Abstract
The activation of the human polyomavirus BK causes polyomavirus-associated nephropathy in immunocompromised humans. Studies of the virus have been restricted since the virus DNA replication is species specific. Cell-based and cell-free DNA replication systems, including the BK virus (BKV) monopolymerase DNA replication system using purified proteins, reproduce the species specificity (28). Therefore, the major host proteins comprising this assay, DNA polymerase α-primase (Pol-prim) and replication protein A (RPA), were intensively studied here. We demonstrate that Pol-prim plays a major role in the species specificity of BKV DNA replication. Both large subunits p180 and p68 of the enzyme complex have central functions in modulating the host specificity. Recently, an inhibitory activity of BKV DNA replication was described (C. Mahon, B. Liang, I. Tikhanovich, J. R. Abend, M. J. Imperiale, H. P. Nasheuer, and W. R. Folk, J. Virol. 83:5708-5717, 2009), but neither mouse Pol-prim nor mouse RPA diminishes cell-free BKV DNA replication. However, the inhibition of BKV DNA replication in mouse extracts depends on sequences flanking the core origin. In the presence of human Pol-prim, the inhibitory effect of mouse cell factors is abolished with plasmid DNAs containing the murine polyomavirus early promoter region, whereas the late enhancer region and the core origin are supplied from BKV. Thus, BKV replication is regulated by both Pol-prim, as a core origin species-specific factor, and inhibitory activities, as origin-flanking sequence-dependent factor(s).
BK virus (BKV) is a human polyomavirus that was first isolated in the 1970s (15). Up to 90% of adults have serologic evidence of exposure to BKV, but in most humans the virus remains latent (25, 26). Almost all disease accompanied by BKV reactivation has been found in immunocompromised patients (22). In recent years, BKV has been associated with nephropathy (polyomavirus-associated nephropathy, or PVAN) in up to 10% of renal transplant patients. Once established, the disease results in allograft loss in 45 to 70% of the patients (18). Importantly, BKV preferentially replicates in human cells and less well in cells of other primates, and the virus is highly tumorigenic in rodents (21, 41, 44). This fact and the lack of sustainable viral replication in rodents or other convenient, experimental animal models have been an enormous setback to the study of PVAN.
As with other members of the Polyomaviridae family, BKV virions are nonenveloped icosahedral particles with a diameter of 45 nm that contain a circular double-stranded DNA genome of 5.3 kb (1). In BKV and in other polyomaviruses, three genomic areas have been distinguished: (i) a noncoding control region including the origin of viral DNA replication, (ii) the early genes encoding large and small T antigens (TAgs), and (iii) the late genes which code for the capsid proteins VP-1, VP-2, and VP-3 and the agnoprotein (22).
BKV DNA replication is similar to that of all other members of the Polyomaviridae family and requires only one viral protein, the multifunctional large TAg, whereas all other replication factors are supplied by the host (13, 14, 28, 39, 47). As the first step, TAg binds to the core origin, which contains the early palindrome, an AT-rich sequence, and the TAg binding site II, which consists of two pairs of G(G/A)GGC pentanucleotides. In the presence of ATP, TAg forms a double hexamer and partially melts the early palindrome (EP) and untwists the AT-rich sequence of the BKV core origin (5, 6, 14). Then the TAg double hexamers bidirectionally unwind the viral replication origin, which requires ATP hydrolysis. In the following process the two hexamers remain associated with each other, with the separated single-stranded DNA (ssDNA) threading through the hexameric channels (14). The viral core origin is sufficient to constitute a functional replication origin, but the presence of auxiliary domains increases its activity 5- to 100-fold in vivo (16, 30). After the viral TAg unwinds the core origin and its flanking sequences, replication protein A (RPA), the main eukaryotic ssDNA-binding protein, covers the resulting stretches of ssDNA, whereas topoisomerase I releases the resulting torsional stress and enhances initiation of DNA replication (5, 7, 43). Then, DNA polymerase α-primase (Pol-prim) is loaded onto this TAg-RPA-topoisomerase 1-DNA complex, yielding a functional initiation complex. In the following step, Pol-prim synthesizes short RNA primers at the origin, and these RNA primers are elongated by the DNA polymerase function of the enzyme complex (9, 35, 47). After a polymerase switch from Pol-prim to DNA polymerase δ (Pol δ) with the help of RPA, replication factor C (RFC), and proliferating cell nuclear antigen (PCNA), processive DNA synthesis is completed by Pol δ in association with PCNA, the sliding clamp, on the leading strand (38, 51, 54, 59). Lagging-strand synthesis is discontinuous, and multiple initiation events catalyzed by Pol-prim must take place. Again, after the elongation of the RNA primers by Pol-prim, DNA synthesis is switched to Pol δ, which then synthesizes the complete Okazaki fragments. The maturation of these Okazaki fragments requires the collaboration of RNase H, PCNA, flap endonuclease 1 (Fen-1), Pol δ, and DNA ligase I to establish a continuous strand also on the lagging strand (9, 19, 20, 51, 55).
TAg functions in infected cells rely heavily on specific associations with host proteins; for example, TAg interacts with RPA, Pol-prim, and topoisomerase I to replicate viral DNA. Selective interactions with the host p180 and p48 subunits of Pol-prim were shown to be responsible for species-specific replication of simian virus 40 (SV40) and murine polyomavirus (mPyV) DNAs, respectively (8, 47, 50). The subunits of Pol-prim are highly conserved since 88, 80, 89, and 90% of the amino acids are identical between human and murine p180, p68, p58, and p48, respectively. Biochemical studies have shown that TAg interacts independently with all four subunits of Pol-prim (8, 12, 57). Moreover, the p180, p58, and p48 subunits of Pol-prim also physically bind to RPA (7, 11, 57). RPA and TAg binding sites in the Pol-prim complex are essential for SV40 DNA replication in vitro since the presence of an excess of these purified binding peptides diminishes viral DNA replication in vitro (52, 53). Interestingly, species specificity requires the viral origin of DNA replication, whereas physical protein-protein interactions of purified protein complexes are not host specific in the absence of viral origin DNA (29, 42).
Consistent with other polyomaviruses, analyses of BKV TAg-dependent DNA replication recently revealed that BKV DNA cannot be replicated in murine cells and that cell extracts are able to mimic this behavior (28). Furthermore, a BKV DNA replication system with the purified human proteins Pol-prim, RPA, topoisomerase I, and BKV TAg was inhibited by murine extracts, whereas SV40 DNA replication was not. Further investigations revealed that the presence of inhibitory activities (IAs) in extracts from murine cells blocks BKV DNA replication at an early step of TAg-mediated unwinding of the BKV origin of replication. Detailed analyses using the BKV monopolymerase DNA replication system, which we report here, show that Pol-prim functions as a species-specific factor associated with core origin functions. In addition, we reveal that the inhibitory activities in murine extracts, which are associated with origin-flanking sequence-dependent factor(s), regulate BKV DNA replication in murine cell extracts in a Pol-prim-independent manner.
MATERIALS AND METHODS
DNA.
pUC18-based plasmid DNAs with complete viral origins included pOriBKV (termed B-B-B, for BKV core region flanked by BKV sequence) (see Fig. 7) containing a fragment of the archetype BKV Dik strain (28), pOriSV40 (SV S strain) (42), and plasmids containing BKV-mPyV chimeric origins (P-B-B, B-B-P, and P-B-P, for the BKV [B] core region flanked by BKV and mPyV [P] sequences) (28). The pUC18 plasmid without an insert served as a negative control (pOri−) for cell-free DNA replication. All DNAs for replication assays were purified with Midiprep kits (Qiagen, Germany).
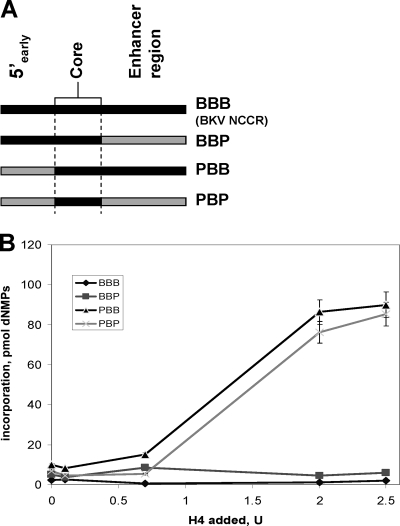
FIG. 7.
Factors interacting with BKV core origin-flanking sequences modulate inhibition of BKV DNA replication in vitro. (A) The complete BKV (B-B-B) or BKV-mPyV chimeric origins (P-B-B, B-B-P, and P-B-P, as indicated; early origin region, core origin, and late origin regions are depicted from left to right and were derived from BKV [B] or mPyV [P] origin) were utilized to investigate the influence of mouse factors on the species specificity of BKV DNA replication in mouse extracts. The various origin constructs are depicted in panel A. NCCR, noncoding control region. BKV DNA was replicated in extracts from nonpermissive mouse cells in the presence of human Pol-prim. (B) The incorporation of dNMPs into DNA templates containing a complete BKV (B-B-B) or BKV-mPyV chimeric origins (P-B-B, B-B-P, and P-B-P) in the presence of 200 ng of recombinant BKV TAg, 100 μg of FM3A mouse extract, and the indicated amount of recombinant human Pol-prim (0.0, 0.7, 2.0 and 2.5 units). Incorporation of dNMPs into DNA was measured by scintillation counting. The DNA synthesis was determined in duplicates and repeated three times. The average of these experiments and the standard deviations are presented.
Protein purification.
Human recombinant topoisomerase I (48) and RPA (36, 40) were expressed and purified, and their concentrations and activities were determined as previously described (3, 27). Expression and purification of human and mouse Pol-prim as well as different hybrids were performed as previously described (50). Briefly, High Five (Invitrogen) insect cells (3.0 × 109) were infected with 10 PFU of each recombinant baculovirus per cell and incubated at 27°C for 48 h. Pol-prim complexes were purified by phosphocellulose chromatography, followed by immunoaffinity chromatography (49). Immobilized monoclonal antibody SJK 287-38 was used to purify recombinant Pol-prim complexes containing mouse p180. SJK 237-71 coupled to Sepharose 4B was used to purify recombinant Pol-prim containing human p180. DNA polymerase assays and DNA primase assays were performed as previously described (4, 17, 34).
The BKV TAg was expressed and purified as previously described (28). Briefly, High Five insect cells (Invitrogen) were infected with the recombinant baculovirus (10 PFU per cell). Infected cells were harvested at 48 h, and BKV TAg was purified by immobilized metal affinity chromatography (IMAC) using Talon resin (Clontech). The purity and amount BKV TAg were determined by SDS-PAGE and Coomassie brilliant blue staining.
SV40 TAg was purified from extracts of insect High Five cells infected with recombinant baculoviruses by immunoaffinity chromatography using monoclonal antibody PAb101 coupled to protein A Sepharose (8, 37).
ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were carried out under the buffer conditions used for in vitro DNA replication, as described previously (12, 42). In brief, plastic 96-well microtiter plates (Immuno Maxi Sorb; Nunc) were coated overnight with excess (1 μg) recombinant human or mouse Pol-prim. Unbound protein was washed with phosphate-buffered saline (PBS). Wells were blocked for 2 h with 0.3 ml of 3% bovine serum albumin (BSA) (Sigma) in PBS. After the plates were washed, the indicated amounts (see Fig. 2) of BKV TAg (in 50 μl) were added, and plates were incubated for 1 h at room temperature and then washed with PBS. Polyclonal antibody against SV40 TAg that recognizes BKV TAg, kindly provided by W. Deppert, was added; the plates were incubated overnight at 4°C, washed, and then visualized by incubation with anti-rabbit immunoglobulin serum conjugated with horseradish peroxidase (Jackson Laboratories, Inc.) in the presence of 3% BSA for 1 h, followed by reaction with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Sigma]. The reaction was allowed to proceed for 20 min, and then plates were quantified spectrophotometrically at 410 nm.
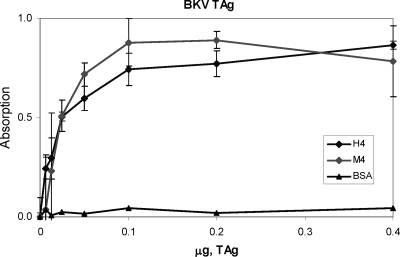
FIG. 2.
Physical interactions of BKV TAg with human and mouse Pol-prim. Purified recombinant Pol-prim complexes (H4 and M4 with all subunits of human or mouse origin, respectively) were immobilized on ELISA plates (0.5 μg per well) and blocked with 3% BSA. BSA alone was used as a negative control. Purified BKV TAg was added in the indicated amounts and incubated for 1 h. After plates were washed, bound TAg was detected using a polyclonal antibody, which was followed by incubation with peroxidase-coupled anti-rabbit antibody and a chromogenic substrate. As an additional control, BKV TAg immobilized on ELISA plates was incubated with the peroxidase-coupled secondary antibody and substrate in parallel; only a background signal was detected (not shown).
DNA synthesis on φX174 ssDNA.
The DNA synthesis on φX174 ssDNA was carried out according to Smith et al. (46). Briefly, the reaction mixture contained the following: 1 μg of φX174 ssDNA (NEB); 20 mM Tris-acetate (pH 7.3); 5 mM magnesium acetate; 20 mM potassium acetate; 1 mM dithiothreitol (DTT); 0.1 mg/ml BSA; 1 mM ATP; 0.1 mM each CTP, GTP, UTP, dATP, dCTP, dGTP, and dTTP; and 0.1 mM [α32P]dCTP (3,000 Ci/mmol; Perkin-Elmer). Comparisons between human and mouse Pol-prim were made by titration, and the amount of enzyme complexes was varied from 0.5 to 2.0 units (3.04 U/μg for human Pol-prim, 2.88 U/μg for mouse Pol-prim, and 1 U to 100 pmol of deoxynucleoside monophosphate [dNMP] incorporation in a 1-h reaction) of each complex per assay. The incorporation of dCMP was determined by acid precipitation of DNA and followed by scintillation counting.
In vitro DNA replication assays.
Replication of DNAs in vitro was assayed as previously described (28) with slight modifications. Briefly, the reaction mixture (40 μl) contained the following: 20 mM HEPES, pH 7.8; 7 mM Mg acetate [MgAc]; 1 mM DTT; 4 mM ATP, 200 μM each CTP, UTP, and GTP; 50 μM dCTP; 100 μM each dATP, dTTP, and dGTP; 40 mM creatine phosphate (pH 7.8); 40 μg/ml creatine kinase; 5 μCi of [α32P]dCTP (3,000 Ci/mmol, Perkin-Elmer); 0.25 μg of test plasmid DNA; FM3A cell extract (100 μg of total protein); 0.2 μg of purified BKV TAg; and purified recombinant human Pol-prim in the indicated amounts (see Fig. 4). After incubation for 60 min at 37°C, reaction products were precipitated with cold 10% (wt/vol) trichloroacetic acid (TCA) containing 2.5% (wt/vol) sodium pyrophosphate, spotted on glass fiber filters (GF/C; Whatman), washed with 1 M HCl, and analyzed by scintillation counting.
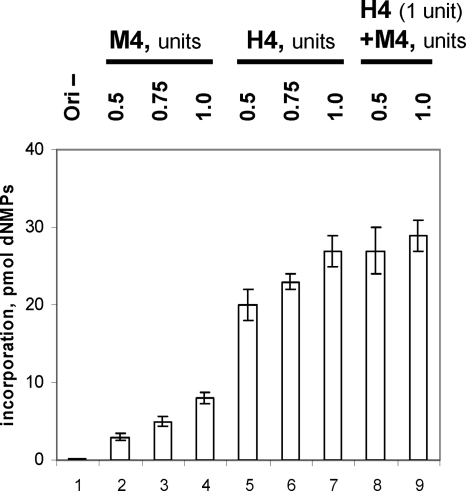
FIG. 4.
Mouse Pol-prim does not inhibit BKV DNA replication in vitro. Incorporation of dNMPs into DNA containing BKV origin of replication in the presence of 200 ng of the purified recombinant BKV TAg, 50 ng of topoisomerase I, 1,000 ng of RPA, and purified human or/and mouse Pol-prim (H4 and M4, respectively), as indicated, was measured. Plasmid without a functional viral origin (Ori−) in the assay with the largest amount of human Pol-prim served as a negative control (bar 1). The effect of mouse Pol-prim on DNA synthesis by human Pol-prim was determined with a BKV origin of replication (bars 8 and 9). After 60 min, DNA was precipitated on GF/C filters, and incorporation of dNMPs by Pol-prim was determined by acid precipitation of newly formed DNA and scintillation counting. DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments and the standard deviations are presented.
Monopolymerase DNA replication assay.
The SV40 and BKV monopolymerase replication assay contained 0.5 μg of pOriBKV DNA (28) or 0.5 μg of pOriSV40 (42); 50 ng of topoisomerase I; 100 ng of Pol-prim; 1 μg of RPA in 30 mM HEPES, pH 7.8; 7 mM MgAc; 0.1 mM EGTA; 0.5 mM DTT; 200 μM each UTP, GTP, and CTP; 4 mM ATP; 100 μM each dATP, dGTP, and dTTP; 10 μM dCTP; 40 mM creatine phosphate; 1 μg creatine kinase; 0.1 mg/ml heat-treated BSA; and 5 μCi of [α32P]dCTP (3,000 Ci/mmol, Perkin-Elmer) in 40 μl. Purified BKV or SV40 TAg (0.2 μg) was added to start the reaction, and after incubation for 60 min at 37°C, the reaction products were precipitated with cold 10% (wt/vol) TCA containing 2.5% (wt/vol) sodium pyrophosphate, spotted on glass fiber filters (GF/C; Whatman), washed with 1 M HCl, and analyzed by scintillation counting.
Initiation of replication of BKV and SV40 DNA.
Initiation reactions were performed as previously described (29, 31, 46) with slight modifications. The initiation reaction mixtures contained 0.25 μg of origin-containing DNA, 1.6 μg of TAg, 1 μg of RPA, 30 mM HEPES-KOH (pH 7.8), 7 mM MgAc, 1 mM EGTA, 1 mM DTT, 0.2 mM UTP, 0.2 mM GTP, 0.01 mM CTP, 4 mM ATP, 40 mM creatine phosphate, 1 μg of creatine kinase, 0.3 μg of topoisomerase I, 0.2 mg/ml of BSA, and 10 μCi of [α-32P]CTP (3,000 Ci/mmol; Perkin-Elmer). Recombinant Pol-prim was added as indicated in the figure legends. Half of the reaction mixture was spotted on DE 81 paper (Whatman), precipitated with cold 10% (wt/vol) TCA containing 2.5% (wt/vol) sodium pyrophosphate, washed with 1 M HCl, and analyzed by scintillation counting. The remaining reaction products were precipitated with ethanol in the presence of 0.8 M LiCl-0.5 mg/ml DNA, redissolved in 45% formamide-5 mM EDTA-0.09% xylene cyanol FF at 65°C for 30 min, heated for 3 min at 95°C, and separated by denaturing 20% polyacrylamide gel electrophoresis for 3 to 4 h at 600 to 800 V as previously described (42). The reaction products were visualized by autoradiography using an FLA-5100 imager (Fuji, Europe).
FM3A extract.
FM3A cell extracts were prepared as previously described (28). Briefly, logarithmically growing FM3A cells in RPMI medium supplemented with 10% fetal bovine serum (FBS) were collected by centrifugation and washed with phosphate-buffered saline and then with hypotonic buffer. Cells were homogenized and NaCl adjusted to 100 mM. Extracts were clarified by centrifugation at 20,000 × g for 30 min and stored at −80°C.
RESULTS
Replication protein A is not a species-specific factor in cell-free BKV DNA replication.
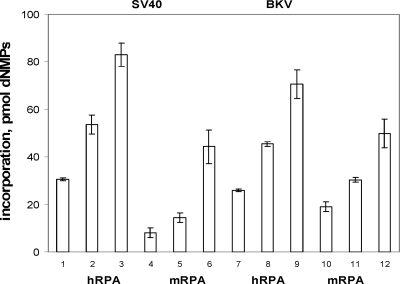
The monopolymerase replication assay was then used to analyze the species specificity of BKV DNA replication in vitro with DNA containing the BKV origin of replication (28). BKV TAg supports DNA replication in vitro and shows specificity for the origin DNA in this assay as well as species specificity since the addition of mouse but not human cell extracts inhibits BKV DNA replication (28). However, whether components of the replication machinery regulate host-specific inhibition of BKV DNA replication remained unknown. Therefore, the question arose as to whether mouse RPA, a host replication factor essential for the initiation of viral DNA replication, would inhibit BKV DNA replication or whether mouse RPA would be able to substitute for human RPA in BKV DNA replication. Preliminary data suggested that RPA is not responsible for inhibition since during the purification of IA from mouse FM3A extracts, RPA and IA were discovered in different purification fractions (data not shown). However, to prove that RPA is not identical with IA, mouse RPA, which was partially purified by ssDNA-cellulose chromatography, was tested in BKV and SV40 DNA replication assays. Mouse RPA is fully active in both systems. Mouse RPA is neither the IA nor the species-specific factor of BKV DNA replication (Fig. 1).
FIG. 1.
Monopolymerase DNA replication in the presence of human and mouse RPA. Incorporation of dNMPs into DNA containing SV40 (bars 1 to 6) or BKV (bars 7 to 12) origin of replication in the presence of 200 ng of the purified recombinant SV40 and BKV TAg, respectively, 50 ng of topoisomerase I, 100 ng of purified human Pol-prim, and increasing amounts of human or mouse RPA (0.5, 1.0, and 1.5 μg), as indicated, was measured as previously described. DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments and the standard deviations are presented.
BKV large T antigen physically and functionally interacts with both human and mouse DNA polymerase α-primase in similar ways.
SV40 TAg has been shown to interact physically with purified Pol-prim and independently with all four Pol-prim subunits (11, 46, 57). In contrast, physical interactions of purified Pol-prim with BKV TAg have not been investigated. Therefore, we tested whether the physical interactions of BKV TAg with the Pol-prim complex are species specific by a modified ELISA (12). The results revealed binding of BKV T TAg to human and mouse Pol-prim complexes with similar efficiencies (Fig. 2). Therefore, BKV large TAg showed no species specificity in physical interactions with Pol-prim on this level. In parallel, SV40 TAg binding to Pol-prim complexes was analyzed, and consistent with previous data, no host specificity was observed (42; also data not shown). The results revealed that human and mouse Pol-prims physically bind to BKV TAg with similarly efficiencies. This raises the question of whether the initiation complex containing mouse Pol-prim and BKV TAg would be functional or could inhibit BKV DNA replication. The latter occurrence was previously seen when peptides containing TAg-binding sites of human Pol-prim or RPA were added to the DNA replication assays prior to the start of the reaction (10, 52).
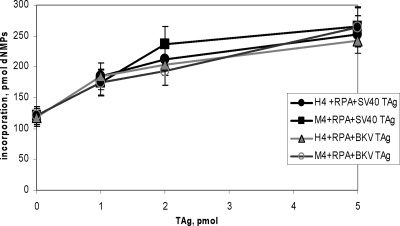
Pol-prim has been shown to be the major host factor responsible for species-specific replication of mPyV and SV40 DNAs in human and mouse cell extracts, respectively (8, 23, 46, 50). The activity of human and mouse Pol-prim was tested in assays with ssDNA as a template (Fig. 3 and data not shown). Pol-prim is able to perform DNA synthesis on ssDNA substrates alone or in the presence of other initiation proteins, e.g., RPA and large TAg. As previously described, RPA inhibits Pol-prim synthesis on ssDNA, but this inhibition is reversed by viral TAg, suggesting that all three proteins together form an active synthesis complex (Fig. 3 and data not shown). This assay provides the evidence for the functional activity of the purified proteins and their ability to cooperate in DNA synthesis. We investigated whether BKV TAg stimulates Pol-prim synthesis on an ssDNA substrate in a species-specific manner. Both SV40 and BKV TAg stimulated mouse and human Pol-prim complexes on φX174 DNA in the presence or absence of RPA (Fig. 3 and data not shown). Consistent with previous SV40 studies, these experiments revealed that BKV TAg behaves in the same way as SV40 TAg by efficiently stimulating the Pol-prim activity but not in a species-specific manner.
FIG. 3.
DNA synthesis on φX174 ssDNA template by recombinant Pol-prim complexes. The recombinant four-subunit human and mouse Pol-prims (0.5 U per incubation) were incubated with φX174 ssDNA in the presence of recombinant purified RPA (1 μg) and increasing amounts of purified recombinant SV40 and BKV TAgs (1 to 5 pmol, as indicated). Incorporation of dNMPs was measured by acid precipitation of newly synthesized DNA and scintillation counting. DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments and the standard deviations are presented.
Mouse DNA polymerase α-primase does not support BKV replication in vitro.
These findings lead to the question of whether mouse or human Pol-prim can replace each other or inhibit the DNA synthesis. Mouse Pol-prim was added to the BKV monopolymerase assay instead of human Pol-prim, but the mouse enzyme complex could not substitute for the human Pol-prim complex (Fig. 4, compare bars 2 to 4 with bars 5 to 7). In comparison to the human enzyme complex, the activity of mouse Pol-prim in the assay with pBKVori plasmid was about 4- to 5-fold lower than that of human Pol-prim (Fig. 4 and 5 A). This lack of replication activity of the mouse Pol-prim cannot be explained by an inactivation process during expression or purification of the mouse enzyme complex since the same fraction was active in mPyV DNA replication in vitro, had activity comparable to that of the human Pol-prim in DNA synthesis on the ssDNA template, and physically and functionally interacted with BKV TAg (Fig. 2 and 3).
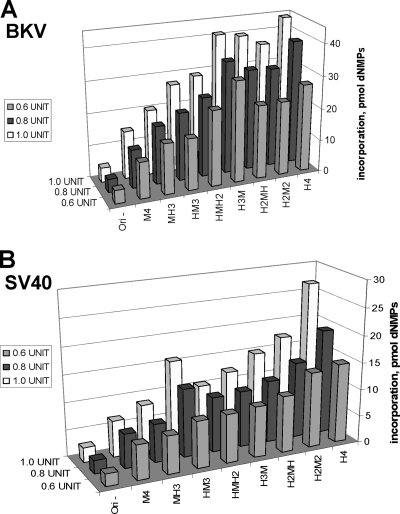
FIG. 5.
Species-specific replication of BKV and SV40 DNAs by human-mouse hybrid Pol-prims. Increasing amounts (0.6, 0.8, and 1.0 U, as indicated) of recombinant human (H4), mouse (M4), or hybrid Pol-prim (MHHH [MH3], HMMM [HM3], HHMM [H2M2], HHHM [H3M], HHMH [H2MH], and HMHH [HMH2]; abbreviations indicate the p180, p68, p58, and p48 subunits in decreasing molecular masses, with M representing a subunit of mouse origin and H representing a subunit of human origin) were used in the monopolymerase replication system. Incorporation of dNMPs into DNA containing BKV (A) or SV40 (B) origin of replication in the presence of 400 ng of the respective viral TAg, 50 ng of topoisomerase I, 1,000 ng of RPA, and indicated amounts of purified Pol-prim complexes was measured by acid precipitation of newly synthesized DNA and scintillation counting. Plasmid without a functional viral origin (Ori−) in the assay with human Pol-prim served as a negative control. DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments, and the standard deviations are presented.
Although the mouse enzyme complex itself was inactive in BKV TAg-dependent DNA replication (Fig. 4, bars 2 to 4), in the presence of the human Pol-prim the mouse Pol-prim did not inhibit BKV DNA synthesis but even slightly stimulated the incorporation of radioactive dNMPs carried out by human Pol-prim (Fig. 4, compare bar 7 with bars 8 and 9). These results suggest that Pol-prim in the mouse replication extracts is not responsible for the inhibition of BKV DNA replication in mouse cell extracts. However, the enzyme complex is a species-specific factor as mouse Pol-prim is not able to efficiently replicate BKV DNA in the presence of BKV TAg, whereas human Pol-prim in the experiments made in parallel is fully active.
BKV DNA replication requires the p180 and p68 subunits of human DNA polymerase α-primase for optimal activity.
It is known that SV40 DNA replication requires only the presence of the large p180 DNA polymerase subunit of human origin in a chimeric Pol-prim complex with the three small subunits of mouse origin to replicate SV40 DNA in mouse extracts in the presence of SV40 TAg (50). In contrast, Pol-prim with the small p48 primase subunit of mouse origin and the three other subunits of human origin is sufficient to allow mPyV DNA replication in human cell extracts in the presence of PyV TAg (8, 23). Since Pol-prim is an important species-specific factor for BKV replication as well, we studied how different mouse-human hybrid Pol-prim complexes containing subunits of both human and mouse origin support BKV DNA replication.
To this end, we expressed in insect cells and then purified six hybrid Pol-prim complexes (MHHH [MH3], HMMM [HM3], HHMM [H2M2], HHHM [H3M], HHMH [H2MH], and HMHH [HMH2]) to near homogeneity. In these designations, H represents a human subunit, and M indicates a subunit of mouse origin; the subunits are noted in order, p180, p68, p58, and p48. BKV and SV40 monopolymerase DNA replication experiments were performed in parallel (Fig. 5) to compare the BKV DNA replication system to the well-studied SV40 system. Mouse Pol-prim is not active in the monopolymerase systems in either the BKV or SV40 system, and the incorporation of radioactive dNMPs on plasmid DNA containing a viral replication origin was close to background level (Fig. 5, M4). Figure 5A shows that the MH3 complex, which consists of mouse p180 and three smaller subunits of human origin, showed some activity, but it was less than 30% of the BKV DNA replication activity with human Pol-prim (H4) (Fig. 5A, compare the three MH3 columns with the H4 columns). These findings suggest a major role of p180 in modulating the species specificity of BKV DNA replication. The importance of the human origin of the p180 and the p68 subunits is underlined by the findings that all hybrid Pol-prim complexes containing the two largest subunits from human (H2M2, H2MH, and H3M) efficiently replicate BKV DNA and reach the level of DNA replication activity of recombinant human Pol-prim having all four subunits of human origin. These results also show that all subunits of Pol-prim contribute to species specificity of replication of BKV origin-containing DNA in the presence of BKV TAg. In contrast to these findings, SV40 DNA replication in vitro with these chimeric Pol-prim complexes reveals a dependence on p180's being of human origin (Fig. 5B) (50). Moreover, the chimeric Pol-prim complexes do not reach the full activity of the recombinant human Pol-prim in the SV40 DNA replication system, whereas H2M2, H2MH, and H3M showed equivalent DNA replication activity in comparison with H4 in the BKV system (compare Fig. 5A and B). These findings suggest that species specificity requirements of BKV DNA replication are less restrictive than those of SV40 DNA replication using the same biochemical assay system.
Initiation of BKV replication by human and mouse DNA polymerase α-primase.
To study the activity of human-mouse hybrid Pol-prims in early stages of DNA replication, we optimized assays for the initiation of BKV and SV40 DNA replication. The primase activities of human and mouse Pol-prim and of a selection of chimeric Pol-prim complexes (H4, M4, H2M2, and MH3) were assessed using an ssDNA substrate. The ranges of primase activity were consistent with previously published data (50). Calculated from these experiments, amounts of Pol-prim used in the initiation assays with viral origins cloned into plasmids were normalized to the same enzyme activity.
The initiation activities of these hybrid Pol-prim complexes were similar to their activities in DNA replication. These data showed that human primase subunits are not essential in both the SV40 and BKV systems. The hybrid H2M2 complex was highly active in the BKV and SV40 systems. The exchange of the large DNA polymerase p180 subunit in a Pol-prim complex of human origin for one of mouse origin caused a reduction in replication levels in both systems. This shows the importance of the p180 subunit in species specificity of both initiation and overall BKV and SV40 replication in vitro. However, it is important to note that hybrid Pol-prim complexes, similar to the human Pol-prim, are more active in the initiation of BKV DNA replication than in the initiation with SV40 DNA (Fig. 6 A).
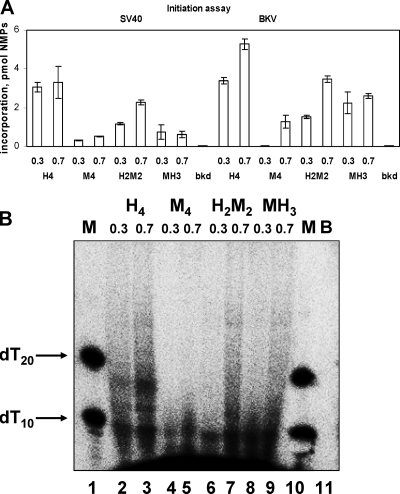
FIG. 6.
Species-specific initiation of SV40 and BKV DNA replication by hybrid Pol-prim complexes. Increasing amounts (0.3 and 0.7 U of Pol-prim determined in the initiation assay on φX174 ssDNA template) of recombinant human (H4), mouse (M4), or hybrid Pol-prim (H2M2 or MH3) were used in the initiation assay on DNA containing SV40 or BKV origin of replication in the presence of 500 ng of the respective recombinant TAg, 1,000 ng of RPA, and 50 ng of topoisomerase I. (A) Incorporation of NMPs into SV40 (bars 1 to 9) or BKV (bars 10 to 18) DNA. Incorporation in the absence of Pol-prim served as a background. (B) RNA primer synthesis products from panel A (probes 10 to 18 for BKV system) were analyzed for the primase products by electrophoresis in denaturing 20% polyacrylamide gels and by autoradiography. Synthesis in the absence of Pol-prim served as a background (B; lane 11). Lanes 1 and 10 contain 5′-end-labeled oligo(dT)10 and oligo(dT)20 as markers (M). The positions of 5′ end-labeled oligo(dT)10 and oligo(dT)20 are marked by arrows.
In addition, initiation products were analyzed by denaturing electrophoresis (Fig. 6B). In these analyses human Pol-prim and the hybrid complex H2M2 were highly active. In contrast, mouse Pol-prim showed very low activity. However, the mouse enzyme complex revealed some basal activity above the background presented in lane 11. As seen by comparing lanes 3, 7, and 9 with lane 5 of Fig. 6B, human and hybrid Pol-prims form longer RNA products than mouse Pol-prim, suggesting that mouse Pol-prim is able to start primer synthesis during the initiation of BKV DNA replication but cannot efficiently elongate these short primers, leading to a later abortion of the BKV DNA replication in vitro.
Interactions of the inhibitory activity with flanking regions of the BKV core origin.
Previous studies have shown that the core origins of SV40 and mPyV but not their flanking regions are responsible for the species specificity of the viral DNA replication (2). Recent results with BKV DNA replication also suggest that the main species-specific function may associate with the core origin (28). The latter idea is supported by the findings presented here that Pol-prim modulates the species specificity of BKV DNA in vitro. The presence of inhibitory activities (IAs) in mouse cell extracts, which block the BKV DNA replication in these extracts supplemented with human Pol-prim (28), raises the question of whether the IAs also interact with the core origin or whether they require different activities of the BKV origin that associate with the early region and the enhancer regions on the late site adjacent to the core origin. The involvement of the core origin-flanking sites would suggest that multiple independent mechanisms are present in mouse cells to control the species specificity of BKV DNA replication, which is different from the current model of species-specific control of polyomavirus DNA replication. In such a context we expected human Pol-prim to be a species-specific function associated with the core origin, and by supplementing mouse cell extracts with hum Pol-prim, we could investigate whether changing the flanking sequences of BKV to mPyV would make these human Pol-prim-supplemented extracts permissive for BKV TAg-dependent replication of chimeric human-mouse polyomavirus DNA in vitro (Fig. 7).
The addition of increasing amounts of human Pol-prim (minimum requirement of 2 U, equivalent to 660 ng of enzyme complex) in the presence of 0.2 μg of BKV TAg allowed mouse crude replication extracts to efficiently replicate DNA containing BKV-mPyV chimeric origins P-B-B and P-B-P, which have the early flanking region of the wild-type BKV origin replaced by the equivalent polyomavirus sequences. In contrast, both BKV wild-type B-B-B and the chimeric B-B-P origin showed background levels of BKV TAg-dependent DNA replication at any concentration of human Pol-prim. These results indicate that BKV DNA replication is independently regulated by both Pol-prim, representing a core origin species-specific factor, and the newly identified inhibitory activities, which require origin-flanking sequences for their action and specifically sequences located in the early region of the BKV origin of replication. These findings yield new insight into the regulation of species specificity of BKV polyomavirus, which differs from that of other Polyomaviridae such as mPyV and SV40.
DISCUSSION
BKV is the etiological agent of PVAN in about 10% of renal transplant patients (18). It has been shown that BKV DNA replication is restricted in murine cells and extracts (28). Moreover, in a cell-free BKV DNA replication system with purified human proteins (monopolymerase replication assay), the presence of an unknown factor(s) from murine cell extracts that inhibit BKV DNA replication was recently described (28). These findings have revealed the molecular basis for a potential explanation for the fact that no mouse model could be established to study virus reactivation accompanying PVAN.
RPA is essential for eukaryotic DNA replication and is known to have some impact on species specificity of SV40 DNA replication (7, 56). In our analyses presented here, mouse RPA can fully substitute for human RPA in BKV DNA replication in vitro, and the two have similar activities. Therefore, mouse RPA is not a species-specific factor and is not the source of the inhibitory activities. Similar to RPA, Pol-prim is essential for eukaryotic DNA replication and is the major species-specific factor of mPyV and SV40 (8, 32, 33, 50). Here, we verified the ability of mouse Pol-prim to functionally and physically interact with BKV TAg, similar to its interaction with SV40 TAg in the SV40 system (10, 53, 57). Our results indicated that mouse Pol-prim is able to substitute for human Pol-prim, binding to BKV TAg with the same efficiency and forming functional complexes in the absence of the replication origin (29, 42).
However, we determined here that purified mouse Pol-prim complex does not support BKV DNA replication, and very low, nearly background levels of dNMP incorporation were detected in BKV DNA replication experiments. This suggests for the first time that the human Pol-prim complex is a species-specific factor for BKV DNA replication. This is in agreement with mPyV and SV40 DNA replication, whereas it is contrary to JC virus (JCV) DNA replication (2, 45, 47). The presence of an inhibitory activity in mouse extracts (28) raises the question of whether mouse Pol-prim could be the possible inhibitor of BKV DNA replication in mouse extracts. The hypothesis would be that mouse Pol-prim could form replication-inactive complexes with BKV TAg instead of human Pol-prim, thus efficiently blocking replication of BKV DNA. However, the present data do not support this hypothesis. The addition of purified mouse Pol-prim to the BKV monopolymerase replication system in the presence of human Pol-prim did not result in any inhibition; in fact, even a slight but reproducible stimulatory effect was observed. The findings presented here indicate that BKV TAg can bind to both human and mouse Pol-prim with equal efficiencies but that replication complexes having human Pol-prim allow initiation and further steps of DNA replication to occur more effectively, shifting the system toward a functional initiation complex containing human Pol-prim.
It is known that SV40 TAg independently interacts with all four subunits of Pol-prim and that these interactions are important for DNA replication and are involved in regulation of the species specificity of SV40 DNA replication (8, 24, 50, 57, 58). To systematically test the role of the individual subunits of Pol-prim in more detail, we expressed and purified different human-mouse chimeric Pol-prim complexes and studied their activities in the monopolymerase replication system. We showed that any hybrid Pol-prim complex containing both human p180 and p68 subunits (H2M2, H3M, and H2MH) retained full activity in BKV DNA replication (i.e., equal to the activity of human Pol-prim complex). This finding suggests that interactions with the two largest subunits of human Pol-prim are the main factor determining the correct positioning of Pol-prim at the origin of replication to initiate BKV DNA replication. This view is consistent with the finding that producing an enzyme complex where the largest subunit is of mouse origin and the smaller subunits are of human origin yields an enzyme complex (MH3) which has the lowest replication activity (about 50% of the H4 replication level). This confirms that the human p180 subunit is important for BKV DNA replication. In contrast, in the SV40 DNA replication system, the HM3 complex has barely any replication activity, and the level of incorporation of dNMPs is close to that of the mouse Pol-prim. These data suggest that a certain similarity in the mechanisms of species specificity in BKV and SV40 DNA replication may exist. However, the comparison of the replication patterns of different hybrid Pol-prim complexes reveals that, unlike SV40 DNA replication, which requires the presence of precisely one human subunit (p180) in the complex, the BKV DNA replication system seems to be less demanding in this regard.
Since the initiation reactions in the mPyV and SV40 DNA replication systems have been shown to be the species-specific reactions (8, 42, 50) and since we wanted to provide more detailed insight into the process of species-specific BKV DNA replication, we reconstituted the initiation reaction of BKV DNA replication in vitro. In these experiments we monitored the level of NMP incorporation and also analyzed the products of primer synthesis. These initiation results and the DNA replication levels reveal that differences in the levels of replication are defined mostly by different abilities to initiate DNA replication. The MH3 complex, in which both primase subunits and p68 are of human origin but p180 is from mouse, has a low initiation level in SV40 initiation assays in vitro and an intermediate level in BKV initiation assays in vitro. In contrast, the Pol-prim complex containing both mouse primase subunits (H2M2) retains more than 50% of the activity of the human Pol-prim complex in initiation of BKV DNA replication. These data suggest that the origin of the primase subunits is only partially important for the primer synthesis activity. We propose a spatial structure of the BKV initiation complex containing Pol-prim, TAg, and probably RPA in which multiple subunits of Pol-prim are involved in correct positioning of primase for effective initiation of DNA replication, which, in turn, requires the presence of human Pol-prim subunits but which is disturbed by the presence of the mouse equivalent subunits. The view is supported by the finding that the primer products synthesized by mouse and hybrid Pol-prim complexes are shorter than those produced by human Pol-prim.
These studies showed the importance of human Pol-prim for BKV DNA replication in vitro. However, they do not give any additional information about the inhibitory factor(s) found in mouse cell extracts. This activity is specific for BKV (28), and it has not yet been reported for another viral system, i.e., not JCV, mPyV, or SV40 (28, 45, 47). As we demonstrated earlier, the inhibitory effect is greater when mouse extract is added during the unwinding step of DNA replication (28). This result suggests that an inhibitory factor(s) may interact or modify the proteins involved in the unwinding reaction or the origin-containing DNA. As inhibitory effects did not persist in a replication system utilizing SV40 TAg and an SV40 origin of replication, whereas inhibition was present with SV40 TAg utilizing a BKV origin (data not shown), we assumed that the inhibitory factor(s) are origin specific, raising the question as to which origin functions are necessary for the inhibition. Earlier, we demonstrated that BKV TAg can recognize different chimeric origins of replication containing the BKV core origin (28). These chimeric origins were previously tested in mouse cells and in mouse cell extracts, but it appears that the core origin-flanking regions did not have an impact on species specificity (2, 28; also data not shown). However, we have shown here that the presence of human Pol-prim is crucial for BKV DNA replication, which means that the analyses mentioned above could not determine whether the presence of PyV origin sequences affected the activity of mouse extracts since they were not supplemented with human Pol-prim to support BKV DNA replication. Therefore, we tested here whether chimeric origins containing BKV core origin and mPyV origin-flanking sequences could support BKV DNA replication in mouse extracts supplemented with human Pol-prim.
Interestingly, in mouse extracts human Pol-prim and BKV TAg cooperated to replicate DNA containing P-B-B and P-B-P chimeric origins, both of which have a mouse polyomaviral early region, whereas the wild-type origin, B-B-B, and a chimeric origin, B-B-P (the latter having a polyomaviral sequence on the late site), did not support DNA replication in vitro. These results suggest that species-specific BKV DNA replication is negatively regulated by a factor(s) that depends on the early region of the BKV replication origin, but this is not due to any of the initiation proteins necessary for the initiation and initial elongation reaction, which take place in the monopolymerase system. Importantly, Pol-prim is absolutely required for BKV DNA replication, and it is a core origin-specific factor and supports BKV DNA replication with both substituted origin-flanking sequences. However, we could show here that, in contrast to mPyV and SV40 DNA replication, two independent mechanisms control the species specificity of BKV DNA replication in vitro. One is the species-specific formation of the initiation complex requiring one or more human subunits in the Pol-prim complex and the BKV core origin. The other regulatory function is modulated by a trans-acting factor(s) interacting with sequences adjacent to the core origin in the early region, blocking BKV DNA replication. This factor(s) is present in extracts from nonpermissive mouse cells but not in human cells. These biochemical studies reveal a novel regulatory mechanism for the control of species-specific replication of polyomavirus DNA and indicate that a dual system exists in the case of BKV. Elucidating the nature of this new regulatory activity may shed light on the mechanism of eukaryotic DNA replication, and efforts to purify the inhibitory activities are ongoing.
Acknowledgments
We thank Michael Carty for critical reading of the manuscript and Wolfgang Deppert for the gift of polyclonal antiserum.
This work was supported by NIH grant R21 AI062848 and Science Foundation Ireland grants to H.P.N. and an NUI Galway College of Science Scholarship and a Thomas Crawfort Hayes Fellowship to I.T.
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Abend, J. R., J. A. Low, and M. J. Imperiale. 2007. Inhibitory effect of gamma interferon on BK virus gene expression and replication. J. Virol. 81:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett, E. R., M. Naujokas, and J. A. Hassell. 1989. Requirements for species-specific papovavirus DNA replication. J. Virol. 63:5371-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialek, G., H. P. Nasheuer, H. Goetz, B. Behnke, and F. Grosse. 1988. DNA polymerase α-DNA primase from human lymphoblasts. Biochim. Biophys. Acta 951:290-297. [DOI] [PubMed] [Google Scholar]
- 4.Bialek, G., H. P. Nasheuer, H. Goetz, and F. Grosse. 1989. Exonucleolytic proofreading increases the accuracy of DNA synthesis by human lymphocyte DNA polymerase α-DNA primase. EMBO J. 8:1833-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowiec, J. A., F. B. Dean, P. A. Bullock, and J. Hurwitz. 1990. Binding and unwinding—how T antigen engages the SV40 origin of DNA replication. Cell 60:181-184. [DOI] [PubMed] [Google Scholar]
- 6.Borowiec, J. A., and J. Hurwitz. 1988. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 7:3149-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broderick, S., K. Rehmet, C. Concannon, and H. P. Nasheuer. 2010. Eukaryotic single-stranded DNA binding proteins: central factors in genome stability. Subcell. Biochem. 50:143-163. [DOI] [PubMed] [Google Scholar]
- 8.Bruckner, A., F. Stadlbauer, L. A. Guarino, A. Brunahl, C. Schneider, C. Rehfuess, C. Previes, E. Fanning, and H. P. Nasheuer. 1995. The mouse DNA polymerase α-primase subunit p48 mediates species-specific replication of polyomavirus DNA in vitro. Mol. Cell. Biol. 15:1716-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgers, P. M. 2009. Polymerase dynamics at the eukaryotic DNA replication fork. J. Biol. Chem. 284:4041-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornreiter, I., W. C. Copeland, and T. S. Wang. 1993. Initiation of simian virus 40 DNA replication requires the interaction of a specific domain of human DNA polymerase alpha with large T antigen. Mol. Cell. Biol. 13:809-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dornreiter, I., L. F. Erdile, I. U. Gilbert, W. D. von, T. J. Kelly, and E. Fanning. 1992. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 11:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dornreiter, I., A. Hoss, A. K. Arthur, and E. Fanning. 1990. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 9:3329-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanning, E., and R. Knippers. 1992. Structure and function of simian virus 40 large tumor antigen. Annu. Rev. Biochem. 61:55-85. [DOI] [PubMed] [Google Scholar]
- 14.Fanning, E., and K. Zhao. 2009. SV40 DNA replication: from the A gene to a nanomachine. Virology 384:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner, S. D., A. M. Field, D. V. Coleman, and B. Hulme. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1:1253-1257. [DOI] [PubMed] [Google Scholar]
- 16.Guo, Z. S., C. Gutierrez, U. Heine, J. M. Sogo, and M. L. Depamphilis. 1989. Origin auxiliary sequences can facilitate initiation of simian virus 40 DNA replication in vitro as they do in vivo. Mol. Cell. Biol. 9:3593-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann, G. R., C. Biebricher, S. J. Glaser, F. Grosse, M. J. Katzameyer, A. J. Lindner, H. Mosig, H.-P. Nasheuer, L. B. Rothman-Denes, A. R. Schäffner, G. J. Schneider, K.-O. Stetter, and M. Thomm. 1988. Twelfth Fritz Lipmann lecture. Initiation of transcription—a general tool for affinity labeling of RNA polymerases by autocatalysis. Biol. Chem. Hoppe Seyler 369:775-788. [PubMed] [Google Scholar]
- 18.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79:1277-1286. [DOI] [PubMed] [Google Scholar]
- 19.Hübscher, U., G. Maga, and S. Spadari. 2002. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 71:133-163. [DOI] [PubMed] [Google Scholar]
- 20.Hübscher, U., H. P. Nasheuer, and J. Syväoja. 2000. Eukaryotic DNA polymerases, a growing family. Trends Biochem. Sci. 25:143-147. [DOI] [PubMed] [Google Scholar]
- 21.Imperiale, M. J. 2000. The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology 267:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, M., J. R. Abend, S. F. Johnson, and M. J. Imperiale. 2009. The role of polyomaviruses in human disease. Virology 384:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kautz, A., A. Schneider, K. Weisshart, C. Geiger, and H. P. Nasheuer. 2001. Different regions of primase subunit p48 control mouse polyomavirus and simian virus 40 DNA replication in vitro. J. Virol. 75:1751-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kautz, A., K. Weisshart, A. Schneider, F. Grosse, and H. P. Nasheuer. 2001. Amino acids 257 to 288 of mouse p48 control the cooperation of polyomavirus large T antigen, replication protein A, and DNA polymerase α-primase to synthesize DNA in vitro. J. Virol. 75:8569-8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles, W. A. 2006. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv. Exp. Med. Biol. 577:19-45. [DOI] [PubMed] [Google Scholar]
- 26.Knowles, W. A. 2001. Propagation and assay of BK virus. Methods Mol. Biol. 165:19-31. [DOI] [PubMed] [Google Scholar]
- 27.Kolpashchikov, D. M., K. Weisshart, H. P. Nasheuer, S. N. Khodyreva, E. Fanning, A. Favre, and O. I. Lavrik. 1999. Interaction of the p70 subunit of RPA with a DNA template directs p32 to the 3′-end of nascent DNA. FEBS Lett. 450:131-134. [DOI] [PubMed] [Google Scholar]
- 28.Mahon, C., B. Liang, I. Tikhanovich, J. R. Abend, M. J. Imperiale, H. P. Nasheuer, and W. R. Folk. 2009. Restriction of human polyomavirus BK virus DNA replication in murine cells and extracts. J. Virol. 83:5708-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melendy, T., and B. Stillman. 1993. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J. Biol. Chem. 268:3389-3395. [PubMed] [Google Scholar]
- 30.Muller, W. J., C. R. Mueller, A. M. Mes, and J. A. Hassell. 1983. Polyomavirus origin for DNA replication comprises multiple genetic elements. J. Virol. 47:586-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami, Y., T. Eki, and J. Hurwitz. 1992. Studies on the initiation of simian virus 40 replication in vitro: RNA primer synthesis and its elongation. Proc. Natl. Acad. Sci. U. S. A. 89:952-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami, Y., T. Eki, M. Yamada, C. Prives, and J. Hurwitz. 1986. Species-specific in vitro synthesis of DNA containing the polyoma virus origin of replication. Proc. Natl. Acad. Sci. U. S. A. 83:6347-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami, Y., C. R. Wobbe, L. Weissbach, F. B. Dean, and J. Hurwitz. 1986b. Role of DNA polymerase α and DNA primase in simian virus 40 DNA replication in vitro. Proc. Natl. Acad. Sci. U. S. A. 83:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasheuer, H. P., and F. Grosse. 1987. Immunoaffinity-purified DNA polymerase α displays novel properties. Biochemistry 26:8458-8466. [DOI] [PubMed] [Google Scholar]
- 35.Nasheuer, H. P., H. Pospiech, and J. Syväoja. 2007. Progress towards the anatomy of the eukaryotic DNA replication fork, p. 27-68. In D. H. Lankenau (ed.), Genome integrity: facets and perspectives. Genome dynamics and stability, vol. 1. Springer, Berlin, Germany. [Google Scholar]
- 36.Nasheuer, H. P., D. von Winkler, C. Schneider, I. Dornreiter, I. Gilbert, and E. Fanning. 1992. Purification and functional characterization of bovine RP-A in an in vitro SV40 DNA replication system. Chromosoma 102:S52-S59. [DOI] [PubMed] [Google Scholar]
- 37.Nesper, J., R. W. Smith, A. R. Kautz, E. Sock, M. Wegner, F. Grummt, and H. P. Nasheuer. 1997. A cell-free replication system for human polyomavirus JC DNA. J. Virol. 71:7421-7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nethanel, T., and G. Kaufmann. 1990. Two DNA polymerases may be required for synthesis of the lagging DNA strand of simian virus 40. J. Virol. 64:5912-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oehlmann, M., C. Mahon, and H. P. Nasheuer. 2007. Comparison of DNA replication in Xenopus laevis and simian virus 40. Adv. Exp. Med. Biol. 604:3-16. [DOI] [PubMed] [Google Scholar]
- 40.Pestryakov, P. E., K. Weisshart, B. Schlott, S. N. Khodyreva, E. Kremmer, F. Grosse, O. I. Lavrik, and H. P. Nasheuer. 2003. Human replication protein A. The C-terminal RPA70 and the central RPA32 domains are involved in the interactions with the 3′-end of a primer-template DNA. J. Biol. Chem. 278:17515-17524. [DOI] [PubMed] [Google Scholar]
- 41.Portolani, M., G. Barbanti-Brodano, and M. L. Placa. 1975. Malignant transformation of hamster kidney cells by BK virus. J. Virol. 15:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider, C., K. Weisshart, L. A. Guarino, I. Dornreiter, and E. Fanning. 1994. Species-specific functional interactions of DNA polymerase alpha-primase with simian virus 40 (SV40) T antigen require SV40 origin DNA. Mol. Cell. Biol. 14:3176-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons, D. T., R. Roy, L. Chen, D. Gai, and P. W. Trowbridge. 1998. The activity of topoisomerase I is modulated by large T antigen during unwinding of the SV40 origin. J. Biol. Chem. 273:20390-20396. [DOI] [PubMed] [Google Scholar]
- 44.Small, J. A., G. Khoury, G. Jay, P. M. Howley, and G. A. Scangos. 1986. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 83:8288-8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, R. W., and H. P. Nasheuer. 2003. Initiation of JC virus DNA replication in vitro by human and mouse DNA polymerase alpha-primase. Eur. J. Biochem. 270:2030-2037. [DOI] [PubMed] [Google Scholar]
- 46.Smith, R. W., C. Steffen, F. Grosse, and H. P. Nasheuer. 2002. Species specificity of simian virus 40 DNA replication in vitro requires multiple functions of human DNA polymerase alpha. J. Biol. Chem. 277:20541-20548. [DOI] [PubMed] [Google Scholar]
- 47.Smith, R. W. P., and H. P. Nasheuer. 2000. Control of papovaviral DNA replication, p. 67-92. In S. G. Pandalai (ed.), Recent research developments in virology, vol. 2. Transworld Research Network, Trivandrum, India. [Google Scholar]
- 48.Soe, K., G. Dianov, H. P. Nasheuer, V. A. Bohr, F. Grosse, and T. Stevnsner. 2001. A human topoisomerase I cleavage complex is recognized by an additional human topoisomerase I molecule in vitro. Nucleic Acids Res. 29:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stadlbauer, F., A. Brueckner, C. Rehfuess, C. Eckerskorn, F. Lottspeich, V. Förster, B. Y. Tseng, and H. P. Nasheuer. 1994. DNA replication in vitro by recombinant DNA-polymerase-α-primase. Eur. J. Biochem. 222:781-793. [DOI] [PubMed] [Google Scholar]
- 50.Stadlbauer, F., C. Voitenleitner, A. Bruckner, E. Fanning, and H. P. Nasheuer. 1996. Species-specific replication of simian virus 40 DNA in vitro requires the p180 subunit of human DNA polymerase alpha-primase. Mol. Cell. Biol. 16:94-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stillman, B. 2008. DNA polymerases at the replication fork in eukaryotes. Mol. Cell 30:259-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taneja, P., I. Boche, H. Hartmann, H. P. Nasheuer, F. Grosse, E. Fanning, and K. Weisshart. 2007. Different activities of the largest subunit of replication protein A cooperate during SV40 DNA replication. FEBS Lett. 581:3973-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taneja, P., H. P. Nasheuer, H. Hartmann, F. Grosse, E. Fanning, and K. Weisshart. 2007. Timed interactions between viral and cellular replication factors during the initiation of SV40 in vitro DNA replication. Biochem. J. 407:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waga, S., G. Bauer, and B. Stillman. 1994. Reconstitution of complete SV40 DNA replication with purified replication factors. J. Biol. Chem. 269:10923-10934. [PubMed] [Google Scholar]
- 55.Waga, S., and B. Stillman. 1998. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 67:721-751. [DOI] [PubMed] [Google Scholar]
- 56.Wang, M., J. S. Park, M. Ishiai, J. Hurwitz, and S. H. Lee. 2000. Species specificity of human RPA in simian virus 40 DNA replication lies in T-antigen-dependent RNA primer synthesis. Nucleic Acids Res. 28:4742-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weisshart, K., H. Förster, E. Kremmer, B. Schlott, F. Grosse, and H. P. Nasheuer. 2000. Protein-protein interactions of the primase subunits p58 and p48 with simian virus 40 T antigen are required for efficient primer synthesis in a cell-free system. J. Biol. Chem. 275:17328-17337. [DOI] [PubMed] [Google Scholar]
- 58.Weisshart, K., P. Pestryakov, R. W. Smith, H. Hartmann, E. Kremmer, O. Lavrik, and H. P. Nasheuer. 2004. Coordinated regulation of replication protein A activities by its subunits p14 and p32. J. Biol. Chem. 279:35368-35376. [DOI] [PubMed] [Google Scholar]
- 59.Yuzhakov, A., Z. Kelman, J. Hurwitz, and M. O'Donnell. 1999. Multiple competition reactions for RPA order the assembly of the DNA polymerase delta holoenzyme. EMBO J. 18:6189-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]