Abstract
Purpose
In vitro supplementation of the bile salt, taurodeoxycholic acid (TDCA), has been shown to stimulate proliferation and prevent intestinal apoptosis in IEC-6 cells. We hypothesize that addition of TDCA to a rodent liquid diet will be protective against induced intestinal injury.
Methods
C57Bl6 mice were fed a liquid diet with or without 50mg/kg/day TDCA supplementation. After 6 days, the mice were injected with LPS (10mg/kg) to induce intestinal injury. Specimens were obtained 24 hours later and evaluated for intestinal apoptosis, crypt proliferation, and villus length. A separate cohort of animals were injected with LPS (25mg/kg) and followed 7 days for survival.
Results
Mice whose diet was supplemented with TDCA had significantly increased survival. After LPS-induced injury, mice supplemented with TDCA showed decreased intestinal apoptosis by both H&E and caspase-3. They also had increased intestinal proliferation by BrdU-staining and increased villus length.
Conclusions
Dietary taurodeoxycholic acid supplementation alleviates mucosal damage and improves survival after LPS-induced intestinal injury. TDCA is protective of the intestinal mucosa by increasing resistance to injury-induced apoptosis, stimulating enterocyte proliferation and increasing villus length. TDCA supplementation also results in an increased survival benefit. Therefore, bile acid supplementation may potentially protect the intestine from injury or infection.
Keywords: bile salts, taurodeoxycholic acid (TDCA), intestinal injury, apoptosis, proliferation
INTRODUCTION
The small intestine requires constant turnover of its mucosal surface in order to maintain an intact mucosa. An intact mucosa is required for digestive and secretory functions as well as the formation of a barrier against noxious substances within the intestinal lumen. The mucosal integrity of the small intestine is maintained by a strict balance of proliferation, differentiation, and apoptosis (1,2). In fact, the entire intestinal epithelium is replaced every 3-5 days as its lumen undergoes constant turnover (3). This mucosal integrity can be directly perturbed in diseases that involve intestinal injury including necrotizing enterocolitis and inflammatory bowel disease. Recent data suggest that the mucosal integrity of the intestine can also be important for diseases that are remote from the intestine. Animal models of sepsis from pneumonia and endotoxemia show that the crypt cells of the intestine undergo increased apoptosis and decreased proliferation even when there is no primary disease of the intestine (4-6). There is also evidence that when intestinal apoptosis is suppressed in transgenic mice that overexpress the antiapoptotic protein Bcl-2, survival from sepsis is increased in models of Pseudomonas pneumonia (7) and sepsis from cecal ligation and puncture (8).
Bile salts are normally found within the intestinal lumen. The primary function of bile salts is to aid in the absorption of lipids and lipid-soluble vitamins (9). However, recent studies have shown that bile salts have other biologic effects independent of their role in digestion. The bile salt deoxycholic acid has been shown to increase proliferation and induce apoptosis in colon cancer cell lines (10,11). The bile salt taurodeoxycholic acid (TDCA) has been associated with increased growth of esophageal mucosa in a rabbit explant model (12). Our lab has previously shown that intestinal epithelial cells supplemented with TDCA show increased cellular restitution, increased proliferation, and protection from apoptosis (13-15). Therefore, we chose to look at the in vivo effects of bile acid supplementation in an animal model of endotoxemia. We hypothesized that adding TDCA to the enteral diet of injured mice could protect the intestinal mucosa in vivo by decreasing apoptosis and increasing proliferation. We also chose to follow these animals after acute injury to determine whether this proposed protection resulted in increased survival.
MATERIALS AND METHODS
Animals
C57Bl/6J male mice used in this study were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed in a standard facility, kept on a 12 hour light-dark cycle, and allowed to acclimate to their environment for at least 5 days. The mice received water and mouse chow ad libitum until the time of experimentation. During experimentation, the mice were place on a liquid rodent diet (Micro-Stabilized Rodent Liquid Diet LD101; Purina Mills, St. Louis, MO) with or without the addition taurodeoxycholic acid (TDCA, T0557, Sigma-Aldrich, St. Louis, MO). All studies were approved by the University of Maryland School of Medicine Animal Studies Committee (IACUC protocol #0807008 and #1108007) and in accordance with the National Institute of Health laboratory animals use guidelines.
Diet Formulation
Six to 8 week-old mice (n=6) were given free access to the liquid diet after sham operation. For 7 days, the intake of each mouse was monitored and recorded to evaluate its daily intake. The mice ate an average of 9.8 ± 0.2mL per day even when more food was available (data not shown). Therefore, the TDCA supplemented diet was then made so that the concentration of TDCA given per day was based on an estimated intake of 10mL per mouse.
Previous studies in both humans and rats have shown a wide dose range (6-150 mg/kg/day) when bile salts are supplemented to the diet (16-19). There were no studies specific to mice at the time of our initial experimentation; therefore, we tried a few different doses including 20, 50, or 100mg/kg/day of TDCA supplementation. The dose of 50mg/kg/day resulted in the best results when comparing levels of apoptosis and proliferation (data not shown). This dose was used for all subsequent experiments.
Intestinal Injury Model and Survival Model
Six to eight week-old mice (n = 5-10 per group) underwent isoflurane anesthesia and sham laparotomy. Post operatively they were given a liquid diet with or without the addition of TDCA at a dose 50mg/kg/day. On post-operative day 6, mice were injected intraperitoneally with 10mg/kg dose of lipopolysaccharide (LPS from E. coli 055:B5, 62326, Sigma-Aldrich) or an equivalent volume of normal saline (NS) for sham animals. Twenty-four hours later mice were sacrificed under isoflurane anesthesia and the intestine, liver, and lungs were harvested.
A separate cohort of mice (n=25 per group) was used to evaluate survival differences. Mice were given the liquid diet with or without TDCA supplementation at 50mg/kg/day for 7 days. After 7 days, they were injected intraperitoneally with a 25mg/kg dose of LPS. Mice were followed another 7 days for survival and were kept on the same liquid diet as given before the injury. All experiments were repeated and found to be reproducible.
Intestinal Injury Quantification
Hematoxylin and eosin (H&E) and caspase-3 staining was used to identify and quantify intestinal epithelial apoptosis in 100 well-oriented crypt-villus units. H&E-stained sections were evaluated for morphological changes including cell shrinkage with condensed nuclei (pyknosis) and nuclear fragmentation (karyorrhexis) in a blinded fashion. Active caspase-3 staining was used to assess functional assessment of cellular death and was performed as previously described (20). Briefly, paraffin-embedded slides were deparaffinized, rehydrated, and underwent blocking of endogenous peroxidase activity before being immersed in citric acid based antigen unmasking solution (Vector; Burlingame, CA) and heated in a microwave for 15 minutes. Sections were blocked with 50% goat serum (Vector Laboratories; Burlingame, CA) after cooling to 42 °C. Slides were then incubated overnight with rabbit polyclonal anti-active caspase-3 (1:100; Cell Signaling Technology; Danvers, MA) at 4°C. On the subsequent day, sections were incubated with goat anti-rabbit biotinylated secondary antibody (1:200; Vector Laboratories) for 30 minutes followed by incubation with Vectastain Elite avidin-biotin-peroxidase complex reagent (Vector Laboratories) for 30 minutes. Slides were developed with diaminobenzidine and counterstained with hematoxylin. Quantification of brown-stained apoptotic cells was done in a blinded manner.
Intestinal Proliferation Quantification
In order to label S-phase cells, mice were injected with 5-bromo-2′deoxyuridine (BrdU; 200μL intraperitoneal injection per animal; Invitrogen Corporation; Carlsbad, CA) 90 minutes prior to sacrifice. BrdU staining was later detected via immunohistochemistry as previously described (20). Briefly, paraffin-embedded slides were deparaffinized, rehydrated, and underwent blocking of endogenous peroxidase activity before being immersed in citric acid based antigen unmasking solution (Vector; Burlingame, CA) and heated in a microwave for 15 minutes. Sections underwent protein block (Dako; Carpinteria, CA) after cooling to 42 °C. Slides were then incubated overnight with rat monoclonal anti-BrdU (1:500; Accurate Chemical & Scientific) at 4°C. On the subsequent day, slides were incubated with goat anti-rat secondary antibody (1:500; Accurate Chemical & Scientific) for 30 minutes. Slides were later incubated with Streptavidin/horseradish peroxidase (Dako) for 60 minutes. Sections were developed with diaminobenzidine and counterstained with hematoxylin. Quantification of intestinal proliferation was done in a blinded manner in 100 well-oriented jejunal crypt-villus units.
Intestinal Morphology Evaluation
Villus length was measured using the Nikon Eclipse TE 300 inverted microscope (Nikon Instruments Inc., Melville, NY). Twelve well-oriented villi were measured from the villus tip to the crypt neck on H&E stained slides. Measurements were in micrometers and the reader was blinded as to which diet the animals had received.
Quantification of Liver and Lung Injury
The liver and lung were stained with H&E after being embedded in paraffin (n = 4-5 per group). Sections were evaluated by a pathologist blinded to what group the animals belonged. Liver inflammation was quantified by counting PMN infiltration. Ten high power fields (HPF's) were counted and the number of PMN's was recorded. The average number of PMN's was then assigned to that animal. The lung slides were given a grade from 1 to 4 for level of acute interstitial inflammation which was based on the amount of neutrophils present in the alveolar septa.
Statistical analysis
One-way analysis of variance (ANOVA) with Newman-Keuls Multiple Comparison Post-Test was used when 3 or more groups were compared. A log rank test was used to analyze survival curves. All data are presented as mean ± SEM and were analyzed using the Prism 5.0 statistical program (GraphPad Software, San Diego, CA). Significance was considered with a p-value of less than 0.05.
RESULTS
TDCA Supplementation Improves Survival
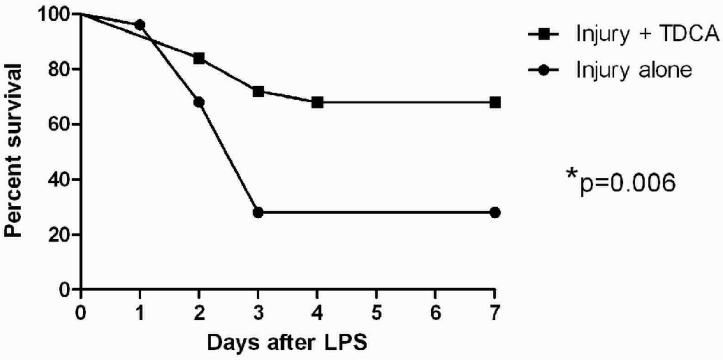
Mice that received a diet supplemented with TDCA (50 mg/kg/day) had significantly improved survival after 25 mg/kg intraperitoneal injection of LPS over those that received non-supplemented diets (68% vs. 28% survival; Fig. 1). Of the mice that died, all did so within 4 days of receiving the LPS. On days 1 to 4, all of the living mice appeared clinically ill (e.g., decreased food intake, decreased movement in the cage, and huddled up together with other mice). The mice that lived beyond the initial 4 days all seemed to recover to their pre-injection activity. However, among the living mice, those that received the TDCA supplemented diet seemed to recover quicker from the insult compared to those animals that did not receive the TDCA. Similar results were reproduced on 3 separate survival trials in order to ensure that the results were accurate.
FIG 1. TDCA Supplementation Improves Survival After LPS-Induced Injury.
Mice were given a diet with or without TDCA supplementation for 7 days prior to injury. They were then given an intraperitoneal injection of LPS at 25mg/kg. Mice were followed for 7 days after the injury, given the same diet as before the injury, and survival was monitored. Animals that received the TDCA supplemented diet had significantly increased survival when compared to those that had control diets.
TDCA Supplementation Decreases Intestinal Injury
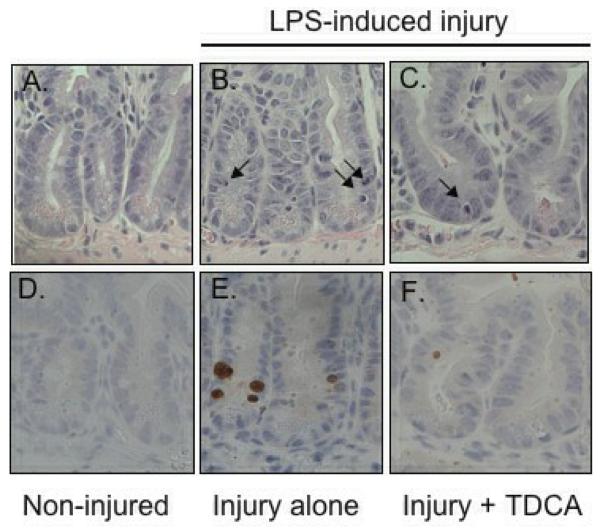
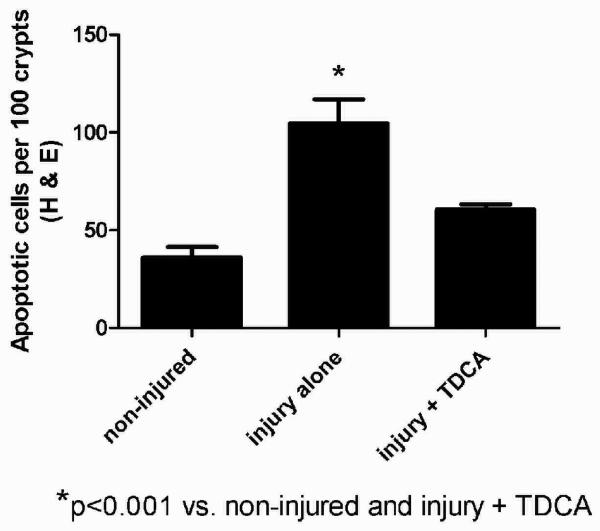
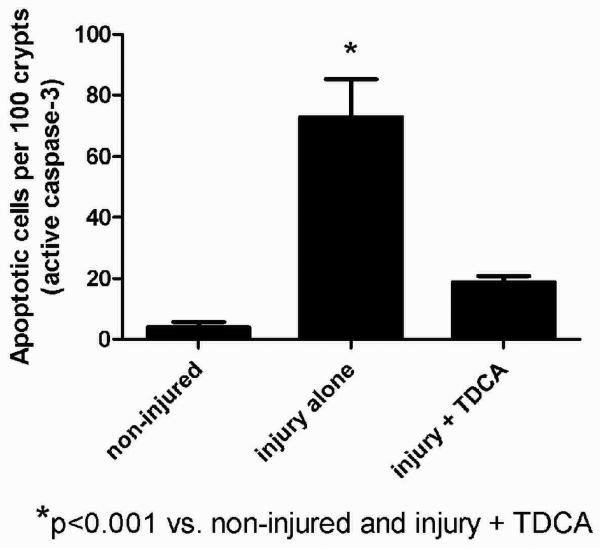
A separate cohort of mice received the same liquid diet with or without the addition of 50mg/kg/day of TDCA. Intestinal apoptosis was measured 24 hours after an intraperitoneal injection of either NS or LPS. As expected, crypt apoptosis was significantly increased in those animals that received LPS when compared to NS by both H&E (104.7 ± 12.3 vs. 36.0 ± 5.5 per 100 crypts; Fig. 2A-B, G) and active caspase-3 staining (72.8 ± 12.5 vs. 4.0 ± 1.8 per 100 crypts; Fig. 2D-E, H). With the addition of TDCA to the liquid diet, crypt apoptosis in mice given LPS was significantly decreased when measured by both H&E (60.5 ± 2.7 per 100 crypts; Fig. 2C, G) and caspase-3 (18.7 ± 2.1 per 100 crypts; Fig. 2F, H). Interestingly, there was no statistical significance between the levels of apoptosis in non-injured mice and those mice that underwent LPS-induced injury with TDCA supplementation. These data show that adding TDCA to the diet is protective of LPS-induced intestinal injury.
FIG 2. TDCA Supplementation Reverses LPS-Induced Intestinal Apoptosis.
Apoptosis was quantified in 100 crypts using both H&E (arrows, A-C) and active caspase-3 staining (brown stained cells, D-F). Animals that received LPS (10mg/kg IP) had significantly increased levels of crypt apoptosis by both staining methods. With the addition of TDCA to the diet, mice that received LPS had levels of apoptosis comparable to non-injured mice (G-H).
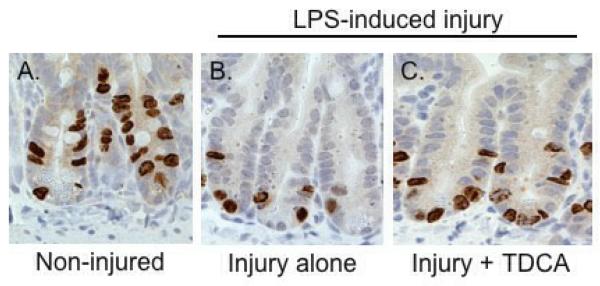
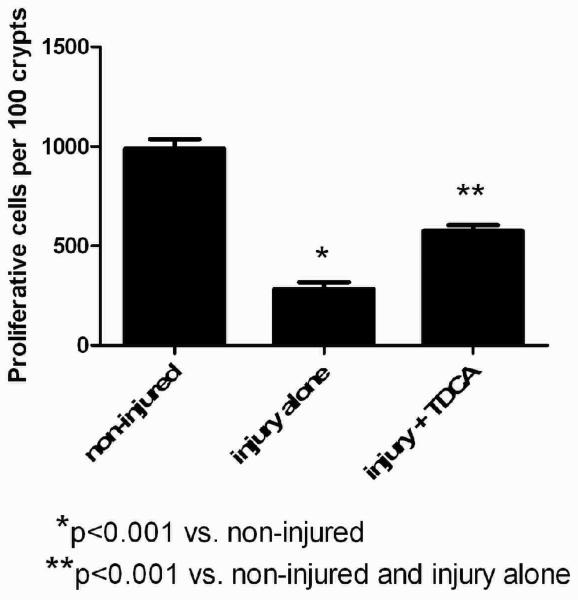
TDCA Supplementation Increases Intestinal Proliferation
Mice that received intraperitoneal LPS had a significantly decreased number of S-phase cells when compared to those that received NS (282.1 ± 35.0 vs. 989.0 ± 46.9 per 100 crypts; Fig. 3A-B, D). This shows that intestinal proliferation is significantly decreased in the presence of LPS-induced intestinal injury. When TDCA is added to the diet in mice that received LPS there is a significant increase in intestinal proliferation compared to the mice receiving LPS without TDCA supplementation (574.0 ± 29.9 per 100 crypts; Fig. 3C, D). This shows that the addition of TDCA to enteral feeding can significantly increase intestinal proliferation in the setting of intestinal injury.
FIG 3. TDCA Supplementation Partially Ameliorates LPS-Induced Reduction of Intestinal Proliferation.
S-phase cells stain brown after BrdU injection and were quantified in 100 crypts (A-C). Animals that received LPS had significantly fewer proliferative cells than non-injured mice. The addition of TDCA to the diet was able to ameliorate this decrease but it did not reach baseline levels of non-injury proliferation (D).
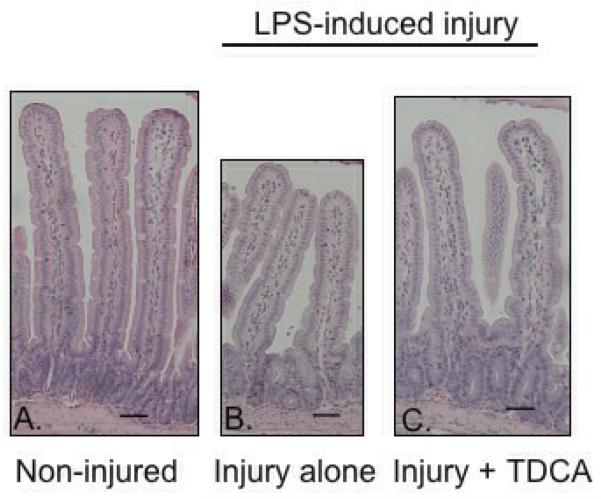
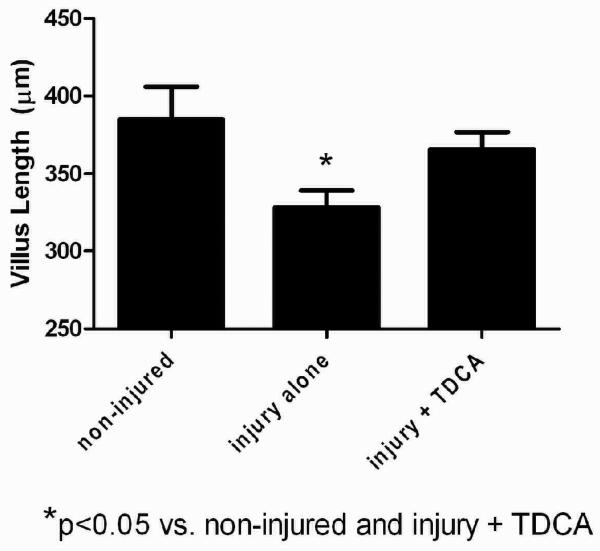
TDCA Supplementation Increases Villus Length
Villus length was significantly decreased in mice that received LPS when compared to those that received NS (328.3 ± 10.7μm vs. 385.2 ± 20.7μm; Fig. 4A-B, D). When mice received TDCA supplementation, however, the LPS-induced loss of villus length was diminished (365.7 ± 11.1μm; Fig. 4C, D). Interestingly, there was no significant difference between mice who received TDCA and LPS when compared to non-injured mice. These data further support TDCA as a protective agent in LPS-induced intestinal injury.
FIG 4. TDCA Supplementation Restores LPS-Induced Reduction in Villus Length.
Villus length was measured in 12 healthy jejunal villi per mouse. Representative pictures are shown at 10x magnification. The black bars each measure 50μm (A-C). Mice that underwent LPS-induced injury had significantly shorter villi. This effect reversed with the addition of TDCA to the diet (D).
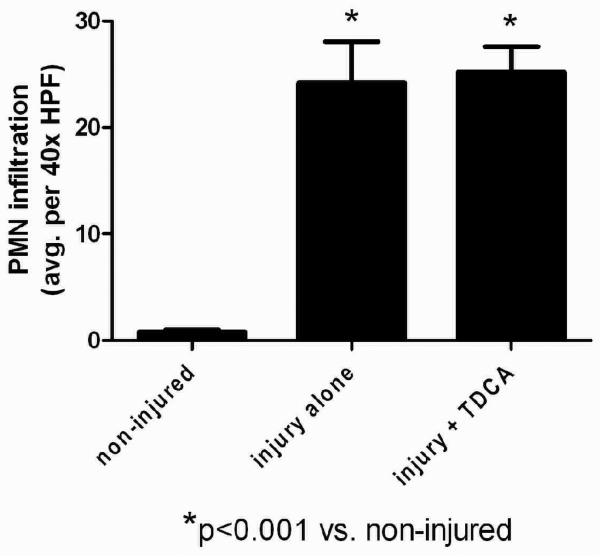
TDCA Supplementation Showed No Protection in Liver Injury
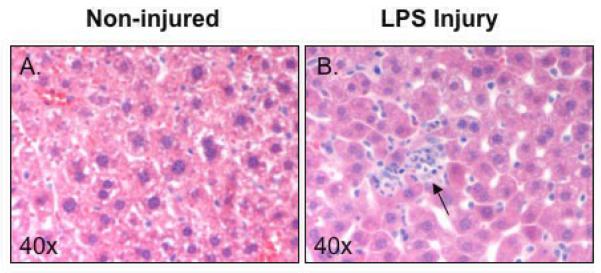
Acute liver injury was quantified and the average number of PMN's per HPF was applied to each animal in the different groups. As expected, non-injured mice showed very minimal inflammation and those receiving systemic LPS showed markedly increased PMN infiltration (Fig. 5A-B). Liver in mice that received LPS had significantly increased PMN infiltration when compared to the livers in mice that received NS (24.2 ± 3.9 vs. 0.8 ± 0.2 PMN's; Fig. 5C). However, unlike the intestinal injury, mice that received TDCA were not protected from LPS-induced liver injury (25.2 ± 2.4 PMN's; Fig. 5C).
FIG 5. TDCA Supplementation is Not Protective in LPS-Induced Liver Injry.
Liver injury was measured by the number of PMN infiltration. Representative pictures are shown at 40x magnification. Mice that received NS showed little inflammation (A) while mice that received LPS showed marked inflammation (arrow, B). The addition of TDCA to the diet did not result in improvement of this liver injury (C).
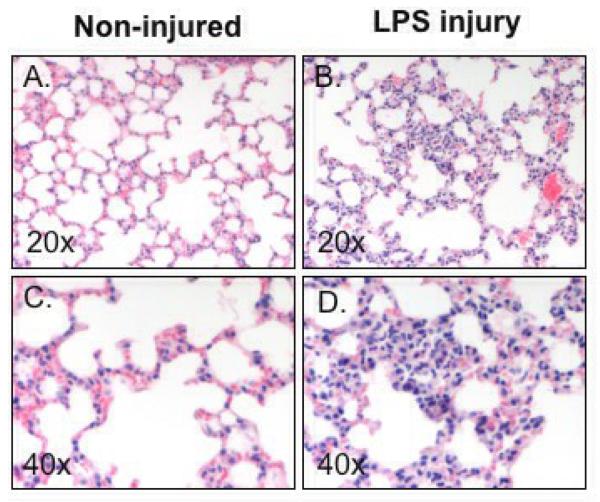
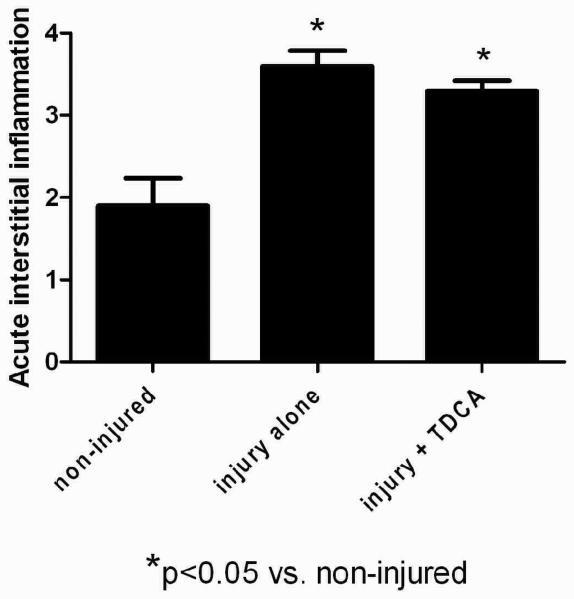
TDCA Supplementation Showed No Protection from Lung Injury
Acute lung injury was assessed by the amount of neutrophils present in the alveolar septa and a grading system of 1 through 4 was applied. Non-injured mice showed very little neutrophil infiltration while those receiving systemic LPS showed marked infiltration (Fig. 6A-D). From this we see that LPS-induced injury is systemic and the lungs in mice that receive LPS have significantly more inflammation when compared to the mice that received NS (3.6 ± 0.2 vs. 1.9 ± 0.3 average score; Fig. 6E). However, unlike the intestinal injury, mice who received TDCA and LPS-induced injury were not protected from this lung injury (3.3 ± 0.1 average score; Fig. 6E). These data, along with the liver injury data, show that the LPS-induced injury is a systemic process but that the protective effects of TDCA supplementation are limited to the intestine.
FIG 6. TDCA Supplementation Has No Effect on LPS-Induced Lung Injury.
Lung injury was scored from 1 to 4 based upon the amount of neutrophil infiltration into the alveolar septa. Mice that did not receive LPS had very little inflammation whereas mice that received systemic LPS had marked inflammation (A-D). The addition of TDCA to the diet did not result in significant improvement of this lung injury (E).
DISCUSSION
This study demonstrates that TDCA supplementation to the diet of mice results in protection from injury-induced intestinal apoptosis, diminished crypt cell proliferation, and decreased villus height. This protection of the intestinal mucosa correlates with a marked increase in animal survival when lethal doses of LPS are given. This is the first study to date that shows bile acid supplementation to be beneficial in critical illness in vivo. Importantly, we see that the survival benefit due to TDCA supplementation is associated with intestinal protection and not protection from systemic insult.
Our data support the growing evidence that suggests that intestinal injury and impaired intestinal barrier is the “motor” of the septic state (21,22). This theory stems from that fact that during critical illness the small intestinal absorptive and barrier functions are damaged. This leads to bacterial translocation which is the first step towards proinflammation in the “gut-liver-lung axis” (23). Research suggests that in order to maintain the gut barrier function, the focus should be on maintaining the integrity of the gastrointestinal mucosa (24).
Previous studies on cirrhotic rats have shown enteral bile acid administration to be associated with decreased bacterial overgrowth and decreased bacterial translocation (19). Our study focused on previously healthy mice that underwent a septic insult. We were able to show that enteral bile acid administration resulted in maintained integrity of the intestinal mucosa which translated into increased survival. More studies are needed to determine whether this is associated with decreased bacterial translocation or some other mechanism.
Although this study shows a correlation between TDCA supplementation and increased protection after intestinal injury, there are a few limitations. First, the mice were given the TDCA supplementation in a liquid diet. Although we estimated that each animal received 50mg/kg/day, this was not measured precisely; mice may have received more or less of the daily dose depending on how much food they consumed. Another possible limitation is that the mice were treated with the TDCA supplementation before the time of injury. In clinical practice, however, patients usually present at the time of injury. However, supplemental bile salts may be beneficial to prevent or minimize intestinal injury in high risk disease processes or in conditions were bile salt concentrations are low. Separate studies were done on non-injured mice (data not shown) given diets with or without TDCA supplementation. In these studies, there was a slight increase in proliferative cells but no difference seen in apoptotic cells or villus height. This shows that the effect we see in the current study is secondary to the protective effect of bile salts during intestinal injury and not simply the effect of bile salts on normal intestine. More studies are needed to further our knowledge regarding timing of the bile salt-induced protection.
Despite these limitations, this study shows that TDCA supplementation is protective from intestinal injury and leads to increased survival when used in the treatment of systemic shock. Importantly, we demonstrate that TDCA protection is limited to the intestine and not other organ systems. This study gives further credence to the theory that protection of the intestine is essential in critical illness.
Enteral supplemention of bile salts resulted in clearly beneficial effects where there was normal bile salt homeostasis. There are many conditions where bile salt production, secretion, and absorption are abnormal such as cholestasis, liver failure, short gut syndrome and malabsorbtion from a variety of causes. Bile salts may be critical for intestinal mucosal integrity in these conditions.
Results from our study could also be expanded to other diseases of intestinal injury, like necrotizing enterocolitis or inflammatory bowel disease. In premature infants, duodenal bile salt concentrations are significantly decreased, and the concentrations increase as postnatal age increases (25). Further, in premature low birth weight infants there is low hepatic bile acid clearance (26). There is also a physiologic cholestasis in the newborn (27). Therefore, the intestinal mucosa of the premature infant, at high risk for NEC, is exposed to less bile salt than the full term neonate. Further studies are necessary to determine if simple measures such as enteral supplementation of bile salts in premature infants could diminish the incidence of NEC, or prove beneficial for patients with intestinal injury as part of their spectrum of disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol. 1997;273:G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 4.Coopersmith CM, Stromberg PE, Davis CG, et al. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med. 2003;31:1630–1637. doi: 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 5.Potoka DA, Upperman JS, Zhang XR, et al. Peroxynitrite inhibits enterocyte proliferation and modulates Src kinase activity in vitro. Am J Physiol Gastrointest Liver Physiol. 2003;285:G861–G869. doi: 10.1152/ajpgi.00412.2002. [DOI] [PubMed] [Google Scholar]
- 6.Cinel I, Buyukafsar K, Cinel L, et al. The role of poly(ADP-ribose) synthetase inhibition in preventing endotoxemia-induced intestinal epithelial apoptosis. Pharmacol Res. 2002;46:119–127. doi: 10.1016/s1043-6618(02)00075-0. [DOI] [PubMed] [Google Scholar]
- 7.Coopersmith CM, Stromberg PE, Dunne WM, et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 8.Coopersmith CM, Chang KC, Swanson PE, et al. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30:195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann AF. Intestinal absorption of bile acids and biliary constituents. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 3rd edition Raven Press; New York, NY: 1994. pp. 1845–1867. [Google Scholar]
- 10.Cheng K, Raufmann JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Martinez JD, Stratagoules ED, LaRue JM, et al. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31:111–118. doi: 10.1080/01635589809514689. [DOI] [PubMed] [Google Scholar]
- 12.D'Addio V, Sayles JM, Alvarez C, et al. Bile: a trophic factor for rabbit esophageal epithelium in vitro. Surg Forum. 1996;647:125–127. [Google Scholar]
- 13.Toledo A, Yamaguchi J, Wang JY, et al. Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-kappa B. Dig Dis Sci. 2004;49:1664–1671. doi: 10.1023/b:ddas.0000043383.96077.99. [DOI] [PubMed] [Google Scholar]
- 14.Strauch ED, Wang JY, Bass BL. Bile salt stimulates intestinal epithelial cell migration through TGFbeta after wounding. J Surg Res. 2001;97:49–53. doi: 10.1006/jsre.2001.6110. [DOI] [PubMed] [Google Scholar]
- 15.Strauch ED, Bass BL, Rao JN, et al. NF-kappaB regulates intestinal epithelial cell and bile salt-induced migration after injury. Ann Surg. 2003;237:494–501. doi: 10.1097/01.SLA.0000060459.03270.E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheen-Chen SM, Chen HS, Ho HT, et al. Effect of bile acid replacement on endotoxin-induced tumor necrosis factor-alpha production in obstructive jaundice. World J Surg. 2002;26:448–450. doi: 10.1007/s00268-001-0247-5. [DOI] [PubMed] [Google Scholar]
- 17.Schneider SM, Joly F, Gehrardt MF, et al. Taurine status and response to intravenous taurine supplementation in adults with short-bowel syndrome undergoing long-term parenteral nutrition: a pilot study. Br J Nutr. 2006;96:365–370. doi: 10.1079/bjn20061826. [DOI] [PubMed] [Google Scholar]
- 18.Emmett M, Guirl MJ, Santa Ana CA, et al. Conjugated bile acid replacement therapy reduces urinary oxalate excretion in short bowel syndrome. Am J Kidney Dis. 2003;41:230–237. doi: 10.1053/ajkd.2003.50012. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo-Zuniga V, Bartoli R, Planas R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–557. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- 20.Clark JA, Clark AT, Hotchkiss RS, et al. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30:36–42. doi: 10.1097/shk.0b013e31815D0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 22.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- 23.Pugin J, Chevrolet JC. The intestine-liver-lung axis in septic syndrome. Schweiz Med Wochenschr. 1991;121:1538–1544. [PubMed] [Google Scholar]
- 24.Barber AE, Jones WG, Minei JP, et al. Bacterial overgrowth and intestinal atrophy in the etiology of gut barrier failure in the rat. Am J Surg. 1991;161:300–304. doi: 10.1016/0002-9610(91)91148-c. [DOI] [PubMed] [Google Scholar]
- 25.Boehm G, Braun W, Moro G, et al. Bile acid concentrations in serum and duodenal aspirates of healthy preterm infants: effects of gestational and postnatal age. Biol Neonate. 1997;71:207–214. doi: 10.1159/000244419. [DOI] [PubMed] [Google Scholar]
- 26.Yamato Y, Kimura A, Inoue T, et al. Fetal bile acid metabolism: analysis of urinary 3beta-monohydroxy-delta(5) bile acid in preterm infants. Biol Neonate. 2001;80:19–25. doi: 10.1159/000047114. [DOI] [PubMed] [Google Scholar]
- 27.Strandvik B, Wahlen E, Wikstrom SA. The urinary bile acid excretion in healthy premature and full-term infants during the neonatal period. Scand J Clin Lab Invest. 1994;54:1–10. doi: 10.3109/00365519409086503. [DOI] [PubMed] [Google Scholar]