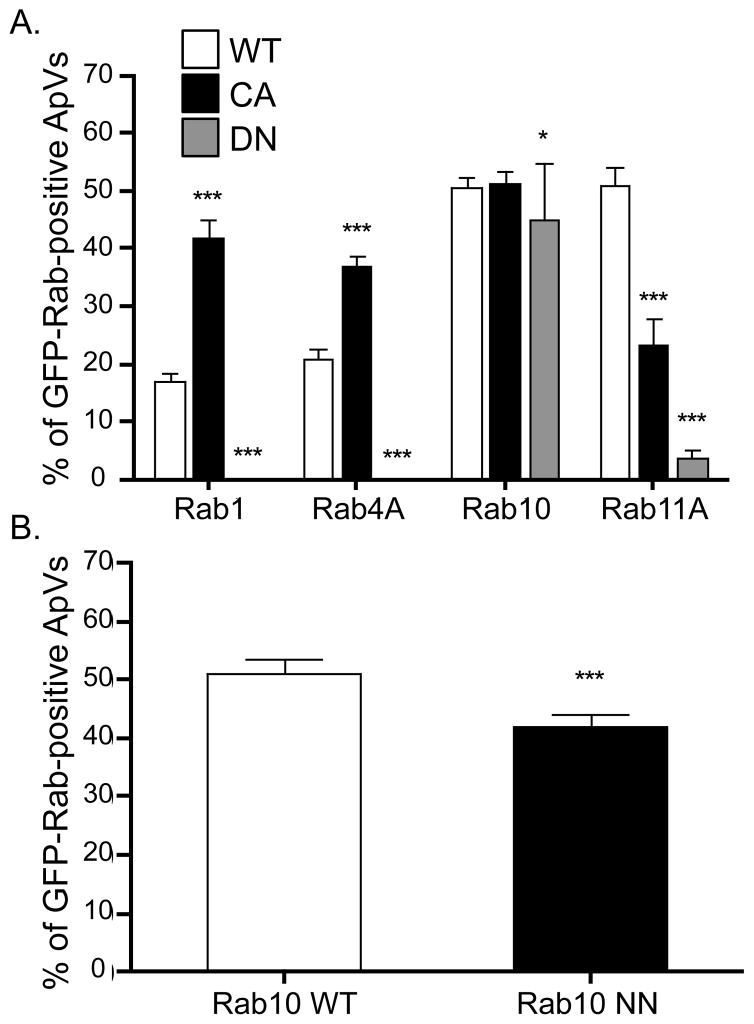
Because Rab GTPases interact with their cognate effector proteins at target membranes in a guanine nucleotide-dependent manner (Stenmark, 2009), we were interested in whether GFP-Rab1, GFP-Rab4A, GFP-Rab10, or GFP-Rab11A interacts with the AVM in a similar manner. To investigate this, we assessed the intracellular locations of constitutively active GTPase-deficient (GFP-Rab1AQ70L, GFP-Rab4AQ67L, GFP-Rab10Q68L, & GFP-Rab11AQ70L) and dominant negative GDP-restricted (GFP-Rab1AS25N, GFP-Rab4AS22N, GFP-Rab10T23N, and GFP-Rab11AS25N) GFP-Rab proteins in infected cells. A. phagocytophilum infected HL-60 cells were nucleofected to express each GFP-Rab. Five h post-nucleofection, the cells were fixed, screened with anti-Msp2 (P44) to denote Anaplasma inclusions, and examined for GFP-Rab recruitment to the ApV. As shown in Figure 6A, the wild type and GTPase-deficient forms of GFP-Rab1, GFP-Rab4A, and GFP-Rab11A localize to the ApV. Notably, considerably higher percentages of ApVs were positive for GFP-tagged, constitutively active forms of Rab1 and Rab4 than their wild-type counterparts. In contrast, the GDP-restricted forms of GFP-Rab1, GFP-Rab4A, and GFP-Rab11A localized extremely poorly to or did not localize to the ApV. Overexpression of GDP-restricted Rab GTPases had no aberrant effects on the percentage of infected cells, numbers of ApVs per cell, or ApV gross morphology (data not shown). In contrast to the guanine nucleotide-dependent recruitment exhibited by GFP-Rab1, GFP-Rab4A, and GFP-Rab11A, GFP-Rab10 localization to the ApV is guanine nucleotide-independent. Indeed, wild type, constitutively active GTPase-deficient, and dominant negative GDP-restricted GFP-Rab10 fusions all localize to the ApV with comparable efficiencies (Figure 6A). Furthermore, the ability of GFP-Rab10N122I, which is unable to bind guanine nucleotides, is only modestly reduced (Figure 6B).
Figure 6. Localization of GFP-Rab1, GFP-Rab4A, & GFP-Rab11A to the ApV is guanine nucleotide-dependent, while GFP-Rab10 association with the ApV is guanine nucleotide-independent.
(A) A. phagocytophilum infected HL-60 cells expressing GFP-fusions of wild-type (WT), constitutively active GTPase-restricted (CA), and dominant negative GDP-restricted (DN) forms of Rab1, Rab4A, Rab10, and Rab11A were assessed for GFP-Rab recruitment to the ApV, which was demarcated by anti-Msp2 (P44) staining of intravacuolar bacteria. The number of GFP-positive ApVs was divided by the total number of ApVs, which was multiplied by 100 to determine the percentage of ApVs to which each GFP-Rab GTPase localized. Results are presented as the mean percentages (±SD) of ApVs exhibiting GFP-Rab GTPase recruitment and are derived from at least three experiments. A total of at least 642 bacterial inclusions were examined for recruitment of each GFP-Rab. (B) Comparison of the colocalization of GFP-Rab10 WT and GFP-Rab10N122I, which is a nucleotide-free mutant that is incapable of binding GTP or GDP (no-nucleotide, NN), to the ApV. Results are presented as the mean percentages (±SD) of ApVs exhibiting GFP-Rab GTPase recruitment and are derived from two experiments. Totals of 363 and 332 inclusions were examined for HL-60 cells expressing GFP-Rab10 and GFP-Rab10N122I, respectively.
Selective recruitment of GFP- or RFP-Rab GTPases to the ApV is dependent on A. phagocytophilum protein synthesis
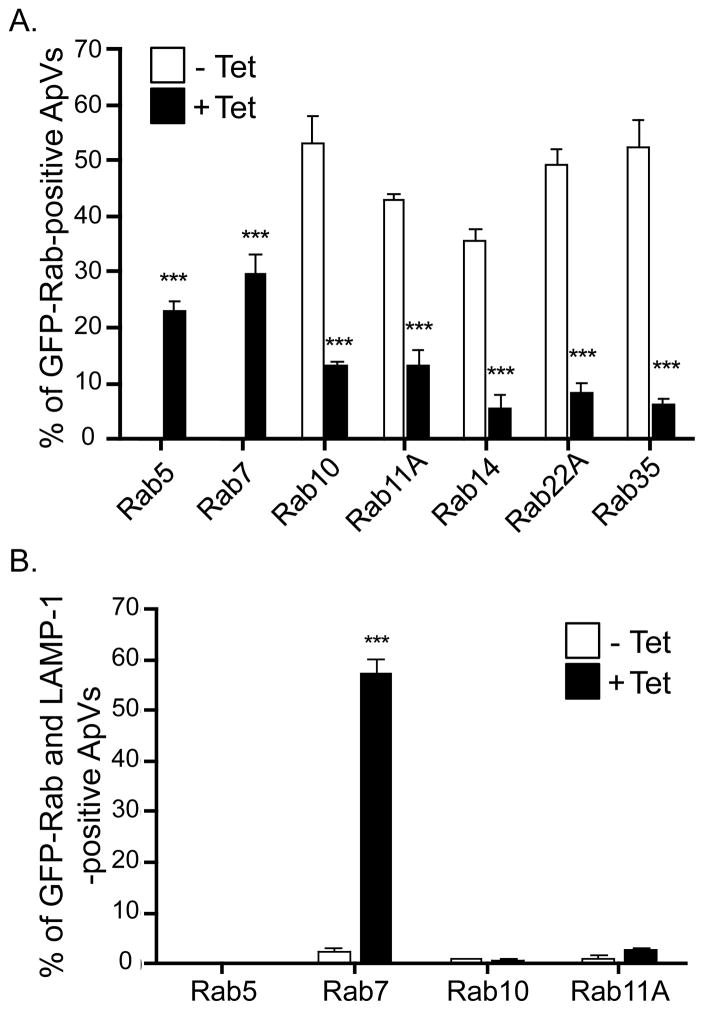
Because tetracycline treatment of A. phagocytophilum infected host cells culminates in A. phagocytophilum delivery to lysosomes (Gokce et al., 1999), we reasoned that bacterial protein synthesis is critical for the ApV to selectively recruit recycling endosome-associated Rabs and prevent maturation of the ApV along the endosomal-lysosomal pathway. Rab5 and Rab7 are found on endosomes and lysosomes, respectively (Stenmark, 2009), and do not normally associate with the ApV (Table 1). To test our hypothesis, we treated A. phagocytophilum infected HL-60 cells expressing GFP-Rab5, GFP-Rab7, GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, and GFP-Rab35 with tetracycline or vehicle control for 30 min. The cells were fixed, screened with anti-Msp2 (P44) to denote bacterial inclusions, and examined for GFP-Rab localization to the ApV. With the exception of GFP-Rab5 and GFP-Rab7, all GFP-Rabs examined associated with the ApV in control cells (Figure 7A). GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, and GFP-Rab35 localization to the ApV was significantly reduced in tetracycline-treated cells. Concomitant with this phenomenon, the ApV became positive for GFP-Rab5 and GFP-Rab7 following the addition of tetracycline, which suggests that de novo bacterial protein synthesis is necessary for the ApV to intercept recycling endosome traffic and to block recruitment of Rab GTPases that promote endosomal maturation and lysosomal fusion. Indeed, 57.4 ± 3.8% of GFP-Rab7-positive ApVs were also positive for the lysosomal marker, LAMP-1 (Figure 7B). As expected, LAMP-1 localizes only to Rab7-positive vacuoles (Stenmark, 2009). Even though the lumens of GFP-Rab7-positive vacuoles were positive for Msp2 (P44) signal, intact organisms were rarely discernible (data not shown).
Figure 7. Selective recruitment of GFP- or RFP-Rab GTPases to the ApV is dependent on bacterial protein synthesis.
A. phagocytophilum infected HL-60 cells were transiently nucleofected with DNA constructs encoding the indicated GFP- or RFP-Rab GTPase. At 5 h post-nucleofection, tetracycline or vehicle control (70% ethanol) was added and incubation was continued. At 30 min, the cells were fixed and examined for GFP- or RFP-Rab GTPase association with the ApV. Intravacuolar A. phagocytophilum organisms were denoted by indirect immunofluorescence staining of Msp2 (P44). (A) GFP- or RFP-Rab localization to the ApV in the absence (−Tet) or 30 min post-addition of tetracycline (±Tet). Up to 817 bacterial inclusions were examined for GFP-Rab GTPase association per condition. Results are the mean (±SD) of at least three experiments. (B) GFP-Rab GTPase-positive A. phagocytophilum inclusions were examined for lysosomal fusion 30 min following the addition of tetracycline or vehicle control using a mAb directed against the lysosomal marker, LAMP-1. Up to 406 bacterial inclusions were examined for the localization of each GFP-Rab GTPase. Results are the mean (±SD) of at least three experiments. Statistically significant (* P<0.05; **P<0.01; ***P<0.001) values are indicated.
Discussion
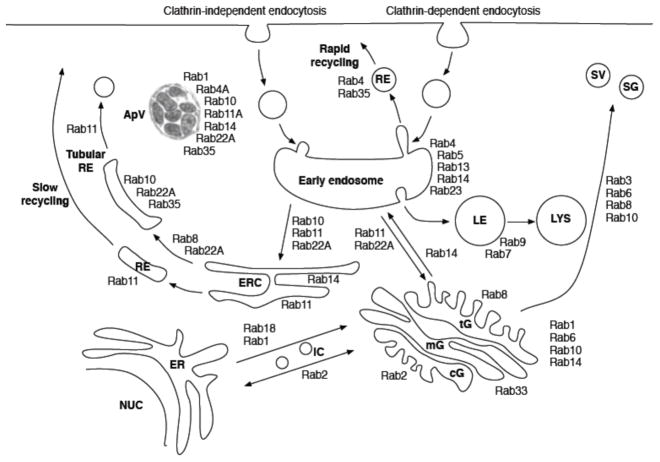
Our data demonstrate that the ApV predominantly hijacks Rab-GTPases that regulate the slow clathrin-independent endocytic recycling pathway (summarized in Figure 8). The high degrees of colocalization with the ApV exhibited by GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, and GFP-Rab35 and the absence of GFP-Rab8 association with the ApV strongly suggest that the ApV hijacks the endocytic recycling compartment and tubular recycling endosomes. Doing so conceivably provides A. phagocytophilum with several intracellular survival advantages. A. phagocytophilum lacks genes necessary for synthesizing 16 amino acids (Hotopp et al., 2006). Since endogenous host proteins that are critical for amino acid uptake are brought into host cells via the slow clathrin-independent endocytic pathway (Eyster et al., 2009), intercepting this pathway is a possible means by which the bacterium acquires amino acids. A. phagocytophilum incorporates cholesterol into its cell wall (Xiong et al., 2009, Lin et al., 2003), presumably to compensate for its lack of peptidoglycan (Hotopp et al., 2006). Since cholesterol is highly abundant in clathrin-independent endocytic recycling endosomes (Sandvig et al., 2008, Mayor et al., 2007), the bacterium may hijack tubular recycling endosomes as a means for obtaining cholesterol. Continual delivery of recycling endosomes to the ApV conceivably provides an unlimited supply of host membrane material to allow for expansion of the AVM, which would be necessary to accommodate growing intravacuolar bacterial populations. Lastly, by coating the AVM with recycling endosome-associated Rab GTPases, the ApV camouflages itself as a recycling endosome, which is key to preventing maturation along the endosomal pathway and avoiding fusion with lysosomes. Support for this premise is evidenced by the lack of GFP-Rab7 association with the ApV, which is in agreement with the report that the ApV avoids lysosomal fusion (Gokce et al., 1999). Additional support is provided by the observed rapid loss of GFP-tagged slow clathrin-independent endocytosis-associated Rabs from the ApV with concomitant acquisition of GFP-Rab5, GFP-Rab7, and LAMP-1 upon the addition of tetracycline.
Figure 8. Model indicating the Rab GTPases and host cell membrane traffic pathways that are hijacked by the A. phagocytophilum-occupied vacuole (ApV).
ER, endoplasmic reticulum; cG, cis-Golgi; ERC, endocytic recycling center; IC, pre-Golgi intermediate compartment; LE, late endosome; LYS, lysosome; mG, medial-Golgi; NUC, nucleus; RE, recycling endosome; SG, secretory granule; SV, synaptic vesicle; tG, trans-Golgi. This figure is partially adapted from Figure 1 presented by Grant and Donaldson.
The ApV’s hijacking of recycling endosome traffic is consistent with the observation that that MHC class I and class II molecules, which are recycling endosome cargo, are found on the AVM (Amigorena et al., 1994, Mott et al., 1999, Neefjes et al., 1990). Further evidence that the ApV primarily acquires recycling endosome-derived traffic and does not acquire Golgi-derived vesicles comes from our observation that an intact Golgi network is not required for GFP-Rab10 or GFP-Rab14 to traffic to or maintain association with the ApV. This implies that GFP-Rab10 and GFP-Rab14 likely associate with the ApV by virtue of the ApV intercepting recycling endosome-derived traffic. BFA does not inhibit ApV recruitment of GFP-Rab10 and GFP-Rab14. This is consistent with a previous report that Golgi-derived markers are absent from the AVM and BFA exerts no aberrant effect on A. phagocytophilum intracellular survival (Mott et al., 1999). However, it cannot be completely ruled out that GFP-Rab10 and GFP-Rab14 are recruited to the ApV from cytosolic pools.
The ApV rapidly recruits recycling endosome-associated Rab GTPases and Rab1 very soon, if not immediately following bacterial entry and, with the exception of Rab14, which begins to dissociate by 24 h, continually recruits recycling endosome-associated Rabs throughout the duration of A. phagocytophilum intravacuolar development. At 4 h post-bacterial addition, the percentage of ApVs that are positive for GFP-Rab14 greatly exceeds those of all other ApV-associated GFP-Rabs, which indicates that Rab14 is the first Rab recruited to and/or it more stably associates with the bacterial inclusion. Initial hijacking of Rab14 may destine the ApV along the slow clathrin-independent endocytic recycling pathway. The fact that the ApV is positive for GFP-Rab14, but is negative for GFP-Rab5 supports previous observations that the ApV does not resemble an early endosome (Mott et al., 1999, Webster et al., 1998). Perhaps the ApV hijacks Rab14 from a cytosolic pool or from an as yet defined Rab14-positive vacuole that is intermediate between early endosome and the endocytic recycling compartment. By 24 h, the number of GFP-Rab14-positive ApVs had declined, while the numbers of ApVs positive for GFP-Rab10, GFP-Rab11A, RFP-Rab22A, and GFP-Rab35 had increased. Since the latter 4 Rabs are found on slow recycling endosomes and slow tubular recycling endosomes, while Rab14 is found on early endosomes and the endocytic recycling compartment, these data suggest that the ApV recruits traffic from the endocytic recycling compartment, slow recycling endosomes, and slow tubular recycling endosomes through 18 h post-infection, but draws less from the endocytic recycling compartment at 24 h post-infection. How the ApV interacts with the recycling endosomes and membrane trafficking pathways beyond 24 h of infection cannot be extrapolated from this study.
Endogenous Rab14 localizes to a similar percentage of ApVs as its GFP-tagged counterpart. Attempts to localize endogenous Rab11 to the ApV in infected HL-60 cells were unsuccessful. Similarly, attempts to localize endogenous Rab4, Rab6, and Rab11 to the chlaymdial inclusion were also not feasible, even though their GFP-tagged counterparts localize to the chlaymdial inclusion (Rzomp et al., 2003). The inability to detect certain endogenous Rab GTPases at pathogen-occupied vacuolar membranes may be due to the fact that the levels of endogenous Rabs that are recruited to inclusions are too low to detect by indirect immunofluoresence microscopy or may simply reflect a limitation of available antibodies.
The ApV is fairly unique with respect to the composition of Rabs that it acquires and/or the fact that most ApV-recruited Rabs are retained for the duration of the infection. The ApV most closely resembles the Mycobacteria pathogen vacuole (MPV; occupied by M. tuberculosis), which recruits Rab11, Rab14, and Rab22 to obtain nutrients from the clathrin-independent recycling endosome pathway (Roberts et al., 2006, Kumar et al., 2009, Brumell et al., 2007, Kyei et al., 2006). Unlike the MPV, which also acquires Rab5 (Kelley et al., 2003), the ApV actively blocks Rab5 localization (Mott et al., 1999). The early and intermediate stages of the Salmonella-containing vacuole (SCV; occupied by S. enterica serovar Typhimurium) hijacks Rab4 and Rab11, respectively, along with other Rabs associated with early endosomes, late endosomes, and pre-Golgi intermediate compartments that do not associate with the ApV (Smith et al., 2005, Smith et al., 2007, Steele-Mortimer et al., 1999). In contrast to inclusions occupied by Chlamydia spp., which recruit both recycling endosome- (Rab4, Rab11, Rab10) and secretory pathway (Rab1, Rab6, and Rab10)-associated Rabs, the ApV does not intercept Golgi-derived traffic (Cortes et al., 2007, Rzomp et al., 2006, Rzomp et al., 2003). Furthermore, intercepting Golgi-derived traffic is essential for chlamydial (Lipinski et al., 2009, Heuer et al., 2009), but not A. phagocytophilum intracellular survival (Mott et al., 1999).
Fluorescent protein-tagged Rab10, Rab11A, Rab14, Rab22A, and Rab35 localize to no more than 55.9 ± 2.89% of ApVs. Also, not all ApVs became positive for GFP-Rab5 or GFP-Rab7 upon the addition of tetracycline. Both phenomena are likely because endogenous Rabs are recruited to those ApVs to which the fluorescent protein-tagged Rabs have not localized. The presence of GFP-Rab4A on fewer ApVs than GFP-Rab10-, GFP-Rab11A-, GFP-Rab14, RFP-Rab22A-, or GFP-Rab35-positive ApVs indicates that the ApV intercepts rapid recycling endosomes but at a lower frequency than it intercepts slow tubular recycling endosomes. GFP-Rab1, which is normally found on endoplasmic reticulum exit sites and pre-Golgi intermediate compartments (Plutner et al., 1991), localized to no more than 6.8 ± 0.8% of bacterial inclusions at any examined time point. Moreover, GFP-Rab2, which is found on pre-Golgi intermediate compartments (Tisdale et al., 1996), does not associate with the ApV. Thus, we can infer that a small percentage of ApVs hijack vesicles that emanate from endoplasmic reticulum exit sites. The low degree of GFP-Rab1 and GFP-Rab4A association with the ApV indicates that acquiring endogenous Rab1-positive endoplasmic reticulum-derived vesicles and/or Rab4A-positive rapid recycling endosomes either does not play a major role in A. phagocytophilum intracellular survival or that Rab1 and Rab4A rapidly cycle on and off the AVM and their transient associations cannot be accurately represented by the “snapshot” analyses utilized in this study. Alternatively, Rab1- or Rab4A-positive ApVs could have different complements of Rab GTPases and may have different intracellular fates. Despite the low percentages of ApVs to which GFP-Rab1 and GFP-Rab4A localize, these are likely real events since the GTPase-deficient versions of both GFP-Rab1 and GFP-Rab4A each localize to significantly greater percentages of ApVs than their wild-type counterparts. This is presumably because the GTPase deficient Rabs are unable to cycle off the AVM once associated
All GFP-Rab GTPases examined in this study are found on the ApV shortly, if not immediately following bacterial entry into HL-60 cells. Also, with the exception of GFP-Rab1, which localizes to very few inclusions throughout infection, and GFP-Rab14, which initially associates to a much greater percentage of ApVs than any other GFP- or RFP-Rab and begins to dissociate by 24 h, the GFP- and RFP-Rabs that are recruited to the ApV are found on increasing numbers of inclusions throughout the course of infection. This is in contrast to Rab GTPase recruitment to other pathogen-occupied vacuoles, such as those occupied by Chlamydia spp. and S. enterica serovar Typhimurium, which differentially recruit Rab GTPases over the duration of bacterial intravacuolar development (Rzomp et al., 2003, Smith et al., 2007).
The observations that GTP-bound forms, but not GDP-bound forms of GFP-Rab1, GFP-Rab4A, and GFP-Rab11A associate with the ApV indicate that these Rabs localize to the ApV as they would to their normal host cell target organelles. These GTP-bound Rabs may directly associate with the ApV by virtue of their interactions with A. phagocytophilum-encoded proteins presented on the AVM that mimic effectors of Rab1, Rab4, and/or Rab11. Alternatively, they may indirectly interact with the ApV through host-derived Rab effectors that are presented by the AVM by virtue of their interactions with Anaplasma-derived AVM proteins. Constitutively active GTPase-deficient GFP-Rab11A localizes to significantly fewer ApVs than wild-type GFP-Rab11A. We speculate that this is because a majority of GTP-locked GFP-Rab11A irreversibly associates with the membranes of recycling endosomes and the endocytic recycling compartment, which would effectively remove GTP-locked GFP-Rab11A from the cytosolic pool to be hijacked by the ApV. This premise is supported by the presence of considerably larger GFP-Rab11A-positive vacuoles in HL-60 cells expressing GTPase-deficient Rab11A as compared to HL-60 cells expressing wild-type Rab11A (data not shown).
Our observations that the GDP-locked and nucleotide-free forms of GFP-Rab10 exhibit no and minimal defects, respectively, in localization to the ApV is striking and strongly suggests that A. phagocytophilum-derived proteins, presumably located on the AVM, mimic host GEF, GDI, and/or GAP activities by regulating guanine nucleotide exchange on Rab10 to directly control Rab10 membrane cycling on/off the AVM. The precedents for such a remarkable phenomenon have been established by the identification of two L. pneumophila inclusion membrane proteins, DrrA/SidM and LepB. DrrA/SidM exhibits dual functionality as a GDF to displace Rab GDI such that Rab1 can be inserted into the Legionella containing vacuole membrane (LCVM) and as a GEF to stimulate Rab1 activation (Machner et al., 2006, Ingmundson et al., 2007, Murata et al., 2006). LepB inactivates and removes Rab1 from the LCVM by functioning as a GAP to stimulate GTP hydrolysis (Ingmundson et al., 2007). High affinity binding to the nucleotide-free or GDP-bound form of a Rab GTPase is a characteristic feature of GDF/GEFs, while high affinity binding to the GTP bound form is a signature of GAPs (Brumell et al., 2007, Stenmark, 2009). Thus, the association of nucleotide-free and GDP-bound GFP-Rab10 suggests that one or more A. phagocytophilum-derived proteins exhibit GDF/GEF activity. An alternative, albeit less likely, possibility is that the ApV indirectly mediates Rab10 membrane cycling by hijacking one or more host-derived Rab10-specific GDIs, GEFs, and/or GAPs. However, association of nucleotide-free and GDP-bound GFP-Rab10 with the ApV, which is inconsistent with how Rabs associate with eukaryotic target membranes, strongly argues in favor of these phenomena being dependent on bacterial-encoded AVM proteins.
To determine if any A. phagocytophilum proteins exhibit primary amino acid sequence homology to eukaryotic GAPs, GEFs, and GDIs that interact with ApV-associated Rabs or to known bacterial PVM and effector proteins, we searched each protein’s sequence against the annotated A. phagocytophilum proteome (Hotopp et al., 2006). No A. phagocytophilum protein exhibits primary sequence similarity to any known eukaryotic or bacterial pathogen Rab ligand (data not shown). This is not surprising since Legionella- and Chlamydia-derived Rab ligands and GEF/GDI/GAP mimics exhibit no primary amino acid sequence homology to any mammalian Rab effector, GAP, or GEF (Cortes et al., 2007, Ingmundson et al., 2007, Machner et al., 2006, Murata et al., 2006, Rzomp et al., 2006, Schlumberger et al., 2005). Indeed, most bacterial effectors that mimic the activities of eukaryotic cellular proteins do so in the absence of detectable amino acid similarity (Galan, 2009). Thus, the A. phagocytophilum proteins that presumably regulate Rab10 membrane cycling are likely GEF/GFI/GAP homologs in functionality, but not in primary amino acid sequence.
This work advances our understanding of a key strategy by which A. phagocytophilum facilitates its survival within myeloid host cells. It actively controls the biogenesis of the vacuole in which it resides by selectively hijacking Rab GTPases that predominantly associate with recycling endosomes. In doing so, the ApV effectively disguises itself as a recycling endosome, which is requisite for avoiding endosomal maturation and lysosomal fusion. This strategy also likely contributes to the bacterium’s acquisition of host membrane material, amino acids, and cholesterol, each of which are important for bacterial growth and/or cell wall stability. This premise warrants further investigation. It will also be critical to identify and functionally assess the bacterial proteins that interact with each ApV-specific Rab GTPase and those that regulate AVM cycling of Rab10. Further dissection of the mechanism of selective recruitment of Rab GTPases to the ApV will not only continue to enhance our understanding of how this unique pathogen survives within the primary effector cell of microbial killing, but will also lead to a greater comprehension of a paradigm exemplified by many intravacuolar pathogens – subversion of host membrane traffic to construct a permissive niche.
Experimental Procedures
Cultivation of uninfected and A. phagocytophilum infected HL-60 cells
HL-60 cells were maintained and Anaplasma phagocytophilum strain NCH-1 was cultured in HL-60 cells as described (Carlyon et al., 2002).
Plasmids
Constructs encoding GFP-Rab5 and GFP-Rab7 were kindly provided by Anthony Nicola (Virginia Commonwealth University). Constructs encoding human GFP-Rab1, GFP-Rab4A, GFP-Rab6A, GFP-Rab10, and GFP-Rab11A have been previously described (Rzomp et al., 2003). pEGFP-Rab2A, pEGFP-Rab3A, pEGFP-Rab8A, pEGFP-Rab14 and pEGFP-Rab18 were constructed by cloning the coding regions of the respective human gene contained on BamHI/XhoI fragments (Missouri S&T cDNA Resource Center) into the BglII and SalI sites of pEGFPC1 (Clontech, Palo Alto, CA). pEGFP-Rab33A was constructed by cloning the coding region of human Rab33A contained within an EcoRI/XhoI fragment on pCDNA3.1-Rab33A (Missouri S&T cDNA Resource Center) into the EcoRI and SalI sites of pEGFPC2. pEGFP-Rab35 was constructed by PCR amplification using a 5′ gene-specific primer designed with a 5′ EcoRI site and a 3′ gene-specific primer with a XhoI site (Table 2). The PCR product was digested with EcoRI and XhoI and cloned into the EcoRI and SalI sites of pEGFPC2. PCR amplifications were performed using HiFidelity Platinum Taq polymerase (Invitrogen, Carlsbad, CA), and the fusion construct was confirmed by DNA sequencing (BioResource Center, Cornell University, Ithaca, NY). Human Rab13, Rab20, Rab22A, Rab23, and Rab27A coding sequences were PCR amplified from cDNA templates purchased from Open Biosystems (Thermo Scientific, Huntsville, AL). Mouse Rab9A coding sequence was PCR amplified from a construct encoding GST-tagged Rab9A. Primers used for amplifying the Rab9A, Rab13, Rab 20, Rab22A, Rab23, and Rab27 coding sequences are listed in Table 2. PCR products were first TA cloned into pGEM-T (Promega, Madison, WI) before digestion with either BamHI or SmaI. BamHI- or SmaI-digested Rab DNA fragments were ligated in-frame into BglII- or SmaI-linearized vector pTagRFP-TC (a gift from Bret Lindenbach, Yale University, New Haven, CT), which was created by replacing EGFP in the Clontech vector EGFPC3 with a photostable TagRFP-TC (Shaner et al., 2008). The construction of pEGFP-Rab4AS22N and pEGFP-Rab4AQ67L has been described (Rzomp et al., 2006). pEGFP-Rab10T23N, pEGFP-Rab10Q68L, pEGFP-Rab10N122I, pEGFP-Rab11AS25N, pEGFP-Rab1AS25N and pEGFP-Rab1AQ70L were constructed using QuikChange (Stratagene, La Jolla, CA) using each respective wild-type fusion as template with the mutagenic oligonucleotides listed in Table 2. pEGFP-Rab11AQ70L was constructed by PCR amplification using a 5′ gene-specific primer designed with a 5′ EcoRI site and a 3′ gene-specific primer with a XhoI site (Table 2). The PCR product was digested with EcoRI and XhoI and cloned into the EcoRI and SalI sites of pEGFPC2.
Table 2.
Oligonucleotides used to amplify select Rab GTPases
| Rab GTPase | Oligonucleotidesa or source of GFP-Rab construct |
|---|---|
| Rab9A | 5′-GTGGATCCb ATGGCAGGAAAATCGTCTCT-3′ 5′-AGGGATCCTCAACAGCAAGATGAGTTTG-3′ |
| Rab10S22N | 5′-CCGGAGTGGGGAAGAACTGCGTCCTTTTTC-3′ 5′-CGAAAAAGGACGCAGTTCTTCCCCACTCCG G-3′ |
| Rab10Q68L | 5′-GGATACAGCAGGCCTGGAGCGATTTCAC-3′ 5′-GTGAAATCGCTCCAGGCCTGCTGTATCC-3′ |
| Rab10N122I | 5′ GAAAGAATGTTACTAGGAATCAAGTGTGATATGGAC-3′ 5′-GTCCATATCACACTTGATTCCTAGTAACATTCTTTC-3′ |
| Rab11AS25N | 5′-GGTGTTGGAAAGAATAATCTCCTGTCTCG-3′ 5′-CGAGACAGGAGATTATTCTTTCCAACACC-3′ |
| Rab13 | 5′-GTGGATCCATGGCCAAAGCCTACGACCA-3′ 5′-AGGGATCCTCAGCCCAGGGAGCACTTGT-3′ |
| Rab20 | 5′-GTGGATCCATGAGGAAGCCCGACAGCAA-3′ 5′-AGGGATCCTCAGGCACAACACCCAGATC-3′ |
| Rab22A | 5′-GTGGATCCATGGCGCTGAGGGAGCTCAA-3′ 5′-AGGGATCCTCAGCAGCAGCTCCGCTTTG-3′ |
| Rab23 | 5′-TCCCCCGGGc AATGTTGGAGGAAGATATGGA-3′ 5′-TCCCCCGGGTTAGGGTATGCTACAGCTGC-3′ |
| Rab27A | 5′-GTGGATCCATGTCTGATGGAGATTATGA-3′ 5′-AGGGATCCTCAACAGCCACATGCCCCCTT-3′ |
| Rab35 | 5′-GAATTCd ATGGCCCGGGACTACGACC-3′ 5′-CTCGAGe TTAGCAGCAGCGTTTC-3′ |
The first and second oligonucleotides listed for each Rab gene correspond to sense and antisense strand, respectively.
BamHI site.
SmaI site.
EcoRI site.
XhoI site.
Nucleofection
Uninfected or A. phagocytophilum infected HL-60 cells (2 × 106) were transiently transfected with 2 μg of endofree purified DNA (Qiagen Endofree Maxiprep kit, Valencia, CA) using the Nucleofector II device (Lonza, Cologne, Germany), Nucleofector solution V, and T-19 program, according to the manufacturer’s protocol. Following nucleofection, the cells were transferred into 12-well plates containing 1.5 mL Iscove’s Modified Dulbecco’s Eagle Media (IMDM; Invitrogen) supplemented with 10 % fetal bovine serum and incubated at 37°C.
Assessment of GFP-Rab recruitment to the ApV
Asynchronous A. phagocytophilum infected HL-60 cells were nucleofected with a panel of representative GFP- or RFP-tagged Rab plasmid DNA. The nucleofected cells were incubated for 5 hours at 37°C in 5% CO2 in a humidified incubator. Cells were then washed twice with PBS, cytocentrifuged onto glass slides using a 200 μl volume of 3.5 × 104 cells at 70 g for 2 min in a Cytospin 4 centrifuge (Thermo Electron, Pittsburgh, PA), fixed in 2% paraformaldehyde in PBS for 20 min and permeabilized in ice-cold methanol for 30 seconds. A. phagocytophilum Msp2 (P44) was detected by sequentially staining for 30 min each with mAb 20B4 (kindly provided by J. Stephen Dumler of John Hopkins University, Baltimore, MD) and Alexa Fluor 594-conjugated goat anti-mouse IgG or Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen). Coverslips were mounted with ProLong Gold Antifade (Invitrogen) and the slides were examined by immunofluorescence microscopy (Carlyon, 2005). To accurately confirm fluorescent protein-tagged Rab GTPases to the ApV, nucleofected A. phagocytophilum infected HL-60 cells were examined by laser-scanning confocal microsocpy using a TCS-SP2 AOBS inverted confocal microscope (Leica Microsystems, Bannockburn, IL). Some images were acquired by spinning disc confocal microscopy using a BX51 microscope (Olympus, Center Valley, PA) affixed with an Olympus disk spinning unit and an Orca-R2 CCD camera (Hammamatsu, Japan). Images were processed using the Slidebook software package (Intelligent Imaging Innovations, Denver, CO). To determine the percentage of bacterial inclusions exhibiting fluorescent protein-tagged Rab association, the number of ApVs (as denoted by the detection of intravacuolar Msp2 [P44]) was divided by the total number of ApVs and multiplied by 100.
To assess for fluorescently tagged-Rab GTPase recruitment to the ApV at 4, 6, 8, and 12 h post infection, uninfected HL-60 cells were first nucleofected with plasmids encoding GFP- or RFP-tagged Rab GTPases. Five hours post-nucleofection, the cells were incubated with host cell-free A. phagocytophilum organisms then washed twice with PBS after 40 min and centrifuged at 300 g for 5 min to remove unbound bacteria. At the appropriate time points, the cells were fixed and labeled with antibodies as described above. Because we observed that GFP signal begins to fade in nucleofected cells between 18 and 24 h, it was necessary to infect HL-60 cells for an appropriate time period prior to nucleofection in order to more accurately assess GFP- or RFP-Rab recruitment to the ApV at time points beyond 12 h. Accordingly, HL-60 cells were incubated first with host cell-free A. phagocytophilum organisms then washed twice with PBS after 40 min to remove unbound bacteria. Seven h post-infection, the cells were nucleofected with GFP- or RFP-Rab DNA, and then assessed at 5, 11, and 17 h post-nucleofection, which corresponded to 12, 18, and 24 h post-infection, respectively. Cells that were nucleofected prior to infection and cells that were infected prior to nucleofection exhibited nearly identical numbers of bound A. phagocytophilum organisms at the beginning of each time course (data not shown), which confirmed that the infection rates for each set proceeded comparably. Likewise, examination of cells that were nucleofected prior to infection and cells that were infected prior to nucleofection at 12 h post-infection revealed highly similar rates of GFP- or RFP-Rab GTPase recruitment to the ApV for all GFP- and RFP-Rabs examined.
Assessment of endogenous Rab14 recruitment to the ApV
A. phagocytophilum infected HL-60 cells were fixed and screened with mAb 20B4 and rabbit polyclonal antisera against human Rab14 (Aviva Systems Biology LLC, San Diego, CA) followed by Alexa Fluor 594-conjugated goat anti-mouse IgG and Alexa Fluor 488-conjugated goat anti-rabbit IgG, respectively. Cells were examined by spinning disk confocal microscopy and images were acquired using Slidebook. The percentage of endogenous Rab14-positive ApVs was determined as described above.
BFA or tetracycline treatment of A. phagocytophilum infected HL-60 cells expressing GFP-Rab GTPases
To determine whether continued association of GFP-Rab10 or GFP-Rab14 to the ApV is dependent on an intact Golgi apparatus, A. phagocytophilum infected HL-60 cells were nucleofected to express GFP-Rab10 or GFP-Rab14. Two h post-nucleofection, BFA (EMD Biosciences, San Diego, CA) in methanol was added to a final concentration of 1 μg/ml for 3 h. Methanol alone served as a vehicle control. To determine whether an intact Golgi apparatus is required for initial GFP-Rab10 or GFP-Rab14 recruitment to the ApV, BFA was added to uninfected HL-60 cells immediately following nucleofection. After 3 h, host cell-free A. phagocytophilum organisms were added. After 2 h (5 h post-nucloefection), the cells were washed to remove unbound bacteria followed by 4 h continued incubation in the presence of BFA. To determine whether selective Rab recruitment to the ApV is bacterial protein synthesis-dependent, A. phagocytophilum infected HL-60 cells were nucleofected to express GFP-Rab5, GFP-Rab7, GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A, or GFP-Rab35. Five h post-nucleofection, tetracycline (Sigma, St. Louis, MO) solubilized in 70% ethanol was added to a final concentration of 10 μg/ml for 30 min. Ethanol alone served as a vehicle control. After the appropriate incubation period in the presence of BFA, tetracycline, or vehicle control, aliquots were removed, processed, and examined by confocal microscopy for GFP- or RFP-Rab GTPase association with the ApV. To determine if tetracycline treatment promotes ApV-lysosomal fusion, A. phagocytophilum infected HL-60 cells expressing GFP-or RFP-Rabs of interest were screened with a LAMP-1-specific mAb (clone H4A3; University of Iowa Developmental Studies Hybridoma Bank, Iowa City) followed by anti-mouse IgG conjugated to Alexa Fluor 594 and examined by immunofluorescence microscopy.
Statistical analyses
The Student’s t test (paired) performed using the Prism 4.0 software package (Graphpad; San Diego, CA) was used to assess statistical significance. Statistical significance was set at p < 0.05.
Acknowledgments
We thank J. Stephen Dumler of The Johns Hopkins University for providing anti-Msp2 (P44) mAb 20B4; Anthony Nicola for donating plasmids encoding GFP-Rab5 and GFP-Rab7; and Aaron Wolen for assistance in preparing Figures 1 and 8. Laser-scanning confocal microscopy was performed at the VCU Department of Neurobiology and Anatomy Microscopy Facility, which is supported in part with funding from NIH-NINDS Center core grant 5P30NS047463. The H4A3 mAb developed by J. Thomas August and James E. K. Hildreth was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Supported by grants from the NIH (AI072683 to JAC and AI073831 to MAS) and the National Research Fund for Tick-Borne Diseases (to JAC).
References
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174:6364–6372. doi: 10.4049/jimmunol.174.10.6364. [DOI] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell JH, Scidmore MA. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev. 2007;71:636–652. doi: 10.1128/MMBR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon JA, Abdel-Latif D, Pypaert M, Lacy P, Fikrig E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect Immun. 2004;72:4772–4783. doi: 10.1128/IAI.72.8.4772-4783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes C, Rzomp KA, Tvinnereim A, Scidmore MA, Wizel B. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun. 2007;75:5586–5596. doi: 10.1128/IAI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, et al. Discovery of New Cargo Proteins that Enter Cells through Clathrin-Independent Endocytosis. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze HJ, Stoorvogel W, Strous GJ, Slot JW, Bleekemolen JE, Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988;107:2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce HI, Ross G, Woldehiwet Z. Inhibition of phagosome-lysosome fusion in ovine polymorphonuclear leucocytes by Ehrlichia (Cytoecetes) phagocytophila. J Comp Pathol. 1999;120:369–381. doi: 10.1053/jcpa.1998.0287. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, et al. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJdo JW, Mueller AC. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect Immun. 2004;72:5392–5401. doi: 10.1128/IAI.72.9.5392-5401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijdo JW, Sun W, Zhang Y, Magnarelli LA, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley VA, Schorey JS. Mycobacterium’s arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol Biol Cell. 2003;14:3366–3377. doi: 10.1091/mbc.E02-12-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EE, Horgan CP, Adams C, Patzer TM, Ni Shuilleabhain DM, Norman JC, McCaffrey MW. Class I Rab11-Family Interacting Proteins are binding targets for the Rab14 GTPase. Biol Cell. 2009 doi: 10.1042/BC20090068. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5:593–601. doi: 10.1016/j.chom.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB, Vergne I, Chua J, Roberts E, Harris J, Junutula JR, Deretic V. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. Embo J. 2006;25:5250–5259. doi: 10.1038/sj.emboj.7601407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski AR, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, Heuer D. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog. 2009;5:e1000615. doi: 10.1371/journal.ppat.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead AR, Rzomp KA, Scidmore MA. The Rab6 effector Bicaudal D1 associates with Chlamydia trachomatis inclusions in a biovar-specific manner. Infect Immun. 2007;75:781–791. doi: 10.1128/IAI.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J, Rikihisa Y, Tsunawaki S. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect Immun. 2002;70:1359–1366. doi: 10.1128/IAI.70.3.1359-1366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J Biol Chem. 2001;276:23607–23615. doi: 10.1074/jbc.M101034200. [DOI] [PubMed] [Google Scholar]
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, et al. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EA, Chua J, Kyei GB, Deretic V. Higher order Rab programming in phagolysosome biogenesis. J Cell Biol. 2006;174:923–929. doi: 10.1083/jcb.200603026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol. 2007;9:891–909. doi: 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Rzomp KA, Moorhead AR, Scidmore MA. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74:5362–5373. doi: 10.1128/IAI.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun. 2003;71:5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Torgersen ML, Raa HA, van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol. 2008;129:267–276. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger MC, Hardt WD. Triggered phagocytosis by Salmonella: bacterial molecular mimicry of RhoGTPase activation/deactivation. Curr Top Microbiol Immunol. 2005;291:29–42. doi: 10.1007/3-540-27511-8_3. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Lin MZ, McKeown MR, Steinbach PA, Hazelwood KL, Davidson MW, Tsien RY. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods. 2008;5:545–551. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Cirulis JT, Casanova JE, Scidmore MA, Brumell JH. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J Biol Chem. 2005;280:24634–24641. doi: 10.1074/jbc.M500358200. [DOI] [PubMed] [Google Scholar]
- Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, et al. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol. 2007;176:263–268. doi: 10.1083/jcb.200611056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther. 2009;7:709–722. doi: 10.1586/eri.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Balch WE. Rab2 is essential for the maturation of pre-Golgi intermediates. J Biol Chem. 1996;271:29372–29379. doi: 10.1074/jbc.271.46.29372. [DOI] [PubMed] [Google Scholar]
- Troese MJ, Carlyon JA. Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand 1. Infect Immun. 2009;77:4018–4027. doi: 10.1128/IAI.00527-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- Webster P, IJdo JW, Chicoine LM, Fikrig E. The agent of Human Granulocytic Ehrlichiosis resides in an endosomal compartment. J Clin Invest. 1998;101:1932–1941. doi: 10.1172/JCI1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Lin M, Rikihisa Y. Cholesterol-dependent anaplasma phagocytophilum exploits the low-density lipoprotein uptake pathway. PLoS Pathog. 2009;5:e1000329. doi: 10.1371/journal.ppat.1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]