Abstract
Context
Persons living with AIDS today remain at elevated cancer risk. Highly active antiretroviral therapy (HAART), widely available since 1996, prolongs life, but immune function is not fully restored.
Objectives
To assess long-term cancer risk among persons with AIDS relative to the general population and the impact of HAART on cancer incidence.
Design, Setting, and Subjects
Records of 263,254 adults and adolescents with AIDS (1980-2004) from 15 U.S. regions were matched to cancer registries to capture incident cancers during years 3-5 and 6-10 after AIDS onset.
Main Outcome Measure
Standardized incidence ratios (SIRs) assessed risks relative to the general population. Rate ratios (RRs) compared cancer incidence before and after 1996 to assess the impact of availability of HAART.
Results
Risk was elevated for the two major AIDS-defining cancers, Kaposi sarcoma (SIRs 5321 and 1347 in the 3-5 and 6-10 year periods, respectively) and non-Hodgkin lymphoma (SIRs 32 and 15). Incidence of both malignancies declined in the HAART era (1996-2006). Risk was elevated for all non-AIDS defining cancers combined (SIRs 1.7 and 1.6 in years 3-5 and 6-10, respectively), and for the following specific non-AIDS-defining cancers: cancers of the oral cavity/pharynx, tongue, anus, liver, larynx, lung/bronchus, and penis, and Hodgkin lymphoma. Anal cancer incidence increased between 1990-1995 and 1996-2006 (RR 2.9, 95%CI 2.1-4.0), as did Hodgkin lymphoma (RR 2.0, 95%CI 1.3-2.9).
Conclusion
Among people who survived an AIDS diagnosis for several years or more, we observed high risks of AIDS-defining cancers and increasing incidence of anal cancer and Hodgkin lymphoma.
Introduction
Persons infected with human immunodeficiency virus (HIV) have an increased risk of cancer.1,2 Advanced HIV infection is characterized by profound immunosuppression (i.e., acquired immunodeficiency syndrome [AIDS]), a risk factor for a number of malignancies. Three cancers are AIDS-defining: Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), and cervical cancer.3 These cancers are caused by oncogenic viruses, specifically, Kaposi sarcoma-associated herpesvirus for KS, Epstein Barr virus for the major AIDS-defining NHL subtypes (diffuse large B cell NHL and central nervous system [CNS] NHL), and human papillomavirus (HPV) for cervical cancer.4-6 HIV-infected people also have an elevated risk for other virus-related cancers (e.g., anal cancer related to HPV, liver cancer related to hepatitis B and C viruses).5-7 In addition, HIV-infected people have a higher prevalence of lifestyle-associated risk factors for cancer including smoking and alcohol abuse.8,9
Use of highly active antiretroviral therapy (HAART) among persons with HIV/AIDS can lead to partial immune restoration and prolonged survival.10,11 Despite declines in cancer incidence attributable to the widespread introduction of HAART in 1996, cancer risk among HIV-infected people remains elevated during the HAART era.2,12-14 Of note, prior studies of cancer risk in HIV-infected people have largely been limited to the first 1-5 years after an AIDS diagnosis.1,2,14-16 As persons with AIDS continue to live longer, the number of people surviving for many years after an AIDS diagnosis will increase. This growing population comprises individuals who have lived for a prolonged period with immune suppression. The effects of these chronic immune disturbances, HAART, and long-term infection with oncogenic viruses are unknown. Estimates of cancer risk are needed to inform both public health programs and clinicians treating this population.
To assess the long-term cancer risk among persons with AIDS, we analyzed data from the HIV/AIDS Cancer Match Study, a large, representative cohort of persons with AIDS in the U.S. Previous analyses included persons diagnosed with AIDS during 1978-1996 with cancer information up to 5-years after AIDS,15 and persons diagnosed with AIDS between 1980-2004 with cancer information up to 2-years after AIDS.2 In the current study, we evaluated the period 3-10 years after AIDS onset, to assess cancer risk relative to the general population, and to determine the impact of HAART on cancer incidence over time.
Methods
Study design
The HIV/AIDS Cancer Match Study links records of persons with HIV or AIDS diagnosed between 1980 and 2008 from 15 U.S. states and metropolitan regions to corresponding cancer registry records using a probabilistic algorithm.1 Cancer registry coverage varies by region but is complete through 2004-2006 for several registries. Following linkage of registry databases, only de-identified data were retained for analyses. Institutional review boards at participating sites approved the study.
The current study focuses on cancer risk late after an AIDS diagnosis, specifically for the period 3-10 years after AIDS onset. AIDS onset was defined using the 1993 Centers for Disease Control and Prevention definition.3 The study was restricted to the most common racial/ethnic categories (non-Hispanic whites, non-Hispanic blacks, and Hispanics) to allow for stable estimates of expected cancer counts. Children less than 15 years of age were excluded. The current study was therefore limited to adults and adolescents who had AIDS onset in 1980-2004 and whose follow-up during the 3-10 year period after AIDS onset overlapped with cancer registry coverage (N=263,254).
Malignancies reported to cancer registries were coded according to the International Classification for Diseases for Oncology.17 Cancers were categorized by site and histology using the Surveillance, Epidemiology, and End Results (SEER) program's “site recode with Kaposi sarcoma and mesothelioma.”18 This classification was slightly modified by grouping some rare cancer types and expanding others (e.g., NHL) into subtypes of special interest. We considered people at risk for each cancer if they had not previously had that cancer.
Statistical methods
We initially evaluated cancer risk separately for the periods 3-5 and 6-10 years after AIDS onset. For each period, cancer cases and person-time were tabulated from the start of the period or the beginning of cancer registry coverage (whichever occurred later) until the first of the following: the end of the period, end of cancer registry coverage, or death (as ascertained from the HIV/AIDS registries at the time of the match). For each cancer, we present the standardized incidence ratio (SIR), defined as the ratio of observed to expected number of cancer cases, as a measure of risk relative to the general population. Expected counts were obtained by applying gender, age, race/ethnicity, calendar year, and registry-specific incidence rates to person-time among people with AIDS. Because virtually all KS and CNS NHL cases in the general population are AIDS-related, we used expected counts for these cancers from SEER registries prior to the epidemic (1973-1979).19 We calculated two-sided exact Poisson 95% confidence intervals (CIs) for the SIRs.
Similarly, for the combined period 3-10 years after AIDS onset, we evaluated changes in cancer incidence between two attained calendar periods: 1990-1995 (pre-HAART era) and 1996-2006 (HAART era). These “attained” calendar periods correspond to those current years regardless of how long an individual had had AIDS, providing that the onset of AIDS was within 3-10 years previously. In these analyses, we calculated incidence rates as the observed counts divided by person-time at risk. Changes in incidence between the calendar periods were then assessed using a rate ratio (RR) obtained from Poisson regression. The Poisson models were adjusted for attained age, race/ethnicity, and gender/mode of HIV exposure (men who reported male-to-male sex [MSM] alone or with injection drug use [IDU], all other men, and females). To account for under- or over-dispersion in the Poisson models, the standard errors of the coefficients were adjusted by Pearson's chi-square statistic divided by the degrees of freedom. When a RR was zero or undefined due to an empty cell, we calculated a one-sided exact 95% CI. We also present SIRs for the combined 3-10 year period restricted to the HAART era, as a measure of cancer risk relative to the general population.
For selected cancers with substantial changes in incidence and sufficient sample size, we calculated incidence as a function of individual attained calendar years (1990-2006). We used joinpoint regression to fit log-linear Poisson models for these annual rates, which translated into annual percentage changes across calendar time.20 All joinpoint models manifested adequate fit according to the Hosmer-Lemeshow statistic. In the figures illustrating these fitted trends, we present only annual percentage changes that differed significantly from zero (P<.05). We used the Joinpoint Regression Program (National Cancer Institute, Rockville, Maryland) for joinpoint analyses.
A consideration in evaluating cancer risk late after AIDS onset is that people with AIDS, after an extended period, may have migrated out of the cancer registry region. Persons with AIDS in our study were known to reside in the cancer registry coverage area at the time of AIDS diagnosis, but we had no data on subsequent out-migration. Prior studies have demonstrated that among persons with AIDS who had died, as many as 10% had moved from their area of AIDS diagnosis before death.21,22 Similar migration rates have been noted among the general population.23 If a person had moved out of the cancer registry coverage area, his cancer would not have been ascertained, leading to a reduction in the observed cancer count but not in the estimated follow-up time, and thus causing an under-estimation of cancer risk. To correct this bias, we adjusted for potential out-migration by incrementally discounting each subject's person-time to reach a total reduction of 10% by 10 years after AIDS diagnosis. All SIR and incidence measures utilized these adjusted person-time estimates. Results were similar in a sensitivity analysis in which we discounted the person-time by a factor of 20% (data not shown).
Results
Demographic characteristics of the 263,254 persons with AIDS included in the study are presented in Table 1. Most were male (80.1%), and the median age at AIDS onset was 36 years. Similar proportions of subjects were non-Hispanic white (39.7%) and non-Hispanic black (40.1%), and 20.2% were Hispanic. Almost half (44.8%) reported MSM and 24.8% had IDU as their mode of HIV exposure (Table 1). CD4 counts at AIDS onset were higher for subjects who contributed follow-up to the 6-10 year period than for those who contributed follow-up only to the 3-5 year period (median 144 vs. 132 cells/μL), consistent with better immune status at AIDS onset conveying longer survival. Additionally, few persons diagnosed with AIDS in 1980-1989 survived long enough to provide follow-up in the 6-10 year period. Also, some people diagnosed with AIDS in 1996-2004 did not have enough time to be followed into the 6-10 year period. As a result, most observation time in the 6-10 year period was provided by people diagnosed with AIDS in 1990-1995 (Table 1).
Table 1. Demographic characteristics of persons with AIDS in the United States, 1980-2004 (N=263,254).
| Subjects contributing follow-up time for specified periods after AIDS onset | |||
|---|---|---|---|
| Characteristic | Overall: Years 3-10 after AIDS | Years 3-5 after AIDS | Years 6-10 after AIDS |
| Total No. | 263,254 | 261,282 | 135,163 |
| Sex, n (%) | |||
| Male | 210,841 (80.1) | 209,119 (80.0) | 109,133 (80.7) |
| Female | 52,413 (19.9) | 52,163 (20.0) | 26,030 (19.3) |
| Age in years at AIDS onset, n (%) | |||
| 15-29 | 46,059 (17.5) | 45,425 (17.4) | 25,656 (19.0) |
| 30-39 | 121,845 (46.3) | 120,963 (46.3) | 63,890 (47.3) |
| 40-49 | 71,074 (27.0) | 70,739 (27.1) | 34,924 (25.8) |
| 50+ | 24,276 (9.2) | 24,155 (9.2) | 10,693 (7.9) |
| Median | 36 | 36 | 36 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 104,402 (39.7) | 103,406 (39.6) | 55,790 (41.3) |
| Non-Hispanic black | 105,761 (40.1) | 104,957 (40.2) | 52,354 (38.7) |
| Hispanic | 53,091 (20.2) | 52,919 (20.2) | 27,019 (20.0) |
| Mode of HIV exposure, n (%) | |||
| MSM | 118,042 (44.8) | 116,957 (44.8) | 62,696 (46.4) |
| IDU | 65,157 (24.8) | 64,777 (24.8) | 33,778 (25.0) |
| MSM and IDU | 16,341 (6.2) | 16,120 (6.2) | 9,037 (6.7) |
| Heterosexual | 11,650 (4.4) | 11,582 (4.4) | 6,118 (4.5) |
| Other/unknown | 52,064 (19.8) | 51,846 (19.8) | 23,534 (17.4) |
| CD4 count at AIDS onset (cells/μL), n (%) | |||
| 0-99 | 74,656 (28.4) | 75,845 (29.0) | 35,121 (26.0) |
| 100-199 | 81,885 (31.1) | 81,603 (31.2) | 45,409 (33.6) |
| 200+ | 33,045 (12.6) | 32,989 (12.6) | 19,609 (14.5) |
| Missing | 73,668 (27.9) | 70,845 (27.2) | 35,024 (25.9) |
| Median | 132 | 132 | 144 |
| Calendar year at AIDS onset, n (%) | |||
| 1980-1989 | 28,318 (10.8) | 26,868 (10.3) | 12,025 (8.9) |
| 1990-1995 | 128,296 (48.7) | 127,774 (48.9) | 83,564 (61.8) |
| 1996-2004 | 106,640 (40.5) | 106,640 (40.8) | 39,574 (29.3) |
Table 1 notes
In the 3-10, 3-5 and 6-10 years columns, subjects are included if they contributed person-time at-risk for cancer during those periods after AIDS onset, respectively.
Abbreviations: MSM, male-to-male sex; IDU, injection drug use.
Risks for the three AIDS-defining cancers were significantly elevated in years 3-5 and 6-10 after AIDS onset (Table 2). KS risk was extremely high in both time periods (SIRs 5321 and 1347 respectively), and NHL risk was also elevated in both periods (SIRs 32 and 15, respectively). Likewise, risks were substantially higher than in the general population for the AIDS-defining NHL subtypes, namely diffuse large B-cell NHL, Burkitt NHL, and especially CNS NHL (Table 2). Cervical cancer risk was increased after AIDS during years 3-5 (SIR 5.6) and years 6-10 (SIR 3.6).
Table 2. Cancer risk in people with AIDS for the period 3-10 years after AIDS onset.
| Years 3-5 after AIDS | Years 6-10 after AIDS | |||||
|---|---|---|---|---|---|---|
| Cancer type | No. cases | SIR | 95%CI | No. cases | SIR | 95%CI |
| All cancer | 9053 | 6.1 | (6.0-6.3) | 3476 | 3.0 | (2.9-3.1) |
| AIDS-defining cancers | ||||||
| Kaposi sarcoma | 3136 | 5321 | (5137-5511) | 615 | 1347 | (1243-1458) |
| Non-Hodgkin lymphoma | 3345 | 32 | (31-33) | 1048 | 15 | (14-16) |
| Diffuse large B-cell NHL | 1618 | 44 | (42-46) | 555 | 23 | (21-25) |
| Burkitt NHL | 95 | 29 | (23-35) | 52 | 23 | (17-30) |
| CNS NHL | 989 | 2005 | (1882-2134) | 182 | 474 | (408-548) |
| Other/unspecified NHL | 1632 | 25 | (24-27) | 441 | 10 | (9-11) |
| Cervix | 101 | 5.6 | (4.5-6.8) | 38 | 3.6 | (2.6-5.0) |
| Non-AIDS-defining cancers | ||||||
| Oral cavity/pharynx | 199 | 1.9 | (1.6-2.1) | 149 | 1.8 | (1.5-2.1) |
| Lip | 14 | 5.4 | (2.9-9.0) | 5 | 2.6 | (0.8-6.0) |
| Tongue | 27 | 1.8 | (1.2-2.5) | 34 | 2.7 | (1.9-3.8) |
| Esophagus | 29 | 1.4 | (0.9-1.9) | 25 | 1.5 | (1.0-2.2) |
| Stomach | 35 | 1.2 | (0.8-1.7) | 23 | 1.0 | (0.7-1.5) |
| Small intestine | 5 | 0.8 | (0.3-1.8) | 1 | 0.2 | (0-1.1) |
| Colon/rectum | 129 | 1.0 | (0.8-1.2) | 98 | 0.9 | (0.7-1.1) |
| Anus | 219 | 27 | (24-31) | 267 | 40 | (35-45) |
| Liver | 86 | 3.7 | (3.0-4.6) | 95 | 4.5 | (3.7-5.5) |
| Pancreas | 22 | 0.8 | (0.5-1.3) | 25 | 1.1 | (0.7-1.7) |
| Larynx | 67 | 2.7 | (2.1-3.4) | 61 | 3.1 | (2.4-4.0) |
| Lung/bronchus | 531 | 3.0 | (2.8-3.3) | 357 | 2.6 | (2.3-2.9) |
| Bones/joints | 5 | 1.3 | (0.4-3.0) | 0 | 0 | (0-1.4) |
| Soft tissue | 23 | 1.6 | (1.0-2.5) | 14 | 1.4 | (0.8-2.4) |
| Melanoma of the skin | 58 | 1.2 | (0.9-1.5) | 38 | 1.0 | (0.7-1.3) |
| Breast | 68 | 0.6 | (0.5-0.8) | 36 | 0.5 | (0.3-0.7) |
| Uterus | 7 | 0.6 | (0.2-1.1) | 4 | 0.4 | (0.1-1.1) |
| Ovary | 14 | 1.5 | (0.8-2.6) | 3 | 0.5 | (0.1-1.5) |
| Vagina/vulva | 13 | 1.7 | (0.0-9.3) | 10 | 6.7 | (3.2-12.3) |
| Prostate | 130 | 0.5 | (0.4-0.6) | 111 | 0.5 | (0.4-0.6) |
| Testis | 26 | 0.9 | (0.6-1.2) | 14 | 0.8 | (0.4-1.3) |
| Penis | 7 | 3.2 | (1.3-6.6) | 14 | 8.5 | (4.6-14.2) |
| Bladder | 28 | 0.9 | (0.6-1.4) | 20 | 0.8 | (0.5-1.3) |
| Kidney | 30 | 0.6 | (0.4-0.9) | 31 | 0.8 | (0.5-1.1) |
| Brain | 16 | 0.6 | (0.4-1.0) | 13 | 0.7 | (0.4-1.2) |
| Thyroid | 20 | 0.7 | (0.5-1.10 | 12 | 0.6 | (0.3-1.0) |
| Hodgkin lymphoma | 184 | 9.1 | (7.8-11) | 145 | 12 | (9.7-14) |
| Nodular sclerosis HL | 50 | 4.9 | (3.7-6.5) | 38 | 6.2 | (4.4-8.5) |
| Mixed cellularity HL | 61 | 15 | (11-19) | 41 | 17 | (12-23) |
| Other HL | 73 | 12 | (9.4-15) | 66 | 16 | (13-21) |
| Multiple myeloma | 31 | 1.6 | (1.1-2.2) | 10 | 0.6 | (0.3-1.2) |
| Lymphocytic leukemia | 15 | 2.7 | (1.5-4.5) | 2 | 0.5 | (0.1-1.9) |
| Myeloid/monocytic leukemia | 49 | 2.5 | (1.9-3.4) | 13 | 1.0 | (0.5-1.6) |
| Mesothelioma | 2 | 0.8 | (0.1-2.9) | 2 | 1.0 | (0.1-3.7) |
| Miscellaneous | 186 | 2.8 | (2.4-3.3) | 220 | 2.5 | (2.2-2.8) |
| Poorly specified | 316 | 7.3 | (6.5-8.7) | 119 | 3.7 | (3.1-4.4) |
| All non-AIDS-defining cancers | 2155 | 1.7 | (1.6-1.8) | 1656 | 1.6 | (1.6-1.7) |
Table 2 notes
Bolded values are significant at P<.05.
Abbreviations: CI, confidence interval; CNS, central nervous system; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; SIR, standardized incidence ratio.
There were 551,326 person-years at-risk for cancer among subjects during years 3-5 after AIDS diagnosis, and 593,796 person-years at-risk for cancer during years 6-10 after AIDS diagnosis.
The overall NHL category includes diffuse large B-cell NHL, Burkitt NHL and other/unspecified NHL. The CNS NHL category includes any type of NHL with ICD-O topography codes C710-C729. The poorly specified category includes cancers of any topography with a poorly specified histology (ICD-O histology codes 8000-8005). The all non-AIDS cancers category includes miscellaneous cancers and excludes both AIDS-defining cancers and poorly specified cancers.
Among non-AIDS-defining cancers (Table 2), risks were elevated in both the 3-5 and 6-10 year periods for cancers of the oral cavity/pharynx, tongue, anus, liver, larynx, lung/bronchus, penis, and for Hodgkin lymphoma. Compared to the general population, risks for anal cancer were especially high (SIRs 27 in the 3-5 year period, 40 in the 6-10 year period). Among Hodgkin lymphoma subtypes, risk was most elevated for the mixed cellularity subtype (SIRs 15 in years 3-5, 17 in years 6-10). Other malignancies for which risk was increased in only one of the two periods after AIDS onset included cancers of the lip and vagina/vulva, multiple myeloma, lymphocytic leukemia, and myeloid/monocytic leukemia (Table 2). In both time periods, risk was lower than in the general population for cancers of the breast and prostate, and during years 3-5 after AIDS for kidney cancer. Overall, for all non-AIDS cancers combined, risk was significantly elevated after AIDS in both years 3-5 (SIR 1.7) and years 6-10 (SIR 1.6).
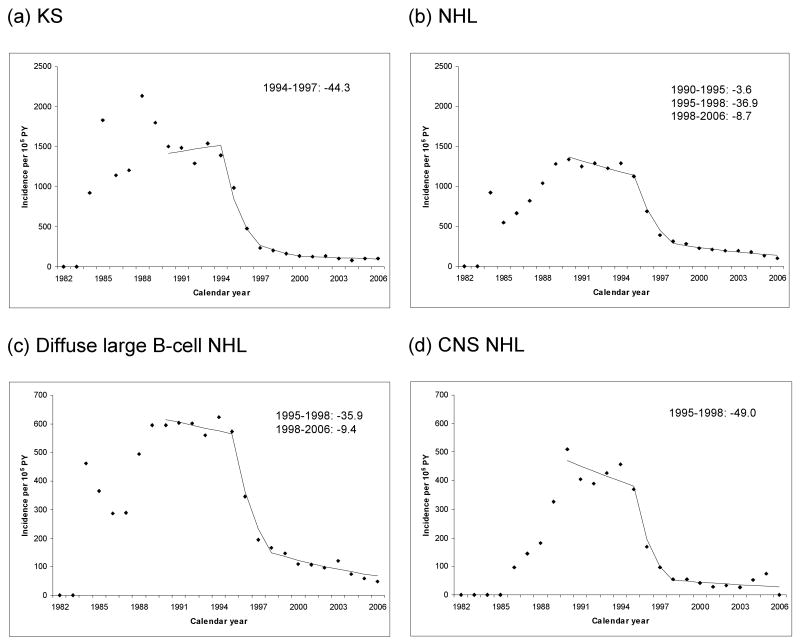
Table 3 compares cancer incidence in the 3-10 years after AIDS for the pre-HAART era (1990-1995) and HAART era (1996-2006). KS incidence declined by 80% (RR 0.2, 95%CI 0.2-0.2) between the pre-HAART and HAART eras. A joinpoint model fitted to the incidence data revealed that this change corresponded to a steep decline during 1994-1997 (44.3% per year, Figure 1A). Similarly, NHL incidence was 70% lower in the HAART era (RR 0.3, 95%CI 0.2-0.3) than in the pre-HAART era, and the joinpoint model indicated that this corresponded to a 36.9% annual reduction during 1995-1998 (Figure 1B). Declines were similar in magnitude for diffuse large B-cell NHL and CNS NHL (Table 3, Figures 1C and 1D). In contrast, the change in Burkitt NHL incidence was weaker (RR 0.6, 95%CI 0.4-1.0), and cervical cancer incidence did not change significantly (RR 0.8, 95%CI 0. 5-1.2). During the HAART era, persons with AIDS continued to have significantly higher risk for all AIDS-defining cancers than people in the general population (SIRs, Table 3).
Table 3. Changes in cancer risk in the HAART era (1996-2006).
| Incidence, per 105 person-years | ||||||
|---|---|---|---|---|---|---|
| Cancer type | Pre-HAART era | HAART era | RR | 95%CI | SIR in the HAART era | 95%CI |
| All cancer | 2968 | 989 | 0.3 | (0.3-0.4) | 3.1 | (3.0-3.2) |
| AIDS-defining cancers | ||||||
| Kaposi sarcoma | 1282 | 190 | 0.2 | (0.2-0.2) | 1584 | (1486-1687) |
| Non-Hodgkin lymphoma | 1226 | 306 | 0.3 | (0.2-0.3) | 15 | (14-16) |
| Burkitt NHL | 22 | 14 | 0.6 | (0.4-1.0) | 26 | (20-32) |
| Diffuse large B-cell NHL | 590 | 156 | 0.3 | (0.3-0.3) | 22 | (20-23) |
| CNS NHL | 414 | 63 | 0.2 | (0.1-0.2) | 592 | (527-662) |
| Other NHL | 614 | 136 | 0.2 | (0.2-0.3) | 11 | (9.8-12) |
| Cervix | 16 | 16 | 0.8 | (0.5-1.2) | 5 | (4.0-6.2) |
| Non-AIDS-defining cancers | ||||||
| Oral cavity/pharynx | 35 | 40 | 1.0 | (0.7-1.4) | 1.8 | (1.5-2.0) |
| Lip | 4 | 2 | 0.6 | (0.2-2.0) | 3.6 | (1.7-6.8) |
| Tongue | 2 | 8 | 2.9 | (1.1-7.7) | 2.3 | (1.6-3.2) |
| Esophagus | 5 | 6 | 0.9 | (0.4-2.0) | 1.4 | (0.9-2.0) |
| Stomach | 5 | 7 | 1.0 | (0.5-2.4) | 1.2 | (0.8-1.7) |
| Small intestine | 1 | 1 | 1.0 | (0.1-11) | 0.4 | (0.1-1.2) |
| Colon/rectum | 24 | 26 | 1.0 | (0.7-1.4) | 0.9 | (0.8-1.1) |
| Anus | 22 | 62 | 2.9 | (2.1-4.0) | 32 | (29-36) |
| Liver | 10 | 23 | 1.9 | (0.9-3.9) | 4.4 | (3.6-5.2) |
| Pancreas | 2 | 6 | 2.2 | (0.4-13) | 1.0 | (0.7-1.4) |
| Larynx | 9 | 15 | 1.3 | (0.8-2.3) | 3.0 | (2.3-3.7) |
| Lung/bronchus | 98 | 100 | 0.8 | (0.6-0.9) | 2.6 | (2.4-2.8) |
| Bones/joints | 0 | 1 | ∞ | (0.2-∞) | 1.1 | (0.3-2.8) |
| Soft tissue | 4 | 4 | 0.9 | (0.3-2.4) | 1.4 | (0.9-2.2) |
| Melanoma of the skin | 10 | 10 | 1.1 | (0.5-2.3) | 1.1 | (0.8-1.4) |
| Breast | 5 | 13 | 1.8 | (1.0-3.2) | 0.7 | (0.5-0.8) |
| Uterus | 1 | 1 | 1.4 | (0.3-7.4) | 0.5 | (0.2-1.0) |
| Ovary | 2 | 2 | 0.5 | (0.2-1.6) | 1.0 | (0.5-1.9) |
| Vagina/vulva | 1 | 3 | 1.8 | (0.4-7.7) | 6.7 | (3.9-11) |
| Prostate | 13 | 30 | 1.6 | (1.1-2.3) | 0.5 | (0.5-0.6) |
| Testis | 5 | 4 | 1.3 | (0.6-2.8) | 0.7 | (0.5-1.1) |
| Penis | 1 | 3 | 3.7 | (0.5-29) | 5.0 | (2.5-8.9) |
| Bladder | 4 | 6 | 1.2 | (0.5-2.9) | 0.9 | (0.6-1.3) |
| Kidney | 6 | 7 | 0.9 | (0.4-2.1) | 0.7 | (0.5-1.0) |
| Brain | 4 | 3 | 0.7 | (0.3-1.9) | 0.6 | (0.3-1.0) |
| Thyroid | 3 | 3 | 1.2 | (0.5-3.0) | 0.7 | (0.4-1.0) |
| Hodgkin lymphoma | 20 | 41 | 2.0 | (1.3-2.9) | 11 | (10-13) |
| Nodular sclerosis HL | 7 | 11 | 1.5 | (0.8-2.9) | 5.0 | (3.7-6.7) |
| Mixed cellularity HL | 5 | 13 | 2.4 | (1.2-5.1) | 21 | (16-26) |
| Other HL | 8 | 17 | 2.0 | (1.1-3.8) | 15 | (12-18) |
| Multiple myeloma | 7 | 4 | 0.5 | (0.2-1.0) | 0.7 | (0.4-1.1) |
| Lymphocytic leukemia | 4 | 1 | 0.4 | (0.1-1.4) | 1.7 | (0.8-3.3) |
| Myeloid/monocytic leukemia | 8 | 7 | 0.8 | (0.4-1.5) | 2.1 | (1.5-2.9) |
| Mesothelioma | 1 | 0 | 0.2 | (0.1-1.1) | 0 | (0-1.5) |
| Miscellaneous | 70 | 62 | 0.8 | (0.5-1.1) | 2.6 | (2.3-2.9) |
| Poorly specified | 111 | 30 | 0.2 | (0.2-0.3) | 3.4 | (2.9-4.0) |
| All non-AIDS-defining cancers | 332 | 448 | 1.2 | (1.0-1.3) | 1.6 | (1.6-1.7) |
Table 3 notes
Data are for the combined period 3-10 years after AIDS onset. During the pre-HAART era (calendar years 1990-1995) there were 166,365 person-years of follow-up, and during the HAART era (calendar years 1996-2006) there were 718,401 person-years of follow-up. The rate ratio (RR) compares incidence between the two periods, adjusted for attained age (15-34, 35-44, 45+ years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic), and gender/mode of HIV exposure (males who reported male-to-male sex [MSM] alone or with injection drug use [IDU], all other males, and females).
Bolded values are significant at P<.05.
Abbreviations: CI, confidence interval; CNS, central nervous system; HAART, highly active antiretroviral therapy; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; SIR, standardized incidence ratio.
The overall NHL category includes diffuse large B-cell NHL, Burkitt NHL and other/unspecified NHL. The CNS NHL category includes any type of NHL with ICD-O topography codes C710-C729. The CNS NHL category includes any type of NHL with topography codes C710-C729. The poorly specified category includes cancers of any topography with a poorly specified histology (ICD-O histology codes 8000-8005). The all non-AIDS cancers category includes miscellaneous cancers and excludes both AIDS-defining cancers and poorly specified cancers.
Figure 1.
Incidence of selected AIDS-defining malignancies as a function of calendar year. The panels show cancer incidence during the period 3-10 years after AIDS onset, as a function of attained calendar year. The points correspond to the individual year estimates, while the lines correspond to results from the joinpoint regression. Annual percentage change is indicated for calendar years where the change was significantly different from zero (P<.05). Panels correspond to (a) Kaposi sarcoma; (b) non-Hodgkin lymphoma; (c) diffuse large B-cell non-Hodgkin lymphoma; (d) central nervous system non-Hodgkin lymphoma. Abbreviation: PY, person-years.
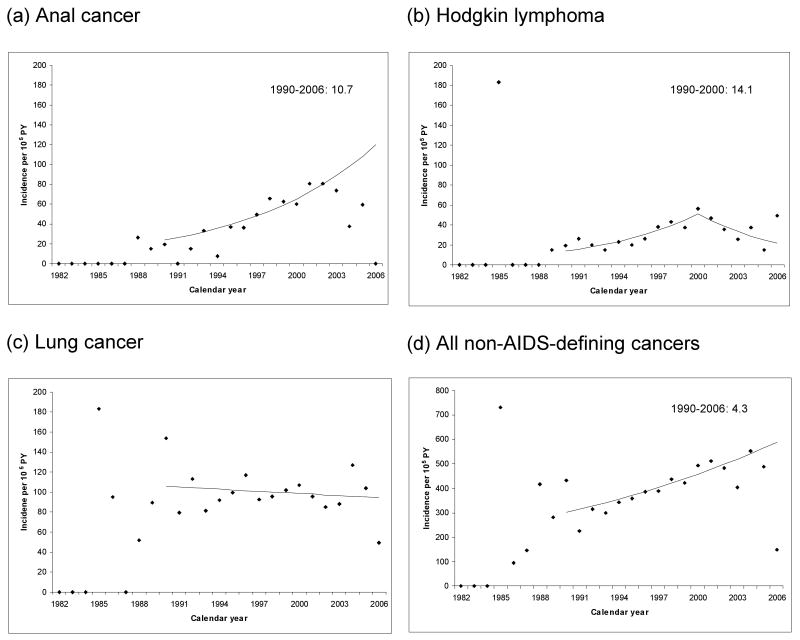
Among non-AIDS-defining malignancies, anal cancer exhibited a three-fold increase in incidence between the pre-HAART and HAART eras (RR 2.9, 95%CI 2.1-4.0, Table 3). During the HAART era, anal cancer risk remained significantly elevated relative to the general population (SIR 32). The joinpoint model for anal cancer demonstrated a 10.7% annual increase in incidence over the entire 1990-2006 period, despite a suggested decline in the most recent years (Figure 2A). In a separate analysis among men, anal cancer incidence increased 11.3% (95%CI 5.9%-16.8%) per year during the HAART era (1996-2006). Incidence of local anal cancer increased significantly (14.1%, 95%CI 7.3%-21.3%) among men during the same years. Regional and distant anal cancer increased annually among men during the same period (5.7%, 95%CI -1.0% to 12.9%), although this increase was not significant. There were no anal cancer cases among women prior to 1996. During 1996-2006, anal cancer increased 4.3% (95%CI -10.5% to 21.5%) per year among women.
Figure 2.
Incidence of selected non-AIDS-defining malignancies as a function of calendar year. The panels show cancer incidence during the period 3-10 years after AIDS onset, as a function of attained calendar year. The points correspond to the individual year estimates, while the lines correspond to results from the joinpoint regression. Annual percentage change is indicated for calendar years where the change was significantly different from zero (P<.05). Panels correspond to (a) anal cancer; (b) Hodgkin lymphoma; (c) lung cancer; (d) all non-AIDS-defining cancers. Abbreviation: PY, person-years.
Hodgkin lymphoma incidence increased significantly between the pre-HAART and HAART eras (RR 2.0, 95%CI 1.3-2.9, Table 3). The Hodgkin lymphoma joinpoint model showed a 14.1% annual increase during 1990-2000, with no significant change subsequently (Figure 2B). The increase in Hodgkin lymphoma incidence was most apparent for the mixed cellularity subtype. During the HAART era, risk of Hodgkin lymphoma remained significantly elevated relative to the general population (SIR 11).
Incidence of lung cancer, the most common non-AIDS-defining cancer, was marginally lower in the HAART era than in the pre-HAART era (RR 0.8, 95%CI 0.6-0.9; Table 3). However, this decline over time was not manifest in the joinpoint model (Figure 2C). There were increases in the HAART era for other less common non-AIDS-defining cancers including cancers of the tongue (RR 2.9) and prostate (RR 1.6).
Overall, the incidence of all non-AIDS cancers increased 20% between the pre-HAART and HAART eras (RR 1.2, 95%CI 1.0-1.3; P=.006). This increase corresponded to a 4.3% annual increase during 1990-2006, although there was a suggestion that incidence declined in the most recent few years (Figure 2D). During the HAART era, people with AIDS had a 60% higher risk for non-AIDS cancers than the general population (SIR 1.6, 95%CI 1.6-1.7).
Comment
This study represents one of the longest and most complete follow-up of persons with AIDS with respect to cancer risk. Our population-based data demonstrated that people with AIDS in the U.S. continue to be at elevated risk for a spectrum of AIDS-defining and non-AIDS-defining cancers years after AIDS diagnosis. While the incidence of KS and NHL has declined substantially in the HAART era, the incidence of non-AIDS-defining cancers overall has risen. Two malignancies in particular, anal cancer and Hodgkin lymphoma, have increased in incidence in recent calendar years.
Among HIV-infected individuals, HAART use has been associated with major decreases in KS and NHL risk, and these decreases have translated into declines in incidence measurable at the population level.2,14,24,25 We demonstrated that while persons with AIDS remain at considerable risk for KS, incidence of this malignancy first started to decline in 1995, possibly due to use of early combination antiretroviral therapy (e.g., dual nucleoside therapy), and continued to decline during the first years when HAART was available. Likewise, steep declines in the incidence of diffuse large B-cell NHL and CNS NHL may be partly explained by introduction of HAART in 1996. HIV-induced immunosuppression, indicated by low CD4 count, is directly related to risk of KS and these NHL subtypes, and declining incidence in people with longstanding AIDS is plausibly linked to the effectiveness of HAART in improving immune function.26 In contrast, we noted a less dramatic decline in the incidence of Burkitt NHL, a subtype whose incidence is not closely associated with CD4 count.26 Despite overall declines in NHL incidence, NHL was the most common malignancy during the HAART era.
Notable excess risks were observed for cancers of anus, penis, cervix, vagina and vulva, and certain sites in the head and neck (oral cavity, pharynx, tongue), all of which can be attributed to persistent infection with oncogenic HPV subtypes.5,27 Anal cancer risk was strongly elevated up to 10 years after AIDS onset and, consistent with other reports,12,28,29 we found that anal cancer incidence increased in the HAART era. This rise was likely not due only to increased anal cancer screening, which has been recommended for HIV-infected MSM,30 because the increasing trend was observed for both men and women, and for regionally advanced disease (which would be detected without screening) as well as for localized disease. Instead, the continued rise of anal cancer incidence in recent years, even in the presence of widespread HAART use, suggests that the key steps susceptible to immune control might have occurred years earlier, and that prolonged survival among people with AIDS has now allowed for the manifestation of invasive cancer. While some investigators have advocated anal cancer screening among HIV-infected people,30,31 it remains controversial.32
A similar explanation related to the long latency of the carcinogenic effects of HPV may explain why cervical cancer incidence in women has not declined in the HAART era. HIV-infected women should be screened annually for cervical cancer.33 A prophylactic HPV vaccine is now available to prevent cervical infection, however its impact will not be realized for several decades, and its efficacy in preventing infection in HIV-positive women or in preventing anal infection is unknown.34
Hodgkin lymphoma risk was also substantially elevated among people with AIDS, and we noted a doubling in Hodgkin lymphoma incidence in this population in the HAART era. Our joinpoint model demonstrated that this increase was largely due to a significant rise throughout 1990-2000. Mixed cellularity Hodgkin lymphoma was the most common subtype and in the context of AIDS, is often associated Epstein Barr virus infection.1,35 Interestingly, Biggar et al. reported that AIDS-related immunosuppression appears to have a non-linear effect on Hodgkin lymphoma risk. Specifically, Hodgkin lymphoma risk increases as the CD4 count declines, reaching a peak when the CD4 count is approximately 225 to 249 cells/μL, and then falls again at very low CD4 counts.35 A subsequent study did not confirm this non-linear relationship.36 Nonetheless, among people with advanced AIDS, it is possible that use of increasingly effective HIV therapies during the HAART era has resulted in a shift in immune function associated with higher Hodgkin lymphoma risk.
Importantly, we observed a 20% overall increase in the incidence of non-AIDS-defining cancers in this population. Because cancer risk was calculated as a rate (i.e., number of events per unit of follow-up time), this rise in incidence cannot be explained simply by a decline in AIDS-related mortality and a consequent increase in time at risk for cancer. The median attained age increased from 33 to 44 years over the 24-year calendar period of the study, reflecting aging of the U.S. AIDS population with improved survival. Nonetheless, our multivariable analyses comparing cancer risk in different calendar periods adjusted for attained age. Thus, the rate ratios that we report correspond to changes in cancer risk that cannot be explained by aging within the cohort. Instead, we believe that the recent overall increase in incidence of non-AIDS-defining cancers is largely driven by the rises in anal cancer and Hodgkin lymphoma incidence noted above. Indeed, when those malignancies were excluded, the change in incidence of all non-AIDS-defining cancers between the pre-HAART and HAART eras was no longer apparent (RR 1.0, 95%CI 0.9-1.1).
Other specific non-AIDS-defining cancers deserve brief comment. Lung cancer was the most frequent non-AIDS-defining malignancy, accounting for 41% of all non-AIDS cancers. While lung cancer incidence declined slightly with widespread HAART use, persons with AIDS remain at higher risk for lung cancer than the general population. Among HIV-infected individuals, the high risk of lung cancer is partly explained by tobacco use, but HIV may amplify the carcinogenic effects of smoking.37 Liver cancer risk was elevated among people with longstanding AIDS, reflecting the carcinogenic effects of hepatitis C and B viruses, and alcohol use.7 Finally, people with longstanding AIDS had a lower risk of prostate and breast cancers than the general population, as has been noted in other studies,16,38 and we found that prostate cancer incidence increased between 1990-1995 and 1996-2006. These patterns could reflect hormonal effects of HIV infection or other factors that may have changed over time, or could partly reflect low rates of screening compared with the general population.38
Strengths of our study include its large size and population-based nature, representing all major U.S. regions and demographic groups affected by the AIDS epidemic, as well as the long period of evaluation of cancer risk (up to 10 years after AIDS onset). While we could not verify that people with AIDS remained in the cancer registry surveillance catchment areas, we adjusted for plausible rates of out-migration. A limitation of our study is that we lacked individual-level data on important cancer risk factors such as smoking and HAART use. Nonetheless, our results accurately reflect the aggregate impact of these factors and changes in their prevalence over time at the population level.
In summary, individuals with AIDS remain at substantially increased risk for cancer for up to 10 years after AIDS onset. Declines in KS and NHL incidence in recent years can be attributed to introduction and wide availability of HAART, but the continuing occurrence of these cancers in people with AIDS points to the need for improvements in access to HAART, more effective regimens targeting drug-resistant HIV strains, and perhaps interventions to boost immune restoration even with HAART use.39-41 Of concern, we also observed an increasing incidence of anal cancer and Hodgkin lymphoma. As persons with AIDS continue to live longer after an AIDS diagnosis and as they age, it is possible that cancer risk will increase further.
Acknowledgments
We thank the staff at the HIV/AIDS and cancer registries at the following locations: Colorado, Connecticut, Florida, Illinois, Georgia, Louisiana, Massachusetts, Michigan, New Jersey, New York, New York, San Diego and San Francisco, California, Seattle, Washington, Texas and Washington, D.C. We also thank Mr. Tim McNeel (Information Management Systems, Rockville, MD) for database management.
Funding/Support: Support for this study was provided by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Footnotes
All authors declare no conflicts of interest.
Dr. Simard had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Goedert JJ, Cote TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;20(351):1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 4.IARC Monographs on the evaluation of carcinogenic risks to humans: Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus 8. Vol. 70. France: Lyon: 1997. [PMC free article] [PubMed] [Google Scholar]
- 5.IARC Monographs on the evaluation of carcinogenic risks to humans: human papillomaviruses. Vol. 90. France: Lyon: 2005. [Google Scholar]
- 6.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Giordano TP, Kramer JR. Does HIV infection independently increase the incidence of lung cancer? Clin Infect Dis. 2005;40:490–491. doi: 10.1086/427028. [DOI] [PubMed] [Google Scholar]
- 9.Chander G, Josephs J, Fleishman JA, et al. Alcohol use among HIV-infected persons in care: results of a multi-site survey. HIV Med. 2008;9:196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li TS, Tubiana R, Katlama C, Calvez V, Ait MH, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–1686. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 11.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;20(148):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 13.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 15.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 16.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. International Classification of Diseases for Oncology. 3rd. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.SEER Cancer Statistics Review, 1975-2002. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 19.Chaturvedi AK, Mbulaiteye SM, Engels EA. Underestimation of relative risks by standardized incidence ratios for AIDS-related cancers. Ann Epidemiol. 2008;18:230–234. doi: 10.1016/j.annepidem.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Buehler JW, Frey RL, Chu SY. The migration of persons with AIDS: data from 12 states, 1985 to 1992. AIDS Mortality Project Group. Am J Public Health. 1995;85:1552–1555. doi: 10.2105/ajph.85.11.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris NS, Dean HD, Fleming PL. Characteristics of adults and adolescents who have migrated from place of AIDS diagnosis to place of death, United States, 1993-2001. AIDS Educ Prev. 2005;17:39–48. doi: 10.1521/aeap.2005.17.Supplement_B.39. [DOI] [PubMed] [Google Scholar]
- 23.U.S.Census Bureau. Domestic net migration in the United States: 2000 to 2004. Available at: http://www.census.gov/prod/2006pubs/p25-1135.pdf.
- 24.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi's sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21 1:S34–41. [PubMed] [Google Scholar]
- 25.Kirk O, Pedersen C, Cozzi-Lepri A, et al. Non-Hodgkin lymphoma in HIV-infected patients in the era of highly active antiretroviral therapy. Blood. 2001;98:3406–3412. doi: 10.1182/blood.v98.12.3406. [DOI] [PubMed] [Google Scholar]
- 26.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;20(99):962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 27.Del Mistro A, Chieco BL. HPV-related neoplasias in HIV-infected individuals. Eur J Cancer. 2001;37:1227–1235. doi: 10.1016/s0959-8049(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 28.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 29.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007;165:1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 30.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;19(281):1822–1829. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 31.Chin-Hong PV, Berry JM, Cheng SC, et al. Comparison of patient- and clinician-collected anal cytology samples to screen for human papillomavirus-associated anal intraepithelial neoplasia in men who have sex with men. Ann Intern Med. 2008;149:300–306. doi: 10.7326/0003-4819-149-5-200809020-00004. [DOI] [PubMed] [Google Scholar]
- 32.Katz KA, Clarke CA, Bernstein KT, Katz MH, Klausner JD. Is there a proven link between anal cancer screening and reduced morbidity or mortality? Ann Intern Med. 2009;150:283–284. doi: 10.7326/0003-4819-150-4-200902170-00020. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 34.The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 35.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clifford GM, Rickenbach M, Lise M, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113:5737–5742. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 37.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;20(24):1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 38.Goedert JJ, Schairer C, McNeel TS, Hessol NA, Rabkin CS, Engels EA. Risk of breast, ovary, and uterine corpus cancers among 85,268 women with AIDS. Br J Cancer. 2006;95:642–648. doi: 10.1038/sj.bjc.6603282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. J Acquir Immune Defic Syndr. 2000;25:115–123. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]