Abstract
While human observational studies and animal studies report a neuroprotective role for estrogen therapy in stroke, the multicenter placebo-controlled Women's Health Initiative (WHI) study concluded that hormone therapy increased the risk for stroke in postmenopausal women. The present study therefore tested the hypothesis that estrogen replacement would increase the severity of a stroke-like injury in females when this replacement occurs after a prolonged hypoestrogenic period, such as the menopause or reproductive senescence, but not when given to females that were normally cycling immediately prior to the hormone replacement. Two groups of female rats were used: multiparous females with normal but lengthened estrus cycles (mature adults), and older multiparous females currently in a persistent acyclic state (reproductive senescent). Animals were either used intact, or were bilaterally ovariectomized and immediately replaced with a 17β-estradiol pellet or control pellet. Animals were subject to a forelimb placing test (a test for sensorimotor deficit) and thereafter to middle cerebral artery occlusion (MCAo) by stereotaxic injection of the vasoconstrictive peptide endothelin-1, adjacent to the MCA. One week after stroke, behavioral tests were performed again. Cortical and striatal infarct volume, measured from brain slices, was significantly greater in intact reproductive senescent females as compared to intact mature adults. Furthermore, estrogen treatment to ovariectomized mature adult females significantly reduced the cortical infarct volume. Paradoxically, estrogen treatment to ovariectomized reproductive senescent females significantly increased cortical and striatal infarct volumes as compared to control pellet replaced senescent females. Significant post-stroke behavioral deficit was observed in all groups on the side contralateral to the lesion, while senescent females also exhibited deficits on the ipsilateral side, in the cross-midline forelimb placement test. Using an animal model that approximates the natural ovarian aging process, these findings strongly support the hypothesis that the effectiveness of estrogen therapy in protecting brain health may depend critically on the time of initiation with respect to a female's reproductive status.
Keywords: 17β-Estradiol, Reproductive senescence, Endothelin-1, Middle cerebral artery occlusion, Stroke, Forelimb placing test
1. Introduction
Stroke is a disease of the elderly, the third major cause of death and the second major cause of dementia in the US. Although men are more likely to suffer a stroke, the lifetime risk for stroke is greater in women due to their longer lifespan, and more women are likely to have fatal strokes (reviewed in Bushnell, 2008). A recent study indicates that women in the 45−54 age range, which corresponds to the perimenopause, are at a higher risk for stroke (Towfighi et al., 2007), suggesting that declining levels of ovarian steroid hormones alter the risk for neurovascular disease. Declining levels of ovarian steroid hormones also alter stroke outcomes, such that in postmenopausal women stroke-associated disability and fatality are worse compared to men (Roquer et al., 2003; Niewada et al., 2005; Di Carlo et al., 2003; Hochner-Celnikier et al., 2005). Sex differences in stroke severity have also been reported in the experimental literature. Occlusion of the middle cerebral artery, which is the most typical experimental stroke model, results in a smaller infarct in young adult female rats (Alkayed et al., 1998) and mice (Park et al., 2006) as compared to their age-matched young adult males. Furthermore, this sex difference in post-stroke infarct volume is abolished when the female rats and mice were ovariectomized (Alkayed et al., 1998; Park et al., 2006), underscoring the importance of gonadal steroids in the neuroprotective effect seen in younger females.
In women, estrogen treatment enhances recovery from traumatic brain injury following cerebral ischemia (Paganini-Hill, 1995; Schmidt et al., 1996) and continued use of estrogen reportedly reduces the risk of stroke (Falkeborn et al., 1993; Longstreth et al., 1994; Paganini-Hill et al., 1988). On the other hand, a randomized, double-blind, placebo-controlled trial of 17β-estradiol therapy to post-menopausal women (Women's Estrogen for Stroke Trial; WEST) reported no improvement due to estrogen, and in fact reported an increased risk of fatal stroke and worse neurological outcomes (Viscoli et al., 2001). Similarly, the Women's Health Initiative (WHI) study also reported that estrogen and estrogen ± progestin treatment increased the risk for stroke (Wassertheil-Smoller et al., 2003).
In animal studies, the effects of estrogen treatment are typically tested in ovariectomized young females, and exogenous estrogen treatment to ovariectomized young females prior to stroke injury dramatically reduces infarct volume following focal cerebral ischemia in rats (Simpkins et al., 1997; Dubal et al., 1998; Rusa et al., 1999) mice (Sawada et al., 2000) and gerbils (Jover et al., 2002). Furthermore, post-stroke (Yang et al., 2003) estrogen replacement has been shown to be effective in reducing the size of the infarct. The role of estrogen treatment to older females is less well understood. In older animals, the sex difference in stroke-related lesions (seen in younger animals) is lost (Alkayed et al., 2000). However, estrogen replacement to aged female rats reduces infarct size (Alkayed et al., 2000; Dubal and Wise, 2001; Toung et al., 2004). In these studies, females were approximately 16 months old or between 9 and 12 months old suggesting that these females were likely acyclic, although they were not characterized by vaginal cytology. Acyclic female rats can present a heterogenous profile, with an initial stage of persistent estrous and an eventual progression to a state of persistent diestrus, where plasma estrogen levels are low or undetectable (Huang et al., 1978; Wise and Ratner, 1980; LeFevre and McClintock, 1988). Thus, these previous MCA occlusion studies may represent a mixed population of aging females, with varying plasma estradiol levels. In the present studies, the goal was to examine a defined group of senescent females that had virtually undetectable levels of estrogen, which would be more representative of the postmenopausal female.
Previous work from this laboratory has shown that there are constitutive differences in the forebrain of persistently diestrus older (reproductive senescent) female rats as compared to young normally cycling females, which may alter the risk and severity of stroke. Specifically, the blood–brain barrier is more permeable in these acyclic females, and estrogen replacement decreased barrier permeability in the hippocampus of younger females but, paradoxically, increased barrier permeability in older acyclic females (Bake and Sohrabji, 2004). Additionally, estrogen treatment to reproductive senescent females exacerbated N-methyl-d-aspartate (NMDA)-induced local inflammation in the forebrain as compared to their younger counterparts (Nordell et al., 2003). The present study used an endothelin-1 (ET-1)-induced MCA occlusion model to examine the extent of tissue damage in these two groups at 1 week following post-stroke injury. Our data indicates that ET-1-induced experimental stroke resulted in a cortical–striatal infarct and loss of sensorimotor function assessed by a vibrissae-evoked forelimb placement task, when measured 7 days post-injury. However, the volume of the infarct was significantly larger in reproductive senescent females. Furthermore, estrogen's effect on the lesion size is modulated by the reproductive age of the animal, such that estrogen reduced the size of the cortical infarct in mature adult females but increased the size of the cortical and striatal lesion in the reproductive senescent females.
2. Methods
2.1. Animals
Sprague–Dawley rats were used in these studies. All animals were purchased from Harlan Laboratories (IN), as proven breeders with four pregnancies (6−7 months, 230−320 g; n = 24) or as retired breeder females (9−11 months; 280−350 g, n = 25). Mature and senescent females were smeared daily to determine cycle length or acyclicity. All animals were maintained in a constant 12-h dark:12-h light cycle. Food and water were available ad libitum. Once estrus cyclicity/acyclicity was established, rats were either used intact or were subject to ovariectomy.
2.2. Estrous cycle determination
Vaginal smears were obtained daily between 10:00 and 11:00 a.m. Smears, obtained with a cotton swab, were placed on a slide and later examined under a microscope (Olympus, Leeds Instruments, TX; 20× objective) and staged according to commonly accepted criteria. The estrous cycle has four stages: proestrus (round nucleated epithelial cells), estrus (enucleated cornified cells) metestrus, (proportional numbers of leukocytes and cornified cells) and diestrus (few cells, predominantly leukocytes, with the presence of thick mucus). Smears were obtained over 14−20 day period and rats that persisted in any one stage for 7 days were considered acyclic. Reproductive senescent animals were included in the study if they were in constant diestrus.
2.3. Ovariectomy
Animals were anesthetized with xylazine (200 mg/kg)/ketamine (10 mg/kg), and bilateral ovariectomy was performed using a dorsal midline incision, using our previously established procedures (Jezierski and Sohrabji, 2000). Ovaries were removed and 17β-estradiol pellets (1.0 mg) 60-day time-release or control pellets (Innovative Research, Sarasota, FL) were placed subcutaneously before closing the incision. The time-release pellets were identical to those used in previous studies (Jezierski and Sohrabji, 2001; Nordell et al., 2003) and were designed to maintain a plasma hormone level of 60−80 pg/ml. Previous studies have shown that a stable level of plasma estradiol is maintained at 3, 4 and 6 weeks, although a recent study reports a high supraphysiologic burst of hormone levels soon after pellet implantation (Singh et al., 2008). Controls received an empty pellet consisting of the same matrix material but devoid of hormone. At sacrifice, the uterus was removed, cleaned of fat and weighed. Uterine weights and plasma follicle-stimulating hormone (FSH) levels were used to determine the effectiveness of estrogen replacement.
2.4. Intracerebral transient MCAo
Animals were subject to stereotaxic surgery to occlude the middle cerebral artery (MCAo). Deeply anesthetized animals were placed in a Kopf stereotaxic apparatus and a midline incision was made in the scalp. The scalp flaps were retracted to expose the skull and a small hole was drilled on the left side, at the coordinates given below. MCA occlusion was induced by microinjecting 3 μl of a 0.5 μg/μl solution of endothelin-1 (American Peptide Company INC, CA), as described in published studies (Biernaskie et al., 2001; Luke et al., 2004). ET-1 was injected at the rate of 1.0 μl/2 min, and the syringe was slowly removed 3 min after the injection in order to minimize backflow. The injection was delivered via a 10-μl Hamilton syringe with a 26 s/2 in./2S needle, and directed adjacent to the MCA at stereotaxic coordinates +0.9 mm anterior, +3.4 mm lateral relative to Bregma and at a depth of −8.5 mm from the dural surface. During surgery, the rats were maintained at 37 °C throughout, and respiratory rate and oxygen saturation were constantly monitored using the Mouse Oximeter (STARR Life Sciences Corp., PA) (see Table 1). The ET-1 model causes a gradual constriction of the vessel which last approximately 16 h in the cortex and 7 h in the striatum, hence cerebral blood flow (CBF) is not measured in these animals. Control animals were injected with saline instead of ET-1. Animals were allowed to recover under heat lamps and returned to the animal holding rooms once completely recovered from anesthesia. All animals were monitored daily. For edema analysis, animals were terminated at 24 h, 3, 7 and 14 days after MCAo. For all other analysis, animals were terminated at 7 days post-MCAo.
Table 1.
Mean oxygen saturation and respiratory rate recoirded during MCA occlusion surgery
| Physiological parameters monitored | RS-Intact | MA-Intact | RS-O | RS-E | MA-O | MA-E |
|---|---|---|---|---|---|---|
| Oxygen saturation | 92.58 ± 2.05 | 90.96 ± 1.86 | 89.24 ± 2.75 | 91.49 ± 1.88 | 89.25 ± 2.28 | 88.87 ± 4.81 |
| Respiratory rate | 64.25 ± 3.40 | 60.18 ± 21.92 | 66.58 ± 9.70 | 73.99 ± 17.11 | 58.69 ± 11.16 | 58.87 ± 9.49 |
2.5. Edema analysis
Animals were terminated 1−14 days after MCAo (n = 3−4 for each time point) by anesthetic overdose and the brain was rapidly removed. The brain was dissected as follows: the olfactory bulbs anteriorly and the cerebellum and spinal cord posteriorly were dissected and discarded. The left (occluded) and right (non-occluded) hemispheres were then separated by a midline incision through the corpus callosum. Each hemisphere was weighed and then placed in an oven at 80 °C for 48 h. Thereafter the hemispheres were reweighed and the weights recorded. Water volume in each hemisphere was estimated by the following equation [wet wt – dry wt]/wet wt and the left (occluded) hemisphere value was normalized to the right (non-occluded) hemisphere. A control group injected with saline served as baseline.
2.6. TTC staining
Animals were overdosed on anesthetic and the brains rapidly removed from the skull. Brains were placed in a brain mold (BrainTree, MA) and hand-sectioned into 2-mm coronal slices. Slices within the following rostral and caudal measurements were analyzed: −2.00 mm and +4.00 mm from Bregma, respectively. Slices were collected on ice and then incubated in a 2% TTC solution at 37 °C for 20 min and later post-fixed with 4% paraformaldehyde for 5 min at room temperature. Subsequently, the slices were photographed using a Nikon E950 digital camera attached to a dissecting microscope. Infarct volume was determined from digitized images using the Quantity One software package (Bio-Rad, CA). Typically three such slices were used for analysis. The area of the infarct was measured in all slices as well as the area of the contralateral hemisphere. In each case, the infarct area of two adjacent slices was averaged and then multiplied by the thickness of the slice, and values across all slices were added to derive the volume of the infarct. A similar approach was used to determine the volume of the non-occluded hemisphere. The volume of the infarct was then normalized to the volume of the contralateral (non-occluded hemisphere). The volume of the cortical and striatal infarct was measured separately in each case. To ensure reliable and consistent detection of the infarct zone, images were digitally converted to black and white and magnified, and all traces were performed by the same author (AS).
2.7. Behavioral testing
The vibrissae-elicited forelimb placing test (Woodlee et al., 2005) was used both before and after the MCAo surgery to assess the functional effects of the neurovascular injury in the ovariectomized/estrogen or ovariectomized/control-replaced rats. Animals were subject to same-side placing trials and cross-midline placing trials elicited by stimulating the ipsiand contra-lesional vibrissae. During the same-side forelimb placing trials, the animal was gently held such that all four limbs were free to move. The animal's ipsi-lesional vibrissae were brushed against the edge of a table to elicit a forelimb placing response, which typically consisted of the forelimb ipsi-lateral to the stimulated vibrissae. Ten trials were performed before the same was repeated for the contra-lesional vibrissae.
Cross-midline placing test
In the cross-midline placing trials, the animal was held gently by the upper body such that the ipsi-lesional vibrissae lie perpendicular to the tabletop and the forelimb on that side is gently restrained as the vibrissae was brushed on the top of the table to evoke a response from the contralateral limb and vice versa. Between each trial the animal was allowed to rest all four limbs briefly on the tabletop to help relax its muscles. Trials in which the animal seemed to struggle or make premature forelimb movements were not counted.
The vibrissae-elicited forelimb placing test was carried out before ovariectomy, before MCAo and after MCAo surgeries. A pretest was performed 2 days before ovariectomy to help acclimatize the animal to the testing procedure and this noticeably reduced struggling on the testing day. The schedule was as follows: behavioral test the day before ovariectomy, pretested 3 days before MCAo, and then tested 2 days prior to the MCAo. Animals were allowed to rest the day before surgery. The last testing was performed on the 7th day after surgery. Each testing and pretesting session consisted of 40 trials per animal, 10 trials each for the same-side placing (left and right) and 10 trials each for left and right cross-midline placing. Each trial is rapid and the entire procedure on a single day is approximately 20 min/rat.
Scoring during the trials was done by an experimenter blind to the animal's group assignment, and was based on a 4-point scale. A vibrissae-elicited response of the forelimb that included a brisk forward and upward movement that ended in the paw pads making a flat, full contact with the tabletop was scored as 3. If there was a slow or sluggish movement of the limb forward and upward, resulting in just the toes or claws contacting the tabletop then it was given a score of 2. When there was a limb movement forward but not upward and the paw made no contact with the tabletop then the trial was scored 1. If the limb did not move in response to stimulation then the trial was given a score 0. Trials scored as a 2 or 3 were counted as a successful “placing” and the once scored 1 or 0 were considered unsuccessful (Woodlee et al., 2005).
2.8. Statistical analysis
Time course of edema was analyzed by one-way ANOVA and planned post hoc comparisons. The effect of stroke injury on the forelimb placement test was analyzed by paired t-test (pre-MCAo and post-MCAo). Group differences in the volume of the infarct were analyzed by a two-way ANOVA for age (mature adults and reproductive senescent females) and region (cortex and striatum), the latter coded as a repeated measure. For estrogen treatment studies, group differences were analyzed separately for mature and senescent females by two-way ANOVA coded for estrogen treatment and region (cortex, striatum, as a repeated measure). For the edema assays, a mix of mature and senescent females was used, with three to four animals per time point. For the mature and senescent comparisons, four animals were used per group, and for the estrogen replacement assays, five to seven animals were used per group. All data in histograms is presented as mean ± S.E.M. for each group. SPSS software (Chicago, IL) was used for statistical analysis.
3. Results
3.1. ET-1-induced infarct
Injections of ET-1 resulted in an infarct that was localized to the cortex and striatum while no infarct was seen in animals injected with saline (Fig. 1a). Since the stereotaxic taxic coordinates targeted the proximal MCA, the lesion is similar in topology to one that occurs with intraluminal suture occlusion of the MCA. The edema time-course analysis (Fig. 1b) indicated that fluid accumulation peaked soon after the surgery and decreased thereafter. Fluid accumulation was estimated by the difference between wet weight and dry weight of the stroke-injured hemisphere normalized to the non-injured hemisphere. Fluid accumulation was significantly greater than saline controls at 24 and 72 h (p < 0.05). At 7, 10 and 14 days after ET-1 injections, fluid accumulation was no different from saline controls. All subsequent analysis was performed at day 7 after ET-1 injections. Key physiological measures (respiratory rate and oxygen saturation) were monitored for all animals under surgery and no group differences were observed (Table 1).
Fig. 1.
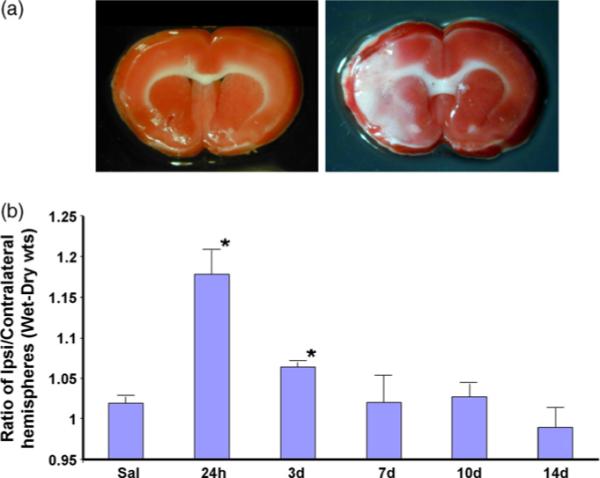
Infarct location and edema in ET-1-induced MCAo. (a) Brain slices, from animals injected with either saline or ET-1 directed towards the MCA, were stained with TTC. Saline injected animals had no visible infarct as seen by the even red stain in both hemispheres, while ET-1 injected animals had unstained areas in the striatum and cortex, indicative of cell loss. (b) Edema analysis: animals were injected with saline or ET-1 and the brains analyzed for fluid accumulation at various time points later. Fluid accumulation, expressed as a ratio of wet wt–dry wt of the left (occluded) and right (non-occluded) hemispheres, was significantly increased in 24 h and 3 days after ET-1 injection. n = 3−4 animals per time point. *p < 0.05.
3.2. Effect of ovarian aging on infarct size resulting from the MCA occlusion
In the first study, mature adults were compared to reproductive senescent animals. Adult rats undergo a normal 4−5 day estrous cycle, and over time, this cycle becomes more irregular and eventually ceases. Reproductive senescent animals were acyclic and in constant diestrus for at least 7 days before the start of the experiment. These animals had 5+ prior pregnancies and were 10−12 months of age at the start of the experiment. Mature adults had 4 pregnancies and were 6−7 months of age. Based on daily vaginal smears, mature adult animals progressed through the various stages of the estrus cycle, and the mean length of the estrus cycle, measured as the length of time (in days) to pass through all stages of the cycle, was 8.17 (±2.64) days.
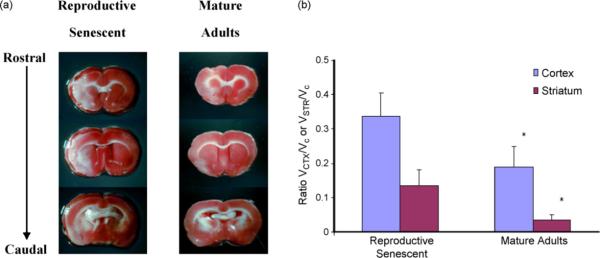
All animals underwent ET-1-induced MCA occlusion and were sacrificed 7 days later. Cortical and striatal infarcts were seen at both ages. Reproductive senescent rats sustained greater cortical and striatal infarcts than mature adult rats following experimental stroke injury (Fig. 2; main effect of age F(1,6)13.11, p < 0.05).
Fig. 2.
Infarct size as a function of reproductive age. Reproductive senescent and mature adult females received stereotaxic injections of ET-1 injections to the MCA. (a) Cortical and striatal lesions were seen in both groups on the left hemisphere as indicated by the regions unstained by TTC. No lesion was seen in the contra-lateral (non-occluded) hemisphere. (b) Quantification of the infarct volume indicated that both the cortical and striatal lesion was significantly greater in the reproductive senescent females as compared to the mature adult females. Histogram depicts mean±S.E.M. of the ratio of the infarct volume to the contra-lateral hemisphere. n = 4 animal per group, *p < 0.05.
3.3. 17β-estradiol replacement studies
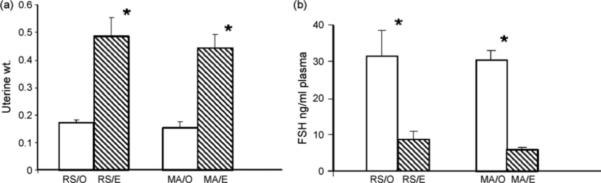
In order to determine the effect of estrogen on infarct size in mature and senescent animals, females in each group (identified as before) were ovariectomized and replaced with estrogen or control pellets. These pellets routinely elevate plasma estradiol levels to 60−80 pg/ml (Jezierski and Sohrabji, 2001; Bake and Sohrabji, 2004). Hormone replacement was confirmed by uterine weights and plasma levels of FSH, which are cumulative indicators of estrogen stimulation. As shown in Fig. 3a, estrogen replacement significantly increased uterine weight of both mature and senescent females (main effect of hormone treatment, F(1,19): 39.23, p < 0.05). The uterine weights of mature and senes-cent females were no different in the ovariectomized and hormone-replaced conditions (F(1,19): 0.005, p > 0.05). High plasma levels of FSH is also a good indicator of hypoestrogenecity and, as shown in Fig. 3b, estrogen replacement also reduced the levels of plasma FSH in both mature and senescent females (F1,15: 34.238, p < 0.05).
Fig. 3.
Estrogen replacement to ovariectomized mature and senescent females. (a) Estrogen replacement (E) to ovariectomized females significantly increased uterine weight as compared to control ovariectomized (O) females in both reproductive senescent (RS) and mature adult (MA) groups (*p < 0.05). (B) Plasma FSH levels were significantly suppressed in ovariectomized animals replaced with estrogen as compared to control ovariectomized animals in both reproductive senescent and mature adult groups. *p < 0.05.
3.4. Infarct size
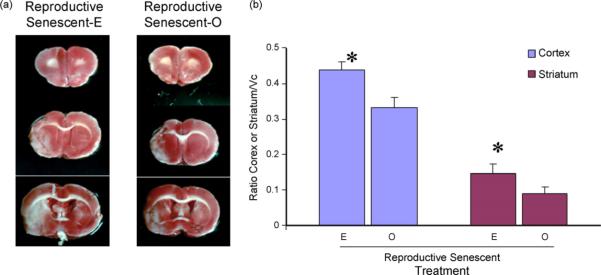
Animals were subject to MCA occlusion at 3 weeks after ovariectomy (and pellet replacement) and survived for 7 days thereafter. The size of the infarct, measured as before, shows that estrogen replacement to reproductive senescent females increases the size of the infarct (Fig. 4a; main effect of hormone, F(1,11): 5.058, p < 0.05). The increased infarct size was not restricted to a specific region; both the cortical and striatal infarct were significantly larger in the estrogen-treated group (F(1,11):373.3, p < 0.05).
Fig. 4.
Effects of estrogen on infarct size in reproductive senescent females: ET-1 injections resulted in cortical and striatal lesions as indicated by the serial TTC-stained sections (a). The size of the infarct in the cortex and the striatum was significantly larger in the estrogen-treated (E) reproductive senescent females as compared to control ovariectomized (O) females. Histogram depicts mean±S.E.M. of infarct volume as a ratio of the contra-lateral hemisphere. n = 5−7 in each group, *p < 0.05.
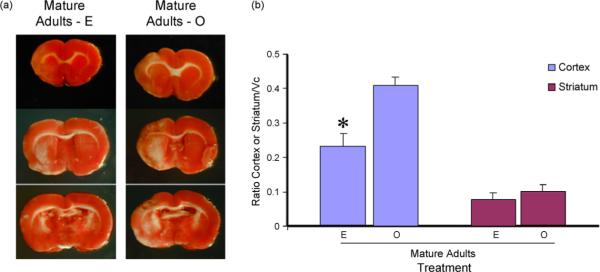
On the other hand, estrogen treatment reduced the infarct size in mature adult females (Fig. 5; F(1,8):10.61, p < 0.05). However, this effect was mainly restricted to the cortex (interaction effect F(1,8): 16.009, p < 0.05); while estrogen had no effect on the proportion of the striatal lesion.
Fig. 5.
Effects of estrogen on infarct size in mature adult females: ET-1 injections resulted in cortical and striatal lesions as indicated by the serial TTC-stained sections (a). The size of the infarct in the cortex was significantly reduced in the estrogen treated mature adult as compared to the control-replaced females. No change was detected in the volume of the striatal lesion. Histogram depicts mean±S.E.M. of infarct volume as a ratio of the contra-lateral hemisphere. n=5 in each group, *p < 0.05.
3.5. Vibrissae-evoked paw-placing reflex
Vibrissae-elicited forelimb placement test was performed prior to ovariectomy, then before stereotaxic surgery and finally at day 7 after surgery. Forelimb placing was completely accurate prior to ovariectomy and prior to stereotaxic surgery, which occurred 3 weeks later. Post-MCA occlusion performance therefore was compared to the score obtained just prior to stereotaxic surgery. The same-side paw-placing reflex task was significantly affected by vessel occlusion in both senescent and mature adult females. In both groups (Fig. 6a and b), animals performed 90% or better before MCAo, but showed a significant loss of paw-placing behavior when the vibrissae contralateral to the lesion side was stimulated after MCAo. No loss of paw-placing behavior was seen when the ipsi-lesional vibrissae was stimulated. Estrogen treatment did not affect forelimb placement at either age group.
Fig. 6.
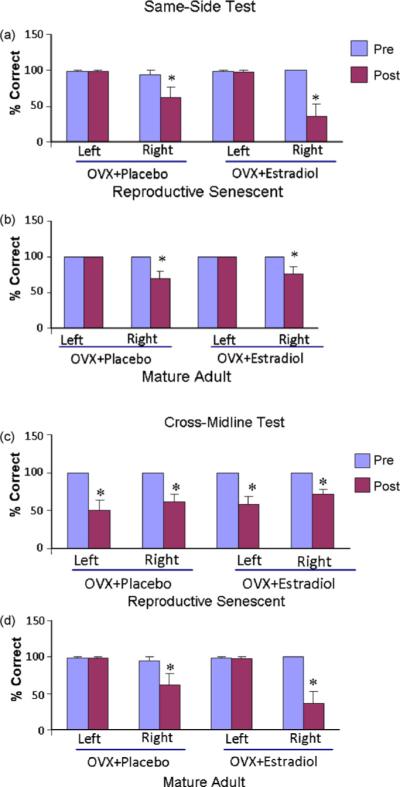
Vibrissae-elicited forelimb placement test in ET-1-induced injury. All groups were tested prior to and after ET-1 injections on the same-side and cross-midline tasks. ET-induced MCA occlusion significantly affected paw placement on the contra-lesional side (*p < 0.05), but not on the ipsilesional side when compared to prior performance in both (a) reproductive senescent and (b) mature adult females. Estrogen replacement did not affect performance in either group. In the cross-midline test, MCA occlusion significantly affected paw placement on the contra-lesional and ipsi-lesional side in the reproductive senescent females (c), while in the mature adult females (d) MCA occlusion significantly affected paw placement on the contra-lesional but not the ipsi-lesional side. *p < 0.05 as compared to the performance prior to the ET-1 injections.
The cross-midline task was also affected by the occlusion however this task revealed an age-related outcome (Fig. 6c and d). As shown in Fig. 6c, in the senescent females, both the ipsi-lesional and contra-lesional paw placement was reduced by 40−60% by MCA occlusion (p < 0.05). In the mature adult group (Fig. 6d), however, there was a significant loss of right forelimb placement (p < 0.05) but no loss of placement of the left forelimb.
4. Discussion
The present study shows that acyclic reproductive senes-cent female rats sustain greater cortical and striatal damage than mature adult females when subject to a middle cerebral artery occlusion. Furthermore, estrogen-replaced reproductive senescent female rats sustained larger cortical and striatal infarcts than their control group, while estrogen-treated mature adults had reduced cortical damage compared to age-matched controls. This data is consistent with other studies that report a neuroprotective role for estrogen following a stroke-like injury in adult female rats (Simpkins et al., 1997; Dubal et al., 1998; Rusa et al., 1999), however it contradicts other studies where middle-aged females were also reported to improve with estrogen treatment (Dubal and Wise, 2001; Alkayed et al., 2000).
In the present study, when intact female rats were subject to MCAo, the reproductive senescent group endured significantly more tissue damage than the mature adult female both in the cortex and the striatum, indicating that the severity of stroke injury is modified by reproductive age. Although several factors may influence this outcome, one strong possibility is that endogenous estrogen functions as a neuroprotective factor. For example, serum estrogen levels are inversely correlated with ischemic stroke damage (Liao et al., 2001) and treatment of female mice with an estrogen receptor antagonist significantly enhanced stroke infarct size (Sawada et al., 2000). Increased cortical and striatal damage has also been reported in intact rats that received aromatase inhibitor following cerebral ischemia (McCullough et al., 2003). Thus, endogenous estrogen levels might be a key factor in protecting the mature adults against tissue damage following MCAo, in contrast to the hypoestrogenic condition in the acyclic females.
The role of estrogen replacement in experimental stroke is well studied and 17β-estradiol has been shown to reduce cell death due to focal ischemia (Simpkins et al., 1997; Dubal et al., 1998) and global ischemia (Hurn et al., 1995; Jover et al., 2002; Shughrue and Merchenthaler, 2003). However, the role of estrogen as a neuroprotectant is not as well studied in acyclic female rats. Estradiol has been reported to reduce infarct size in young and middle-aged females (Dubal and Wise, 2001), however these animals were characterized by age rather than vaginal cytology. Hence, although they are likely to be acyclic (due to advanced age), these animals could be in constant estrus (with measurable estrogen levels) or constant diestrus (a persistent low estrogen state). The type of acyclic stage does matter, as demonstrated in a behavioral study where estrogen replacement was shown to preserve scopolamine-induced cognitive impairment in the older females who were in constant estrus, but was ineffective in older females who were in constant diestrus (Savonenko and Markowska, 2003). The reproductive senescent females in the present study were a more homogenous group since they were characterized as persistently diestrus by daily vaginal smears. A further difference between the previous stroke studies and the current one is the model employed to occlude the MCA. The previous studies used an intraluminal occlusion model with a tightly controlled window of ischemia reperfusion, while the ET-1-induced occlusion (in the present studies) has a more gradual profile, where ischemia occurs over a 12−16-h period (Biernaskie et al., 2001). However, the younger group in this study (the mature adults) was protected by estrogen similar to the widely used intraluminal model, suggesting that the model to produce MCAo may not be the critical variable.
Another likely reason for the difference in outcome of estrogen treatment to senescent females is the duration of post-stroke survival. In both Alkayed et al. (2000) and Dubal and Wise (2001) animals were tested 22−24 h after MCAo, while the present study was tested 7 days post-stroke. This choice was based on the time course of edema analysis where fluid accumulation was noticeably increased 24 h–3 days post-stroke and subsided by 7 days. Ischemia and post-ischemic reperfusion adversely affects the fine network of cerebral capillaries and subsequently alters the blood–brain barrier leading to the formation of edema. In the ET-1 model, tissue swelling occurs as early as 1−2 h after application (Macrae et al., 1993) and, as shown here, is elevated 24 and 72 h later, similar to that observed with the intraluminal suture MCAo model (Tanaka et al., 2007). Hence estimates of tissue damage at early time points after MCAo likely reflect not only cell damage but also tissue distension due to fluid accumulation. Thus our estimate of tissue damage at 7 days post-MCAo is less likely to reflect fluid distension and more representative of tissue damage. An important avenue for future studies is a more focused analysis of estrogen's effects on the temporal resolution of edema and tissue damage. Brain edema is the cardinal complication of ischemic stroke and directly impairs clinical outcomes (Rosenberg et al., 1996). In fact, long-term neurological outcome of up to at least 7 days have been recommended in preclinical experimental studies in addition to acute phase evaluation within 3 days after MCAo (STAIR, 1999; Gladstone et al., 2002) in studies seeking to develop therapeutic interventions. Hence it may be the case that at early time points there is a protective effect of estrogen on both tissue or edema formation, but the resolution of the injury over time is less well regulated by estrogen in older females.
It should be noted, however, that estrogen replacement is not universally neuroprotective. For example, in the intraluminal suture MCAo stroke model, 17β-estradiol replacement increased the volume of the infarct in young adult Sprague–Dawley rats (Gordon et al., 2005), as well as in the normotensive strain WKY (Carswell et al., 2004). Similarly, a brief (5 min) four-vessel occlusion model, produced a much larger hippocampal injury in intact and estrogen-replaced ovariectomized females as compared to ovariectomized females (Harukuni et al., 2001). Furthermore, low physiological levels of 17β-estradiol offered no neuro-protection when given after the onset of an ischemic event (Dubal et al., 1998). In a review, Macrae and Carswell (2006) suggest that the neuroprotective effect of estrogen is more evident in transient models of ischemia but may be less effective in permanent ischemic models. Hence in a severe ischemic injury where the MCA (and the bilateral common carotid) was occluded for 3 h and the tissue examined at 3 days, there were no gender differences in infarct size and no reduction of the infarct due to intravenous or subcutaneous 17β-estradiol (Vergouwen et al., 2000).
The present study underscores the importance of “timing” in the context of estrogen replacement. Estrogen treatment to females before the onset of acyclicity, such as the mature adult female, is neuroprotective, while estrogen treatment to acyclic females, i.e. without recent cyclic exposure to ovarian hormones such as the reproductive senescent females, is deleterious. This is also supported by a recent study where estrogen replacement after a prolonged period of estrogen deprivation (due to ovariectomy) does not protect against focal ischemic injury (Suzuki et al., 2007). While the aforementioned study confirms the importance of timing in estrogen therapy, it differs from the present model where estrogen therapy to the senescent females is actively neurotoxic. This difference is a crucial one in light of the WHI data, where estrogen therapy to postmenopausal women actually increases the risk for ischemic stroke (Rossouw, 2002; Wassertheil-Smoller et al., 2003). Similarly, early and prolonged loss of ovarian hormones due to oophorectomy has been shown to increase the risk for dementia and Parkinson's disease (Rocca et al., 2007a,b). In fact, progressively longer periods of estrogen deprivation (1 week versus 4 weeks of OVX) also increase the size of the infarct (Fukuda et al., 2000).
Although the present study was not designed to address this issue, the mechanism underlying estrogen's dimorphic effects on these two populations (mature and senescent females) may be related to the physiological reorganization that occurs following a prolonged period of hormone deprivation. Our previous studies have shown that estrogen receptor (ER)-alpha is significantly upregulated in the senes-cent female (Jezierski and Sohrabji, 2001). This receptor upregulation might be a response to a declining estrogenic stimulus from the ovaries, but, unintentionally, provides a unique physiological substrate for exogenous estrogen. In a recent study, using a cell line engineered to conditionally express ER-alpha, we reported that at low levels of receptor expression, estradiol treatment was pro-trophic while at high levels of ER-alpha expression, the same concentration of estradiol was no longer trophic and actively suppressed the survival kinases, ERK and Akt (Bake et al., 2008). Thus, one possible mechanism underlying estrogens neurotoxic effects in the senescent female could be due to an interaction between exogenous hormone and supraphysiologic receptor substrates.
The ET-1-induced MCAo model used here was first developed by Sharkey et al. (1993), where this vasoconstrictive peptide is applied/injected adjacent to the MCA, and is a less invasive procedure which nevertheless produces ischemic damage comparable to other MCAo models. Cerebral blood flow reduction which is seen 2−4 h after ET-1 injection, is rapid but not immediate (Macrae et al., 1993) and returns to baseline at 16−22 h post-ET-1 injection (Biernaskie et al., 2001), and, hence, more closely mimics the human stroke profile. Post-surgical complications such as feeding difficul-ties were not observed and mortality is low (Sharkey and Butcher, 1995). Behavioral studies have reported long-term reaching deficits in the staircase task (Marston et al., 1995) and vibrissae-elicited forelimb placement test (Woodlee et al., 2005). The latter test was used as an indicator of stroke injury in the present study. While both mature adults and the reproductive senescent females displayed motor deficits in the contra-lesional arm in both the same-side and cross-midline test post-surgery, irrespective of estrogen treatment, senescent females also showed a functional deficit in the ipsi-lesional arm in the cross-midline test. The latter test is indicative of more severe loss of interhemispheric sensori-motor integration or poorer recovery. Since the tests were performed before MCAo and then 7 days later, the behavioral data cannot distinguish between these two possibilities, but it does underscore the fact that the senescent brain has worse outcomes as compared to the mature adult females. A long-term study indicated that forelimb placing recovered by post-op day 28 in the cross-midline test while it remained impaired up to 128 days in the same-side test (Woodlee et al., 2005). With the growing interest in behavioral recovery after stroke and the possibility of endogenous neurogenesis after ischemia (Liu et al., 1998; Sharp et al., 2002) a long-term testing period would be very valuable for assessing the impact of stroke-type injury on mature adult and senescent groups.
In conclusion, the present studies indicate that reproductive aging impacts the severity of tissue damage in an animal model of stroke and further shows that estrogen's ability to regulate tissue damage is dependent on the reproductive age of the organism. Although this study examines the severity of stroke and not susceptibility or risk, it nevertheless concurs with the findings of the WEST and WHI, where estrogen treatment has more severe vascular consequences in older acyclic females.
Acknowledgements
The authors are grateful to Dr. I. Mhairi MacRae (University of Glasgow, UK) and Dr. Theresa Jones (University of Texas, Austin, TX) for expert advice on the endothelin-1 stroke model. This work was supported by NIH AG19515 and AG028303 to FS.
Footnotes
Disclosure statement
All animal work was performed in accordance with institutional and NIH guidelines for the humane treatment of animals in research.
Conflict of interest
The authors have no financial or personal conflicts of interest related to this work.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17beta-estradiol differentially regulates blood–brain barrier permeability in young and aging female rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Bake S, Ma L, Sohrabji F. ER-alpha overexpression suppresses estrogen-mediated VEGF expression and activation of survival kinases. Endocrinology. 2008;149:3881–3889. doi: 10.1210/en.2008-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn. Reson. Med. 2001;46:827–830. doi: 10.1002/mrm.1263. [DOI] [PubMed] [Google Scholar]
- Bushnell CD. Stroke and the female brain. Nat. Clin. Prac. Neurol. 2008;4:22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J. Cereb. Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, Baldereschi M, Gandolfo C, Candelise L, Ghetti A, Maggi S, Scafato E, Carbonin P, Amaducci L, Inzitari D. Stroke in an elderly population: incidence and impact on survival and daily function. The Italian Longitudinal Study on Aging. Cerebrovasc. Dis. 2003;16:141–150. doi: 10.1159/000070594. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J. Cereb. Blood Flow. Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- Falkeborn M, Persson I, Terent A, Adami HO, Lithell H, Bergstrom R. Hormone replacement therapy and the risk of stroke. Follow-up of a population-based cohort in Sweden. Arch. Intern. Med. 1993;153:1201–1209. [PubMed] [Google Scholar]
- Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, Hall ED. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke. 2000;31:155–160. doi: 10.1161/01.str.31.1.155. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Macrae IM, Carswell HV. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res. 2005;1036:155–162. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Hurn PD, Crain BJ. Deleterious effect of beta-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900:137–142. doi: 10.1016/s0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- Hochner-Celnikier D, Manor O, Garbi B, Chajek-Shaul T. Gender gap in cerebrovascular accidents: comparison of the extent, severity, and risk factors in men and women aged 45−65. Int. J. Fertil. Womens Med. 2005;50:122–128. [PubMed] [Google Scholar]
- Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103:1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Littleton-Kearney MT, Kirsch JR, Dharmarajan AM, Traystman RJ. Postischemic cerebral blood flow recovery in the female: effect of 17 beta-estradiol. J. Cereb. Blood Flow Metab. 1995;15:666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Brain Res. Mol. Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol. Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J. Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol. Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Liao S, Chen W, Kuo J, Chen C. Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci. Lett. 2001;297:159–162. doi: 10.1016/s0304-3940(00)01704-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women. A population-based case–control study. Ann. Intern. Med. 1994;121:168–173. doi: 10.7326/0003-4819-121-3-199408010-00002. [DOI] [PubMed] [Google Scholar]
- Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- Macrae IM, Carswell HV. Oestrogen and stroke: the potential for harm as well as benefit. Biochem. Soc. Trans. 2006;34:1362–1365. doi: 10.1042/BST0341362. [DOI] [PubMed] [Google Scholar]
- Macrae IM, Robinson MJ, Graham DI, Reid JL, McCulloch J. Endothelin-1-induced reductions in cerebral blood flow: dose dependency, time course, and neuropathological consequences. J. Cereb. Blood Flow Metab. 1993;13:276–284. doi: 10.1038/jcbfm.1993.34. [DOI] [PubMed] [Google Scholar]
- Marston HM, Faber ES, Crawford JH, Butcher SP, Sharkey J. Behavioural assessment of endothelin-1 induced middle cerebral artery occlusion in the rat. Neuroreport. 1995;6:1067–1071. doi: 10.1097/00001756-199505090-00029. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol. Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A. Estrogen replacement therapy and stroke. Prog. Cardiovasc. Dis. 1995;38:223–242. doi: 10.1016/s0033-0620(95)80014-x. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Ross RK, Henderson BE. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988;297:519–522. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J. Cereb. Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Ahlskog JE, Elbaz A, Grossardt BR, McDonnell SK, Schaid DJ, Maraganore DM. Risk of cognitive impairment or dementia in relatives of patients with Parkinson disease. Arch. Neurol. 2007a;64:1458–1464. doi: 10.1001/archneur.64.10.1458. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007b;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J. Cereb. Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Rossouw JE. Effect of postmenopausal hormone therapy on cardiovascular risk. J. Hypertens. (Suppl. 20) 2002:62–65. [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182, 780 exacerbates ischemic injury in female mouse. J. Cereb. Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Eber B, Schumacher M, Freidl W. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J. Am. Geriatr. Soc. 1996;44:307–313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Butcher SP. Characterisation of an experimental model of stroke produced by intracerebral microinjection of endothelin-1 adjacent to the rat middle cerebral artery. J. Neurosci. Methods. 1995;60:125–131. doi: 10.1016/0165-0270(95)00003-d. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Ritchie IM, Kelly PA. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J. Cereb. Blood Flow Metab. 1993;13:865–871. doi: 10.1038/jcbfm.1993.108. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Liu J, Bernabeu R. Neurogenesis following brain ischemia. Brain Res. Dev. Brain Res. 2002;134:23–30. doi: 10.1016/s0165-3806(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116:851–861. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Stroke (Dallas) 1999;30:2752. [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Koizumi C, Marumo T, Omura T, Yoshida S. Serum S100B indicates brain edema formation and predicts long-term neurological outcomes in rat transient middle cerebral artery occlusion model. Brain Res. 2007;1137:140–145. doi: 10.1016/j.brainres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J. Cereb. Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Anderson RE, Meyer FB. Gender differences and the effects of synthetic exogenous and non-synthetic estrogens in focal cerebral ischemia. Brain Res. 2000;878:88–97. doi: 10.1016/s0006-8993(00)02713-x. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wise PM, Ratner A. LHRH-induced LH and FSH responses in the aged female rat. J. Gerontol. 1980;35:506–511. doi: 10.1093/geronj/35.4.506. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp. Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann. N. Y. Acad. Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]