Abstract
Purpose
Complement may play a role in the clinical response to rituximab and other monoclonal antibody–based therapies of cancer. The purpose of this study was to explore the relationship between the C1qA[276] polymorphism and the clinical response to rituximab in patients with follicular lymphoma.
Experimental Design
Genotyping for C1qA[276A/G] was done in 133 subjects with follicular lymphoma treated with single-agent rituximab, and correlation with clinical response was done using Cox regression analysis.
Results
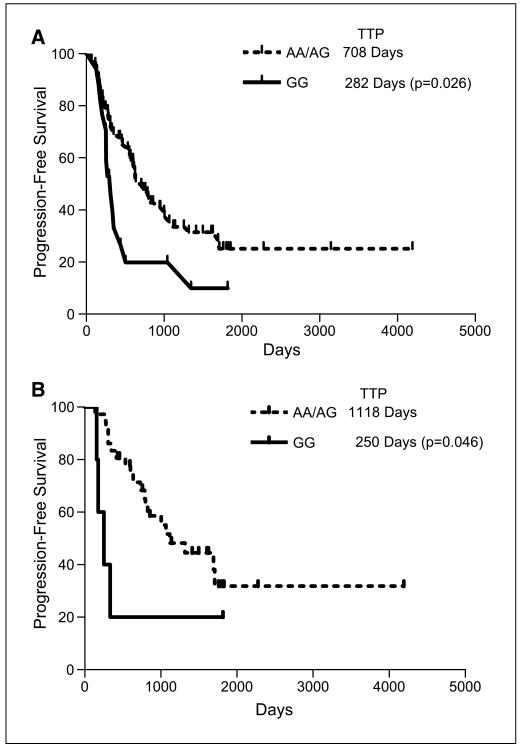
Prolonged remission was observed among subjects that responded clinically to rituximab therapy and were carriers of the A allele compared with homozygous G subjects. Homozygous G subjects had a time to progression of 282 days, whereas A-allele carriers had a time to progression of 708 days [hazard ratio, (HR), 2.5; 95% confidence interval (95% CI), 2.0-3.1; P = 0.02]. Among subjects who achieved complete remission, homozygous G subjects had a time to progression of 250 days, whereas A-allele carriers had a time to progression of 1,118 days (HR, 4.5; 95% CI, 4.1-4.8, P = 0.04).The difference persisted after controlling for CD32 and CD16 polymorphisms. In patients who responded to rituximab used as first-line agent, a linear trend was observed among the C1qA[276] genotypes, with homozygous A subjects achieving complete response at a higher rate compared with heterozygous or homozygous G subjects.
Conclusions
Our findings indicate that polymorphisms in the C1qA gene may affect the clinical response and duration of response to rituximab therapy of follicular lymphoma. These results could have direct implications on designing antibodies with improved efficiency and enhance our understanding of the role of complement in monoclonal antibody therapy.
Rituximab, a chimeric anti-CD20 monoclonal antibody, has become a mainstay in the therapy of B-cell non–Hodgkin's lymphoma since it was introduced in 1997. Used either alone or in combination with other agents, rituximab results in high response rates and some long-term remissions in patients with follicular lymphoma (1–4). The overall response rate in previously treated follicular lymphoma patients receiving rituximab monotherapy is 50% to 60%, including <20% of patients who achieve complete response. Responders have a median time to progression of 12 to15 months (4, 5). Patients who relapse after a first therapy with rituximab may be retreated with comparable response rates and possibly an extended duration of remission (6, 7). When used as first-line therapy for follicular lymphoma, patients' response rates are higher (73%), with about one third of all patients achieving complete response (1, 8, 9).
Despite its certain clinical value, the mechanisms responsible for the clinical antitumor effect of rituximab are not clear. The efficacy of anti-CD20 therapy with rituximab may be mediated through a combination of factors, which include complement activation (10–13). A number of groups, however, have found no evidence that complement is required for the antitumor effect of rituximab in C3- and C4-deficient rodent models (14, 15). Therapeutic activity of rituximab against murine cells expressing human CD20 was absent in syngeneic knockout mice lacking C1q, whereas depletion of natural killer cells, neutrophils, or the use of athymic nude mice did not affect the therapeutic activity of the drug (12). Nevertheless, in this xenograft model, the target cells express little of the complement-neutralizing molecules CD55 and CD59. Furthermore, the level of complement fixation or expression of the complement-neutralizing molecules CD55 and CD59 do not correlate with clinical response to rituximab (16, 17). The importance of antibody-dependent cellular cytotoxicity and natural killer cell activation for therapeutic effect of anti-CD20 antibodies is supported by clinical studies regarding the polymorphisms of Fc receptors and their affinity for monoclonal antibodies (18–20). Finally, the direct cell killing of B cells by anti-CD20 monoclonal antibodies has been reported (21–24). Apoptosis induced by anti-CD20 monoclonal antibodies, however, remains a controversial issue. Other reports suggest that many B-cell lines or primary lymphoma cells are insensitive to rituximab direct killing (13, 25).
Translational Relevance
Rituximab, a chimeric anti-CD20 monoclonal antibody that binds both malignant and normal B cells, is widely used as a component therapy for B-cell lymphoma and chronic lymphocytic leukemia. Despite its certain clinical value, the mechanisms responsible for the antitumor effect of rituximab remain unclear. Both antibody-dependent cellular cytotoxicity and complement fixation seem to play a role. We recently showed that the fixation of complement by rituximab-coated B cells limits natural killer cell activation and antibody-dependent cellular cytotoxicity. Both C1q and C3 were required for this inhibitory effect. Here we show that a polymorphism located in the second exon of the C1qA gene associates with duration of response to single-agent rituximab in patients with follicular lymphoma. In particular, subjects homozygous for the C1qA[276] G allele, that our preliminary data indicate may correlate with higher serum levels of C1q, had a significantly shorter time to progression than A carriers. In addition to suggesting a potential use for the C1qA[276A/G] polymorphism as an outcome predictor in rituximab therapy of follicular lymphoma, these results could have a significant impact on our understanding of the role of complement in immunotherapy, and, in turn, could make possible the selection of monoclonal antibodies with engineered function and improved clinical response.
Until recently, the primary function of C1q was felt to be the activation of the complement cascade in the presence of immune complexes. We now know that C1q, formed by six trimers of A, B and C chains, is a versatile molecule that can bind to a variety of immune and nonimmune molecules (26). C1q has been shown to play an important role in the clearance of apoptotic bodies (27–30). In addition, specific surface receptors for C1q, C1qR, are expressed on a wide range of cell types (31). Those individuals who are congenitally deficient in C1q have a high incidence of autoimmune disorders (32–35). Initially thought to be the effect of high consumption in circulating immune complexes, low levels of complement and C1q component were more recently shown to rather cause the specific pathologic processes of autoimmune diseases (34–37). We previously reported that individuals with the A allele at the C1qA[276A/G] polymorphism have lower C1q protein levels, and a higher incidence of subacute cutaneous lupus erythematosus (38). Our results have been recently confirmed in an independent study among patients with congenital complement deficiencies (39).
The induction of autoimmunity and the induction of an active antitumor response have some similarities (40–43). Because complement seems to be a critical modulator of the immune system's tolerance to self-antigens (44), genetic polymorphisms that correlate with altered complement deficiencies and autoimmunity are logical targets for association studies in clinical outcome of cancer. Indeed, we observed in patients with breast cancer a significant correlation between C1qA gene polymorphisms and the rate of tumor dissemination (45). These data are consistent with the hypothesis that less active complement may result in enhanced cellular immunity and improved clinical outcome.
Taken together, these results suggest a number of hypotheses on how a C1qA polymorphism might have impact on response to rituximab therapy. Individuals with the C1qA[276] A allele could have lower levels of complement-mediated lysis, and therefore a poorer response rate to rituximab. Alternatively, impaired clearance of apoptotic tumor cells due to inefficient complement opsonization in individuals with C1qA[276] A allele could lead to improved cellular immunity against the lymphoma and therefore result in prolonged response to therapy. To begin assessing these hypotheses, we evaluated whether the C1qA[276] polymorphism correlates with either response rate or duration in patients with follicular lymphoma treated with rituximab monotherapy.
Materials and Methods
Study population
The C1qA[276A/G] genotype was determined in 133 subjects with follicular lymphoma treated with rituximab monotherapy at the Stanford Medical Center, the Mayo Clinic, and the University of Iowa from 1993 to 2007. The 84 patients treated at the Stanford Medical Center represent the population previously explored for the impact of CD16 polymorphisms on response to rituximab (20). The 33 subjects treated at the Mayo Clinic were part of a study conducted in the North Central Cancer Treatment Group (8). The University of Iowa patients represented all follicular lymphoma patients treated with single-agent rituximab monotherapy for whom sera and detailed response and follow-up data were available. Sixty-two patients received rituximab as their first-line therapy. Seventy-one patients received rituximab for relapsed lymphoma after prior chemotherapy. A prerequisite for inclusion in this study was that the subjects did not receive chemotherapy within two months before initiation of the rituximab therapy and no maintenance therapy was administered. Patients received four weekly infusions of 375 mg/m2 rituximab monotherapy using standard infusion guidelines. The study was conducted according to protocols approved by the institutional review boards at the University of Iowa, the Mayo Clinic, and Stanford University, and informed consent was obtained from all patients before blood sample collection.
Assessment of clinical response and duration of response to rituximab
Pretreatment staging consisted of physical examination; complete blood count; blood chemistry including lactic dehydrogenase; computed tomography scans of the chest, abdomen and pelvis; and bone marrow aspiration and biopsy. Follicular Lymphoma International Prognostic Index scores were not calculated as the preponderance of patients were treated at time of relapse. Rituximab response was determined by physical examination, computed tomography scanning, and pathology for bone marrow involvement if previously involved between 1 and 3 mo after last rituximab administration, and every 3 mo for the first year and every 6 mo thereafter until progression. The clinical response was then scored according to the International Workshop Response Criteria (46). The median length of follow-up after rituximab was 53 mo. Duration of response was defined as the time interval in days from the end of the rituximab therapy until progression of the disease or the last follow-up in patients who remained in partial or complete remission. Clinical management and assessment of disease status was done by individuals blinded to the C1q genotype.
Specimen collection and processing
Peripheral blood samples from all lymphoma patients were obtained before the initiation of rituximab therapy. Genomic DNA was purified using the PAXgene Blood DNA extraction kit according to the manufacturer's instructions (Qiagen).
Analysis of C1qA[276A/G] polymorphism
Determination of the C1qA[276A/G] polymorphism was done blindly on coded specimens by restriction-fragment length polymorphism analysis as previously described (38). Briefly, the genomic DNA region containing the polymorphism was amplified by PCR and the amplicon purified with available commercial PCR product purification kits. Restriction-fragment length polymorphism analysis was done by enzymatic digestion with ApaI restriction endonuclease (New England Biolabs). Restriction digest fragments were separated in 2.5% agarose gels.
Statistical analysis
Subjects who had the 276 A/A or A/G genotype were designated as A carriers. Genotype and clinical response were compared using Cox regression analysis. The clinical responses of subjects with various C1qA[276] genotypes were compared using two-sided Fisher's exact test. In addition to the C1qA[276A/G] polymorphism, CD16 and CD32 polymorphisms were considered and evaluated. Time to relapse curves were estimated using the Kaplan-Meier method. This study was designed as a retrospective cohort study. The time of entry considered in the statistical analysis was the date of completion of rituximab treatment. Time to relapse was measured in days from the completion of therapy to disease progression or most recent follow-up for patients still in remission. Cox regression was used to estimate the odds ratio of relapse after monoclonal antibody therapy associated with the C1qA[276] genotype. P values from Cox regression were based on the likelihood ratio test. Ninety-five percent confidence intervals (95% CI) for odds ratios were based on the normal approximation. Statistical analysis and the Kaplan-Meier curves were generated using the GraphPad Prism version 4 software (GraphPad Software Inc., 2003). A Cox proportional hazards regression model was used for multivariate analysis of complement, CD16 and CD32 polymorphisms (R Foundation for Statistical Computing, 2007). The correlation between the complement and the CD16 and CD32 polymorphisms was estimated with Fisher's exact test.
Results
Patient characteristics
The demographics and general characteristics of patients in this study are summarized in Table 1. Enrolled in the study were 67 female and 66 male patients. The median age at diagnosis was 54 years. Ninety-five percent of patients had grade 1 or 2 disease, and 97% had advanced stage. Bone marrow was involved by lymphoma in 97 patients (75%) at the time of treatment. No correlation was observed between gender, age at diagnosis, histology, bone marrow involvement, or clinical staging and the C1qA[276A/G] polymorphism. The frequency of the C1qA[276A] allele among all patients with lymphoma was 0.56, whereas the frequency of the C1qA[276G] allele was 0.44. Thirty-nine patients (29%) were homozygous A, 24 patients (18%) were homozygous G, and 70 patients (53%) were heterozygous. The average heterozygosity computed from the variation data obtained from the study of 260 chromosomes in the National Center for Biotechnology Information SNP database shows that for the C1qA[276A/G] polymorphism the Hardy-Weinberg probability is 0.8 with an average of 0.561 for C1qA[276A] and 0.439 for C1qA[276G]. Thus, the overall allelic distribution in follicular lymphoma patients was not statistically different from the allelic distribution in the general population. In addition, the follicular lymphoma population enrolled in this study was in Hardy-Weinberg equilibrium with regard to the C1qA[276] polymorphism.
Table 1.
Patient characteristics according to C1qA[276]polymorphism
| Characteristic | C1qA[276] genotype | All patients (%) | ||
|---|---|---|---|---|
| AA (%) | AG (%) | GG (%) | ||
| Sex | ||||
| Female | 20 (51) | 37 (53) | 10 (42) | 67 (50) |
| male | 19 (49) | 33 (47) | 14 (58) | 66 (50) |
| Age, y | ||||
| <65 | 25 (64) | 51 (73) | 17 (71) | 93 (70) |
| >65 | 14 (36) | 19(27) | 7 (29) | 40 (30) |
| Pathology | ||||
| Grade 1 (FSC) | 23 (59) | 44 (63) | 19 (79) | 86 (64) |
| Grade 2 (FM) | 16 (41) | 21 (30) | 4 (17) | 41 (31) |
| Grade 3 (FLC) | 0 (0) | 5 (7) | 1 (4) | 6 (5) |
| Stage | ||||
| II | 1 (3) | 2 (3) | 1 (4) | 4 (3) |
| III | 10 (26) | 13 (19) | 7 (29) | 30 (23) |
| IV | 28 (71) | 55 (78) | 16 (67) | 99 (74) |
| Bone marrow biopsy | ||||
| Negative | 10 (26) | 15 (22) | 7 (30) | 32 (25) |
| Positive | 28 (74) | 53 (78) | 16 (70) | 97 (75) |
Abbreviations: FSC, follicular small cleaved cell lymphoma; FM, follicular mixed small and large cell lymphoma; FLC, follicular large cell lymphoma.
Response rate to rituximab therapy according to the C1qA[276] genotype
We found no association between the C1qA[276] polymorphism and the overall response rate (complete response, complete response/unconfirmed, partial response) to rituximab (Table 2). Among 39 patients with homozygous A genotype, 27 (69%) responded to rituximab, whereas 46 of 70 heterozygous (66%) and 17 of 24 homozygous G patients (71%) responded to rituximab monotherapy used either first line or after chemotherapy relapse. In patients who received rituximab as first-line therapy, 15 of 19 (79%) homozygous A, 28 of 36 (78%) heterozygous, and 6 of 7 (86%) homozygous G patients responded to treatment. In patients who received rituximab at relapse following chemotherapy, 12 of 20 (60%) homozygous A, 18 of 34 (53%) heterozygous, and 11 of 17 (65%) homozygous G patients responded to monoclonal antibody treatment.
Table 2.
Response rate to up-front rituximab and rituximab as second-line agent
| Therapy | Best response to rituximab | C1qA[276] genotype | |||
|---|---|---|---|---|---|
| AA (%) | AG (%) | GG (%) | Total (%) | ||
| Up-front | Complete response (CR, CRu) | 10 (53) | 12 (33) | 1 (14) | 23 (37) |
| Partial response (PR) | 5 (26) | 16 (45) | 5 (71) | 26 (42) | |
| Overall response | 15 (79) | 28 (78) | 6 (86) | 49 (79) | |
| Total | 19(100) | 36 (100) | 7 (100) | 62 (100) | |
| Second-line | Complete response (CR, CRu) | 4 (20) | 8 (24) | 4 (24) | 16 (23) |
| Partial response (PR) | 8 (40) | 10 (29) | 7 (41) | 25 (35) | |
| Overall response | 12 (60) | 18 (53) | 11 (65) | 41 (58) | |
| Total | 20 (100) | 34 (100) | 17 (100) | 71 (100) | |
| Overall | Complete response (CR, CRu) | 14 (36) | 20 (29) | 5 (21) | 39 (29) |
| Partial response (PR) | 13 (33) | 26 (37) | 12 (50) | 51 (39) | |
| Overall response | 27 (69) | 46 (66) | 17 (71) | 90 (68) | |
| Total | 39(100) | 70 (100) | 24 (100) | 133 (100) | |
Abbreviation: CRu, complete response/unconfirmed.
However, there was a trend toward a higher percentage of homozygous A patients achieving a complete response when compared with heterozygous or homozygous G subjects. Overall, 14 of 39 (36%) homozygous A, 20 of 70 (29%) heterozygous, and 5 of 24 (21%) homozygous G patients achieved a complete response among patients who responded to rituximab. The trend seems to be largely due to a better complete response rate among patients who received up-front monoclonal antibody therapy. In this group, 10 of 19 (53%) homozygous A and only 12 of 36 (33%) heterozygous and 1 of 7 (14%) homozygous G subjects achieved a complete response to rituximab. A χ2 test for trend analysis showed a trend for A carriers to achieve a complete response after up-front rituximab therapy at higher rates than G carriers (χ2, 3.7; P = 0.053). When rituximab was given to patients who relapsed after standard chemotherapy, the fraction of subjects who achieved complete response was similar regardless of the C1qA[276] genotype: 33%, 44%, and 36% for the A/A, A/G, and G/G genotypes, respectively.
Relapse rate after rituximab therapy correlates with C1qA[276] genotype
During the first year after completion of rituximab therapy, subjects were evaluated at 3, 6, 9, and 12 months. At 3 months after completion of therapy patients were evaluated for best response and at later time points for relapse. The relapse rate results for the first follow-up year are summarized in Table 3. We observed that the rate of relapse was significantly higher in homozygous G subjects than in carriers of the A allele. In the first year of follow-up, 12 of 17 (71%) homozygous G patients relapsed, whereas only 21 of 73 (29%) of carriers of heterozygous and homozygous A subjects did so (P = 0.002). The correlation between the C1qA[276] genotype and the relapse rates after rituximab was more evident among patients who achieved a complete response after rituximab. In this group, 4 of 5 (80%) homozygous G patients relapsed by the end of the first year, whereas only 6 of the 34 (18%) A-allele carriers did so. The rates of relapse were significantly higher for homozygous G patients at earlier time points as well: 40% versus 3% at 6 months and 60% versus 3% at 9 months for GG versus A carriers, respectively. No significant difference in the relapse rates has been observed between homozygous A and heterozygous subjects.
Table 3.
Relapse rate after rituximab therapy according to C1qA[276] genotype: first year follow-up
| Best response→ | All responders | P* | Complete responders | P* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A carrier | GG | A carrier | GG | |||||||||||
| Interval after mAb therapy (mo) | Relapsed | Ongoing | % | Relapsed | Ongoing | % | Relapsed | Ongoing | % | Relapsed | Ongoing | % | ||
| 1-3 | 0 | 73 | 0 | 0 | 17 | 0 | 0 | 34 | 0 | 0 | 5 | 0 | ||
| 6 | 8 | 65 | 11 | 3 | 14 | 17 | 0.43 | 1 | 33 | 3 | 2 | 3 | 40 | 0.04 |
| 9 | 14 | 59 | 19 | 7 | 10 | 41 | 0.06 | 1 | 33 | 3 | 3 | 2 | 60 | 0.004 |
| 12 | 21 | 52 | 29 | 12 | 5 | 71 | 0.002 | 6 | 28 | 18 | 4 | 1 | 80 | 0.01 |
Abbreviation: mAb, monoclonal antibody.
Two-sided Fisher's exact test, comparing homozygous G with A carrier (AA+AG). Percentages represent % relapsed from all patients under clinical evaluation at each time point.
Duration of response to rituximab correlates with C1qA[276] genotype
We next examined the relationship between the C1qA[276A/G] polymorphism and the overall duration of rituximab response (Fig. 1). The A carriers who responded clinically to rituximab therapy had a median progression-free survival of 708 days versus 282 days for the homozygous G subjects [hazard ratio (HR), 2.5; 95% CI = 2.0-3.1; P = 0.026]. Among those subjects who achieved complete remission, the A carriers had time to progression of 1,118 days whereas G/G subjects had a time to progression of 250 days (HR, 4.5; 95% CI, 4.1-4.8; P = 0.046).
Fig. 1.
Overall progression-free survival in C1qA[276] G homozygous versus A carrier subjects with follicular lymphoma who responded to rituximab treatment. Progression-free survival log-rank curves were plotted by C1qA[276] AA and AG versus GG genotype. International Workshop Response Criteria were used to estimate complete clinical remission after one round of anti-CD20 therapy. A, patients who achieved partial or complete response (PR, CR, CRu). B, patients who achieved complete response (either CR or CR/unconfirmed).TTP, time to progression.
Multivariate analysis for C1qA[276A/G], FcγRIIIa (CD16) and FcγRIIa (CD32) polymorphisms
We used the Cox proportional hazards regression to evaluate whether the C1qA[276] polymorphism (A carrier versus GG homozygous) is an independent prognostic factor from the CD16 (158 valine V or phenylalanine F) and CD32 (131 histidine H or arginine R) polymorphisms. Both CD16VV and CD32HH genotypes have been found to associate with higher affinity to human IgG and improved response rates and duration of response to rituximab therapy. When only the C1qA[276] polymorphism is included in the Cox model, the A carrier significantly reduces the risk of relapse in responders by 50% (HR, 0.5; P = 0.026). When C1qA[276] and either CD16 or CD32 polymorphisms are included in the model, the A allele still reduces the risk of relapse by 50% (P = 0.025 or 0.026, respectively). The results remain unchanged when all three polymorphisms are included in the model (P = 0.024). We also used the Cox regression to examine whether CD16 and CD32 are associated with relapse time in the current data set. The results suggest that the CD16VV and CD32HH tend to reduce the risk to relapse, but the effects are not statistically significant in this population (HR, 0.76; P = 0.37 for CD16; and HR, 0.68; P = 0.22 for CD32, respectively).
We also examined the correlation between the C1qA[276] and the CD16 and CD32 genotypes. The odds ratio between C1qA[276] (A carrier versus GG) and CD16 (VV homozygous versus F carrier) from a 2 by 2 contingency table analysis is 1.5 (95% CI, 0.36-9.0; P = 0.75). The odds ratio between C1qA[276] (A carrier versus GG) and CD32 (CD32HH versus R carriers) from a 2 by 2 contingency table analysis is 0.9 (95% CI, 0.23-4.3; P = 0.99). We also used the logistic regression analysis to examine whether the complement polymorphism is related to CD16 and CD32 in a joint fashion. Again, the analysis results show that there is no significant correlation between the C1qA[276A/G] and the CD16 and CD32 polymorphisms (P = 0.56 for CD16 and P = 0.85 for CD32, respectively).
These analyses show that the C1qA[276A/G] polymorphism is a prognosis factor affecting the time to relapse independent of CD16 and CD32. In particular, the A carriers show a prolonged response, whereas the homozygous G patients relapse significantly sooner. Also, there is no significant correlation between the C1qA[276] and the CD16 and CD32 genotypes.
Discussion
The G allele of C1qA[276], which is associated with shorter remissions to rituximab as outlined above, is also associated with higher C1q levels and measurements of complement-mediated lysis based on standard assays (38). This is opposite of what would be expected if the primary role of C1q was to participate in complement-mediated lysis of target cells.
There are a number of possible explanations for our findings. We now know that the effects of complement are much more complex. C1q has been reported to have a critical role in the phagocytosis of apoptotic bodies (27–30). In addition, there is evidence that there is an inverse relationship between C1q and antigen-presenting cell maturation and function (47). The increased incidence of lupus in both C1q-deficient patients and individuals lacking the C1qA[276G] allele provides further evidence of an inverse relationship between C1q activity and adaptive immunity (38, 39). Thus, one possible explanation for our findings is that the C1qA[276] G allele, with its more active complement, opsonizes tumor apoptotic bodies more effectively, thus facilitating their phagocytosis and removal. Rapid clearance of such material could limit the development of a cellular and humoral immune response, and hence results in a shorter progression-free interval. In contrast, in carriers of the A allele, with less complement function, the apoptotic bodies may be less efficiently opsonized by complement, thus providing more tumor cell fragments for dendritic cell– processing and presentation. If this is the case, the effect of the C1q polymorphism on tumor killing may go beyond antibody-based therapy of lymphoma. Indeed, we recently identified the C1qA[276] polymorphism as a significant indicator for improved metastasis-free survival in patients with breast carcinoma (45).
We recently reported that complement may actually interfere with rituximab-based killing. More specifically, we found that fixation of complement by rituximab-coated B cells limits natural killer cell activation and killing because the C3b component of complement interferes with the interaction between rituximab and the CD16 (48). If complement fixation actually limits the efficacy of rituximab, we would expect to observe a better response rate in patients with the C1qA[276] AA and AG genotypes, but it is less clear how this mechanism would explain the prolonged response.
Calreticulin, a natural receptor for C1q expressed by many cell types, has been reported to inhibit angiogenesis and tumor growth through its NH2-terminal region termed vasostatin (49). C1q interacts with calreticulin in the region responsible for the inhibitory effect of calreticulin on epithelial cell development and angiogenesis (50). Thus, C1q activity could affect the tumor microenvironment or angiogenesis with subsequent changes in duration of response.
In this population we have observed no association between the C1qA polymorphism and the overall rate of response to rituximab therapy. Regardless of C1qA[276] genotype, roughly one third of subjects did not respond to monoclonal antibody treatment. A likely explanation for this observation is that the rate of initial response is primarily related to factors intrinsic to the malignant cell itself, such as surface expression of target molecules for monoclonal antibody therapy, and much less to host-innate immune characteristics, including complement. Nonetheless, duration of response relies on the efficient control of residual tumor growth that involves other components of the immune system, including antibody-dependent cellular cytotoxicity, natural killer cells, and cytotoxic T-cells.
We observed a trend for more patients with the C1qA[276] A homozygous genotype to achieve a complete response to up-front rituximab therapy. This trend was not seen in patients who responded to anti-CD20 monoclonal antibody treatment after relapsing from or not responding to previous chemotherapy. In this later group, <25% of subjects achieved complete remission regardless of C1qA[276] genotype. This finding may be due to selection bias, as patients who relapse after chemotherapy are likely to have lymphomas with aggressive behavior that limits the efficacy of second-line rituximab therapy, therefore annulling any role C1q may have in the clinical outcome.
In conclusion, we have shown that a polymorphism in the C1qA[276] locus correlates with duration of rituximab response in follicular lymphoma patients. Although the differences between subjects in remission with different genotypes of C1qA are significant, we need to be cautious in the final interpretation of the results given the small size of the patient population that could be thus far be studied. Therefore, this study should be considered hypothesis-generating, not hypothesis-confirming. Ongoing studies are exploring the impact of this polymorphism in a larger population of lymphoma patients and in individuals with other cancers. In the laboratory, we are exploring the effect of C1q in general, and the C1qA[276] polymorphism in particular, on development of a cellular immune response. Confirmation of the clinical findings outlined above, and a better understanding of the mechanisms responsible, could have a major impact on prognosis and therapy for lymphoma and other malignancies.
Acknowledgments
We thank Christiana Taylor, Laura Jacobus, and Tina Knutson for patient consenting and for the collection and coding of blood specimens and Dr. Brian Smith for statistics assistance and fruitful discussion.
Grant support: NIH grant P50-CA97274-Iowa/Mayo Lymphoma Specialized Programs of Research Excellence program and USPHS grants CA-25224, CA-37404, CA -15083, CA -63826, CA -35195, CA -35267, CA -35101, CS-35431, CA -35090, CA-35113, CA-35415, CA-35448, CA-63848, CA97274, and CA112904 from the National Cancer Institute, Department of Health and Human Services.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Hainsworth JD, Litchy S, Burris HA, III, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:4261–7. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 2.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Marcus RE, Solal-Celigny P, Imrie K, et al. MabThera (rituximab) plus cyclophosphamide, vincristine and prednisone (CVP) chemotherapy improves survival in previously untreated patients with advanced follicular non-Hodgkins lymphoma (NHL) Blood. 2006;108:146a. [Google Scholar]
- 4.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–63. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–43. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 7.Lemieux B, Bouafia F, Thieblemont C, et al. Second treatment with rituximab in B-cell non-Hodgkin's lymphoma: efficacy and toxicity on 41patients treated at CHU-Lyon Sud. Hematol J. 2004;5:467–71. doi: 10.1038/sj.thj.6200559. [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE, Vukov AM, Habermann TM, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–8. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–6. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 10.Caragine TA, Imai M, Frey AB, Tomlinson S. Expression of rat complement control protein Crry on tumor cells inhibits rat natural killer cell-mediated cytotoxicity. Blood. 2002;100:3304–10. doi: 10.1182/blood.V100.9.3304. [DOI] [PubMed] [Google Scholar]
- 11.Caragine TA, Okada N, Frey AB, Tomlinson S. A tumor-expressed inhibitor of the early but not late complement lytic pathway enhances tumor growth in a rat model of human breast cancer. Cancer Res. 2002;62:1110–5. [PubMed] [Google Scholar]
- 12.Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 13.Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro : CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–8. [PubMed] [Google Scholar]
- 14.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcγ receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–53. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida J, Hamaguchi Y, Oliver JA, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–69. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannerji R, Kitada S, Flinn IW, et al. Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistance. J Clin Oncol. 2003;21:1466–71. doi: 10.1200/JCO.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Weng WK, Levy R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood. 2001;98:1352–7. doi: 10.1182/blood.v98.5.1352. [DOI] [PubMed] [Google Scholar]
- 18.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 19.Dall'Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–9. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 20.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Byrd JC, Kitada S, Flinn IW, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–43. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- 22.Cardarelli PM, Quinn M, Buckman D, et al. Binding to CD20 by anti-B1antibody or F(ab')(2) is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51:15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood. 2002;99:1314–9. doi: 10.1182/blood.v99.4.1314. [DOI] [PubMed] [Google Scholar]
- 24.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91:1644–52. [PubMed] [Google Scholar]
- 25.Manches O, Lui G, Chaperot L, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–54. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 26.Kishore U, Ghai R, Greenhough TJ, et al. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol Lett. 2004;95:113–28. doi: 10.1016/j.imlet.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 28.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–9. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 29.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. JExp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghiran I, Tyagi SR, Klickstein LB, Nicholson-Weller A. Expression and function of C1q receptors and C1q binding proteins at the cell surface. Immunobiology. 2002;205:407–20. doi: 10.1078/0171-2985-00142. [DOI] [PubMed] [Google Scholar]
- 32.Ghebrehiwet B, Peerschke EI. Role of C1q and C1q receptors in the pathogenesis of systemic lupus erythematosus. Curr Dir Autoimmun. 2004;7:87–97. doi: 10.1159/000075688. [DOI] [PubMed] [Google Scholar]
- 33.Petry F. Molecular basis of hereditary C1q deficiency. Immunobiology. 1998;199:286–94. doi: 10.1016/S0171-2985(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 34.Hoppenreijs EP, van Dijken PJ, Kabel PJ, Th Draaisma JM. Hereditary C1q deficiency and secondary Sjogren's syndrome. Ann Rheum Dis. 2004;63:1524–5. doi: 10.1136/ard.2003.016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 36.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 37.Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol. 2003;107:140–51. doi: 10.1016/s1521-6616(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 38.Racila DM, Sontheimer CJ, Sheffield A, Wisnieski JJ, Racila E, Sontheimer RD. Homozygous single nucleotide polymorphism of the complement C1QA gene is associated with decreased levels of C1q in patients with subacute cutaneous lupus erythematosus. Lupus. 2003;12:124–32. doi: 10.1191/0961203303lu329oa. [DOI] [PubMed] [Google Scholar]
- 39.Petry F, Loos M. Common silent mutations in all types of hereditary complement C1q deficiencies. Immunogenetics. 2005;57:566–71. doi: 10.1007/s00251-005-0023-z. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll DM. Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci U S A. 1999;96:5340–2. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sioud M. How does autoimmunity cause tumor regression? A potential mechanism involving cross-reaction through epitope mimicry. Mol Med. 2002;8:115–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Ram M, Shoenfeld Y. Harnessing autoimmunity (vitiligo) to treat melanoma: a myth or reality? Ann N Y Acad Sci. 2007;1110:410–25. doi: 10.1196/annals.1423.043. [DOI] [PubMed] [Google Scholar]
- 43.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen CH, Fischer EM, Leslie RG. The role of complement in the acquired immune response. Immunology. 2000;100:4–12. doi: 10.1046/j.1365-2567.2000.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racila E, Racila DM, Ritchie JM, Taylor C, Dahle C, Weiner GJ. The pattern of clinical breast cancer metastasis correlates with a single nucleotide polymorphism in the C1qA component of complement. Immunogenetics. 2006;58:1–8. doi: 10.1007/s00251-005-0077-y. [DOI] [PubMed] [Google Scholar]
- 46.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 47.Castellano G, Woltman AM, Nauta AJ, et al. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103:3813–20. doi: 10.1182/blood-2003-09-3046. [DOI] [PubMed] [Google Scholar]
- 48.Wang SY, Racila E, Taylor RP, Weiner GJ. NK cell activation and antibody dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–63. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pike SE, Yao L, Jones KD, et al. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–56. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuart GR, Lynch NJ, Lu J, et al. Localisation of the C1q binding site within C1q receptor/calreticulin. FEBS Lett. 1996;397:245–9. doi: 10.1016/s0014-5793(96)01156-8. [DOI] [PubMed] [Google Scholar]