Abstract
Atrial fibrillation (AF), the most prevalent sustained cardiac arrhythmia, often coexists with the related arrhythmia atrial flutter (AFL). Limitations in effectiveness and safety of current therapies make an understanding of the molecular mechanism underlying AF more urgent. Genome-wide association studies implicated a region of human chromosome 4q25 in familial AF and AFL, ≈150 kb distal to the Pitx2 homeobox gene, a developmental left–right asymmetry (LRA) gene. To investigate the significance of the 4q25 variants, we used mouse models to investigate Pitx2 in atrial arrhythmogenesis directly. When challenged by programmed stimulation, Pitx2null+/− adult mice had atrial arrhythmias, including AFL and atrial tachycardia, indicating that Pitx2 haploinsufficiency predisposes to atrial arrhythmias. Microarray and in situ studies indicated that Pitx2 suppresses sinoatrial node (SAN)-specific gene expression, including Shox2, in the left atrium of embryos and young adults. In vivo ChIP and transfection experiments indicated that Pitx2 directly bound Shox2 in vivo, supporting the notion that Pitx2 directly inhibits the SAN-specific genetic program in left atrium. Our findings implicate Pitx2 and Pitx2-mediated LRA-signaling pathways in prevention of atrial arrhythmias.
Keywords: atrial flutter, atrial tachycardia, cardiac conduction, left–right asymmetry, homeobox
Atrial fibrillation (AF), the most common adult arrhythmia, increases in prevalence with age, eventually afflicting 5% of the population over age 65 years and 10% of those over age 80 years. Moreover, patients with AF have a significantly increased risk of stroke, heart failure, and dementia (1 –3). Electrical impulses critical for a normal heartbeat are initiated in the sinoatrial node (SAN) or pacemaker region. In AF, rapid and irregular atrial activity overrides normal SAN function, often resulting in irregular impulse conduction to the ventricles. In many cases, ectopic electrical activity originates in the pulmonary veins and may serve to trigger and maintain AF (1, 4). The related arrhythmia, atrial flutter (AFL), displays more regular and organized electrical activity than does AF (5). Significantly, current treatments for AF are suboptimal because of incomplete effectiveness and deleterious side effects. It also has been recognized that untreated AF results in pathologic remodeling that makes AF more likely to recur (6). Thus, it is critically important to uncover the genetic mechanisms underlying AF to aid in patient management and to develop more safe and effective therapies.
The pituitary homeobox (Pitx) family of homeobox genes containing three genes, Pitx1, Pitx2, and Pitx3, is a subgroup within the larger Paired-related superfamily of homeobox genes (7, 8). Pitx2 was identified as the gene mutated in Rieger syndrome I, a haploinsufficient disorder that includes ocular, tooth, and anterior body wall defects as primary characteristics (9). Importantly, the Pitx2 gene encodes three isoforms: Pitx2a, Pitx2b, and Pitx2c. The Pitx2c isoform plays a critical role as a late effector in left–right asymmetry (LRA), a fundamental component of organ morphogenesis in vertebrates. The signaling pathways regulating LRA are initiated in the presomite-stage embryo and are mediated in large part through Nodal signaling. Pitx2c is the major downstream effector of the Nodal pathway (10).
Recent genome-wide association studies identified sequence variants on chromosome 4q25 that were associated with increased risk for AF in multiple human populations (11 –13). Moreover, the 4q25 variants were strongly associated with AF cases diagnosed at an earlier age (<60 years) and with recurrence after ablation therapy (14). In a small Icelandic cohort, the sequence variants also were strongly associated with AFL (11). These correlative sequence variants were found in proximity to Pitx2, suggesting that Pitx2 may be the AF locus in this region. We show that Pitx2c is expressed in the immediate postnatal period in left atrium and pulmonary vein. Moreover, Pitx2null +/− mice that are heterozygous for a Pitx2-null allele that removes all isoform function (15) are prone to atrial arrhythmias. In vivo ChIP assays reveal that Pitx2c binds directly to Shox2, suggesting that Pitx2c directly represses the SAN genetic program in the left atrium. Together, our findings support Pitx2 as a bona fide susceptibility gene for atrial arrhythmias.
Results
Pitx2c Is Expressed in the Postnatal Left Atrium and Pulmonary Vein.
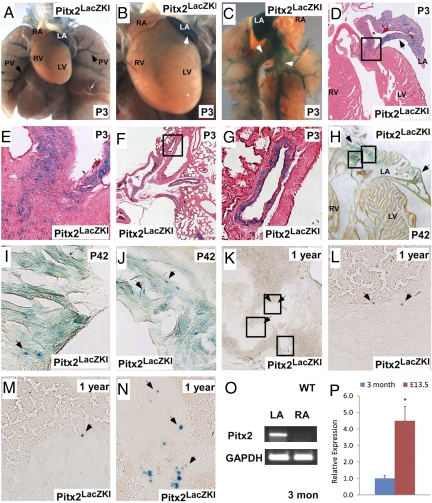
To determine if Pitx2 is expressed in the postnatal heart, we generated a Pitx2 LacZ knockin allele, Pitx2LacZ, to follow Pitx2 expression efficiently (Methods). LacZ staining in postnatal day 3 (P3) pups indicated that Pitx2 was expressed in the left atrium and pulmonary veins (Fig. 1 A–C). Sections confirmed that Pitx2LacZ directed LacZ activity in the left atrium and pulmonary veins (Fig. 1 D–G). Pitx2 expression also was detected in the right ventricle, although this expression was weak (Fig. 1B). By P42, Pitx2 expression was detected in the left atrial myocardium, although at diminished levels (Fig. 1 H–J). At 1 year, Pitx2 expression was found only in rare left atrial myocardial cells (Fig. 1 K–N). To gain insight into postnatal Pitx2 isoform expression, we investigated LacZ expression in a transgenic line, Pitx2ctg3K, in which LacZ is directed by Pitx2c regulatory elements (16). Because this Pitx2c transgene is influenced by copy number and position effect, this experiment provided only qualitative information about Pitx2 isoform expression in left atrium. In 3-month-old Pitx2ctg3K transgenic mice, we found LacZ activity in right ventricle and left atrium, supporting the notion that Pitx2c was expressed in the postnatal left atrium and right ventricle (Fig. S1 A–D). RT-PCR experiments also indicated that Pitx2c was expressed in left atrium (Fig. 1O). Quantitative RT-PCR indicated that Pitx2c expression levels in a 3-month-old left atrium were ≈25% of that found in the embryo 13.5 days postconception (dpc) (Fig. 1P). Together, these findings indicate that Pitx2c is expressed in postnatal left atrium, pulmonary vein, and right ventricle and that Pitx2c expression in left atrium is silenced during aging.
Fig. 1.
Pitx2 is expressed in the left atrium and pulmonary vein of postnatal mice. (A–G) Whole-mount LacZ staining and sagittal sections of Pitx2 LacZ allele at P3. Pitx2 is expressed in the left atrium, right ventricle, and pulmonary vein (arrows). Boxed areas in D and F are shown at higher magnification in E and G, respectively. (H–J) LacZ staining (arrows) on sagittal sections of Pitx2 LacZ allele on P42 shows LacZ activity in the left atrium. Boxed areas in H are shown at higher magnification in I and J. (K–N) LacZ staining (arrows) on sagittal sections of 1-year-old Pitx2 LacZ allele. Pitx2 is expressed in rare left atrial myocardial cells in left atrium. Boxed areas in K are shown at higher magnification in L, M, and N. (Magnification in E, G, I, J and L-N: ×200.) (O) RT-PCR of Pitx2c in WT left atrium and right atrium shows Pitx2c is expressed in left atrium. (P) Quantitative RT-PCR analysis of Pitx2c comparing embryonic and adult stages. Arrows indicate Lac staining. *, statistically significant difference (P < 0.05). LA, left atrium; LV, left ventricle; PV, pulmonary vein; RA, right atrium; RV, right ventricle.
Baseline Electrophysiological Features of Pitx2null +/− Mice.
To test the hypothesis that Pitx2c is a susceptibility gene for atrial arrhythmias such as AF and AFL, we first used conventional six-lead surface ECG and four-lead bipolar intracardiac electrograms to record simultaneously in WT and Pitx2null +/− mice (Fig. S2). Under baseline conditions, there were no differences between Pitx2null +/− and WT mice in heart rate, interval between two consecutive R wave peaks (RR), interval from beginning of the P wave to the peak of the R wave (PR), or in the time elapsing from the beginning of the QRS complex to the end of the T wave (corrected Q-T interval) on surface ECGs (Table 1), with the exception of a small but significant prolongation of the QRS interval in Pitx2null +/− mice. These findings were corroborated using bipolar intracardiac electrogram recordings. Endocardial programmed electrical stimulation was used to determine atrial effective refractory period, atrioventricular nodal effective refractory period, and sinus node recovery time at a basic cycle length of 100 ms. There were no significant differences between Pitx2null +/− and WT mice (Table 1). During these electrophysiological studies, no episodes of spontaneous AF were observed in any of the mice studied.
Table 1.
Baseline cardiac electrophysiological parameters in WT and Pitx2null +/− mice
| ECG intervals (ms) | WT (n = 4) | Pitx2null+/− (n = 6) | Statistics |
| HR | 470.5 ± 36.3 | 487.8 ± 8.6 | NS |
| RR | 129.7 ± 9.4 | 123.2 ± 2.2 | NS |
| PR | 44.9 ± 1.3 | 46.1 ± 0.74 | NS |
| QRS | 9.1 ± 0.2 | 10.7 ± 0.4 | P = 0.05 |
| QTc | 23.5 ± 1.2 | 26.8 ± 1.1 | NS |
| SNRT | 179.8 ± 31.0 | 176 ± 13.5 | NS |
| AVERP | 52.0 ± 2.2 | 56 ± 1.3 | NS |
| AERP | 44.5 ± 0.3 | 47.7 ± 1.4 | NS |
All values expressed mean ± SEM. AERP, atrial effective refractory period; AVERP, atrioventricular nodal effective refractory period; HR, heart rate; NS, nonsignificant; PR, interval from beginning of P waves to the peak of R wave; QRS, duration of interval between beginning of Q wave to peak of S wave; QTc, duration of Q-T interval corrected for heart rate; RR, interval between two consecutive R wave peaks; SNRT, sinus node recovery time.
Pacing-Induced Atrial Arrhythmias in Pitx2null +/− Mice.
Although Pitx2null +/− mice were in normal sinus rhythm at baseline, we wanted to test the notion that Pitx2c haploinsufficiency predisposed mice to atrial arrhythmias. There is precedent for this idea, because a mouse model for a gain-of-function mutation in ryanodine receptor type II (RyR2) provided a substrate for AF that was insufficient to produce spontaneous arrhythmias. RyR2 mutants required a second arrhythmogenic triggering event, in this case activation of Ca2+/calmodulin-dependent protein kinase II, which was uncovered by increased heart rate (17).
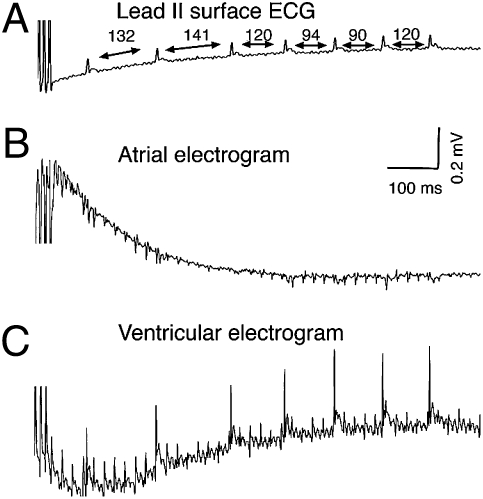
Inducibility of atrial arrhythmias, determined twice in each mouse, was measured with surface leads and an intraatrial catheter using the stimulation protocol previously described (17, 18). Episodes of AFL/tachycardia, defined as rapid but regular atrial rhythm lasting >1,000 ms (19), were observed frequently in Pitx2null +/− mice (Fig. 2 A–C). Based on a-wave morphology and rate on the atrial electrogram, episodes of pacing-induced atrial arrhythmias were observed more frequently in Pitx2null +/− (100%, six of six mice) than in WT mice (one of four mice, Fisher's exact test, P = 0.033). One of the Pitx2null +/− mice showed an atrial arrhythmia only once with an episode lasting for at least 16 s, whereas five of six Pitx2null +/− mice developed arrhythmias following both burst-pacing stimuli. Mean duration of atrial arrhythmias in Pitx2null +/− mice, confirmed by rapid and regular a-wave on atrial electrograms, was 164 ± 131 s. In addition, we observed an episode of AFL/tachycardia in one of the WT mice (although this episode only lasted 6.8 s). Taken together, our findings indicate that Pitx2c haploinsufficiency predisposes mice to atrial arrhythmias, including AFL/tachycardia.
Fig. 2.
Surface ECG (A) and intracardiac electrograms (B and C) revealing an episode of pacing-induced atrial tachycardia in a Pitx2null +/− mouse. (A) Surface ECG showing the last three paced beats of the burst protocol. The tracing reveals the absence of P waves and irregular RR intervals, suggestive of atrial arrhythmia. (B) Atrial electrogram shows the presence of rapid and regular a waves, suggestive of AFL/tachycardia. (C) Ventricular electrograms reveals irregular intervals between v waves.
Pitx2c Inhibits the Pacemaker Gene Program in Left Atrium.
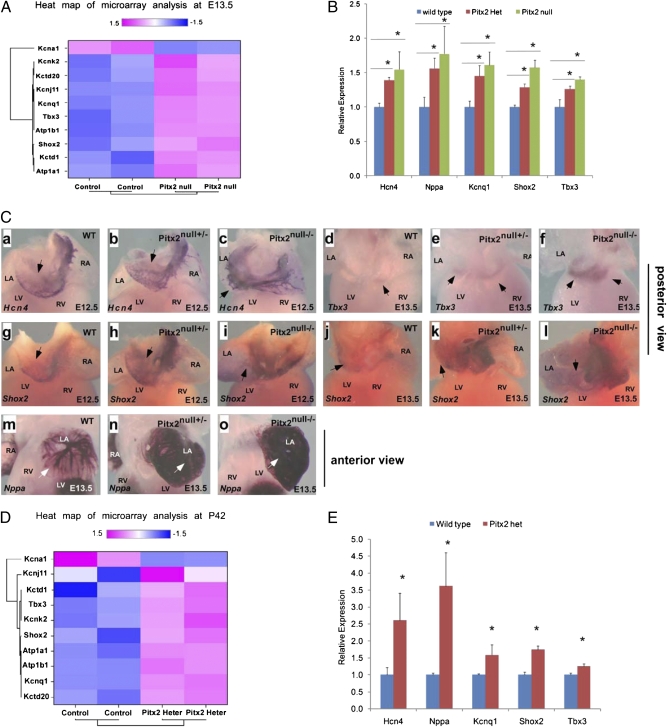
To gain insight into the mechanisms underlying predisposition to AFL/tachycardia, we performed microarray analysis on Pitx2null-mutant and control hearts (Methods and Fig. S3). Interestingly the microarray study revealed that the SAN genes Shox2 and Tbx3, as well as a number of channel genes, were up-regulated in the Pitx2null-mutant embryos when compared with controls (Fig. 3A). Among the up-regulated channel genes was Kcnq1, a potassium-channel gene that has been implicated in familial AF through a gain-of-function mutation (20).
Fig. 3.
Pitx2 inhibits the SAN program. (A). Heat map of microarray data from Pitx2null −/−-mutant embryo hearts compared with WT hearts at 13.5 dpc. All pacemaker genes and ion-channel genes designated to be present at significant levels were identified in the normalized data, followed by log2 transformation, subjected to hierarchical clustering and heat-map generating. (B) Quantitative RT-PCR validation of microarray analysis of Pitx2-mutant hearts compared with WT control hearts at 13.5 dpc. The quantitative real-time RT-PCR analysis showed that Kcnq1, ANF, Hcn4, Shox2, and Tbx3 were up-regulated in Pitx2-mutant hearts. (C). Whole-mount in situ analysis at 12.5 dpc and 13.5 dpc with indicated probes. Genotypes and probes are labeled. Arrows designate areas of relevant gene expression in control and mutant embryos. (D) Heat map of microarray data and (E) quantitative RT-PCR validation of microarray on WT and Pitx2null +/− mouse hearts at P42. Potassium-channel genes including Kcnq1, as well as the SAN genes Shox2 and Hcn4, were up-regulated in the Pitx2null +/−-mutant hearts. In B and E, * indicates statistically significant differences (P < 0.05).
We next used quantitative RT-PCR and whole-mount in situ hybridization to gain further insight into the mechanisms underlying predisposition to AFL/tachycardia in Pitx2c mutants. We examined expression of Hcn4, a hyperpolarization-activated, cyclic nucleotide-gated (HCN) channel that has a critical role in SAN automaticity (21, 22). In 12.5-dpc control embryos, Hcn4 was expressed in the large caval veins posterior to the heart, as well as in the forming SAN region (Fig. 3Ca and Fig. S4 A and B). In Pitx2null+/− mutants, Hcn4 appeared to be modestly up-regulated in the left superior caval vein that also expresses Pitx2c (Fig. 3Cb and Fig. S4 C and D) (23). In Pitx2null −/− embryos, the anatomy of the junction between the large veins and the atrium was disrupted (24, 25), and Hcn4 was expressed continuously in the abnormal vein–atrial junction in Pitx2null−/−-mutant embryos and in the posterior wall of the mutant left atrium (Fig. 3Cc and Fig. S4 E and F). Quantitative RT-PCR indicated that Hcn4 was quantitatively up-regulated in both Pitx2null+/− and Pitx2null−/− mutant embryos (Fig. 3B).
Tbx3 is a T-box gene that is expressed in SAN progenitors and is required for correct separation of the SAN from working atrial myocardium (26). In Pitx2null +/− mutants, Tbx3 expression was more prominent in the left atrioventricular canal, whereas in Pitx2null −/− mutants Tbx3 was expanded and up-regulated in the left atrioventricular canal and in the midline cells at the base of the common venous sinus (Fig. 3 B and Cd–f).
Shox2, a homeodomain containing transcription factor expressed in the developing SAN, has been shown recently to be required for SAN development (27, 28). At both 12.5 and 13.5 dpc in Pitx2null +/− embryos, Shox2 was expressed similarly to control SAN progenitors, although expression was up-regulated in left superior caval vein and interatrial septum of Pitx2null+/− embryos (Fig. 3Cg, h, j, and k and Fig. S4 G–J and M–P). Shox2 up-regulation was confirmed using quantitative RT-PCR (Fig. 3B). In Pitx2null −/− embryos, Shox2 was expanded in the posterior wall of the left atrium and left atrial myocardium (Fig. 3 Ci and l and Fig. S4 K, L, Q, and R). Quantitative RT-PCR also indicated that Shox2 was up-regulated in Pitx2null−/− embryos (Fig. 3B).
In addition to genes identified in the microarray, we also investigated candidate genes in the Pitx2 mutants. Nppa, encoding the atrial natriuretic peptide hormone that regulates intravascular volume, has been reported to be a Pitx2 target and has been implicated in familial AF (29, 30). Whole-mount in situ hybridization and quantitative RT-PCR indicated that Nppa expression was up-regulated in Pitx2null +/− and Pitx2null −/−-mutant embryos (Fig. 3 B and Cm–o).
We next evaluated gene expression in the left atrium of P42 Pitx2null +/− mice that were prone to atrial arrhythmias as shown in Fig. 2. Microarray analysis followed by quantitative RT-PCR verification revealed that genes expanded in the adult heart were similar to those up-regulated in Pitx2null-mutant embryos. A number of potassium-channel genes, including Kcnq1, were up-regulated in the Pitx2null +/− P42 left atrium, as well as the SAN genes Shox2 and Hcn4 (Fig. 3 D and E).
Pitx2c Directly Represses Shox2.
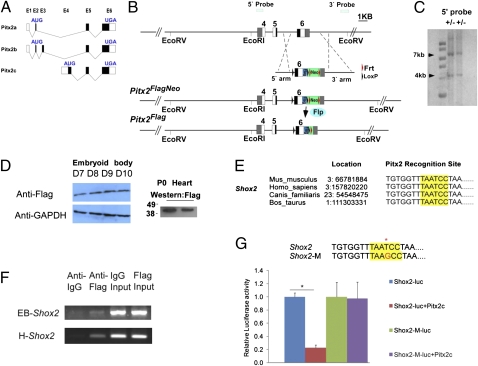
To understand the underlying molecular mechanisms in the predisposition to atrial arrhythmia in Pitx2null +/− mice, we generated an allele of Pitx2, Pitx2Flag (Fig. 4 A–C). We found that Pitx2Flag directed Pitx2c expression in embryoid bodies cultured from 7–10 days (Fig. 4D). In the P0 heart, we observed strong expression of Pitx2c protein in mice that were heterozygous for the Pitx2Flag allele (Fig. 4D).
Fig. 4.
Pitx2 directly represses Shox2. (A) Summary of exon usage by Pitx2 isoforms. AUG and UGA: start and stop codons; E1->E6, exons 1->6. (B) Targeting strategy of generating the Pitx2Flag allele. (C) Southern blots for the Pitx2Flag allele showing the WT 7-kb and mutant 4-kb bands. (D) Western blot showed Pitx2Flag-directed Pitx2 expression in embryoid bodies and P0 heart. D7-D10, culture day 7–10. (E) Alignment of Pitx2 recognition elements in Shox2. (F) In vivo ChIP indicated that Shox2 was bound by Pitx2 in embryoid bodies (EB) and adult left atrium (H). (G) Luciferase activity assay experiments indicated that Pitx2 repressed Shox2 in P19 cells and that this repression was lost when the Pitx2 binding site was mutated. At top, highlight in red indicates point mutation inserted to disrupt Pitx2 binding. * indicates statistically significant differences (P < 0.05).
Bioinformatics analysis revealed a Pitx2c recognition element in the Shox2 gene that was conserved in the mouse, human, dog, and cow genomes (Fig. 4E and Methods). Moreover, in vivo ChIP indicated that the Pitx2c recognition element, located in Shox2 intron 2, was bound by Pitx2c in both embryoid bodies and the adult left atrium (Fig. 4F). Transfection experiments using a 2-kb Shox2 genomic fragment that included the Pitx2c binding site indicated that Pitx2c repressed Shox2 expression in P19 cells and that this repression was lost when the Pitx2c binding site was mutated (Fig. 4G). Together, our findings support the hypothesis that Shox2 is a direct target for Pitx2c repression in the embryonic and adult left atrium.
Discussion
Atrial arrhythmias such as AF and AFL are common human arrhythmias with potentially devastating clinical consequences. Our findings, taken together with previously reported data, indicate that Pitx2c is an important susceptibility gene for atrial arrhythmias that normally represses the pacemaker gene program in the left atrium. We identify Shox2, a critical regulator of the SAN gene program, as a Pitx2c target gene. The observation that Pitx2c is most highly expressed during embryogenesis and in the early postnatal heart suggests that the arrhythmogenic substrate is established relatively early in life.
Pitx2c Directly Inhibits Pacemaker Specification.
Our in vivo ChIP data and transfection experiments indicate that Pitx2c normally silences Shox2 in the left atrium. Previous work has shown that Shox2-mutant mice have defective SAN development with reduced expression of Hcn4 and Tbx3, indicating that Shox2 positively regulates the SAN genetic program. Shox2 functions, at least in part, by inhibiting Nkx2.5 expression in SAN progenitors (Fig. S5) (27, 28). Nkx2.5 promotes the atrial working myocardial program and represses the SAN genetic pathway (31, 32). Transfection experiments revealed that Shox2 directly represses the Nkx2.5 5′ flanking region and that this repression was independent of Shox2 DNA binding activity (28). Although this genetic program is intact on the right side of the embryo, on the left side Pitx2c disrupts the negative cascade by inhibiting Shox2 expression (Fig. S5).
Tbx3 also promotes the SAN phenotype while inhibiting the working atrial myocardial phenotype through both direct and indirect mechanisms. There is strong evidence that Tbx3 binds to and inhibits atrial genes, such as Cx43. Tbx3 also promotes the expression of SAN genes, most likely through a poorly understood indirect mechanism (26). Moreover, recent findings indicate that Tbx3 is genetically downstream of Shox2 (28). Together, our data support a model whereby Pitx2c directly represses Shox2 and inhibits the SAN genetic program in left atrium.
Our findings support the notion that Pitx2c loss of function promotes ectopic automaticity in the left atrium. Under normal conditions, the left atrium should be protected from impulse generation. In Pitx2null+/− mutants, our data reveal that left atrial myocardium expresses genes that are characteristic of the SAN such as Hcn4. We suggest that the Pitx2null+/− null mutant left atrium provides an arrhythmogenic substrate that would enhance other pathologic triggers for atrial arrhythmias.
Atrial Fibrillation and Left–Right Asymmetry: A Direct Molecular Connection.
Our findings indicate that the Pitx2c-mediated LRA signaling cascade inhibits SAN specification by disrupting a negative regulatory transcriptional cascade. Previous work showed that Pitx2c directs formation of left and right anatomic characteristics that separate the systemic and pulmonic circulation that is vital for normal cardiac function. Morphologic abnormalities in Pitx2null homozygous mutants include right atrial isomerism with bilateral venous valves, deficiency of the primary interatrial septum, and anomalous pulmonary venous drainage (31, 33). In Pitx2c −/−-mutant embryos, caval and pulmonary veins drain into an abnormal medial venous sinus. Moreover, there was a deficiency in contribution of the Pitx2-expressing lineage to pulmonary veins in Pitx2c homozygous mutant embryos (25) because of a defect in pulmonary vein precursors (24).
Mommersteeg and colleagues (31) reported that Pitx2c homozygous mutants had a bilateral SAN that expressed SAN markers such as Hcn4. Our findings suggest that Pitx2c regulates the precise location of the SAN by directly inhibiting Shox2, a transcriptional regulator of SAN gene program. Moreover, we show that Pitx2 haploinsufficiency promotes the occurrence of atrial tachyarrhythmias.
Our data uncover a remarkably close connection between LRA and the SAN genetic program. Pitx2c is a direct target of the TGFβ superfamily member Nodal that is expressed transiently at early stages of development (10). Left-sided Pitx2c expression persists in organ primordia well after Nodal has been silenced (34, 35). Furthermore, the identification of Pitx2c as a susceptibility gene for atrial arrhythmias provides deeper insight into the genetic pathways that predispose to AF and AFL. Importantly, the Nodal-Pitx2c signaling cassette is evolutionarily conserved, raising the possibility that Nodal also is implicated in predisposition to atrial arrhythmias. Indeed, mutations in Nodal have been identified in human patients with LRA defects but have not been reported in familial AF (36).
Other Mechanisms Contributing to Pitx2c Predisposition to Atrial Arrhythmia.
In addition to the Shox2-mediated mechanism, we found other gene expression changes in Pitx2c mutants that suggest other contributory mechanisms for predisposition to atrial arrhythmias. Atrial natriuretic peptide, encoded by Nppa, regulates multiple ion channels in atrial cardiomyocytes. An atrial natriuretic peptide gain-of-function mutation that may promote arrhythmias within atrial myocardium has been identified in human patients (29). Our finding that Nppa was up-regulated in Pitx2c mutants suggests that similar mechanisms may contribute to the predisposition to atrial arrhythmia in Pitx2c mutants.
Previous work indicated that a gain-of-function substitution in a conserved serine of Kcnq1 (S140G) results in familial AF (20). Moreover, separate mutations in Kcnq1—a serine-to-proline (S209P) substitution and a valine-to-methionine (V141M) substitution—also were reported in familial AF (37, 38). In vitro experiments indicate that the gain-of-function mutations in Kcnq1 may be caused by defects in channel inactivation (39). Our data indicate that Kcnq1 is up-regulated in Pitx2c mutants. Together these findings suggest that Pitx2c likely has multiple targets that are relevant to the predisposition to atrial arrhythmia.
Methods
Mouse Alleles and Transgenic Lines.
The Pitx2null allele, which removes function of all Pitx2 isoforms, and the Pitx2tg3k transgenic line have been described previously (15, 16).
Real-Time Quantitative PCR.
Total RNA was isolated from embryonic hearts and adult hearts using RNeasy Micro Kit (QIAGEN), followed by RT-PCR using Super ScriptTM II reverse transcriptase (Invitrogen). Real-time thermal cycling was performed using Mx3000P thermal cycler (Stratagene) with SYBR Green JumpStartTM Taq ReadyMixTM (Sigma). Sequences of primers are available upon request.
Cardiac Electrophysiology.
Cardiac electrophysiology was performed as described (40).
Microarray Studies.
Total RNA was extracted from embryonic hearts or P42 hearts and purified using either RNeasy Micro Kit (QIAGEN) for embryonic hearts or TRIzol Reagent (Invitrogen) for postnatal hearts. DNA microarray analysis was performed using the OneArray Mouse Whole Genome Array that interrogated 17,455 unique genes using 29,972 probes (Phalanx Biotech Group). Graphical presentations of the array data are shown in Fig S3.
ChIP.
ChIP analysis was performed using a ChIP assay kit (Upstate).
Generation of Reporter Constructs.
Using rVista2.0 (41) and Ensembl genomic alignments (http://www.ensembl.org/), we analyzed Shox2 genomic sequence and the genomic sequence5 kb upstream of Shox2 to identify conserved Pitx2 binding sites. This analysis was followed by the ChIP analysis, and the Pitx2 biding site “TAATCC” in intron between exon 2 and exon3 of Shox2 was validated.
Site-Directed Mutagenesis.
Site-directed mutagenesis of the Pitx2 binding sites in the Shox2 intron was achieved by using the QuikChange II site-directed mutagenesis kit (Stratagene).
Supplementary Material
Acknowledgments
We thank A. Bradley (Wellcome Trust), and R. Behringer (University of Texas MD Anderson) for reagents, Y. Bai for help with cloning, D. Ai for help with Western blots, and Dr. Jerome Kalifa for discussions. This work was supported by Grants 2R01DE/HD12324-12, R01DE16329, R01HL093484-01 (to J.F.M.), and R01HD052785 (to R.L.J.) from the National Institutes of Health. X.H.T.W. is a W. M. Keck Foundation Distinguished Young Scholar in Medical Research and also is supported by Grants R01HL089598 and R01HL091947 from the National Institutes of Health. J.W. was supported by Grant 09PRE2150024 from the South Central Affiliate of the American Heart Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912585107/-/DCSupplemental.
References
- 1.Haïssaguerre M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, et al. Prevention of atrial fibrillation: Report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Po SS, et al. Rapid and stable re-entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation. J Am Coll Cardiol. 2005;45:1871–1877. doi: 10.1016/j.jacc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 5.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 6.Knollmann BC, Roden DM. A genetic framework for improving arrhythmia therapy. Nature. 2008;451:929–936. doi: 10.1038/nature06799. [DOI] [PubMed] [Google Scholar]
- 7.Schneitz K, Spielmann P, Noll M. Molecular genetics of aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes Dev. 1993;7:114–129. doi: 10.1101/gad.7.1.114. [DOI] [PubMed] [Google Scholar]
- 8.Gage PJ, Suh H, Camper SA. The bicoid-related Pitx gene family in development. Mamm Genome. 1999;10:197–200. doi: 10.1007/s003359900970. [DOI] [PubMed] [Google Scholar]
- 9.Semina EV, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- 10.Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat Rev Genet. 2002;3:103–113. doi: 10.1038/nrg732. [DOI] [PubMed] [Google Scholar]
- 11.Gudbjartsson DF, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 12.Gudbjartsson DF, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kääb S, et al. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur Heart J. 2009;30:813–819. doi: 10.1093/eurheartj/ehn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010;55:747–753. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- 16.Ai D, et al. Nuclear factor 1 and T-cell factor/LEF recognition elements regulate Pitx2 transcription in pituitary development. Mol Cell Biol. 2007;27:5765–5775. doi: 10.1128/MCB.01848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelu MG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verheule S, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrickel JW, et al. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res Cardiol. 2002;97:452–460. doi: 10.1007/s003950200052. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 21.Harzheim D, et al. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J. 2008;27:692–703. doi: 10.1038/emboj.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann S, Stieber J, Stöckl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J. 2007;26:4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco D, et al. Multiple transcriptional domains, with distinct left and right components, in the atrial chambers of the developing heart. Circ Res. 2000;87:984–991. doi: 10.1161/01.res.87.11.984. [DOI] [PubMed] [Google Scholar]
- 24.Mommersteeg MT, et al. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, et al. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development. 2002;129:5081–5091. doi: 10.1242/dev.129.21.5081. [DOI] [PubMed] [Google Scholar]
- 26.Hoogaars WM, et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaschke RJ, et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation. 2007;115:1830–1838. doi: 10.1161/CIRCULATIONAHA.106.637819. [DOI] [PubMed] [Google Scholar]
- 28.Espinoza-Lewis RA, et al. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev Biol. 2009;327:376–385. doi: 10.1016/j.ydbio.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgson-Zingman DM, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganga M, et al. PITX2 isoform-specific regulation of atrial natriuretic factor expression: Synergism and repression with Nkx2.5. J Biol Chem. 2003;278:22437–22445. doi: 10.1074/jbc.M210163200. [DOI] [PubMed] [Google Scholar]
- 31.Mommersteeg MT, et al. Molecular pathway for the localized formation of the sinoatrial node. Circ Res. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 32.Martin JF. Left right asymmetry, the pulmonary vein, and a-fib. Circ Res. 2007;101:853–855. doi: 10.1161/CIRCRESAHA.107.164079. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Liu W, Lu MF, Brown NA, Martin JF. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128:2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- 34.Ryan AK, et al. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- 35.Logan M, Pagán-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94:307–317. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- 36.Mohapatra B, et al. Identification and functional characterization of NODAL rare variants in heterotaxy and isolated cardiovascular malformations. Hum Mol Genet. 2009;18:861–871. doi: 10.1093/hmg/ddn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong K, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Das S, et al. Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation. Heart Rhythm. 2009;6:1146–1153. doi: 10.1016/j.hrthm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Restier L, Cheng L, Sanguinetti MC. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol. 2008;586:4179–4191. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sood S, et al. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loots GG, Ovcharenko I. rVISTA 2.0: Evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–W221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.