Abstract
Background
The FDA currently recommends at least 4 hours of recipient monitoring to detect early infusion reactions; recent catastrophic reactions to “first in man” biological agents have emphasized the importance of this rule for initial studies of new products. The value of such monitoring for better established agents is less obvious.
Methods
We therefore reviewed infusion-related adverse events (AEs) following administration of ex-vivo expanded T cell products (antigen specific CTLs, allodepleted T cells and genetically modified T cells) on Investigational New Drug (IND) studies in our center.
Results
From 1998 to 2008, we infused 381 T cell products to 180 recipients, enrolled on 18 studies, receiving T cells targeting malignancies or post-transplant viral infections. There were no Grade 3-4 infusion reactions during initial monitoring or 24 hour follow-up. Twenty four mild (grade 1-2) adverse events (AEs) occurred in 21 infusions either during or immediately following infusion (up to 6 hours), most commonly nausea and vomiting (10/24; 41.6%), likely due to the DMSO cryoprotectant, and hypotension (20.8%), attributable to diphenhydramine pre-medication. 22 additional non-severe events were reported within 24 hours of infusion, most commonly culture negative fever, chills and nausea. Increased risk of adverse effects was associated with age (IRR 0.98; 95% CI 0.96-1.00; p=0.05), while an increase risk of immediate infusion-related events was higher in patients reporting allergies (IRR 2.72; 95% CI 1.00-7.40; p=0.05); sex, disease type or T cell source (allogeneic or autologous) had no effect on frequency of adverse events.
Discussion
Hence infusion of T cells is safe in the outpatient setting and associated with no severe reactions, so that monitoring for one hour after infusion is likely sufficient. As many of the AEs were attributable to diphenhydramine premedication, a lower dose (0.25mg/kg) should be selected.
Keywords: T cells, Cytotoxic T Lymphocytes, Epstein-Barr virus, Infusion reaction, Adverse events
INTRODUCTION
T cell therapies can benefit a range of disorders including cancer, viral infections, invasive fungal disease, and autoimmune and allergic disorders(1-9). The number of trials exploring this approach has increased substantially over the past decade and the clinical trials database currently lists 180 approved T cell immunotherapy protocols in 30 countries around the world (10). While clinical toxicities and adverse event profiles have been reported for other cell-based infusion products (11;12), there has been no comprehensive evaluation of infusion reactions after administration of ex-vivo manipulated T cells. Infusion of T cells could mediate a panoply of unintended effects, including fever and nonspecific constitutional symptoms, as a consequence of inflammatory mediator release or cytokine secretion, by transmission of infectious agents or following acute lung injury due to their entrapment in the pulmonary vasculature (13).
The FDA currently recommends at least 4 hours of recipient monitoring following administration of ex-vivo expanded T cells and two recent severe adverse events (AEs) after “first in man” biological agents have emphasized the importance of this rule for initial clinical studies using new products. In the first case, a patient received T cells modified to express a chimeric antigen receptor (CAR) specific to the B cell tumor antigen CD19 that also contained a co-stimulatory moiety (CD28). The patient was lymphodepleted with cyclophosphamide prior to the infusion. Within 48 hours the patient developed fever, hypotension, dyspnea, and renal failure, with negative blood cultures, and progressed to a fatal outcome (14). A second patient received T cells transduced with a chimeric antigen receptor targeting HER2/neu containing both the CD28 and 4-1BB costimulatory domains(15). Within 4 hours of infusion, this patient developed rapidly progressive respiratory distress requiring ventilation(15) and subsequently died. Although these events were rare, they emphasize why a high level of monitoring is required for any first in man study; however the relevance for more established T cell infusion products is not known. We therefore retrospectively studied the incidence and severity of immediate and early adverse events occurring in subjects enrolled on 18 different IND studies of ex vivo manipulated T cell cells over a 10 year period at our institution.
METHODS
Patient Details
We reviewed the charts, infusion records, and adverse event reports of 180 patients who received 381 infusions of ex-vivo expanded T cells on IND studies at the Center for Cell and Gene Therapy at Baylor College of Medicine, The Methodist Hospital and Texas Children’s Hospital in Houston, Texas from January 1, 1998 to November 20, 2008. Included in this study were patients who received antigen-specific cytotoxic T cells, allodepleted T cells, or genetically modified T cells, on 18 FDA-approved investigational new drug studies. The infused T cells targeted malignancies or viral infections after hemopoietic stem cell transplant (HSCT). Cell doses were protocol specific and ranged from 104/kg up to 2 × 108/m2. Patients were premedicated with intravenous diphenhydramine (0.5-1mg/kg, with a maximum dose of 50mg) and Tylenol (10mg/kg up to a maximum dose of 625mg) prior to infusion. All cellular products were cryopreserved using 10% DMSO and administered intravenously over 1-15 minutes immediately after thawing. The types of cellular products are summarized in Table 1.
Table 1.
Summary of Studies
| Study Name | T Cell Product | Dose Range (cells/m2) | Indication |
|---|---|---|---|
| ANGEL NCT00058617 | Autologous EBV-specific CTL | 2 × 107 – 2 × 108 | Relapsed EBV-positive Hodgkin’s disease lymphoma (17) |
| ANGELA NCT00058773 | Autologous EBV-specific CTL | 2 × 107 – 2 × 108 | EBV-positive Hodgkin’s disease post autograft (17) |
| SCAEBV NCT00608608 | Autologous EBV-specific CTL | 1 × 107 – 1 × 108 | Severe chronic active EBV infection (16) |
| EUCLID NCT00058604 | Autologous EBV-specific CTL | 2 × 107 – 1 × 108 | Prevention and treatment of EBV lymphoma following solid organ transplantation (21) |
| NPC | Autologous EBV-specific CTL | 2 × 107 – 2 × 108 | Nasopharyngeal carcinoma (18) |
| CLANC NCT00608257 | Autologous EBV-specific CTL | 2 × 107 – 1 × 108 | EBV-positive nasopharyngeal cancer after lymphodepletion (24) |
| NATELLA NCT00682864 | Autologous LMP1/LMP2 specific CTL | 2 × 107 – 2 × 108 | Nasopharyngeal carcinoma |
| NESTLES NCT00085930 | Autologous 14g2a-zeta-CAR-transduced CTL and 14g2a-zeta-CAR-transduced EBV-specific CTL | 2 × 107 | High-risk Neuroblastoma (23) |
| ALASCER NCT00070226 | Autologous or donor derived LMP2a-specific CTL | 2 × 107 – 1 × 108 | EBV-positive lymphoma, Hodgkin’s Disease lymphoepithelioma, or severe chronic EBV (22) |
| ALCI NCT00671164 | Autologous or donor-derived LMP1/LMP2 specific CTL | 2 × 107 – 1 × 108 | EBV-positive lymphoma, lymphoepithelioma, or severe chronic EBV |
| ACDAL NCT00082225 | Autologous or donor-derived LMP2a-specific CTL | 2 × 107 – 1 × 108 | EBV-positive Hodgkin’s disease or non-Hodgkin’s lymphoma after lymphodepletion |
| ETNA NCT00058604 | Donor-derived EBV-specific CTL | 2 × 107 – 1.2 × 108 | Immune reconstitution in allogeneic/mismatch HSCT recipients (26) |
| LYPTAIST NCT00111033 | Donor-derived adenovirus/EBV-specific CTL | 5 × 106 – 1.35 × 108 | Adenovirus and EBV infection post allogeneic HSCT (25) |
| VICTA NCT00078533 | Donor-derived CMV/adenovirus/EBV-specific CTL | 1 × 107 – 1 × 108 | Prophylaxis of CMV, adenovirus and EBV infection post allogeneic HSCT (20) |
| CHALLAH NCT00711035 | Third party allogeneic adenovirus/EBV/CMV-specific CTL | 2 × 107 | Third party CTLs for persistent reactivation/infection with adenovirus/EBV/CMV after HSCT |
| RFT-DGA NCT00622297 | Donor-derived allodepleted T cells | 1 × 104 – 5 × 106* | Immune reconstitution post-haploidentical stem cell transplant (19) |
| RAFAHS NCT00586274 | Donor-derived allodepleted T cells | 1 × 104* | Immune reconstitution post-haploidentical stem cell transplant for Fanconi anemia (19) |
| HIMRFT NCT00586547 | Allodepleted T cells | 1 × 103 – 1 × 104 * | Immune reconstitution post-reduced intensity haploidentical stem cell transplant (19) |
cell doses are expressed per kg
All patients were treated on IRB approved protocols conducted under INDs after approval by the FDA. If T cells were genetically modified or if they were stimulated by antigen presenting cells that were genetically modified the studies were also reviewed by the Recombinant DNA Advisory Committee of the National Institutes of Health and the Institutional Biosafety Committee. This collated analysis, combining data on infusion safety from all 18 studies was also approved by the IRB at Baylor College of Medicine. Adverse events were collected and graded on case report forms using NCI common toxicity criteria (version 2 or 3 depending on when the study was initiated).
Outcome data from some of these studies have been reported (16-26) but a detailed analysis of infusion-related adverse events has not been previously presented.
Statistical methods
We initially used descriptive statistics (mean, standard deviation, median and range) to analyze the data. All patients who received T-cell infusions were included in the analysis. Adverse event data were summarized in the form of tables. Incidence tables were generated to summarize incidence of patients reporting at least one episode of each specific adverse event and incidence of serious adverse events. The total number of episodes for each event reported, the grade and attribution to study therapy of each episode reported were summarized. The incidence of adverse events was also listed by age group, presence of an allergy and source of T cells, and compared by Fisher’s exact test. The association of the incidence of AE with the type of cells, gender, age at infusion and presence of allergy was then further analyzed by the Poisson regression model using the generalized estimation equation (GEE) to account for the correlation of AE within subjects, and we report estimates of the association and the robust 95% confidence intervals. All p-values are 2-sided, with P < 0.05 considered statistically significant. All statistical analyses used R and STATA 9.0 software packages.
RESULTS
Patient Characteristics
A total of 381 T cell products were given to 180 patients (some studies infused more than two products (23) or had more than one infusion) during the ten year period between 1998 to 2008. Patient characteristics are summarized in Table 2. The types of T cell products infused are summarized in Table 3.
Table 2.
Patient Characteristics
| Characteristic | T cell Recipients |
|---|---|
| Age at Infusion | range 9 mo. to 80.4 y mean 26.6 y median 18.5 y |
| Male:female | 109:71 |
| Ethnicity | |
| White | 86 |
| Hispanic | 41 |
| Asian | 25 |
| Black | 20 |
| Others | 8 |
| Primary Diagnosis | |
| Nasopharyngeal carcinoma | 42 |
| Hodgkin’s lymphoma | 28 |
| AML | 18 |
| Non-Hodgkin’s lymphoma | 16 |
| ALL | 15 |
| Neuroblastoma | 14 |
| Post-solid organ transplant | 11 |
| Severe, chronic EBV infection | 10 |
| Lymphoproliferative disorders | 5 |
| Myelodysplastic syndrome | 4 |
| Beta-thalassemia | 3 |
| Hemophagocytic syndromes | 3 |
| Aplastic anemia | 2 |
| CML | 2 |
| Multiple Myeloma | 2 |
| SCID | 2 |
| Fanconi’s anemia | 1 |
| Lymphoepithelioma | 1 |
| Acute Biphenotypic Leukemia | 1 |
Table 3.
Types of Infusion
| Type of T cell Product | |
|---|---|
| Allogeneic | |
| Adenovirus/EBV/CMV-specific CTLs | 24 |
| Adenovirus/EBV-specific CTLs | 14 |
| EBV-specific CTLs | 13 |
| Allodepleted CTLs | 47 |
| LMP2a-specific CTLs | 14 |
| LMP1/LMP2a-specific CTLs | 4 |
| Autologous | |
| EBV-specific CTLs | 139 |
| CAR-transduced EBV-specific CTLs | 15 |
| CAR-transduced OKT3 blasts | 15 |
| LMP2a-specific CTLs | 44 |
| LMP1/LMP2a-specific CTLs | 52 |
Adverse Events Resulting from the Infusion are Non-Severe
Twenty four grade 1-2 adverse events (AEs) occurred in 21 infusions of 22 ex vivo expanded T cell products (one patient was enrolled on a study where two products were administered), giving an incidence of 6.55%, either during infusion or the immediate post-infusion monitoring period (which lasted between 1 – 6 hours). Figure 1 summarizes the observed events. Figure 2 enumerates an additional 22 events (incidence of reported or observed adverse events occurring in the 24 hour window following T cell infusion). Again, no severe adverse events that were related to the T cell products were noted. Overall, we observed a total of 46 immediate (within 24 hours) non-severe adverse events (12.56%) and no severe adverse events following the infusion of ex vivo manipulated T cells. The most common adverse events were nausea/vomiting and hypotension.
Figure 1. Adverse Events During T Cell Infusion/Post-Infusion Monitoring.
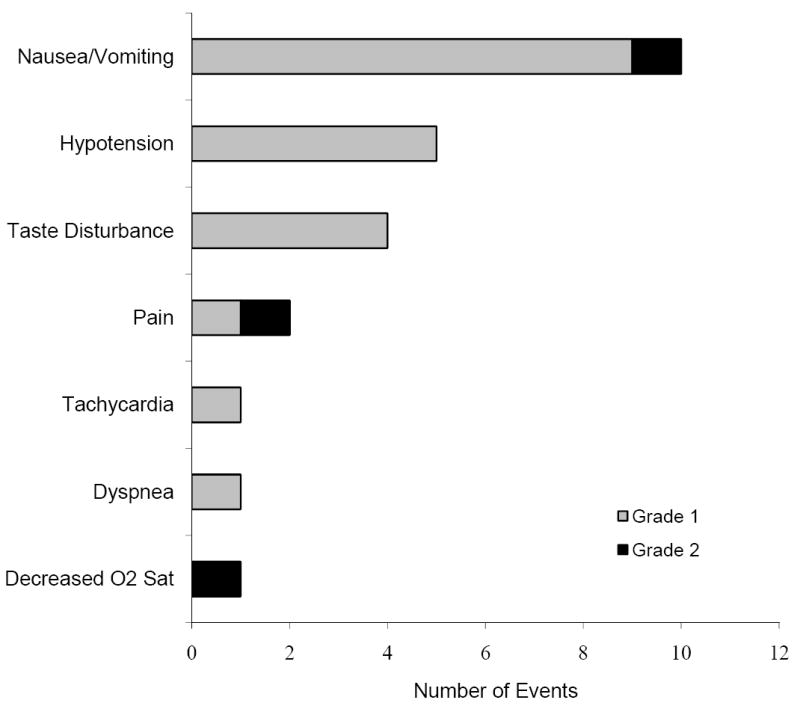
Twenty four adverse reactions during infusion or the immediate post-infusion monitoring period amount to an incidence of 6.5% adverse events in 366 infusions of 381 T cell products. Two grade 3 reactions, 2 grade 2 reactions, and 2 grade 1 reactions were also reported but were considered unrelated to the T cell products. All adverse events that were possibly/definitely related to the T cell infusion were non severe (grade 1-2). Adding the adverse events related to T cell infusion reported within 24 hours results in an incidence of 12.5%.
Figure 2. Adverse Events Reported Within 24 Hours.
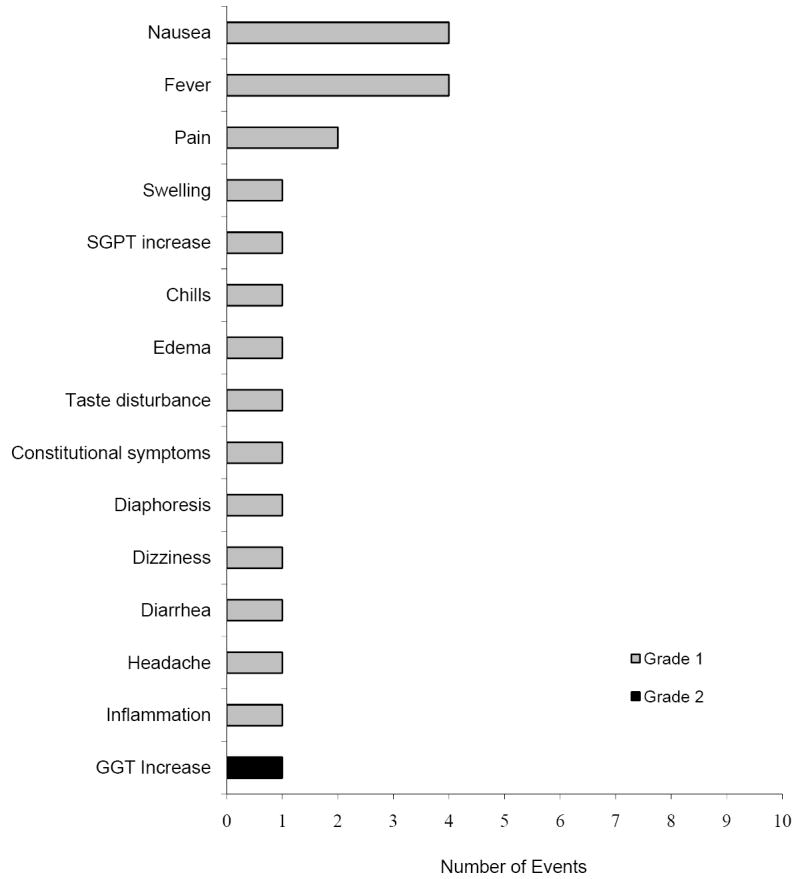
An additional 266 adverse events were reported within a day after infusion. A total of twenty two events possibly or definitely related to the ex vivo manipulated T cell product are listed here (all of which are non-severe/grade 1-2). Both the frequency of each adverse event and the severity of the reaction are noted.
The Majority of Adverse Events are Related to DMSO or Diphenhydramine
All infusions consisted of T cell products suspended in media containing 10% DMSO as a cryoprotectant and the cells were not washed prior to infusions. Subjects received the infusions after premedication with diphenhydramine and acetaminophen, except when contraindicated by the presence of allergies or by concomitant medications that would produce unwanted interactions. As seen in Figure 3, most of the immediate adverse events noted were attributed to DMSO (e.g. nausea/vomiting, taste disturbance) or diphenhydramine (e.g. hypotension, transient hypoxia resulting from sedation).
Figure 3. Adverse Events Attributable to DMSO or Diphenhydramine.
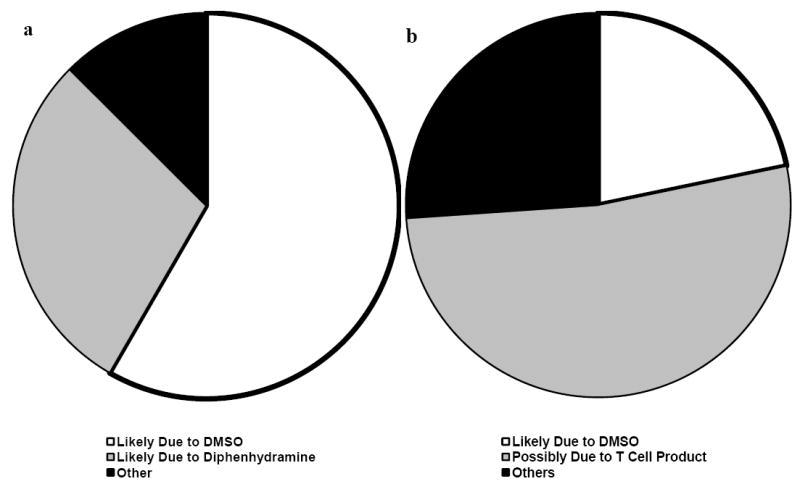
Most of the (a) immediate adverse events seen during infusion and the post-infusion period are attributable to either DMSO or diphenhydramine. There were an additional five adverse events attributable to DMSO seen or reported (b) within 24 hours of infusion, with most events at this time period conceivably resulting from a mild inflammatory process mediated by the T cell products. None of the adverse events were severe enough to be a cause of concern.
Relationship of Adverse Events to Other factors
For analysis, adverse events (AEs) were defined as an event grade 1 or higher, possibly, probably, or definitely related to the T cell infusion. Because all adverse events were non-severe, no attempt was made to distinguish AE grades in the analysis.
Adverse events were first summarized by infusion episode. Fisher’s exact analysis of all T cell product infusions grouped by patient age, patient ethnicity, or cell source did not reveal any associations with increased risks for adverse events. The very young and the very old were no more susceptible to untoward side effects from T cell infusions (Table 4a). Neither the patient’s ethnicity (Table 4b) nor increasing antigenic mismatch between donor and recipient cells (Table 4c & 4d) contributed to a higher incidence of AEs. T cells from both allogeneic and autologous sources resulted in similar rates of adverse events following infusions (Table 4c), and allogeneic cells that were mismatched at more than 2 antigens are no more associated with adverse events than donor T cells matched at 5/6 and 6/6 HLA loci. (Table 4d).
Table 4.
Incidence of Adverse Events Analyzed by T Cell Infusion
| a. Adverse events subgrouped by age | ||||
| No. of AE | Age <18 | Age >18 | ||
| 0 | 152 | 195 | ||
| 92.12 | 90.28 | |||
| 1 | 8 | 16 | ||
| 4.85 | 7.41 | |||
| 2 | 5 | 3 | ||
| 3.03 | 1.39 | |||
| 3 | 0 | 2 | ||
| 0 | 0.93 | |||
| Total | 165 | 216 | ||
| Fisher’s Exact | 0.360 | |||
| b. Adverse events subgrouped by presence of allergies | ||||
| No. of AE | No allergy | With allergy | ||
| 0 | 190 | 157 | ||
| 92.23 | 89.71 | |||
| 1 | 10 | 14 | ||
| 4.85 | 8 | |||
| 2 | 5 | 3 | ||
| 2.43 | 1.71 | |||
| 3 | 1 | 1 | ||
| 0.49 | 0.57 | |||
| Total | 206 | 175 | ||
| Fisher’s Exact | 0.624 | |||
| c. Adverse events subgrouped by T cell source | ||||
| No. of AE | Autologous | Allogeneic | ||
| 0 | 241 | 106 | ||
| 90.94 | 91.38 | |||
| 1 | 17 | 7 | ||
| 6.42 | 6.03 | |||
| 2 | 5 | 3 | ||
| 1.89 | 2.59 | |||
| 3 | 2 | 0 | ||
| 0.75 | 0.93 | |||
| Total | 265 | 116 | ||
| Fisher’s Exact | 0.950 | |||
| d. Adverse events in allogeneic T cell products subgrouped by extent of antigenic mismatch | ||||
| No. of AE | 4/6 | 5/6 | 6/6 | Unspecified |
| 0 | 50 | 6 | 39 | 11 |
| 94.34 | 85.71 | 88.64 | 91.67 | |
| 1 | 1 | 1 | 4 | 1 |
| 1.89 | 14.29 | 9.09 | 8.33 | |
| 2 | 2 | 0 | 1 | 0 |
| 3.77 | 0 | 2.27 | 0 | |
| Total | 53 | 7 | 44 | 12 |
| Fisher’s Exact | 0.458 | |||
Analysis by infusion episodes, however, is not very comprehensive, as AE within a subject are not statistically independent. We thus performed more rigorous data analysis of the data by individual subject. Since the number of AE events from each individual should follow a Poisson distribution, we used a Poisson regression model with a generalized estimating equation (GEE) for correlated count data. We estimated the incidence rate (IR) per subject, and by covariates and in this multivariate Poisson regression model we compared: autologous vs allogeneic stem cell source of T cells, patient gender, age at infusion, and the presence of allergies. We also fitted the Poisson regression model to the level of antigen mismatch for recipients of allogeneic T cells. Stratifying adverse event data by incidence rates per patient results in significantly lower incidences seen as patient age increases. Immediate adverse event rates are also increased in patients with documented allergies. (IRR = incidence rate ratio, AE = adverse event) In our analysis of patients with allergies, we classified patients whose allergy status is undocumented within the allergy group, to make for a more conservative estimate of the effects of allergies and to avoid dropping any patients from our calculations. By this approach we found a significant effect of older age on the probability of both immediate and total adverse events. The presence of allergies also predisposed patients to develop immediate infusion reactions. (Table 5).
Table 5.
Incidence of Adverse Events Analyzed Per Patient
| IRR | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Immediate AE | |||
| Auto vs Allo | 0.27 | 0.07 – 1.11 | 0.07 |
| Male vs Female | 0.59 | 0.23 – 1.52 | 0.28 |
| Age at Infusion | 0.97 | 0.94 – 1.00 | 0.05 |
| Allergy vs No Allergy | 2.72 | 1.00-7.40 | 0.05 |
| AEs After 24 Hours | |||
| Auto vs Allo | 1.32 | 0.34 - 5.05 | 0.69 |
| Male vs Female | 0.49 | 0.19 - 1.27 | 0.14 |
| Age at Infusion | 0.99 | 0.96 - 1.01 | 0.27 |
| Allergy vs No Allergy | 0.77 | 0.31 – 1.87 | 0.56 |
| Total AEs | |||
| Auto vs Allo | 0.58 | 0.22 – 1.54 | 0.28 |
| Male vs Female | 0.56 | 0.29 – 1.11 | 0.1 |
| Age at Infusion | 0.98 | 0.96 – 1.00 | 0.05 |
| Allergy vs No Allergy | 1.41 | 0.73 – 2.71 | 0.31 |
DISCUSSION
Most complex biological products such as ex-vivo expanded T cells are prepared following multi-step procedures – including cell activation, ex vivo expansion using complex media, serum and cytokines, and genetic modification, all of which may increase the risk for subsequent infusion reactions from infection, contamination or hypersensitivity (27). Several studies have previously looked at the safety profile of unmanipulated or minimally manipulated cellular therapies (11;12), but our study was aimed at evaluating the consequences of infusing ex-vivo expanded T cells. Our intent was to provide guidance for immediate safety monitoring after administration of these cellular products. We identified an overall adverse event (AE) incidence rate of 17.3%, and none of these AE’s were severe. This excellent safety record is similar to preliminary experience with other cell-based therapies (28) Of note, the majority of AEs in our analysis could be attributed to either the cryoprotectant (DMSO) or the diphenhydramine used as premedication rather than to the T cells themselves.
Of the adverse events observed, only mild immune/inflammatory responses (constitutional symptoms, mild fever, chills) reported on 10 occasions within 24 hours of T cell infusion are likely mediated by the ex vivo generated T cell products; a similar rate of such symptoms have been reported in studies of other cell products (29). Since we found an increased risk of infusion reactions in recipients with documented allergies, we recommend careful monitoring of those patients who report such predispositions. We observed no differences in adverse event rates if patients are grouped according to cell source used for infusion.
DMSO is a commonly used cryoprotectant, and a variety of methods have been proposed to minimize the adverse effects of this agent. Washing cells prior to infusion is effective, but introduces additional variables into analysis of product functionality that we believed unjustified by the low rate and severity of infusion reactions. Fractionated cell infusions (30) and the use of strawberry flavored lollipops (31) have been shown to decrease the incidence of DMSO-related clinical effects.
The patients in this series received relatively low infusion volumes (0.7mL to 58 mL) and T cell numbers (3 × 105/kg to 2 × 108 cells/m2). While larger quantities, or first in man products may have an entirely different safety profile due, for example, to pulmonary vascular congestion or cytokine storms (14), our experience with these more established, smaller scale infusions, clearly shows immediate safety.
Hence, we propose that one hour monitoring post-infusion, with clear instructions to the patients to report any adverse events they see before the next clinic visit, should be sufficient to maintain this excellent safety record. Indeed, reduction of the dosage of diphenhydramine may reduce adverse events, such as mild hypotension, still further.
Acknowledgments
This work was supported by grants RO1CA061384, PO1 CA94237, P50CA126752 and U54HL081007 from the National Institutes of Health and a SCOR from the Leukemia and Lymphoma Society. We wish to thank all the clinicians involved in care of these patients, the staff in the GMP facilities who manufactured cells and all the research staff who collected data.
Abbreviations
- AEs
Adverse events (AEs)
- CAR
Chimeric antigen receptor
- CTLs
Cytotoxic T Lymphocytes
- DMSO
Dimethyl sulfoxide
- DNA
Deoxyribonucleic acid
- EBV
Epstein-Barr virus
- FDA
Food and Drug Administration
- GEE
Generalized Estimation Equation
- HLA
Human Leukocyte Antigen
- HSCT
Hemopoietic Stem Cell Transplant
- IND
Investigational New Drug
- IRB
Institutional Review Board
Reference List
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008 Apr;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009 Feb 21;373(9664):673–83. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007 Jun;117(6):1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 5.Fujita Y, Rooney CM, Heslop HE. Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant. 2008 Jan;41(2):193–8. doi: 10.1038/sj.bmt.1705906. [DOI] [PubMed] [Google Scholar]
- 6.Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006 Mar 15;107(6):2562–9. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 7.Nicolson KS, Wraith DC. Natural and induced regulatory T cells: targets for immunotherapy of autoimmune disease and allergy. Inflamm Allergy Drug Targets. 2006 Sep;5(3):141–8. doi: 10.2174/187152806778256098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leen AM, Heslop HE. Cytotoxic T lymphocytes as immune-therapy in haematological practice. Br J Haematol. 2008 Oct;143(2):169–79. doi: 10.1111/j.1365-2141.2008.07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkenburg JH, Heslop HE, Barrett AJ. T cell therapy in allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008 Jan;14(1 Suppl 1):136–41. doi: 10.1016/j.bbmt.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Clinical Trials.Gov. June 5; 9 A.D. Available from: URL: http://www.clinicaltrials.gov/ct2/results?term=t+cell+immunotherapy.
- 11.Alessandrino P, Bernasconi P, Caldera D, Colombo A, Bonfichi M, Malcovati L, et al. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant. 1999 Mar;23(6):533–7. doi: 10.1038/sj.bmt.1701609. [DOI] [PubMed] [Google Scholar]
- 12.Cordoba R, Arrieta R, Kerguelen A, Hernandez-Navarro F. The occurrence of adverse events during the infusion of autologous peripheral blood stem cells is related to the number of granulocytes in the leukapheresis product. Bone Marrow Transplant. 2007 Dec;40(11):1063–7. doi: 10.1038/sj.bmt.1705861. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Proposed Approach to Regulation of Cellular and Tissue-Based Products. 1997 February 28; doi: 10.1089/scd.1.1997.6.195. [DOI] [PubMed] [Google Scholar]
- 14.Brentjens RJ, Riviere I, Hollyman D, Taylor C, Nikhamin Y, Stefanski J, et al. Unexpected Toxicity of Cyclophosphamide Followed by Adoptively Transferred CD19-Targeted T Cells in a Patient with Bulky CLL. Molecular Therapy. 2009;17(Suppl 1):S157. [Google Scholar]
- 15.Minutes of the December 1st 2009 RAC meeting. 2009 http://obaodnihgov/rdna_rac/rac_meetingshtml.
- 16.Savoldo B, Huls MH, Liu Z, Okamura T, Volk HD, Reinke P, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002 Dec 1;100(12):4059–66. doi: 10.1182/blood-2002-01-0039. [DOI] [PubMed] [Google Scholar]
- 17.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T Lymphocyte Therapy for Epstein-Barr Virus+ Hodgkin’s Disease. J Exp Med. 2004 Dec 20;200(12):1623–33. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of Nasopharyngeal Carcinoma with Epstein-Barr Virus-specific T Lymphocytes. Blood. 2005 Mar 1;105:1898–904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 19.Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006 Sep 15;108(6):1797–808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Nov;12(10):1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 21.Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006 Nov 1;108(9):2942–9. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007 Oct 15;110(8):2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008 Nov;14(11):1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis CU, Straathof K, Bollard CM, Gerken C, Huls MH, Gresik MV, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009 Mar 12;113(11):2442–50. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplant. Blood. 2009 Aug 21; doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long term outcome of EBV specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2009 Oct 30; doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger SR. Current regulatory issues in cell and tissue therapy. Cytotherapy. 2003;5(4):289–98. doi: 10.1080/14653240310002324. [DOI] [PubMed] [Google Scholar]
- 28.Gastineau DA. Will regulation be the death of cell therapy in the United States? Bone Marrow Transplant. 2004 Apr;33(8):777–80. doi: 10.1038/sj.bmt.1704452. [DOI] [PubMed] [Google Scholar]
- 29.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009 Jun 17; doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 30.Martino M, Morabito F, Messina G, Irrera G, Pucci G, Iacopino P. Fractionated infusions of cryopreserved stem cells may prevent DMSO-induced major cardiac complications in graft recipients. Haematologica. 1996 Jan;81(1):59–61. [PubMed] [Google Scholar]
- 31.Ozdemir E, Akgedik K, Akdogan S, Kansu E. The lollipop with strawberry aroma may be promising in reduction of infusion-related nausea and vomiting during the infusion of cryopreserved peripheral blood stem cells. Biol Blood Marrow Transplant. 2008 Dec;14(12):1425–8. doi: 10.1016/j.bbmt.2008.09.010. [DOI] [PubMed] [Google Scholar]


