Abstract
The cutaneous surface of a normal adult individual contains approximately 20 billion T cells, nearly twice the number present in the entire circulation. Recent studies have demonstrated a role for these cells in both normal immunity and in inflammatory skin diseases such as psoriasis. Regulatory T cells protect against autoimmune reactions to self antigens and assist in the resolution of cutaneous inflammation. However, they can also shield tumors from immune detection, allow latent infections to persist and can dysfunction under the conditions present in inflammatory skin diseases. Th17 T cells protect organisms against extracellular pathogens but also play a key role in the pathogenesis of psoriasis. Evidence suggests that effector memory T cells produced during immune reactions survive and persist long term within the skin, providing local and rapid protection against pathogen reexposure. This review summarizes the current understanding of how skin resident T cells contribute to normal and aberrant immunity in the skin.
INTRODUCTION
T and B lymphocytes rearrange the DNA encoding their antigen receptors and therefore have the capacity to recognize any antigen. These cells also serve as the repositories for immunologic memory; lymphocytes generated during an immune response can persist for decades, providing rapid and specific responses to rechallenge. T and B cells thus provide highly flexible and long lasting immunity.
Naïve T cells are found primarily within the blood and lymph nodes. Expression of the homing addressins L-selectin and CCR7 supports the migration of naïve T cells into lymph nodes where they encounter dendritic cells (DC) bearing antigen derived from the peripheral tissues (Baekkevold et al., 2001; Warnock et al., 1998). When naïve T cells encounter their cognate antigen, they differentiate and gain effector functions including cytotoxicity and cytokine production. During this differentiation process, T cells are imprinted to express tissue specific homing addressins that affect their subsequent migration patterns. In mice, naïve transgenic T cells developed expression of the gut homing addressins α4β7 and CCR9 when they first encountered antigen in the gut draining lymph nodes but expressed the skin homing addressins CLA and CCR4 if they encountered antigen first in the skin draining lymph nodes (Campbell and Butcher, 2002; Campbell et al., 1999). Further studies demonstrated that both DC and lymph node stromal cells have the ability to imprint homing receptor expression on T cells (Edele et al., 2008; Mora et al., 2003; Mora et al., 2005). Effector T cells are thus programmed during differentiation to migrate to the tissue from which their cognate antigen was originally derived.
When confronted by an infectious pathogen, the immune system does two important things: it responds and it remembers. Two different types of T cells are generated during an immune response (Sallusto et al., 1999). Effector memory T cells (TEM) up-regulate expression of tissue homing addressins and develop effector functions. These cells predominate in the blood in the early stages of an immune response, migrate into peripheral tissues and effect clearance of the pathogen (Mackay et al., 1990; Whitton and Zhang, 1995). Following the peak of the immune response, most of these cells disappear from the blood and a second population, central memory T cells (TCM) predominates (Razvi et al., 1995). Like naïve T cells, TCM express the lymph node homing addressins L-selectin and CCR7 and generally lack addressins for peripheral tissues. TCM have lower levels of effector functions but can proliferate vigorously and develop into effector T cells when rechallenged with antigen (Sallusto et al., 2004).
Until fairly recently, it was believed that T cells only entered tissues such as the skin under conditions of active inflammation (Kupper and Fuhlbrigge, 2004). This review will discuss recent findings that skin and other tissues are stably colonized by long-lived populations of memory T cells. These tissue resident T cells provide long lasting, local and rapid responses to pathogen reexposure but can also contribute to inflammatory and autoimmune skin diseases.
Skin resident T cells: hiding in plain sight
It has been known for decades that T cells are present in non-inflamed human skin and it has been proposed that these cells may comprise a skin specific immune system (Bos et al., 1987). Comprehensive study of these T cells and an understanding of their true numbers has been difficult because mechanical or enzymatic dissociation of the skin produces very few cells. Despite this difficulty, studies using histologic methods or skin dissociation have successfully demonstrated that >95% of the T cells in normal skin are CD45RO memory T cells, <5% are naïve, most express CLA, 50% express CCR8 and a subset express CCR7 and CCR10 (Bos et al., 1989; Campbell et al., 2001; Homey et al., 2002; Schaerli et al., 2004).
T cells are arguably the most migratory cells in the body. T cells enter and travel through every human tissue with the likely exception of cortical bone. Unlike neutrophils and monocyte derived macrophages, T cell migration into the tissues is not a one way trip. T cells can migrate into peripheral tissues and then return to the circulation via migration through the lymph nodes. A simple method of isolating skin T cells that took advantage of their tendency to migrate towards chemokines produced by dermal fibroblasts extracted surprising numbers of T cells from normal human skin (Clark et al., 2006b). This observation led to the enumeration of T cells in skin and it was found that normal human skin contains about 1 million T cells/cm2 (Clark et al., 2006a). Extrapolation of this finding suggested that the skin of a normal adult contains approximately 20 billion T cells, nearly twice the number present in the entire blood volume. These T cells co-expressed high levels of the skin homing addressins CLA and CCR4, had a diverse T cell repertoire and were polarized to produce a variety of different cytokines in response to T cell stimulation (Clark et al., 2006a). 80% of T cells in skin lacked expression of CCR7/L-selectin, confirming their identity as effector memory T cells. T cells that did express CCR7 and L-selectin also co-expressed the skin addressins CLA and CCR4, suggesting that they were an intermediate phenotype capable of accessing both the skin and secondary lymphoid organs.
At the time of these studies, it was assumed that TEM primarily remained in the circulation until recruited into the tissues at sites of inflammation. Classical skin homing TEM (CLA+, CCR7/L-selectin−) cannot enter the lymph nodes and as a result, should be found either in the blood or the skin. Enumeration of CLA+ T cells in skin and blood demonstrated that >90% of CLA+ skin homing T cells were present in skin under resting, non-inflamed conditions and that less than 10% were in the peripheral circulation (Clark et al., 2006a). Thus, even in the absence of inflammatory stimuli, the vast majority of skin homing TEM were resident in skin and well placed to respond to local challenges.
Local T cells responses in skin: lessons from psoriasis
In considering the treatment of inflammatory skin diseases, we and others have often focused on inhibiting the migration of T cells into skin. E-selectin is expressed by all postcapillary venules in skin and is upregulated with inflammation (Chong et al., 2004; Kupper and Fuhlbrigge, 2004). By binding to CLA expressed by skin homing T cells, E-selectin supports lymphocyte rolling, the first step of T cell entry into the skin (Berg et al., 1991; Kupper and Fuhlbrigge, 2004). It therefore came as a surprise to find that blockade of E-selectin, which should block migration of T cells into skin, was ineffective in the treatment of psoriasis, a T cell mediated inflammatory skin disease (Bhushan et al., 2002).
This result was made more intelligible by an elegant series of experiments in mice grafted with human psoriatic skin. Boyman et al. found that normal appearing, non-lesional skin from patients with psoriasis developed full blown psoriatic lesions when transplanted onto immunodeficient mice but skin taken from a normal patients did not (Boyman et al., 2004). Development of the psoriatic lesion was dependent on the activation and local proliferation of a population of autoreactive T cells transferred with the initial skin graft. These T cells were themselves stimulated into action by the local production of IFNα by plasmacytoid DC (pDC), likely produced in response to the trauma of transplantation (Nestle et al., 2005).
This series of experiments showed two important things about skin resident T cells. First, T cells present in normal appearing skin were able to give rise to a full psoriatic lesion in the absence of T cell recruitment from blood. Thus, T cells resident in even normal appearing skin can initiate full blown immune responses and although migration of T cells into the skin from the blood occurs in many inflammatory conditions, it may not always be required. In light of these experiments, the failure of E-selectin blockade to control psoriasis became understandable. Autoreactive T cells capable of initiating psoriasis reside within the normal appearing skin of patients with psoriasis. Preventing the entry of additional T cells into skin does not address the threat posed by the lymphocytes already present.
The second lesson from these experiments is that autoreactive T cells in the skin behave very differently depending on their local inflammatory environment. Autoreactive T cells exist in normal appearing skin from psoriatic patients but these cells remain quiescent despite living side by side with APC expressing and capable of presenting autoantigens. Only when skin APC are activated to produce IFNα do these autoreactive T cells proliferate, produce cytokines, and initiate an active psoriatic lesion. This finding highlights the importance of understanding how T cells are activated by innate immune cells within the skin and explains why TNF antagonists (etanercept, infliximab, adalimumab) and integrin antibodies that disrupt APC/T cell crosstalk (efalizumab, alefacept) are effective in psoriasis whereas E-selectin blockade is not (Bhushan et al., 2002; Griffiths, 2004).
Additional studies in mice and humans have confirmed that T cells can become activated, proliferate and carry out effector functions locally within the skin. As is described more fully below, HSV-specific T cells in mice and humans remained localized around latently infected nerves and prevented HSV reactivation (Wakim et al., 2008b; Zhu et al., 2007). In patients immunized with BCG and challenged with a PPD, effector T cells proliferated within the skin at the PPD site (Vukmanovic-Stejic et al., 2006). In summary, all elements necessary for a memory T cell response - T cells and APC – are resident within human skin and their interaction can give rise to full secondary immune responses within the skin.
Regulatory T cells in the skin: the good, the bad and the ineffective
The immune system is faced with the difficult problem of mounting immune responses to dangerous pathogens while maintaining tolerance to the body’s own tissues and to harmless or commensal organisms. Regulatory T cells (Tregs) are one of many mechanisms developed by the immune system to enforce tolerance to harmless and self antigens. Tregs are critical to the development and maintenance of self-tolerance and function by suppressing the activation, cytokine production and proliferation of other T cells (Sakaguchi, 2005). These cells are characterized by high expression of the transcription factor FOXP3 and by their ability to suppress T cell responses in vitro.
We have found that between 5–10% of the T cells resident in normal human skin are FOXP3+ Tregs and that these cells proliferate under conditions similar to those found in inflamed skin (Clark et al., 2008; Clark and Kupper, 2007). This suggests that local proliferation of Tregs in the skin may serve as a brake for cutaneous inflammation. Indeed, Tregs were observed to locally proliferate within human skin during DTH reactions and increased numbers of Tregs were found in the skin lesions of contact dermatitis and resolving fixed drug eruptions (Teraki and Shiohara, 2003; Vukmanovic-Stejic et al., 2008)
Recent experiments in mice support the idea that Tregs decrease inflammatory tone in the skin. Mice with mutations in FOXP3 lack Tregs and develop widespread and lethal autoimmunity, a condition that can be prevented by the transfer of wild type Tregs (Khattri et al., 2003). These mice were reconstituted with Tregs that lacked 1,3-fucosyltransferase VII (FuT7), an enzyme necessary for formation of E-selectin ligands (Malý et al., 1996). These Tregs functioned normally but had a selective inability to migrate into the skin. These reconstituted mice had decreased inflammation in the liver and lungs, consistent with normal homing of Tregs to these sites, but they had marked skin inflammation. These mice were kept in germ-free facilities and not exposed to pathogens or other inflammatory stimuli, suggesting that the activity of Tregs is needed to control inflammation even in normal, non-challenged skin. These experiments also showed that Tregs need to be present within the skin in order to exert their anti-inflammatory effects, as opposed to suppressing skin inflammation by acting in the skin draining lymph nodes. This work suggests that Tregs play a role in maintaining homeostasis in normal skin and that they function by locally suppressing the activity of other T cells resident in skin.
For each mechanism of immune tolerance, there are cancers and pathogens that co-opt it to escape immune detection. Tregs are expanded in patients with many types of cancer and are also often recruited into the tumors themselves (Baecher-Allan and Anderson, 2006). 50% of the T cells present in human squamous cell carcinomas of the skin (SCC) are FOXP3+ Tregs and increased Tregs have also been observed in basal cell carcinomas, primary melanoma and melanoma metastases (Ahmadzadeh et al., 2008; Kaporis et al., 2007; Mourmouras et al., 2007). Topical therapy of human SCC with imiquimod, a topical immunomodulator and TLR7 agonist, reduced the % and suppressive function of Tregs, suggesting that it may be useful in reversing Treg induced tumor tolerance (Clark et al., 2008). Tregs also enable latent infection and induce reactivation of cutaneous leishmaniasis and paracoccidiomycosis in mice and likely play a similar role in humans (Belkaid et al., 2002; Xu et al., 2003).
Tregs have the capacity to be potently anti-inflammatory but they are ineffective under certain conditions. Tregs can suppress the activity of T cells which have received a low to mid strength T cell receptor (TCR) signal but cannot suppress T cells that receive a high avidity TCR signal, such as those observed in memory responses to dangerous pathogens (Baecher-Allan et al., 2002). Tregs therefore act as the immunologic equivalent of a high band pass filter, suppressing T cell responses with TCR avidities below a certain threshold and allowing those with higher avidity signals to proceed. Local production of the cytokine IL-6 also renders T cells resistant to the suppressive effects of Tregs in mice and preliminary work suggests this is also true in humans (Goodman et al., 2007; Korn et al., 2007). Expression of the chemokine receptor CCR5 is important for Treg migration and suppressive ability; Tregs that lack CCR5 are less effective in mouse models of paracoccidioidomycosis, leishmaniasis and graft vs. host disease (Moreira et al., 2008; Wysocki et al., 2005; Yurchenko et al., 2006). Lastly, Tregs can be outnumbered. In a mouse model of sarcoma, it was the percentage of Tregs that determined if a tumor would be tolerated or destroyed by the immune system (Bui et al., 2006).
Considerable numbers of Tregs are present in the skin lesions of psoriasis but these cells have decreased suppressive activity (Sugiyama et al., 2005). IL-6 is increased in psoriatic skin and this cytokine may interfere with the ability of Tregs to suppress inflammation (Goodman et al., 2007; Grossman et al., 1989) Additionally, patients with psoriasis have fewer CCR5+ Tregs than normal individuals and the CCR5+ Tregs that are present in psoriatic patients have decreased function (Sugiyama et al., 2008). Thus it appears that the Tregs present within psoriatic lesions fail to suppress inflammation because they are less active intrinsically and because their activity is antagonized by the pro-inflammatory cytokine milieu in psoriasis.
Th17 cells in the skin: key players in psoriasis
Th17 cells are a separate lineage of T cells that produce the Th17 cytokines IL-17A, IL-17F, TNFα, IL-21 and IL-22 and depend upon IL-23 for their development, survival and proliferation (Harrington et al., 2005; Park et al., 2005). Th17 cells provide immunity against a variety of extracelluar pathogens, including bacteria such as Klebsiella pneumoniae and fungi such as Cryptococcus neoformans and Candida albicans (Happel et al., 2005; Huang et al., 2004; Kleinschek et al., 2006).
Th17 cells have also been implicated in a variety of inflammatory and autoimmune disorders. IL-17 is increased and Th17 cells are demonstrable in the synovial fluid and tissues of patients with rheumatoid arthritis (Aarvak et al., 1999; Chabaud et al., 1999; Kotake et al., 1999) and in the brain lesions and CSF of patients with multiple sclerosis (Lock et al., 2002; Matusevicius et al., 1999). Polymorphisms in the IL-23R, a receptor required for the development and survival of Th17 cells, are associated with susceptibility to ulcerative colitis and Crohn's disease (Duerr et al., 2006). Th17 cells also contribute to the pathology observed in mouse models of colitis, experimental autoimmune encephalomyelitis and arthritis (Ouyang et al., 2008).
Increasing evidence suggests that Th17 cells are also key players in the pathogenesis of psoriasis. DC and keratinocytes in the skin lesions of psoriasis produce increased amounts of IL-23, a cytokine that supports the development and proliferation of Th17 cells (Kryczek et al., 2008; Lee et al., 2004; Piskin et al., 2006; Wilson et al., 2007; Zaba et al., 2008). Treatment of patients with monoclonal antibodies against IL-12/23p40, a component shared between the IL-12 and IL-23 receptors, led to significant clinical improvements in psoriasis (Kimball et al., 2008; Krueger et al., 2007). Although this finding could not discriminate between causative roles for IL-23 vs. IL-12, a role for IL-23 in psoriasis is supported by genetic studies demonstrating that polymorphisms in the IL-23 receptor and other genes in the IL-23 signaling pathway are associated with psoriasis (Capon et al., 2007; Cargill et al., 2007; Nair et al., 2009; Nair et al., 2008). Th17 cells are demonstrable in psoriatic lesions and are found in higher numbers than in normal human skin (Kryczek et al., 2008; Lowes et al., 2008). Th17 cells produce the cytokine IL-22, a cytokine that induces human keratinocyte proliferation and acanthosis in vitro (Sa et al., 2007). Injection of IL-23 into the skin of mice induced dermal inflammation and epidermal acanthosis reminiscent of the changes seen in psoriasis and these effects that were found to be mediated through production of IL-22 (Chan et al., 2006; Zheng et al., 2007). These findings support a role for IL-23 in supporting the survival, proliferation and function of Th17 T cells that in turn contribute to psoriasis through their production of IL-22 and other inflammatory cytokines. These observations suggest that selective disruption of IL-23 signaling (for example, by targeting IL-23p19 subunit) should lead to reduced Th17 T cells and potentially to long lasting improvements in psoriasis.
Tissue resident T cells: on site immune memory
TCM persist long term in the circulation following resolution of an immune response, a finding that led researchers to propose that TCM are the only T cells responsible for maintaining long term immunologic memory (Lanzavecchia and Sallusto, 2005). This contention is at odds with clear and elegant experiments in mice demonstrating that TEM do persist long term—not in the blood but within the peripheral tissues (Masopust et al., 2001; Reinhardt et al., 2001). In animal models, T cells generated during immune responses in the skin, gut and lungs persist within these tissues and provide protection against re-infection at these sites (Hogan et al., 2001b; Liang et al., 1994; Stittelaar et al., 2005; Xu et al., 2004). A series of elegant studies examining HSV infection of the skin in mice have provided some intriguing details about the nature of these cells. Following infection with HSV, CD8 antigen specific T cells accumulated in the skin near latently infected dorsal root ganglia and suppressed reactivation of the virus (van Lint et al., 2005; Wakim et al., 2008b). Re-infection with HSV led to local proliferation of these resident cells and also recruitment of additional antigen specific T cells from the circulation (Wakim et al., 2008a). There were therefore two populations of antigen specific T cells capable of responding to rechallenge: skin resident T cells that arose and were on site as a result of the primary infection and circulating antigen specific T cells that were newly recruited from the blood. Using GFP-expressing mice and serial transplantation of latently infected ganglia, this group dissected the contributions and characteristics of these two cell types (Gebhardt et al., 2009). Surprisingly, antigen specific tissue resident T cells generated during the primary immune response remained in the same location over 100 days after primary HSV infection. These T cells failed to reenter the circulation and did not even migrate into infected ganglia transplanted in direct approximation. Antigen specific T cells were present in highest numbers at the site of initial infection but were also demonstrable in other locations within the skin. These tissue resident T cells provided enhanced local protection against reinfection with HSV. Skin resident T cells lacked L-selectin and expressed higher levels of the activation antigen CD69 than T cells recruited from blood, a finding that has been replicated in humans (Clark et al., 2006a). These studies demonstrated that a functionally distinct, non-migratory population of skin resident T cells arose following viral infection, persisted long-term within the skin and provided effective protection against local reinfection. In humans, a similar population of HSV-specific T cells was found resident in the skin of military recruits more than two months after clearance of a primary HSV infection and these T cells increased in number during subclinical HSV reactivation. Antigen specific T cells also persisted long term locally in the skin at the site of PPD injection in BCG-vaccinated individuals (Vukmanovic-Stejic et al., 2006).
The finding that skin resident T cells are a sessile, nonmigratory population may help to explain some of the puzzling eruptions we see in dermatology. Fixed drug eruptions (FDE) are local skin lesions that occur following ingestion of a causative drug, spontaneously resolve once the drug is discontinued, then recur in the same location years or even decades later when the drug is taken again. Histologically, this eruption is a cytotoxic reaction against epidermal keratinocytes mediated by a CD8 T cells and indeed, a population of CD8 T cells producing IFNγ and TNFα is found within the epidermis of clinically resolved, hyperpigmented FDE lesions (Shiohara and Moriya, 1997; Teraki et al., 1994; Teraki and Shiohara, 2003). The fixed location of this eruption may reflect the fixed location of a population of drug reactive T cells resident in skin. Similarly, it is striking how psoriatic plaques remain in the same location long-term and tend to recur in the same locations following cessation of suppressive therapy. Given the evidence that autoreactive Th17 cells may drive this disease, it is tempting to speculate that each psoriatic plaque represents a population of autoreactive skin resident Th17 cells. However, changes in other cell types could also mediate this localized behavior, including fixed populations of pathogenic APC or possibly fixed changes in the blood vessels at these sites. Lastly, the different behavior of central memory and skin resident effector memory T cells may underlie the differing clinical manifestations we observe in cutaneous T cell lymphoma. In patients with stage IA mycosis fungoides (MF), malignant T cells are confined to stable patches and plaques on the skin and life expectancy is normal (Kim et al., 2003). In Sézary syndrome, the malignant T cells migrate throughout the entire skin surface, giving rise to erythroderma, and also colonize the blood and lymph nodes. Intriguing new data suggests that the malignant T cells in MF are skin resident effector memory cells, a population expected to remain in a fixed position. However, malignant T cells in Sézary syndrome bear markers suggestive of central memory T cells, a cell type that normally migrates through the blood, lymph nodes, and can also be found in low numbers in normal human skin (Campbell et al., 2009; Clark et al., 2006a). The fact that malignant central memory T cells give rise to diffuse erythroderma and malignant skin resident T cells give rise to fixed plaques of inflamed skin is an eloquent argument in favor of the mobile versus sessile nature of these two lymphocyte subsets.
Other tissues have their own populations of resident T cells that contribute local immune protection. In mice, a population of antigen-specific T cells remained resident in the lung several months after recovery from influenza or Sendai virus infection (Hogan et al., 2001a). It was the number of lung resident pathogen-specific T cells and not the number in the lymph nodes that correlated with protection against re-infection. The gut epithelium in mice is colonized by CD8 memory T cells with substantial lytic activity (Masopust et al., 2001). Murine intraepithelial CD8 cells bearing the αβ TCR and both αβ CD8 chains (comparable to conventional T cells) are scarce in the gut at birth but progressively increase throughout life as antigen-experienced T cells accumulate (Cheroutre, 2004; Cheroutre and Madakamutil, 2004). Gut resident T cells isolated from mice that have recovered from pathogens such as Toxoplasma gondii protect against infection when transferred into naïve animals (Lepage et al., 1998).
Central and tissue resident T cells: an integrated view
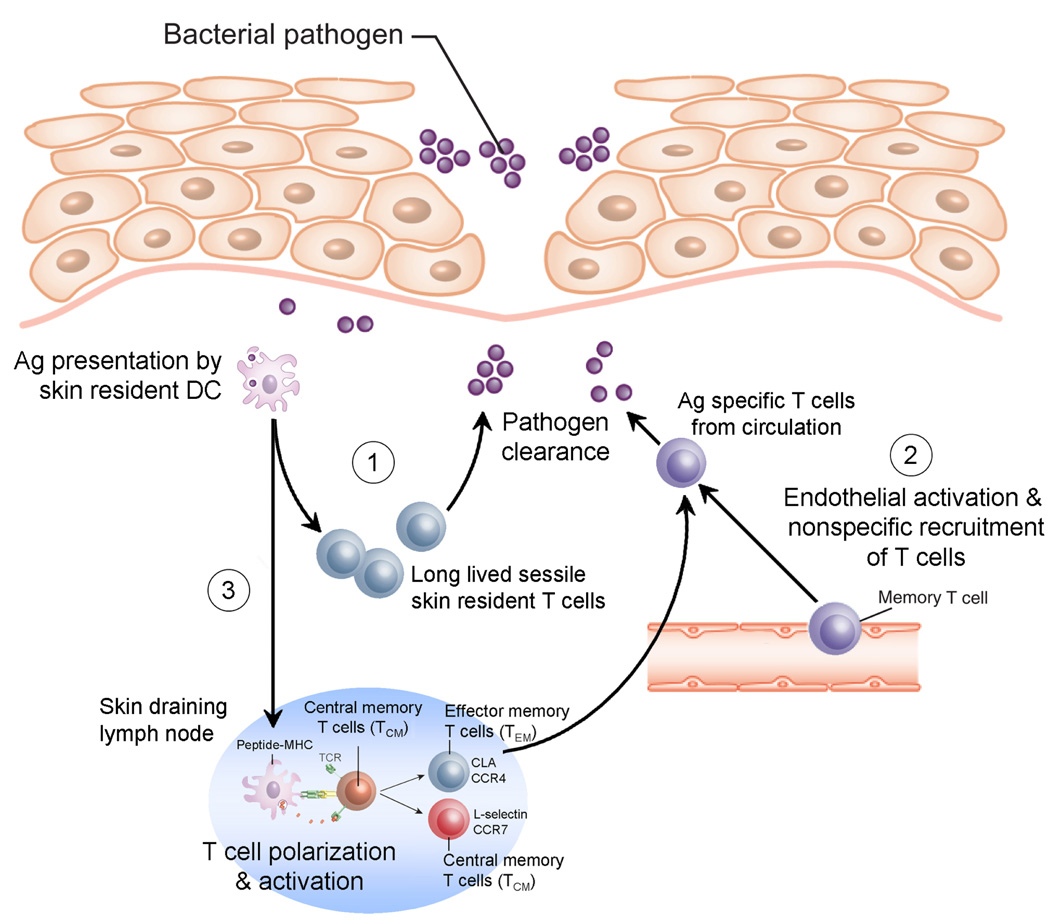
To conceptualize how TEM and TCM contribute to immunity, it may be helpful to describe their contributions to both naïve and memory T cell responses in the skin. During a primary immune response, when the immune system encounters an antigen for the first time, the antigen is taken up by skin resident DC and these cells migrate to the skin draining lymph nodes (Figure 1). Within the lymph nodes, DC present antigen to naïve T cells. Upon recognition of cognate antigen, naïve T cells undergo differentiation and polarization into skin homing TEM and TCM. TEM then migrate through the bloodstream and are distributed to all parts of the skin, although the highest numbers of these cells will be found at the site of pathogen exposure (Gebhardt et al., 2009). These cells effect clearance of the pathogen and then remain resident locally within the skin (Gebhardt et al., 2009). In the early stages of a primary immune response, proliferating T cells are also released from the skin draining lymph nodes and distribute to antigen-free lymph nodes draining other tissues (Liu et al., 2006). T cells continue to proliferate within these new lymph nodes and give rise to new populations of effector T cells that migrate to and take up residence in the gut, lungs and other peripheral tissues. In this way, immunization through the skin actually generates widespread systemic immunity via the generation of disparate populations of tissue resident TEM cells.
Figure 1. Contributions of TEM and TCM to primary immune responses in the skin.
After pathogen exposure, DC take up antigen, migrate to the skin draining lymph nodes and present it to naïve T cells. Naïve T cells that recognize their cognate antigen undergo differentiation and polarization in to skin homing effector memory T cells (TEM) and central memory T cells (TCM). TEM leave the lymph nodes, enter the circulation and migrate into the skin where they effect clearance of the pathogen. TEM colonize all areas of the skin but are found in highest numbers at sites of pathogen exposure. In the early stages of the immune response, proliferating T cells are also released from the skin draining lymph nodes and migrate to antigen-free lymph nodes draining other peripheral tissues. T cells continue to proliferate within these lymph nodes, giving rise to new populations of TEM that home to gut, lungs and other peripheral tissues. In this way, immunization through the skin gives rise to a diverse population of tissue resident T cells that provide systemic immune protection.
In a memory immune response, T cell responses can be divided into three distinct stages (Figure 2). First, local re-exposure to a pathogen leads to antigen uptake and local antigen presentation by tissue resident DC. These DC stimulate the proliferation and effector functions of antigen specific skin resident T cells located in the skin, leading to rapid neutralization of the pathogen (Wakim et al., 2008b). Second, local inflammation leads to up regulation of vascular adhesion receptors on the skin endothelium, leading to nonspecific recruitment of T cells from the circulation. Only a minority of these T cells will be antigen specific, yet the small numbers of antigen specific cells that do enter the skin under these conditions have been shown to contribute to immune responses (Wakim et al., 2008a). Third, migration of antigen laden DC to the skin draining lymph nodes will lead to stimulation of TCM and the subsequent production of large numbers of skin homing TEM. These TEM will then migrate through the bloodstream, enter areas of inflamed skin, and affect clearance of the pathogen.
Figure 2. The role of TEM and TCM in recall immune responses.
Memory immune responses can be divided into three stages. First, DC take up antigen following pathogen re-exposure and present it to TEM resident locally within the skin. These cells proliferate and effect clearance of the pathogen. Second, inflammation leads to endothelial activation and nonspecific recruitment of T cells from the blood. The small numbers of antigen-specific T cells recruited into the skin in this way can also participate in clearance of the pathogen. Third, DC carry endocytosed antigen to the skin draining lymph nodes where it is presented to TCM. These TCM then give rise to new populations of skin homing TEM that migrate to the skin and clear the infection.
T cells have the flexibility to respond to any antigen, to migrate to any tissue, and to produce a plethora of cytokines and effector functions fine tuned to efficiently eliminate pathogens and tumors. Although tissue resident T cells have been described in gut, lung and skin, it is likely that all tissue types have to some degree their own populations of resident T cells. By populating both the peripheral tissues and the lymph nodes with distinct types of memory T cells, the adaptive immune system manages to provide both flexibility and the ability to neutralize pathogens rapidly.
Human skin is populated by 20 billion T cells, charged with the responsibility of locally defending the skin against tumors and pathogens while maintaining tolerance to self antigens exposed during injury. The evolution of this system of local T cell deposition, combined with amount of energy it takes to generate and maintain these cells, make it clear that defending the skin is a high priority for the immune system. However, skin resident T cells can dysfunction; these cells can fail to suppress pathogenic inflammation, shield tumors and infections with T cells designed to limit autoimmunity and generate autoreactive T cells that can initiate psoriasis and other inflammatory skin diseases. Studies of the antigen specificity of skin resident T cells and how they are activated in skin are likely to identify ways that T cell function can be enhanced in tumors and inhibited in inflammatory skin disorders. Because immune reactions in skin can be visually observed, sampled and manipulated with topical medications, the skin provides an accessible site in which to study human immune responses. Novel therapies arising from an understanding of T biology in skin should be broadly applicable to pathogenic immune states in other tissues.
ACKNOWLEDGEMENTS
Research by the author described in this review was carried out with the support and advice of Dr. Thomas S. Kupper, Chairman of Dermatology at Brigham and Women’s Hospital. The authors would like to thank Adam Calarese and Dr. Michael Lichtman for providing helpful suggestions and editorial comments. The author is supported by NIH grants 1K08AI060890-01A1, a Translational Research Award from the Leukemia and Lymphoma Society, a New Investigator Award from the Scleroderma Foundation and a Clinical Investigator Award from the Damon Runyon Cancer Research Foundation.
Abbreviations used
- CLA
cutaneous lymphocyte antigen
- pDC
plasmacytoid dendritic cell
- SCC
squamous cell carcinoma
- Tregs
regulatory T cells
REFERENCES
- Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 Is Produced by Some Proinflammatory Th1/Th0 Cells But Not by Th2 Cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJ, Jr, Wunderlich JR, Merino MJ, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Seminars in cancer biology. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. The Journal of experimental medicine. 2001;193:1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. The Journal of experimental medicine. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan M, Bleiker TO, Ballsdon AE, Allen MH, Sopwith M, Robinson MK, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. The British journal of dermatology. 2002;146:824–831. doi: 10.1046/j.1365-2133.2002.04743.x. [DOI] [PubMed] [Google Scholar]
- Bos JD, Hagenaars C, Das PK, Krieg SR, Voorn WJ, Kapsenberg ML. Predominance of "memory" T cells (CD4+, CDw29+) over "naive" T cells (CD4+, CD45R+) in both normal and diseased human skin. Archives of dermatological research. 1989;281:24–30. doi: 10.1007/BF00424268. [DOI] [PubMed] [Google Scholar]
- Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, Kapsenberg ML. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J Invest Dermatol. 1987;88:569–573. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. The Journal of experimental medicine. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer research. 2006;66:7301–7309. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. The Journal of experimental medicine. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, et al. CCR7 Expression and Memory T Cell Diversity in Humans. J Immunol. 2001;166:877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Soler D, Clark RA, Kupper TS. Malignant clonal T cells in leukemic (L-)CTCL/Sezary syndrome (SS) exhibit a unique cell surface profile: high and uniform expression of CCR4 and central memory markers CCR7, L-selectin, and CD27. J Invest Dermatol. 2009;129:S54. [Google Scholar]
- Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Human genetics. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. American journal of human genetics. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis and rheumatism. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. The Journal of experimental medicine. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annual review of immunology. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- Cheroutre H, Madakamutil L. Acquired and natural memory T cells join forces at the mucosal front line. Nat Rev Immunol. 2004;4:290–300. doi: 10.1038/nri1333. [DOI] [PubMed] [Google Scholar]
- Chong BF, Murphy J-E, Kupper TS, Fuhlbrigge RC. E-Selectin, Thymus- and Activation-Regulated Chemokine/CCL17, and Intercellular Adhesion Molecule-1 Are Constitutively Coexpressed in Dermal Microvessels: A Foundation for a Cutaneous Immunosurveillance System. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006a;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006b;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. The Journal of experimental medicine. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Goodman W, Massari J, McCormick T, Cooper K. Does IL-6 in psoriatic lesions reverse the ability of regulatory T cells to suppress effector T cell proliferation? J Invest Dermatol. 2007;127:S125. Poster 749. [Google Scholar]
- Griffiths CE. T-cell-targeted biologicals for psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:157–161. doi: 10.2174/1568010043343912. [DOI] [PubMed] [Google Scholar]
- Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. The Journal of experimental medicine. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001a;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. The Journal of experimental medicine. 2001b;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nature medicine. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. The Journal of infectious diseases. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Kaporis HG, Guttman-Yassky E, Lowes MA, Haider AS, Fuentes-Duculan J, Darabi K, et al. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol. 2007;127:2391–2398. doi: 10.1038/sj.jid.5700884. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of dermatology. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Archives of dermatology. 2008;144:200–207. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature medicine. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. The New England journal of medicine. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Current opinion in immunology. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. The Journal of experimental medicine. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage AC, Buzoni-Gatel D, Bout DT, Kasper LH. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J Immunol. 1998;161:4902–4908. [PubMed] [Google Scholar]
- Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature medicine. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Mackay C, Marston W, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malý P, Thall AD, Petryniak B, Rogers CE, Smith PL, Marks RM, et al. The [alpha](1,3)Fucosyltransferase Fuc-TVII Controls Leukocyte Trafficking through an Essential Role in L-, E-, and P-selectin Ligand Biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Science. Vol. 291. New York, NY: 2001. Preferential localization of effector memory cells in nonlymphoid tissue; pp. 2413–2417. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, et al. Multiple sclerosis. Vol. 5. Houndmills, Basingstoke, England: 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis; pp. 101–104. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin-and gut-associated lymphoid tissues. The Journal of experimental medicine. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira AP, Cavassani KA, Massafera Tristao FS, Campanelli AP, Martinez R, Rossi MA, et al. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol. 2008;180:3049–3056. doi: 10.4049/jimmunol.180.5.3049. [DOI] [PubMed] [Google Scholar]
- Mourmouras V, Fimiani M, Rubegni P, Epistolato MC, Malagnino V, Cardone C, et al. Evaluation of tumour-infiltrating CD4+CD25+FOXP3+ regulatory T cells in human cutaneous benign and atypical naevi, melanomas and melanoma metastases. The British journal of dermatology. 2007;157:531–539. doi: 10.1111/j.1365-2133.2007.08057.x. [DOI] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-[kappa]B pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Ruether A, Stuart PE, Jenisch S, Tejasvi T, Hiremagalore R, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-{alpha} production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- Razvi ES, Jiang Z, Woda BA, Welsh RM. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. The American journal of pathology. 1995;147:79–91. [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature immunology. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, et al. A skin-selective homing mechanism for human immune surveillance T cells. The Journal of experimental medicine. 2004;199:1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara T, Moriya N. Epidermal T cells: their functional role and disease relevance for dermatologists. J Invest Dermatol. 1997;109:271–275. doi: 10.1111/1523-1747.ep12335465. [DOI] [PubMed] [Google Scholar]
- Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. Journal of virology. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, McCormick TS, Massari J, Shimada S, Cooper KD. CCR5 expressing CD4+CD25high regulatory T cells are both numerically and functionally impaired in patients with psoriasis vulgaris. J Invest Dermatol. 2008;127 [Google Scholar]
- Teraki Y, Moriya N, Shiohara T. Drug-induced expression of intercellular adhesion molecule-1 on lesional keratinocytes in fixed drug eruption. The American journal of pathology. 1994;145:550–560. [PMC free article] [PubMed] [Google Scholar]
- Teraki Y, Shiohara T. IFN-gamma-producing effector CD8+ T cells and IL-10-producing regulatory CD4+ T cells in fixed drug eruption. The Journal of allergy and clinical immunology. 2003;112:609–615. doi: 10.1016/s0091-6749(03)01624-5. [DOI] [PubMed] [Google Scholar]
- van Lint AL, Kleinert L, Clarke SR, Stock A, Heath WR, Carbone FR. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. Journal of virology. 2005;79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, et al. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MH, Akbar AN. Mantoux Test as a model for a secondary immune response in humans. Immunology letters. 2006;107:93–101. doi: 10.1016/j.imlet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol. 2008a;181:5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Science. Vol. 319. New York, NY: 2008b. Dendritic cell-induced memory T cell activation in nonlymphoid tissues; pp. 198–202. [DOI] [PubMed] [Google Scholar]
- Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. The Journal of experimental medicine. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton JL, Zhang J. Principles of cytotoxic T lymphocyte induction and recognition. Current topics in microbiology and immunology. 1995;202:247–259. doi: 10.1007/978-3-642-79657-9_16. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, et al. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. The Journal of experimental medicine. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. Psoriasis Is Characterized by Accumulation of Immunostimulatory and Th1//Th17 Cell-Polarizing Myeloid Dendritic Cells. J Invest Dermatol. 2008;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. The Journal of experimental medicine. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]