Abstract
Novel nanomaterials are being developed to improve diagnosis and therapy of diseases through effective delivery of drugs, biopharmaceutical molecules and imaging agents to target cells in disease sites. Such diagnostic and therapeutic nanomaterials, also termed “nanomedicines”, often require site-specific cellular entry to deliver their payload to subcellular locations hidden beneath cell membranes. Nanomedicines can employ multiple pathways for cellular entry, which are currently insufficiently understood. This review, first, classifies various mechanisms of endocytosis available to nanomedicines including phagocytosis and pinocytosis through clathrin-dependent and clathrin-independent pathways. Second, it describes the current experimental tools to study endocytosis of nanomedicines. Third, it provides specific examples from recent literature and our own work on endocytosis of nanomedicines. Finally, these examples are used to ascertain 1) the role of particle size, shape, material composition, surface chemistry and/or charge for utilization of a selected pathway(s); 2) the effect of cell type on the processing of nanomedicines; 3) the effect of nanomaterial-cell interactions on the processes of endocytosis, the fate of the nanomedicines and the resulting cellular responses. This review will be useful to a diverse audience of students and scientists who are interested in understanding endocytosis of nanomedicines.
1. Introduction: Cellular entry of nanomedicines
A new era of nanomedicine that uses devices of nanoscale size to address urgent needs for improved diagnosis and therapy of diseases is being etched in the 21st century. Polymeric micelles, quantum dots, liposomes, polymer-drug conjugates, dendrimers, biodegradable nanoparticles, silica nanoparticles, etc. are few examples of nanoparticulate materials researched in laboratories, undergoing preclinical development, or already used in clinic [1–6]. These nanomaterials, collectively called “nanomedicines”, can deliver low molecular mass compounds, proteins and recombinant DNAs to focal areas of disease or to tumors to maximize clinical benefit while limiting untoward side effects. Such nanomedicines are also expected to drastically improve early diagnosis through molecular imaging techniques. A quintessential feature of such modalities is their ability for site-specific delivery, not only to the desired organ, but also to a targeted sub-cellular compartment. Hence, a new paradigm for drug delivery and nanomedicine requires nanomaterials to differentially interact with the surface of their target cells and undergo intracellular trafficking that would lead to determined locations inside cells [7]. Consequently, the interest to intracellular trafficking of nanomaterials has skyrocketed.
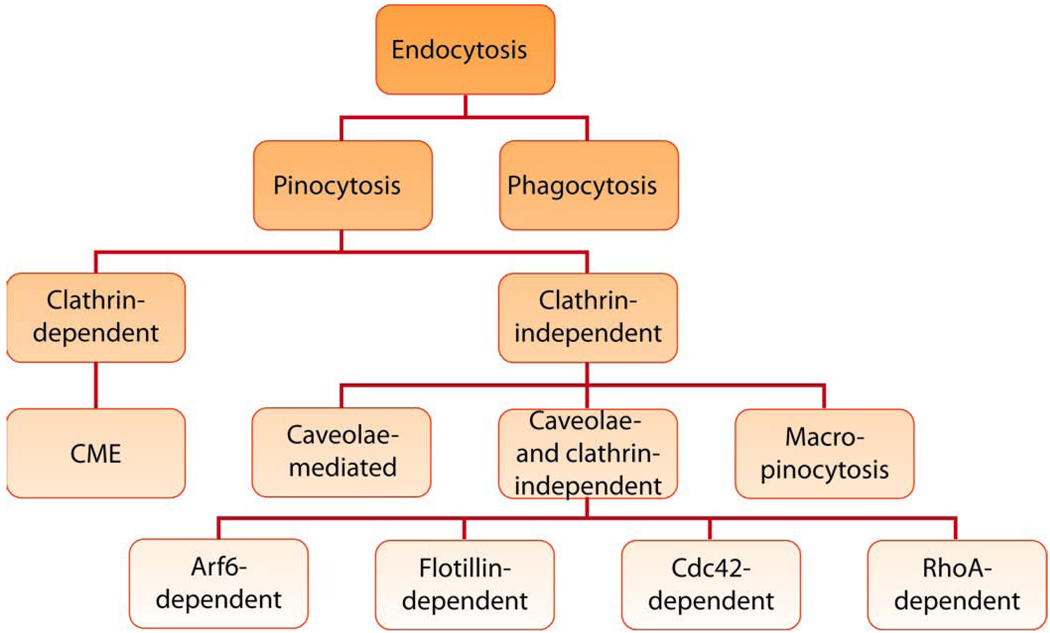
A nanoparticle placed in the external milieu of a cell can interact with the exterior of the plasma membrane, which can lead to this nanoparticle entry inside the cell through a process termed “endocytosis” (Fig. 1). Endocytosis involves multiple stages. First, the cargo is engulfed in membrane invaginations that are pinched off to form membrane-bound vesicles, also known as endosomes (or phagosomes in case of phagocytosis). Cells contain heterogeneous populations of endosomes equipped with distinct endocytic machinery, which originate at different sites of the cell membrane. Second, the endosomes deliver the cargo to various specialized vesicular structures, which enables sorting of cargo towards different destinations. Finally, the cargo is delivered to various intracellular compartments, recycled to the extracellular milieu or delivered across cells (a process known as “transcytosis” in polarized cells). Generally, endocytosis can be divided into two broad categories - phagocytosis (the uptake of large particles) and pinocytosis (the uptake of fluids and solutes). Phagocytosis was originally discovered by Ilya Mechnikov as a process by which macrophages engulf particles as large as 20 µm [8]. This process is characteristic of specialized professional phagocytes, such as, macrophages, neutrophils, monocytes and dendritic cells. Pinocytosis in contrast is present in all types of cells and has multiple forms depending on the cell origin and function. Several different classifications of pinocytosis have been proposed. The most recent approach is based on the proteins involved in different endocytic pathways (Fig. 2). In this approach pinocytosis is classified as clathrin-dependent endocytosis (also known as clathrin-mediated endocytosis (CME)) and clathrin-independent endocytosis [9]. The clathrin-independent pathways are further classified as 1) caveolae-mediated endocytosis, 2) clathrin- and caveolae-independent endocytosis and 3) macropinocytosis. Clathrin- and caveolae-independent pathways are sub-classified as Arf6-dependent, flotillin-dependent, Cdc42-dependent and RhoA-dependent endocytosis [10]. Another classification of endocytosis, based on materials interaction with cellular membrane (receptor-mediated, adsorptive, fluid phase), has also been used to describe cellular entry of nanomaterials. However, this classification is less precise and often mistakenly used interchangeably with the previously described classification (e.g., receptor-mediated endocytosis is sometimes confused with CME). We, therefore, urge the nanomedicine community to utilize the classification based on endocytosis proteins that is being actively researched and have recently emerged in the endocytosis field (Fig. 2).
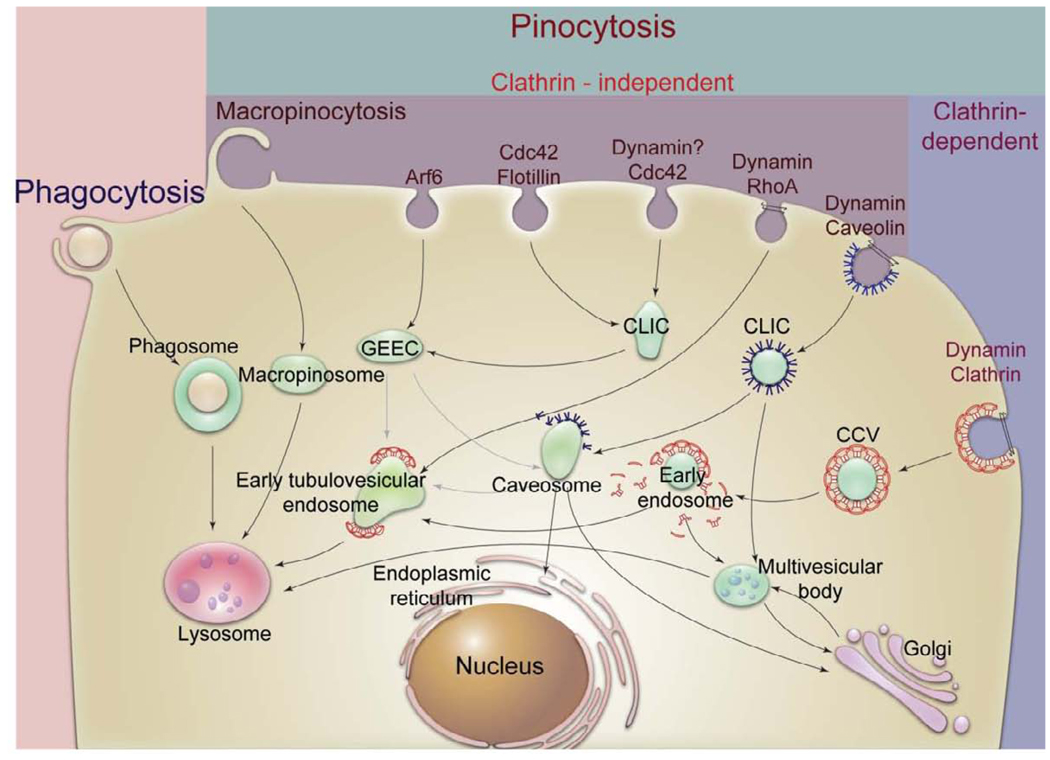
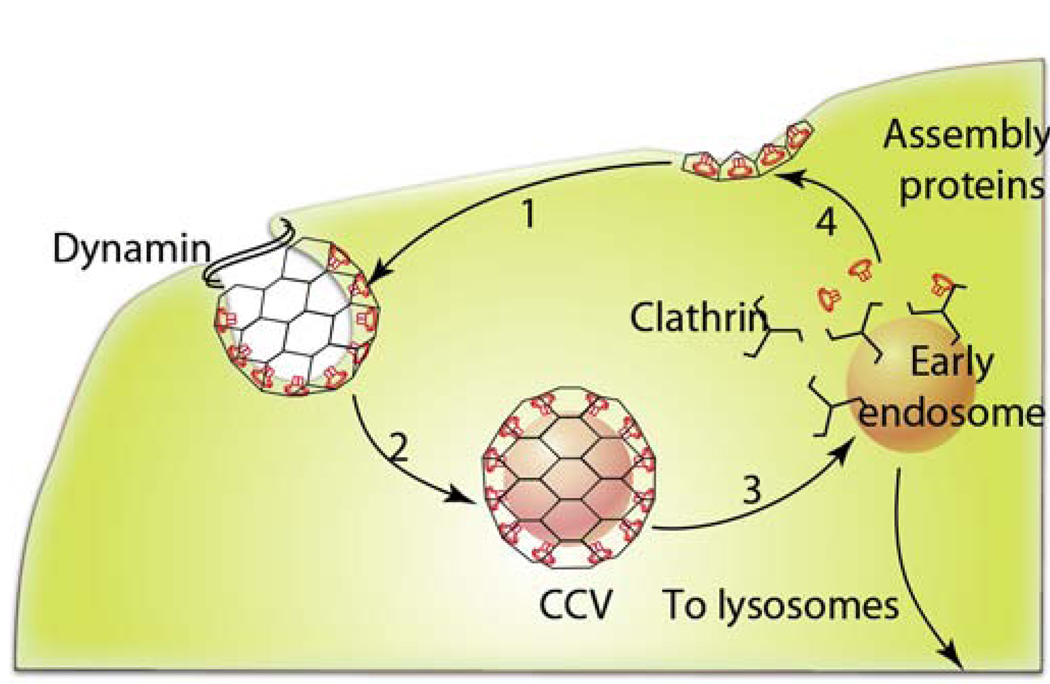
Fig. 1. Different mechanisms of endocytosis.
There are multiple pathways for cellular entry of particles and solutes. The picture of endocytosis trafficking is actively researched and evolving [11], [12]. In all cases the initial stage of endocytosis proceeds from the plasma membrane portals of cellular entry and involves engulfment of cargo into intracellular vesicles. The second stage often involves sorting of the cargo through endosomes. It is followed by the final stage during which the cargo is delivered to its final destination, recycled to extracellular milieu or delivered across cells (not shown). The figure is a simplified representation of complex trafficking mechanisms and their cross-talks. More details for stages of phagocytosis and CME are presented in Fig. 3 and Fig. 5. Abbreviations are: CCV, clathrin coated vesicles, CLIC, clathrin-independent carriers; GEEC, GPI-anchored protein-enriched compartment; GPI, glycophosphatidylinositol, MVB, multivesicular body.
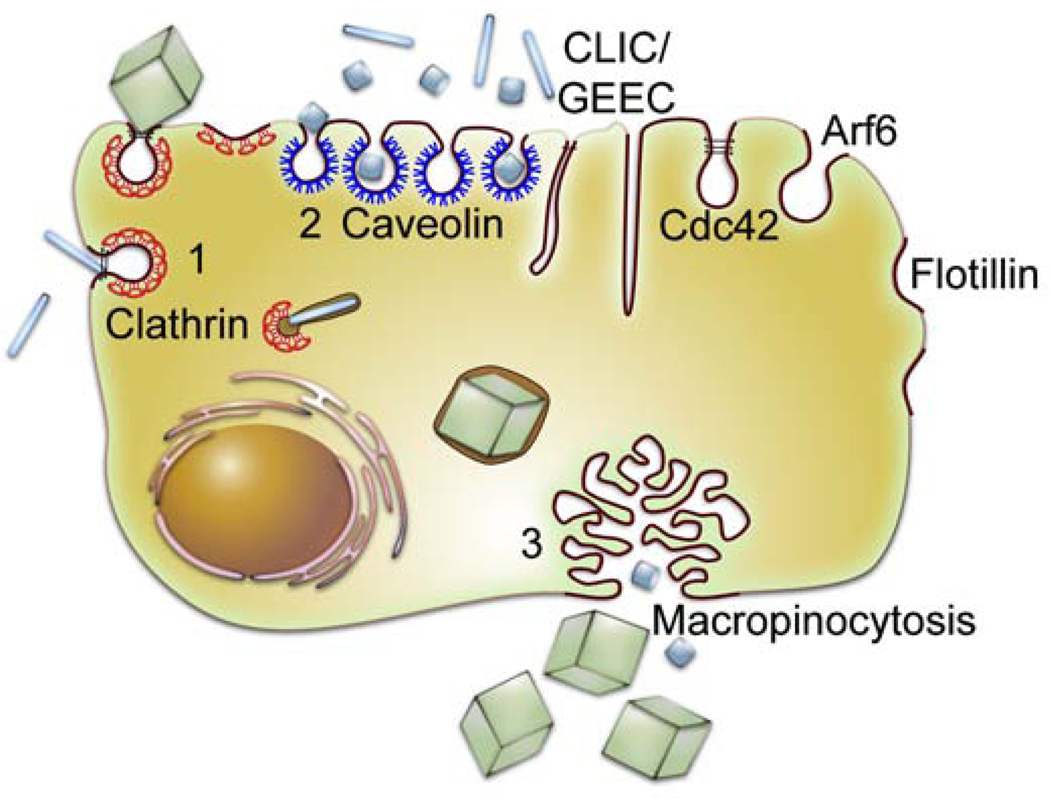
Fig. 2.
Classification of endocytosis based on endocytosis proteins that are involved in the initial entry of particles and solutes.
This papers overviews different endocytosis pathways utilized by various nanomaterials to enter cells and attempts to make some general conclusions about the preferred trafficking routes based on the nanomaterial structure and properties. We briefly describe phagocytosis and focus in detail on various pinocytosis mechanisms including clathrin-dependent and clathrin-independent pathways. The current experimental tools to study endocytosis of nanomaterials are discussed followed by specific examples from recent literature and our own work on selected nanomaterials that have shown to employ different pathways. These examples are used to ascertain several key questions, such as: i) whether particle size, shape, material composition, surface chemistry and/or charge are responsible for employment of a selected pathway; ii) how are nanomaterials processed in different cell types; iii) how do nanomaterial-cell interactions influence cell trafficking and whether such interactions can impact nanomaterial fate and cellular responses? Of course, in view of great diversity of nanomaterials, multiplicity and complexity of cellular pathways, and often missing comprehensive experimental data our generalization cannot be complete. Nevertheless, we believe that our analysis with help both established nanomedicine investigators and those who are just entering this exciting field to understand its current state.
2. Tools to dissect intracellular trafficking
Most current methods to study intracellular trafficking of nanomaterials involve either 1) colocalization of nanomaterials with specific endocytosis markers and structures or 2) exclusion of specific endocytosis mechanisms by inhibitors of endocytosis or cell mutants. In case of colocalization studies different markers can be used. For example, in so-called “pulse-chase” design, proteins, such as transferrin or cholera toxin B (CTB), with known trafficking pathways are exposed to cells simultaneously or before the nanomaterial (“pulse”) and their inclusion or exclusion from the same vesicles is detected at different time points (“chase”). The most widespread current method for detection involves attaching different fluorescent probes to the protein and nanomaterial that allows analyzing distribution of color or fluorescence resonance energy transfer (FRET) throughout the cell compartments. The advantage of such approach is that it allows using live cell imaging by confocal microscopy, which arguably causes least artifacts. The disadvantage is that such endocytosis markers are rarely selective with respect to specific trafficking pathways and may utilize different endocytosis mechanisms in different cell types. Therefore, as an alternative or supplementary approach, cells can be transfected with constructs containing proteins that reside in specific endocytosis vesicles or intracellular organelles, which are fused with fluorescent proteins, such as the Green Fluorescent Protein (GFP). Examples include rab5 in early endosomes [13], caveolin-1 in caveolae [14], human Golgi-resident enzyme N-acetylgalactosaminyltranferase-2 in Golgi [15], lysosomal association protein 1 (Lamp1) in lysosomes [16], signal sequence of calreticulin and endoplasmic reticulum (ER) retention signal KDEL in ER [17], leader sequence of E1 alpha pyruvate dehydrogenase in mitochondria [18], myristoyl/palmtoylation sequence from Lck tyrosine kinase in plasma membrane [19] and actin or tubulin in cytoskeleton [20]. In addition to the fusion proteins there are a variety of small molecular mass probes, which can stain specific cellular organelles. The examples of such probes include weakly basic amines such as Lysotracker™ that selectively accumulate in lysosomes, fluorescently labeled glibenclamide that binds to the sulphonylurea receptors of ATP-sensitive K+ channels in ER, the mitochondrial dye MitoTracker™ [21] and others. Importantly, colocalization of nanomaterials with fusion proteins and molecular probes can be also explored in live cells. Another way of studying colocalization is immunocytochemistry applied to fixed cells. This method allows employing specific antibodies to different proteins present along the endocytic vesicles and organelles. It considerably supplements the available fusion proteins and molecular probes. Furthermore, in this case the detection can use fluorescent probes, enzymes, gold nanoparticles and many other labels, which also broadens the spectrum of available visualization tools. Apart from confocal microscopy, the electron microscopy is also highly useful as it allows visualizing nanomaterials coupled with electron dense labels in different vesicular structures under very high resolution [22]. Atomic Force Microscopy (AFM) has also been used recently to demonstrate the interactions of nanomaterials with the cell membrane [23].
Exclusion of specific endocytosis mechanisms is a distinct and powerful technique to elucidate endocytosis. This can be achieved, for example, using various pharmacologic inhibitors of endocytosis that include chemical or biological agents [24–25]. Examples of chemical inhibitors include K+ depletion of sucrose for CME, methyl-beta-cyclodextrin (MβCD) and other cholesterol depletory compounds for caveolae, as well as other compounds described in Table 1. One issue with chemical inhibitors is that they are rarely selective and often disturb multiple endocytosis pathways [25]. Therefore, it is essential to utilize such inhibitors in combination with endocytosis markers as positive controls and other methods described above to validate the inhibitory mechanism and effective inhibitor concentration in the particular cell types. As an alternative the use of siRNA that can knock down specific endocytic proteins is becoming increasingly popular [14]. Similarly, the dominant negative transfected mutants have been used extensively to exclude specific endocytosis pathways [26–27]. Some of such mutants are listed in Table 1. In the case of mutants an additional advantage is possibility to co-express GFP or other reporter, which allows visualizing the transfected cell population in live cells. In this case the non-transfected cells serve as an internal control. It is useful also to employ in parallel both the siRNA gene knock-out as well as gene overexpression in mutants to minimize the artifacts associated with each method. One concern, however, with both siRNA and mutant-based techniques is that the transfection agents used to deliver siRNA or gene into cells may also disturb the trafficking pathways present in naïve cells. This can be overcome by utilizing stably transfected cells or knock-out cell lines derived from knock-out animals. An example of such knock-out cells are caveolae-deficient fibroblasts derived from caveolae −/− mice [28].
Table 1.
Main pathways of pinocytosis are presented along with some examples of their cargoes, endocytic proteins and biological and chemical inhibitors. Abbreviations are: AP2, adaptor protein-2; AP180, adaptor protein-180; Arf (1 or 6), ADP-ribosylation factor; Cdc42, cell division control protein 42; CTB, cholera toxin B; CytoD, cytochalasin D; γc-cytokine receptor, cytokine receptor common gamma chain; DN, dominant negative; FCεR1, fragment crystallizable region epsilon receptor-1;GPCR, G-protein coupled receptors; GPI, glycophosphatidylinositol; IL2Rβ, interleukin-2 receptor beta; KD, knock-down; LatA, latrunculin A; MβCD, methyl-beta-cyclodextrin; MHC, major histocompatibility complex; M6P, mannose-6-phosphate; PP2, (4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine); Rab5, ras-like in rat brain; Rac1, ras-related C3 botulinum toxin substrate 1; RhoA, ras homolog gene family, member A; RTKs, receptor tyrosine kinases; Src, sarcoma (protooncogenic tyrosine kinase), SNX9, sorting nexin-9; SV40, simian virus-40, TfR, transferrin receptor.
| Pinocytosis pathways | Implicated cargoes | Endocytic proteins | Dynamin dependence |
Inhibitor(s) | |
|---|---|---|---|---|---|
| Chemical | Biological | ||||
| CME | RTKs, GPCRs, TfR, M6P, anthrax toxin |
Clathrin, AP2, epsin, SNX9, actin, amphiphysin, Rab5 and many others |
Yes | Hypertonic sucrose, K+ depletion, chlorpromazine, actin polymerization inhibitors (CytoD, LatA) |
AP180 DN, dynamin2 DN |
| Caveolae-mediated endocytosis | CTB, SV40, modified albumin, GPI- anchored protein |
Caveolin, cavin, Src, actin, PKC (many signaling proteins localize on caveolae) |
Yes | Cholesterol depletors (e.g. mβCD, Filipin), genistein, PP2 (src kinase family inhibitor), actin polymerization inhibitors |
dynamin2 DN, cav1 DN, cav1 KD |
| Arf6 | MHC class I proteins, protectin, carboxypeptidase |
Arf6 | No | Cholesterol Depletors, genistein, actin polymerization inhibitors |
Arf6 DN |
| Flotillin | CTB, protectin, proteoglycans | Flotillin 1 and 2, other proteins unclear |
No | Cholesterol depletors, genistein, actin polymerization inhibitors |
|
| Cdc42 | Fluid phase markers, CTB, GPI- anchored protein |
Cdc42, Arf1 | Unclear | Cholesterol depletors, genistein, actin polymerization inhibitors |
Cdc42 DN, Clostridium toxin B (RhoA GTPase inhibitor) |
| RhoA | IL2Rβ, FCεR1, γc-cytokine receptor | RhoA, Rac1 | Unclear | Cholesterol depletors, genistein, actin polymerization inhibitors |
RhoA, Clostridium toxin B |
The cells with excluded endocytosis mechanisms are studied along with the normal cells using a variety of techniques allowing determining intracellular trafficking of nanomaterials. For example, confocal microscopy can be performed after exposure of cells to chemical and biological inhibitors and compared with that of the untreated cells [21]. The confocal microscopy has been coupled with 3D Z-stack imaging analysis to obtain exact location of the studied materials. The uptake of nanomaterials can be also quantified by flow cytometery, fluorescent microscopy or simple radioactivity sampling [29]. Most recently, multiple particle tracking has been used by several investigators to monitor the dynamics of the endocytosis of various nanomaterials in different cells [30]. However, a thorough and conclusive analysis must include multiple approaches to unambiguously dissect intracellular trafficking mechanisms.
3. Phagocytosis
As mentioned already phagocytosis occurs primarily in professional phagocytes, like macrophages, monocytes, neutrophils and dendritic cells. There are suggestions that some other types of cells, referred to as nonprofessional phagocytes, such as fibroblasts, epithelial and endothelial cells, may also display some phagocytic activity, but to a much lower extent [31]. Altogether, the phagocytic pathway of cellular entry consists of three distinct steps (Fig. 3): 1) recognition of the particles by opsonization in the bloodstream; 2) adhesion of the opsonized particles onto the cell membrane; 3) ingestion of the particle by the cells. Opsonization of nanoparticles occurs through adsorption of proteins, such as immunoglubulins (Ig) G (and M), complement components (C3, C4, C5), blood serum proteins (including laminin, fibronectin, etc.) and others. The opsonized particle then attaches to the macrophage surface through specific receptors. For example, Fc receptor (FcR) or complement receptors (CR) can bind respectively to the constant fragment of immunoglobulins or complement molecules adsorbed at the particle [32]. Other receptors that may play role in phagocytosis of nanoparticles include mannose/fructose and scavenger receptors [32–33]. The receptor-ligand interaction leads to signal cascades, which result in actin rearrangement and formation of a phagosome. The phagosome may have different sizes depending of the size of the particles, which can range from as little as few hundred nanometers to dozens of microns [31]. Examples were described with murine bone marrow-derived macrophages of nearly 14 µm ingesting IgG-opsonized latex beads greater than 20 µm in diameter [34]. Phagosome and its contents undergo maturation through a series of fusion and fission events, which lead to the transfer of the cargo to the late endosomes and ultimately lysosomes to form a phagolysosome.
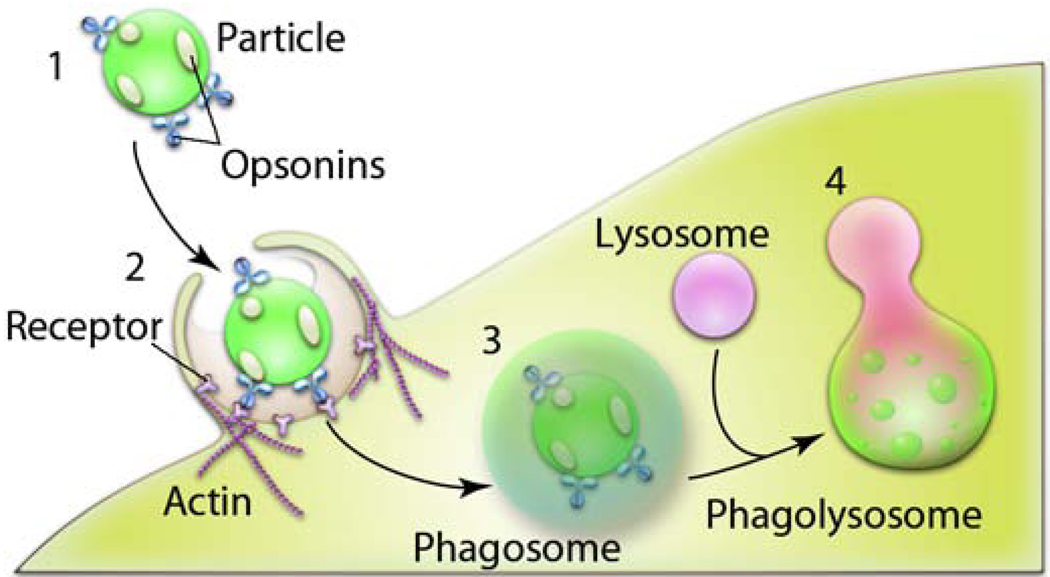
Fig. 3. Stages of phagocytosis of particles.
1) Particles undergo recognition in the bloodstream through opsonization i.e. adsorption of proteins (immunoglubulins (Ig) G (and M), complement components (C3, C4, C5); blood serum proteins (including laminin, fibronectin, etc.). 2) Opsonized particles attach onto the cell membrane through receptors present on the cell surface of a phagocyte. 3) The particles are ingested into phagosomes. 4) The phagosomes mature, fuse with lysosomes and become acidified, leading to the enzyme-rich phagolysosomes where the particles are prone to degradation.
A recent study by Champion et al. described the striking effect of the shape of particles on phagocytosis in alveolar rat macrophages [35]. They have prepared polystryrene (PS) based particles of more than twenty shapes including spheres, rectangles, rods, worms, oblate ellipses, elliptical disks (PS) and UFO-like [35]. The particle sizes were primarily 1 to about 10 µm. However, the size was not a determining factor in phagocytosis [36]. All particle shapes independently of their sizes were capable of initiating phagocytosis in at least one orientation. However, unexpectedly, the crucial role in phagocytosis was played by the local particle shape at the point where the particle was attached to the macrophage [35]. For example, a macrophage attached to a sharper side of the ellipse would internalize this ellipse in a few minutes. In contrast, a macrophage attached to a dull side would not internalize the same ellipse for many hours (Fig. 4). Spheres were internalized from any point of attachment independently of their size. Although, particle size played much lesser role in the initiation of the phagocytosis it could of course affect its completion especially when the particle volume exceeded that of a cell.
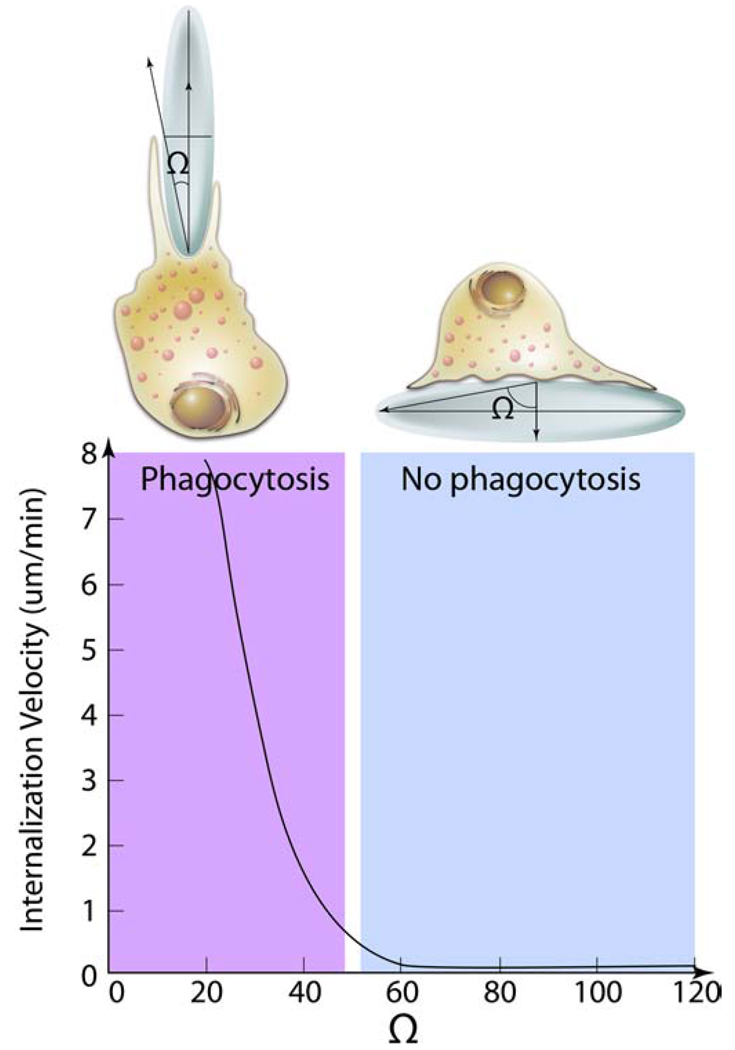
Fig. 4. Effect of particle geometry on phagocytosis.
The entry of a nanoparticles inside macrophages depends on the angle between the membrane normal at the point of initial contact and the line defining the particle curvature at this point (Ω). The internalization velocity is positive at Ω ≤ 45°, which indicates that the particle undergoes internalization. As the angle exceeds critical value ≈ (45°) the internalization velocity is zero, the macrophages lose the ability to entrap particles and start spreading over the particle.
The effect of the geometry in phagocytosis was quantified by measuring the angle between the membrane normal at the point of initial contact and the line defining the particle curvature at this point [36]. If this angle exceeded some critical value (45°) the macrophages would lose the ability to entrap particles and attach to these particles in a process similar to spreading (Fig. 4). The authors suggested that the shape of the particle at the point of attachment defined the complexity of actin structures that would need to be rearranged to allow engulfment. Above the critical point the necessary actin structures could not be created and the macrophages would switch to the spreading behavior. Altogether, this eloquent study clearly demonstrated the relationship between cellular transport by phagocytosis and physical properties of the phagocytosed materials. Further examples of different nanomaterials, which utilize phagocytosis can be found in a recent review by Hillaireau et al. [31]
4. Clathrin-dependent endocytosis
CME is the “classical route” of cellular entry, which is present and inherently active in all mammalian cells. It is responsible for uptake of essential nutrients like cholesterol carried into cells by low density lipoprotein (LDL) via the LDL receptor, or iron carried by transferrin (Tf) via the Tf receptor. These proteins are now commonly used as markers of CME. Other functions of CME include down-regulation of cell signaling by internalization and degradation of receptors and maintaining cellular homeostasis, for example, by trafficking ion pumps [9, 12].
Mechanistically, CME involves engulfment of receptors associated with their ligands to a coated pit. The coated pit forms due to polymerization of a cytosolic protein called clathrin-1, which also requires assembly proteins like AP180 and AP-2. The assembled vesicle (ca. 120 nm) is pinched off from the plasma membrane by a small GTPase, dynamin [37] (Fig. 5). Various accessory proteins like amphiphysin, Eps15 and intersectin, have been shown to act as scaffolds that connect the endocytic machinery with actin cytoskeleton [38–39]. Actin defines spatial regulation and movement of the endocytic vesicle towards the interior of the cells. Within cells the clathrin coat is shed off and the vesicles fuse with the early endosomes where they are sorted to late endosomes/lysosomes, to trans-Golgi network or to the recycling endosomes to be transported back to plasma membrane [40].
Fig. 5. Schematic representation of CME.
1) The assembly proteins, AP-2 and AP180 are targeted to the plasma membrane where they mediate clathrin assembly. Upon that clathrin triskelions polymerize into a polygonal lattice, which helps to deform the plasma membrane into a coated pit. 2) Dynamin, a multidomain GTPase, is recruited to the necks of coated pits, where it assembles into a spiral collar. Upon hydrolysis of GTP dynamic collar promotes scission of the membrane and release of the vesicle known as CCV. 3) The next step involves uncoating of CCV and formation of an early endosomes, which are then routed towards the lysosomes as shown in Fig. 1. 4) The coat constituents are recycled for reuse.
While there are multiple and sometimes conflicting reports on the nanomaterials trafficking pathways, there are few examples in which CME appears to be defined as the most prominent mechanism for the cellular entry. These examples are described below.
4.1 Poly(ethylene glycol)-Polylactide nanoparticles
The biodegradable nanoparticles made of D,L-polylactide (PLA) and poly(ethylene glycol-co-lactide) (PEG-co-PLA) blends are widely explored for drug delivery [41–42]. Due to the presence of the partially hydrolyzed PLA the surface of these nanoparticles is usually negatively charged but can be altered by cationic surfactants such as stearylamine. A recent study, examined the effect of such modifications on the uptake of the nanoparticles (around 100 nm) in fibroblast and epithelial cells [41–42]. In both cell types the cationic particles were more readily accumulated in cells compared to anionic particles. However, the pathways of their entry depended on the cell type. In the polarized MDCK epithelial cells, which are normally devoid of caveolae on the apical surface [43–44], the nanoparticles used CME independently of their charge. In contrast, in non-polarized HeLa cervical cancer cells the anionic particles used multiple pathways (CME and caveolae-mediated), while cationic particles appeared to be restricted to CME as well as to macropinocytosis. Interestingly, the nanoparticles charge also determined their intracellular fate. In MDCK cells the cationic particles was routed for transcytosis, while the anionic particles reached the lysosomes [41–42]. This example clearly shows that the entry and subsequent trafficking of the nanoparticles can depend both on the cell type and the particle charge.
4.2 Poly(lactic-co-glycolic acid) (PLGA) nanoparticles
A close example is nanoparticles made of PLGA, a biodegradable synthetic material approved by the United State Food and Drug Administration (FDA) for therapeutic use [45]. The cellular transport of such nanoparticles was also shown to be both cell type and surface charge dependent [23]. In this case the nanoparticles were relatively large (ca. 300 nm) and heterogeneous (polydispersity index 0.2), which obviously complicated interpretations of their cellular uptake. Nevertheless, the cell dependence of the uptake was shown using basic PLGA nanoparticles, which are negatively charged. In the vascular smooth muscle cells (VSMCs) these particles entered predominantly through the CME, however, in rat corneal epithelial cells they utilized the clathrin- and caveolae-independent pathways [46–47]. Furthermore, in VSMCs 85% of these particles was recycled to the cell surface from the early endosomes [46–47] . The remaining 15% were reported to escape the endosomes and reach the cytosol. To increase the endosomal escape the authors subsequently modified the nanoparticles surface with a cationic polymer, poly(L-lysine) (PLL) [23]. The adhesion of nanoparticles at the cell plasma membrane was quantified by AFM in cancer cells. The PLL modification led to nearly five-fold increase in the interaction force. The modified particles were also more rapidly internalized via CME, which allowed considerably increasing the cellular delivery of a model protein encapsulated in such nanoparticles [23].
4.3 Silica-based nanomaterials (SNTs)
CME was observed for “template synthesized” silica nanotubes (SNTs) [48]. Such SNTs provide unique structural features including inner voids for loading bioactive compounds, end functionalization to control compounds release, and inner and outer surfaces that can be differentially functionalized for targeting and biocompatibility. The cationic SNTs (50 nm in diameter and 200 nm long) functionalized with positively charged aminosilane group at the outer surface were shown to internalize via CME and reach lysosomes in cancer cells [48]. In another study mesoporous silica-based nanoparticles (ca. 110 nm) were shown internalize into mesenchymal stem cells and fibroblasts via a CME but not through caveolae [49].
4.4 Chitosan nanoparticles
The cationic chitosan nanoparticles were shown to utilize CME for entry in respiratory epithelium (A549) and intestinal epithelium (Caco-2) cells [50–51]. The nanoparticles used in this study were large (430 nm) and highly polydisperse (polydispersity index 0.5), which means that large particles co-existed with small ones, capable of entry into small vesicles. The selectivity of this formulation towards CME suggests that in this particular case the chemical composition of the material was more important than its size in defining the CME entry mechanism. However, the self-assembled cationic nanoparticles of hydrophobically modified chitosan (360 nm, zeta potential ca. +22mV) are using multiple pathways for cellular entry including CME, caveolae and macropinocytosis [52]. These nanomaterials possibly interacted with the cell membranes through the 5-β-cholanic acid residues used for hydrophobization of chitosan, which altered their trafficking.
4.5 Surface modified nanoparticles that target CME
A special case is nanomaterials, which are modified to display surface ligands that utilize CME. Such ligands can include, for example, mannose-6-phosphate, Tf, nicotinic acid, etc [53]. Notably, the cellular trafficking of such modified materials may differ from the trafficking of the ligand alone [54]. For example, quantum dots (QDs) (ca. 50 nm) modified with Tf utilized CME as the initial stage of entry, however, in contrast to Tf alone they were not routed to lysosomes and not recycled to the cell surface. Instead these modified QDs resided in the perinuclear endosomes. In another example, QDs (30 nm) modified with Shiga Toxin were entering cells, but unlike the toxin did not accumulate in the Golgi and were routed to the endosomes. Such effect was likely to be due to the ability of the QDs to interfere with the cellular trafficking since they were also shown to alter the transport of unconjugated ligands (Shiga toxin, Ricin B) [54]. Therefore, in attempts to target nanomaterials though specific ligands and cellular pathways one should take into account a possibility that such nanomaterials can alter the normal pathways employed by these ligands. Further examples of those effects are discussed below for unimers and micelles of Pluronic® block copolymers [55].
5. Caveolae-mediated endocytosis
Caveolae are abundant in muscle, endothelial cells, fibroblasts and adipocytes and absent in neurons and leukocytes [12]. They are a subset of lipid rafts, the cholesterol-rich plasma membrane regions that cluster endocytosis and signal transduction functionalities [56]. The definitive characteristic of caveolae is the presence of the hairpin-like membrane protein, caveolin-1, which is necessary for biogenesis of caveolae. Due to this protein caveolae assume their hallmark flask-shaped structure (60–80 nm) and can engulf cargo molecules, which bind to caveolae surface. In addition to caveolin-1, which is present in most cells, there are other isoforms like caveolin-2 or caveolin-3 (specific for muscle) [12]. Other components of the caveolae endocytic machinery include proteins like cavin, which induces membrane curvature, dynamin, which enables vesicle scission, as well as vesicle-associated membrane protein (VAMP2) and synaptosome-associated protein (SNAP), which mediate subsequent vesicle fusion, etc. [57–58]. After budding of the plasma membrane the caveolae vesicles transport and fuse with caveosomes or MVBs that have neutral pH [59]. This pathway appears to be slower compared to the CME in vitro but, importantly, at least in some cases it can bypass lysosomes. Hence, several pathogens including viruses and bacteria exploit this pathway to prevent lysosomal degradation [60]. For the same reason this pathway is believed to be beneficial for cellular delivery of proteins and DNA [61]. The typical molecules to undergo the caveolae-mediated endocytosis are CTB and Shiga toxin. These molecules interact with the glycosphingolpids (GSL) residing in caveolae, such as GM-1 (CTB) and Gb3 (Shiga toxin) [12]. Both CTB and Shiga Toxin are sometimes used as markers for caveolae. However, it should be noted that they are not restricted to caveolae and can also enter through clathrin- and caveolae-independent pathways described below.
Caveolin-1 is a very promiscuous protein, which binds and promotes ordering of multiple molecules including lipids (cholesterol, GSL, etc.), fatty acids, and membrane proteins [60]. Thus, caveolae sequester multiple ligands responsible for cellular signaling and their downstream signaling components in close proximity for efficient signal activation and transduction. Some examples include heterotrimeric G proteins, non-receptor tyrosine kinases, insulin receptor, platelet derived growth-factor receptor, and endothelial nitric-oxide synthase (eNOS) [59]. Many of these molecules seem to directly interact with caveolin. For example, eNOS binds to the caveolin-1 scaffolding domain and remains inactive when bound. The ligands, which disrupt this interaction, enable eNOS activation, which leads to production of nitric oxide (NO) and increases vascular permeability [62].
Several nanomaterials are reported to enter cells via caveolae. This pathway has attracted tremendous attention in nanomedicine, since it has the ability to bypass lysosomes (although there are few exceptions described below). Furthermore, the caveolae-mediated endocytosis is the most prominent transendothelial pathway and thus this route may be employed for trans-vascular delivery of nanomaterials [22].
5.1 Polymeric micelles with cross-linked anionic core
Core-cross linked polymeric micelles (cl-micelles) of poly(ethylene oxide)-b-poly( methacrylic acid) (PEO-b-PMA) copolymer are unique hydrogel-like nanomaterials, which have swollen cores of a cross-linked PMA network surrounded by a PEO shell [63]. At extracellular pH 7.4 they have strong negative charge (zeta potential −18 mV), which decreases at pH 5.0 (−7 mV), while the cl-micelles contract from ca. 150–160 nm to ca. 110 nm. These cl-micelles display selective entry in cancer cells but do not enter normal epithelial cells due to their ability to target differences in endocytosis mechanisms between these cells (Fig. 6) [26]. The internalization of cl-micelles in the cancer cells proceeds predominately through caveolae-mediated endocytosis. In confluent normal epithelial cells this endocytosis route is absent at the apical side [44] and the cl-micelles sequester in tight junction (TJ) regions of the cell membrane without entering the cells. Furthermore, contrary to conventional wisdom in cancer cells the cl-micelles bypass the early endosomes and reach the lysosomes within 30 min. where they release drug in a pH dependent fashion. Hence, micelles loaded with a cytotoxic drug are toxic to cancer cells but not to normal epithelial cells, where they do not enter [26].
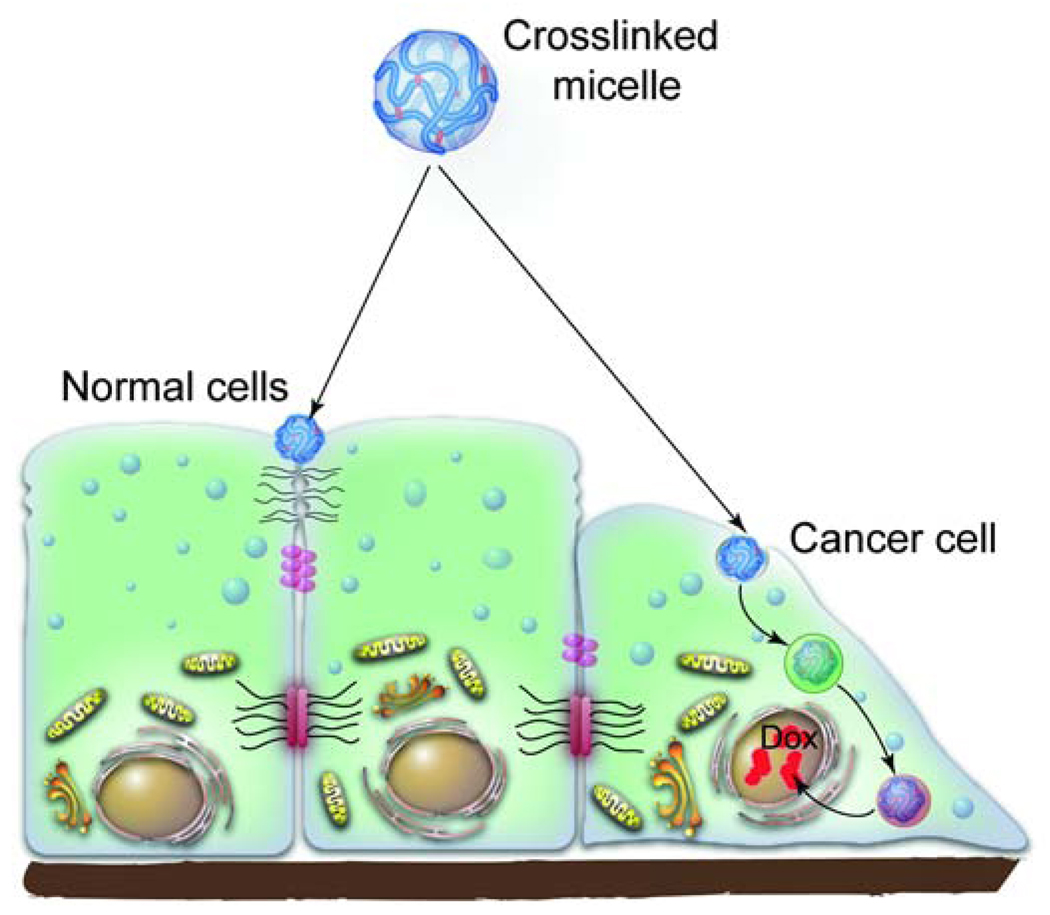
Fig. 6. Pathways of intracellular trafficking of cl-micelles in normal and cancer epithelial cells.
The cl-micelles carrying a drug, doxorubucin (Dox), in normal epithelial cells were shown to sequester at the apical surface of the cell membrane near the TJs. However, during cancer progression the TJs are lost. As a result the cancer epithelial cells internalize the cl-micelles through caveolae. The cl-micelles are then routed to the lysosomes where the drug is released through a pH-dependent mechanism. The released drug accumulates in the nucleus and kills the cancer cells.
5.2 DOXIL®
DOXIL® is a slightly negatively charged PEGylated liposomes (86 nm), which encapsulate doxorubicin hydrochloride and are used to treat patients with metastatic ovarian cancer. Surprisingly, little was known until recently about trafficking of DOXIL® nanoparticles into cells. Our study presents evidence that these nanoparticles utilize caveolae-mediated endocytosis to internalize into epithelial cancer cells [26]. Similarly to the cl-micelles, after the entry through caveolae the DOXIL® nanoparticles are accumulated in lysosomes, where the drug is apparently released [26].
5.3 Polysiloxane nanoparticles
Amphiphilic self-assembled nanoparticles (ca. 100 nm) of poly(3-aminopropyl)siolxane (PAPS) modified with stearic acid residues and galactose were shown to selectively target caveolae in human aortic endothelial cells [64]. Even more interestingly, these nanoparticles can activate eNOS cell signaling and promote NO formation. This strongly suggests that the nanoparticles disrupt eNOS binding to the caveolin-1 scaffolding domains and provides evidence that synthetic nanomaterials can activate signal transduction leading to pharmacological effects due to interactions with specific micro-domains at the cell membrane.
5.4 QDs
The effects of surface modifications on cellular entry were recently examined using CdSe/ZnS core-shell ellipsoid QDs (diameters 6 nm, 12 nm), which were modified with PEG, PEG-amine or poly(acrylic acid) [65]. The resulting QDs-based nanomaterials differed both in surface charge and size, being neutral (45 nm), positive (20 nm) and negative (18 nm), respectively. Of the three materials the negatively charged particles displayed the greatest uptake in skin cells. Moreover, the negatively charged particles entered the cells primarily through caveolae, while the neutral and positive particles did not appear to use this pathway [65].
5.5 Abraxane®
Aside from charge the specific interactions with the receptor located in caveolae may define the cellular entry of nanomaterials. This is a case of Abraxane®, a nanoparticle albumin-bound (nab) form of paclitaxel (130 nm) approved by the FDA for metastatic or relapsed breast cancer [66]. Abraxane® nanoparticles take advantage of caveolae-mediated transcytosis for efficient delivery of the drug to the tumor sites [67]. They bind to gp60, the albumin receptor present in caveolae of endothelial cells, and transport across the vascular walls to the tumor interstitial spaces [68]. After entering the interstitial spaces the Abraxane® nanoparticles are captured by SPARC (secreted protein, acidic and rich in cysteine) that is selectively secreted by the tumors [69–70]. The SPARC-nanoparticles complexes are taken up in tumor cells resulting in selective tumor cytotoxicity.
5.6 Surface-modified nanoparticles that target caveolae
As the significance of caveolae for the delivery of nanomaterials has emerged the attempts have been made to modify the surface of these nanomaterials to target proteins displayed in caveolae. One such target was identified by Oh et al. by in vivo quantitative proteomics of caveolae isolated from tumor lung endothelium [22]. They determined that these caveolae are enriched with aminopeptidase P (APP). Furthermore, the monoclonal antibody to APP was shown to be transported in vivo within seconds across endothelium to the lung tissue. Hence, the colloidal gold nanoparticles modified with antibody to APP were found to concentrate in caveolae of lung microvasculature [22].
Another well known targeting molecule is cyclic RGD peptide, which binds with αvβ3 integrin receptor located in caveolae. Recently, Oba et al. used thiolated c(RGDfK)-PEG-b-PLL copolymer for cellular delivery of the pDNA [71]. This copolymer binds with the DNA molecules through its cationic PLL chains resulting in core-shell polyion complex nanoparticles having core of electrostatically coupled PPL/DNA and a shell of PEG decorated with the cyclic RGD peptide. The core of such nanoparticles was additionally stabilized by cross-linking PLL aminogroups. They were shown to enter cells through caveolae and localize in the perinuclear regions in HeLa cells. The increased entry of the RGD-modified complexes compared to the non-modified analogs was accompanied by a superior DNA transfection efficiency in these cells.
6. Clathrin- and caveolae-independent endocytosis
Cellular entry occurs in cells devoid of both CME and caveolin-1. In such cells clathrin- and caveolae-independent endocytosis with ca. 90 nm vesicles has been shown to carry different cargoes including extracellular fluid, SV40, CTB, glycosylphosphatidylinositol (GPI)-linked proteins, interlukin-2, growth hormones, etc. For these cargoes multiple entry pathways appear to exist, which can be regulated through multiple effectors. Based on the effectors the caveolae- and clathrin- independent pathways are presently classified as Arf6-dependent, flotillin-dependent, Cdc42-dependent and RhoA-dependent [12].All these pathways appear to require specific lipid compositions and are dependent on cholesterol. Most of these pathways are dynamin-independent although the role of dynamin in some pathways is still being researched. While their later stages are not yet clearly identified, they appear to bypasses the rab5-poitive early endosomes. As an example, the GPI-linked proteins are transported though GPI-anchored protein enriched early endosomal compartments (GEECs) having a tubulovesicular morphology. Some of these pathways can also utilize GEEC-independent endosomes and may interplay with clathrin-dependent endocytic compartments. This is the case, for example, of the transport of IL-2Rβ, γc cytokine receptor and the IgE receptor FcεR1 (a major signaling pathway for allergic reactions)[12, 72]. Another subtype is also independent of the GEEC and requires Arf6 positive endosomes involved in recycling towards the plasma membrane. This pathway is utilized by the major histocompatibility class (MHC)-1 protein, which is critical for antigen presentation and immune response [12, 73–74].
There are not many nanomaterials documented to utilize different subtypes of the clathrin- and caveolae-independent endocytosis. The examples include nanoparticles and polymers modified with folate [75] . Folate binds to GPI-anchored folate receptor, FRα, which is overexpressed in tumor cells. The expression of this receptor increases as the cancer stage advances, which may be important for drug targeting [75]. However, the folate entry in cells is complex and along with clathrin- and caveolae-independent endocytosis it can also involve CME in specific cells types[12, 76]. Many nanomaterials and polymers were conjugated with folate including liposomes, protein toxins, biodegradable nanoparticles, and water soluble N-(2-hydroxypropyl)methacrylamide (HPMA). These materials were used targeted intracellular delivery of cytotoxic drugs, imaging agents and also for boosting immune response [75].
7. Macropinocytosis
Macropinocytosis is a special case of clathrin-, caveolae- and dynamin-independent endocytosis, which is initiated by transient activation of receptor tyrosine kinases by growth factors [24]. The receptor activation mediates a signaling cascade that leads to changes in the actin cytoskeleton and triggers formation of membrane ruffles. These membrane ruffles protrude to engulf the surrounding fluid and nutrients in the extracellular milieu [24, 77–78]. They can simply melt with the cell membrane or form an intracellular vacuole, also termed as a macropinosome [79]. The macropinosomes are larger (0.5–10 µm) and distinct from other vesicles formed during pinocytosis. Many particles like bacteria, apoptotic bodies, necrotic cells and viruses can induce the ruffling behavior independently of the growth factors, and internalize in macropinosomes [24]. Selected nanomaterials were assumed to utilize this pathway based on their entry dependence on actin formation inhibitors, such as cytochalasin D [55]. This pathway is possible for virtually any cell with only few exceptions, such as macrophages and brain microvessel endothelial cells. In principle it can internalize large particles with submicron and greater sizes in cells, which lack phagocytosis. In most cases this pathway may serve as a non-specific entry point and it is discussed further for nanomaterials that can utilize multiple mechanisms for cellular entry.
8. Nanomaterials employing multiple pathways for entry
Most nanomaterials have been shown to exploit more than one pathway to gain cellular entry. Some examples of such nanomaterials are discussed below.
8.1 PRINT micro-and nanoparticles
These particles are designed through a top-down lithographic fabrication method called PRINT (Particle Replication In Non-wetting Templates) [80]. PRINT technology results in the formation of nearly monodisperse nanoparticles that have precisely controlled shape, size and charge. In an elegant study Gratton et al., evaluated internalization pathways of three different series of micro- and nano-particles made from the cross-linked PEG-based hydrogels [81]. All materials were obtained by UV-copolymerization of the same mixture of monomers in different PRINT molds and therefore are believed to have the same chemical composition, but different sizes and shapes. The materials were positively charged and had relatively close zeta potentials (ranging from +21mV to +41mV). They included cubic microparticles (2 µm, 3 µm, and 5 µm), cylindrical microparticles (0.5 × 1 µm, 1 × 1 µm) and cylindrical nanoparticles (200 × 200 nm; 100 × 300 nm; 150 × 450 nm). Surprisingly, all these different particles including the largest ones were shown to internalize in HeLa cells (Fig. 7). However, the nanoparticles seemed to enter cells more rapidly than the microparticles. Furthermore, the shape of the nanoparticles also mattered as the extended cylinders (150 × 450 nm) entered faster than the equal cylinders of nearly same volume (200 × 200 nm), or cylinders of lower volume (100 × 300 nm). All nanoparticles appeared to employ multiple pathways of entry including CME, caveolae-mediated endocytosis and to a lesser extent macropinocytosis. It also appeared that CME and caveolae were more prominent for the entry of nanoparticles compared to the microparticles. Interestingly, when the charge of these materials was inverted to negative (−34mV) by acylating their amino groups their entry became negligible.
Fig. 7. Cellular entry of PRINT nano- and microparticles.
PRINT nanoparticles of all shapes utilize multiple pathways to gain cellular entry including CME (1), caveolae-mediated endocytosis (2) and, to a lesser extent, macropinocytosis (3). The shape of the particles appears to be important in regulating the rate of their cellular entry (not shown). Cube-shaped PRINT microparticles also utilize multiple routes of cellular entry but their macropinocytosis appears to be the most prominent.
8.2 Pluronic® block copolymers
Another interesting example is the cellular entry of amphiphilic triblock copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), Pluronic® P85 (P85) [82]. Below the critical micelle concentration (CMC) this copolymer exists as single molecule chains (unimers) and above CMC forms 14.6 nm aggregated micelles with a hydrophobic PPO core and hydrophilic PEO shell. Both the copolymer and micelles are electrostatically neutral. This copolymer was shown to utilize multiple pathways to gain entry in mammalian cells depending on its aggregation state (Fig. 8A) [55]. In particular, P85 unimers internalize predominantly through caveolae-mediated endocytosis, however, in cells devoid of caveolae the copolymer exploits caveolae- and clathrin-independent endocytosis. Interestingly the unimers were shown to bypass early endosomes/lysosomes, transport to endoplasmic reticulum, and eventually reach mitochondria [21]. Furthermore, the copolymer succeeds in entering nearly impenetrable cells, such as primary brain endothelial cells and primary neurons where the copolymer moves anterograde from cell body to axons/dendrites (Fig. 8B). On the contrary P85 micelles internalize exclusively through CME [55]. Furthermore, at concentrations above CMC P85 inhibits caveolae-mediated endocytosis while having little or no effect on CME [55].
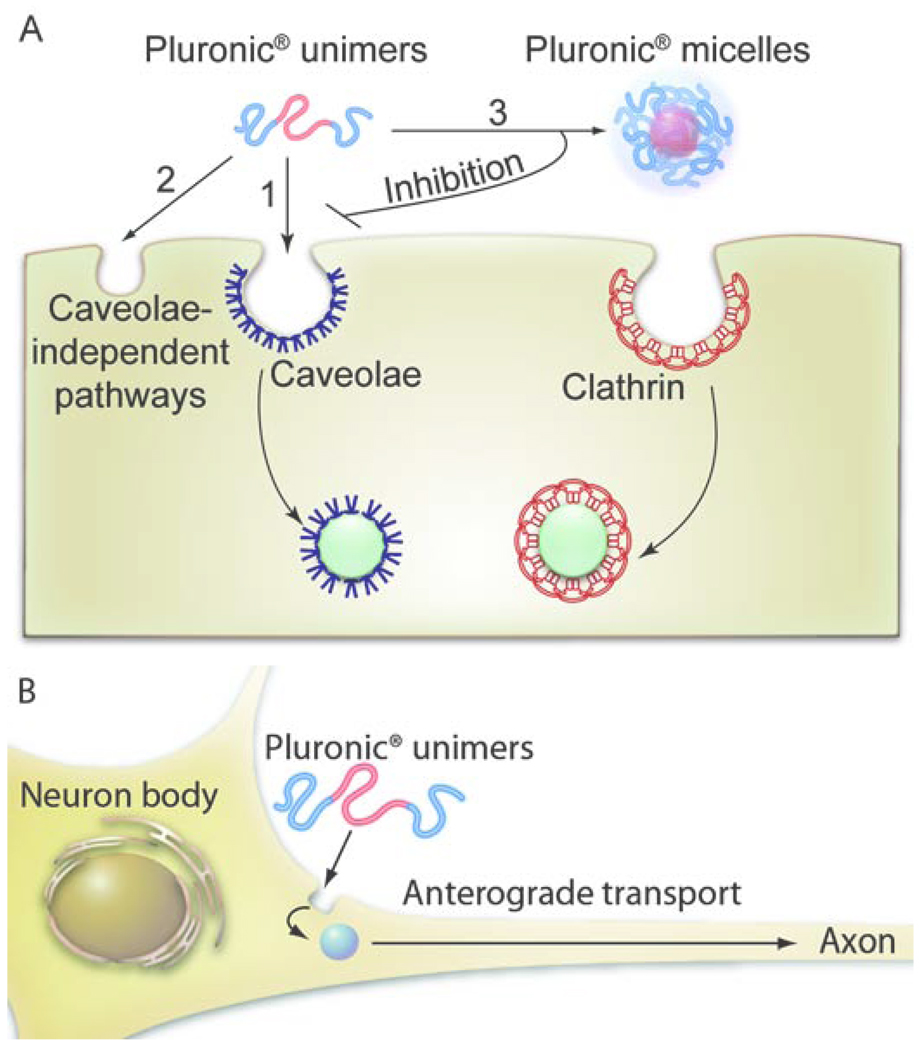
Fig. 8. The entry mechanisms of Pluronic® block copolymers in (A) epithelial cells and (B) neurons.
A. In cells displaying the caveolae pathway Pluronic® P85 unimers enter through caveolae-mediated endocytosis (1). In cells devoid of caveolae, such as confluent MDCK cells, the block copolymer unimers can also enter through caveolae-independent pathways (2). Once the concentration of the block copolymer increases above the CMC the micelles are formed, which enter through the CME (3). Under these conditions the block copolymer inhibits the caveolae-mediated endocytosis. B. In primary neurons (also devoid of the caveolae) the Pluronic® P85 unimers enter the cell body from where they undergo anterograde trafficking to the axons/dendrites.
8.3 PS nanoparticles
Contrary to Pluronic® micelles PS nanoparticles do not disintegrate upon dilution. However, their cellular trafficking can also depend on their size. For example, Lai et al. have recently shown that negatively charged 43 nm PS nanoparticles can enter HeLa cells through CME and reside in the endo-lysosomal compartment [83]. The reduction of the nanoparticle size to 24 nm resulted in a shift of the entry mechanism to clathrin-and caveolae-independent pathway. Furthermore, it also altered the processing of the nanoparticles, which in this case bypassed lysosomes and localized near the perinuclear space. Recent work also evaluated the entry of 200 nm positively charged (amine-modified) or negatively charged (carboxylate-modified) PS nanoparticles [26]. Independent of their charge such nanoparticles were shown to enter caveolae-deficient fibroblasts as well as confluent MDCK cells that lack apical caveolae.
8.4 Poly(amidoamine) (PAMAM) dendrimers
Dendrimers are repeatedly branched, monodisperse and usually highly symmetric compounds, which have been widely researched for delivery of therapeutic and diagnostic agents [84]. They have large number of surface groups that can immobilize drugs, enzymes, targeting moieties or other imaging agents. The amine- modified cationic dendrimers or carboxylate-modified anionic PAMAM dendrimers were shown to utilize multiple routes of entry inside Caco-2 and B16f10 melanoma cells.[85–86]. The entry mechanisms appeared to depend on the dendrimer generation. For example, a lower generation amine-modified dendrimer G2 was entering cells primarily through CME, while the higher generation dendrimer G4 utilized multiple routes [85–86]. The positively charged dendrimers have been shown to enter early endosomes and then route towards the lysosomes within 20 min. [85], [87]. In contrast, the negatively charged dendrimers appeared to accumulate in lysosomes at later points. Finally, in experiments studying the transport of the dendrimers across the polarized cell monolayers both cationic as well as anionic dendrimers were shown to open up the TJ and cross the monolayers by a paracellular route as well as transcellular route [88].
8.5 Non-viral gene delivery agents and DNA
Nanosized complexes of cationic lipids with DNA (“lipoplexes”) or synthetic polycations with DNA (“polyplexes”) have been used for gene delivery. Clearly, the cellular trafficking of such nanomaterials can strongly depend on their composition, size, shape, and surface characteristics. Since the polyelectrolyte complexes of DNA can undergo multiple structural transitions [89] it is hard to precisely control their characteristics, especially in the cellular environment. As a result there are often conflicting reports involving such systems. For example, one study reported that cellular entry of polyethyleneimine (PEI)-based polyplexes depends both on the cell type as well as PEI structure [90]. More specifically, it was shown that polyplex based on branched PEI in the monkey kidney cells (COS-7) predominantly utilize CME [91] while in HeLa cells both CME and caveolae-mediated endocytosis. In contrast, polyplex based on linear PEI appeared to internalize through CME independently of the cell type. To the contrary, another study suggested that linear PEI/DNA polyplex use both pathways while branched PEI/DNA polyplex as well as polyamidoamine (PAMAM) dendrimer/DNA polyplex use only caveolae [86]. This clearly contradicts to another report that branched PEI-based polyplexes use both CME and caveolae-mediated endocytosis [92]. Our own data suggested that polyplexes based on linear PEI or PEG-g-PEI graft copolymer utilize both pathways [93]). Similarly, polyplexes based on PEG-g-PLL graft copolymer were also shown to utilize CME, caveolae-mediated endocytosis and macropinocytosis [94]. Lipoplexes can enter cells through multiple pathways and their entry depends on the lipoplex composition. For example, lipoplexes based on DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl sulfate) [92] and Lipofectamine were shown to internalize by CME. In contrast, DMRIE-C (1,2-dimyristyloxypropyl-3-dimethyl-hydroxy ethyl ammonium bromide)-based lipoplex internalizes through caveolae [95]. However, following the initial internalization stages both pathways appear to converge towards the late endosomes or lysosomes, where DNA delivered with Lipofectamine- and DMRIE-based lipoplexes is found. However, it should be noted that blocking of the lysosomal transport did not improve the transfection yield, which suggests that the DNA may utilize a different pathway to reach its nuclear destination.
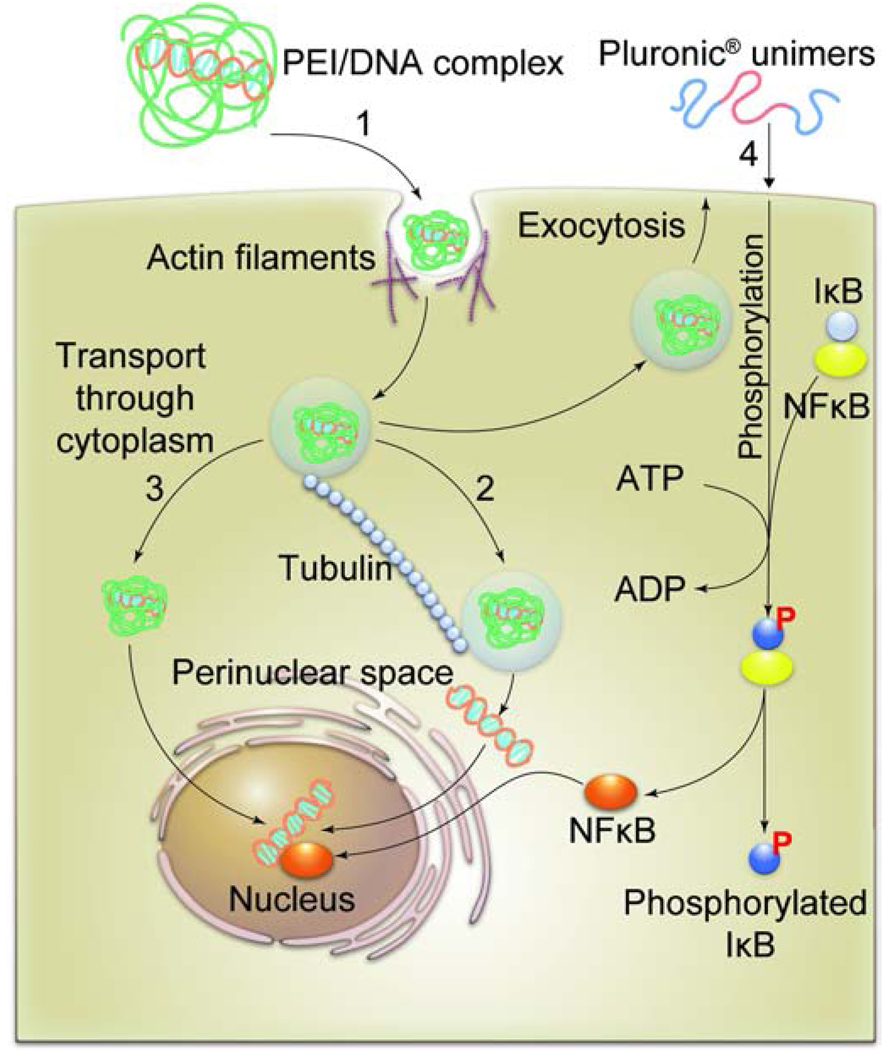
An ideal gene delivery agent after initial uptake must escape degradation in lysosomes, and enable translocation of DNA to the nucleus. In a classic study Bausinger and colleagues followed the intracellular trajectory of linear PEI/DNA polyplexes by fluorescence live cell imaging [96]. They were able to show that at the initial stage polyplexes attached to the cell membrane are co-localized with actin filaments, while at the later stage they appear to move along the microtubules. A similar observation was made by Suh et al. who suggested that branched PEI/DNA polyplexes entrapped in endosomes are transported by motor-protein driven machinery along the microtubules through the cytoplasm towards the nucleus [97]. They also speculate that after reaching the perinuclear space the DNA can diffuse to the nucleus (Fig. 9). This appears to contradict a widespread belief that DNA can be released directly to cytoplasm from endosomal/lysosomal compartments through destabilization of these compartments by PEI (“proton sponge” hypothesis) [98]. The nuclear import of the DNA is cell-cycle dependent and is one of the most challenging barriers for non-viral gene delivery. Surprisingly, it was recently shown that synthetic polymers can help to overcome this barrier through the activation of specific cellular trafficking machinery [93]. In particular, Pluronic® block copolymers added to cells enhance uptake and nuclear import of plasmid DNA delivered with polyplexes (Fig. 9). Notably, this effect appears to be due to the ability of the block copolymers to activate NFκB signaling in the cells. NFκB was shown to bind to cytosolic DNA and facilitate its transport to the nucleus through nuclear import machinery [99]. Consequently, the effects of the Pluronic® copolymer were dependent on the DNA promoter [93]. The copolymer enhanced nuclear import of pDNA containing NFκB binding sites, but had no effect on the import of pDNA without these sites. Interestingly, Pluronics® have been also shown to increase transcription of the DNA delivered into the nucleus in transiently and stably transfected cells [100]. This phenomenon may also involve activation of the NFκB transcription machinery. Overall, the interest in the effects of the Pluronics® on gene delivery has been recently propelled by findings that these copolymers can greatly enhance the delivery of the naked DNA in vivo [101]. Very little is known how the DNA alone is transported into the cells, however, it was shown recently that naked DNA in serosal cells in vivo can internalize through macropinocytosis [102].
Fig. 9. DNA delivery to the nucleus.
1). PEI/DNA complexes internalize into cells utilizing actin-dependent pathway. 2). The endosomes containing PEI/DNA complexes travel inside the cytoplasm along the microtubules and reach the perinuclear space where the DNA is released through an unknown mechanism. 3). An alternative mechanism may involve direct release of PEI/DNA complex from endosomal/lysosomal compartments, followed by the transport of the complex through the cytoplasm and to the nucleus. 4). The DNA import to the nucleus can be enhanced by activating cellular signaling by Pluronic® block copolymers. In this case Pluronics® bind with the cell membranes and activate phosphorylation of IκB by an IκB-kinase (not shown). The phosphorylated IκB dissociates from its complex with NFκB. The released active NFκB enhances transport of DNA into nucleus in a promoter-dependent fashion.
8.6 Surface modified nanoparticles that target multiple pathways
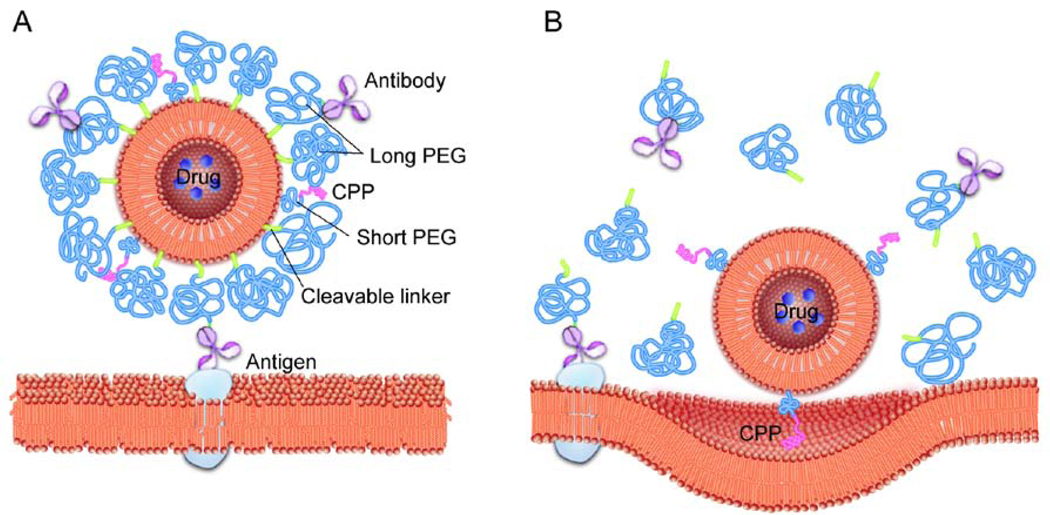
To increase cellular delivery of various nanomaterials such as liposomes, iron nanoparticles, siRNA- and DNA-containing polyplexes, etc., their surfaces were modified with cell penetrating peptides (CPPs), also known as Protein Transduction Domains (PTDs) [103–104]. Such ligands usually employ multiple pathways for intracellular trafficking. They represent small cationic polypeptides of from 10 to 30 amino acids. Some of the best characterized CPPs are TAT peptide, penetratin, transportan, poly-arginine, rabies virus glycoprotein (RVG) peptide, etc. For example, human immunodeficiency virus TAT fusion protein (>30 kDa) and TAT peptides (1 to 5 kDa) enter cells by cholesterol-dependent macropinocytosis as the primary entry mechanism [105–106]. Their transport does not seem to involve CME or caveolae. Yet, since the TAT peptide was shown to enter cells even when macropinocytosis was inhibited, alternative mechanisms were also postulated, which involve direct translocation of the peptide across the plasma membrane [107]. In a recent example, Torchillin et al. modified PEGylated liposomes and PEG-polyphosphatidylethanolamine micelles with 1) the targeting antibodies exposed at the liposome surface above the PEG layer and 2) the TAT peptide hidden within this layer [108]. Such double functionality is believed to ensure delivery of the liposomes to the target cells, where the antibody layer is cleaved and the TAT peptide exposed, which enables subsequent particle delivery into cells (Fig. 10). In another example the RVG peptide was shown to cross the brain endothelial cells and subsequently enter the neuronal cells using nicotinic acetylcholine receptor (nAchR) [109]. This peptide was further fused with nonamer arginine residues to form complexes with siRNA, which could deliver siRNA to the brain and enable protection against fatal viral encephalitis in mice. In a subsequent study, this peptide was conjugated to PAMAM dendrimers through a PEG spacer and then complexed with DNA, yielding polyplexes with RVG functionalities exposed at the surface [110]. These polyplexes were shown to internalize in the brain endothelial cells through CME and caveolae-mediated endocytosis, and deliver the DNA to the brain in vivo. Notably, their transport mechanism appeared to be more dependent on the GABAB receptor than on nAchR.
Fig. 10. Targeting of stimuli-sensitive double-targeted liposome to tumors.
A. The surface of the drug-loaded liposome is modified with a cell-penetrating peptide (CPP) attached via relatively short PEG chains. This peptide is masked by long PEG chains anchored to the liposome surface via. pH-sensitive cleavable links. Some of the long PEG chains are decorated with the antibody specific to the tumor antigen. The antibody is exposed and can bind with the antigen at the tumor cell surface. B. Inside the acidic microenvironment of the tumor the long PEG chains and the antibody conjugates are detached from the liposome resulting in exposure of the CPP. The CPP interacts with the cell membrane and facilitates endocytosis of the drug-loaded liposomes into tumor cells.
In addition to CPPs, various other ligands were used to enhance cellular delivery of both nanoparticles and water-soluble polymers. For example, the HPMA copolymer was recently modified with an antibody against prostate specific membrane antigen (PSMA) [111]. The cellular internalization of such antibody-modified HPMA in the prostate cancer cells overexpressing PSMA was greatly enhanced compared to the copolymer conjugated to a non-specific IgG. The entry of the antibody-HPMA conjugates involved CME, macropinocytosis, as well as clathrin- and caveolae-independent endocytosis. At the later stages of trafficking the conjugate appeared to be routed to the late endosomes. Muro et al., have also showed that in polystyrene particles of micron and submicron sizes of different shapes (spherical or elliptical) can be internalized into endothelial cells by targeting intercellular adhesion molecule 1 (ICAM-1) [112]. The authors suggest such particles enter through caveolae-, clathrin-, and macropinocytosis-independent pathway, which, however depends on actin. Furthermore, the ICAM-1-targeted micron sized particles remained in the prelysosomal compartments whereas the submicron sized particles reached the lysosomes more rapidly. Moreover, it appeared that particle shape influenced the rate of endocytosis, with spheres internalized more rapidly than elliptical disks.
9. Generalizations and future directions
Future drug delivery systems aim to deliver drugs not only precisely to a specific cell population but often to a specific intracellular compartment. Therefore, it has become increasingly necessary to delineate mechanism(s) of intracellular trafficking of synthetic nanomaterials that can serve as the carriers for drugs or themselves act as therapeutic or imaging agents. Furthermore, it is quintessentially important to learn how to direct the nanomaterials towards selected intracellular compartments through engagement of specific cellular trafficking machinery(ies). The growing body of research suggests that nanomaterials transport in cells depends on structure and physicochemical characteristics of nanomaterials (size, shape, charge, hydrophobicity, etc.), biospecific interactions between biological moieties decorating nanomaterials with cells, as well as peculiarities of the in endocytic machinery present in different cell types. The complex interplay of nanomaterial-cell interactions results in intracellular sorting of nanomaterials towards different destinations and can mediate activation of cellular signaling. Given the diversity of nanomaterials and cells used in the trafficking studies the finding of common factors that define intracellular transport of the nanomaterials has become a formidable task. Yet in this paper we make an attempt to delineate such factors and make recommendations for future research.
9.1 Effect of the particle charge
First, the cellular entry definitely depends on the nanomaterial charge. At present the majority of reports suggest that positively charged nanomaterials predominantly internalize through CME with some fraction utilizing macropinocytosis. Examples include cationic nanoparticles of very different origin - stearylamine-coated PEG-co-PLA, PLGA modified with PLL, amino group-modified SNTs, chitosan, etc [41–42] . However, there are some exceptions, most notably, PEI-based polyplexes, which are strongly cationic and yet may utilize multiple pathways including caveolae-mediated endocytosis [92]. It should be noted, however, that such complexes are usually formulated with excess of polycation and in cellular environment may additionally bind serum proteins, which can drastically affect their net charge and composition and alter trafficking. Furthermore, the large excess of polycations used in cell transfection experiments may also disturb the normal cell trafficking mechanism. Another exception involves positively charged PRINT particles which can utilize multiple pathways to gain cellular entry [81].
On the other hand, negatively charged nanoparticles, such as DOXIL®, cl-micelles, and QDs, are more likely to utilize caveolae-mediated endocytosis [26],[65]. Exceptions include some carboxylate-modified PS nanoparticles and negatively charged PLGA nanoparticles, which can enter cells through caveolae-independent pathways [46– 47]. Since cell membranes are generally negatively charged, it is widely believed that negatively charged nanomaterials should internalize slower compared to their positively charged counterparts. This appears to be a case of negatively charged PRINT nanoparticles. Yet a striking exception is negatively charged QD, which were shown to internalize much faster compared to neutral or positively charged QD [65]. It is unclear from literature whether neutral nanomaterials show any preference for specific cellular entry routes. In case of neutral Pluronic® micelles the CME pathway appears to be the main route of entry. However, this may be due to the ability of this block copolymer to inhibit caveolae-mediated endocytosis at micellar concentrations [55].
9.2 Effect of the particle size
It has been long believed that the size of nanomaterials may play a paramount role in their inclusion within different endocytic vesicles that greatly vary in sizes. Specifically, the need to keep particle small, between 10 to 100 nm, to enter the endocytic vesicles was postulated by many and became a foundation for current definition of nanomedicine by various agencies worldwide. However, while the small size may beneficial for a rapid entry into cells there appears to be no size cut off limit up to at least 5 µm to gain cellular entry of some materials through pinocytosis. The largest particles are, perhaps, more likely to enter cells through macropinocytosis as was suggested for PRINT microparticles [81]. Other notable example of size effects is negatively charged PS nanoparticles, which showed CME based entry for 43 nm particles and caveolae-mediated entry for 25 nm particles [83] . A major problem in dissecting the effects of the size is high polydispersity of many nanomaterials. However, as discussed above in some cases, such as highly polydisperse chitosan nanoparticles [51], the size of the particles may play much lesser role in defining the entry pathway than the chemical composition of the nanomaterial.
9.3 Effect of the particle shape
The effects of the particle shape were previously described for phagocytosis [113]. However, to best of our knowledge for pinocytosis the PRINT nanoparticles remain the only clear example of relationship between the particle geometry and cellular entry [81, 114]. Clearly, to define such effects the nearly monodisperse nanoparticles need to be manufactured. Other than shape, however, the compressibility of the material may matter for soft nanoparticles, such as nanogels and the like [114].
9.4 Effect of the cell type
Perhaps, the most exciting, challenging and poorly explored area is the relationship between the nanomaterial cellular trafficking and cell type. Few examples discussed above underscore that the cell type may be critical in defining the nanoparticle entry and final destination in the cells. Notably, it even appears that the differential endocytic pathways in normal and tumor cells: may be a gateway for selective targeting of novel nanomaterials into tumors [26]. Most studies of the nanomaterial trafficking performed so far did not emphasize the link between cell origin and availability of various endocytic pathways present in these cells. At the same time, the known cellular pathways may be differentially presented or even totally absent in selected cell types depending on the cell phenotype, and, in some cases, even growing conditions, such as cell density, presence of growth factors, etc. Obviously, in-depth understanding of the cell biology and it’s relation to nanomaterials science is most critical for advancement of this area of nanomedicine and drug delivery.
9.5 Understanding subcellular trafficking of nanomaterials
Delivery of polymers and nanomaterials to specific intracellular compartments, e.g. different cellular organelles, cytoplasm etc., has become increasingly popular due advent of drugs that target specific regions within the cells. For example, recently, a hexadentate-PLGA copolymer chemically conjugated to a selective MAPK inhibitor was shown to internalize and release drug in the cytosol of tumor cells [115]. Pluronic® block copolymers can reach mitochondria where they exert unique pharmacological activities like ATP depletion in multidrug resistant cancer cells [116]. Some materials like cl-micelles can be routed to lysosomes and employ lysosomal pH as a trigger for release of a cytotoxic drug precisely within the cancer cells [26]. On the contrary, non-viral gene delivery systems, should bypass lysosomes to prevent degradation of DNA, siRNA or antisense oligonucleotides.
Therefore, the studies of cell trafficking should extend beyond the initial entry point of the nanomaterials towards precise definition of the entire nanomaterial cellular itinerary. In such studies one must be careful not to misinterpret the initial stages of entry as a guarantee for the nanomaterial’s final destination. Thus, caveolae-mediated endocytosis is still broadly believed to bypass lysosomes but in certain cases such as cl-micelles the entry through this pathway may destine to the lysosomes [26]. In another case Pluronic® unimers enter through caveolae and then pass through ER to mitochondria – the pathway, which still is quite peculiar for biological molecules [21]. Needless to say the impetus should be given in the future to gain fundamental understanding of sorting of nanomaterials. One might even speculate that certain motif(s) (e.g., hydrophobic or chargeable groups), within the synthetic polymer might govern its sorting to a specific compartment and on removing such motif the trafficking may change. Well known analogs of such motifs are present in proteins (e.g. KDEL amino acid sequence targeting the ER, or GPI anchor on the plasma membrane) [117–118]. However, we also need to realize that synthetic water-soluble polymers and nanomaterials can exploit pathways that are unique for biomacromolecules and are not fully explored.
9.6 Understanding active targeting of nanomaterials
The concept of active targeting of nanomaterials using antibodies, polypeptides, aptamers and other targeting groups, has generated tremendous interest and on a surface appears to be simple [3, 84]. It may be more complicated than it is seems because both water-soluble polymers and nanoparticles are large enough to engage in considerable non-specific surface interactions with cells and organelles that may be comparable with the specific interactions of the ligands attached to polymers and nanomaterials. One obvious remedy could be, firstly, to modify the surface of the nanoparticle with “inert” polymer that would have minimal interactions with the cells, and, secondly, to attach one or multiple targeting ligand(s) at the inert layer to allow for specific binding of the targeting ligands with their receptor [4]. The problem, however, is in identifying such an “inert” polymer to mask the surface of the carrier. The current “gold standard” is PEG but there are clear indications that even this polymer attached to nanomaterials can activate complement, and otherwise engage the body immunity [119].
With this caution, there are several exciting examples of using targeting ligands for delivery of nanomaterials into cells. Some of these examples, e.g. APP-modified gold nanoparticles [22], RVG-peptide/siRNA complexes [109–110], TAT-modified liposomes [104] and others are discussed above. Such biospecific, surface-modified nanomaterials are rapidly internalized in cells. Conventional wisdom assumes that they might follow a similar intracellular itinerary as the ligands used for their modification. However, this might not be the case, as illustrated above by an example of QD, which are modified with Tf or CTB [54]. Furthermore, Pluronic® block copolymers were shown to inhibit caveolae-mediated endocytosis at higher concentrations (0.01% and above) [55]. Even more interestingly they have shown an ability to enhance nuclear import of polyplex-delivered DNA [93]. By interacting with specific membrane microdomains, such as caveolae, polymers and nanomaterials may affect multiple signal transduction pathways. Another example of this sort is polysiloxane nanoparticles that appear to activate of eNOS signaling [64]. These examples suggest that polymers and nanomaterials can also influence intracellular transport. It is expected therefore that introduction of surface modifications in nanomaterial may result in a complex trafficking behavior, as nanomaterials may have an intrinsic ability to affect select cellular functions.
10. Conclusions
Understanding the cellular entry of nanomedicines has become central to the field of drug delivery. Nanomedicines utilize endocytic vesicles or endosomes which in turn employ complex trafficking machinery to sort towards a specific intracellular destination. We categorized nanomedicines that utilize select endocytosis pathways to gain intracellular access. Based on examples reported one can conclude that charge, shape, material composition, and surface chemistry are critical physicochemical parameters that determine cellular entry of nanomedicines through definitive endocytic route(s). Reports now suggest that nanomedicines can also influence the cellular signaling by interacting with membrane micro-domains which home different signaling components (receptors, signal activators, transducers). In addition, biospecific ligands decorated on a nanomedicine to target it towards a select route may actually have different trafficking as compared to the ligand alone. The fledgling cross-disciplinary area of endocytosis of nanomedicines is at the interface of biology and material science and may bring the next wave of significant technological breakthrough. To reach this goal, certain caveats need to be addressed. It should be noted that various laboratories utilize different cell types, use heterogeneous nanomedicines and different tools to study trafficking mechanism-thus obfuscating the efforts to make definite, generalizations. To better understand this area, we recommend that each study use multiple cell types, utilize homogeneous nanomedicines and employ multiple tools to dissect trafficking. Another challenge is that these generalizations are based mostly on in-vitro studies. Thus, developing assays to study the complex process of endocytosis of nanomedicines in-vivo remains a key challenge for the future success of this field. We hope that this review will assist a diverse audience of readers and will facilitate future studies to develop efficient nanomedicines for diagnosis and treatment of cancer and other diseases.
Acknowledgments
We are grateful for the support by the grants from the National Institutes of Health CA89225, CA116591, NS051334 (AVK), and 1P20RR021937 (COBRE Nebraska Center for Nanomedicine), the Department of Defense W81XWH-09-1-0386, USAMRMC 06108004 (AVK), and the UNMC Bukey fellowship (GS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kabanov AV, Alakhov VY. Pluronic (R) block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Critical Reviews in Therapeutic Drug Carrier Systems. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 2.Duncan R. The dawning era of polymer therapeutics. Nature Review Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Advanced Drug Delivery Reviews. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Review Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 5.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM-C, Decuzzi P, Tour JM, Robertson F, Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotechnology. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran L, Knolker H-J, Simons K. Subcellular targeting strategies for drug design and delivery. Nature Review Drug Discovery. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 8.Germain RN. An innately interesting decade of research in immunology. Nature Medicine. 2004;10:1307–1320. doi: 10.1038/nm1159. [DOI] [PubMed] [Google Scholar]
- 9.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 10.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nature Review Molecular Cell Biology. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieffer M, Maritzen T, Haucke V. SnapShot: Endocytic Trafficking. Cell. 2009;137:382.e381–382.e383. doi: 10.1016/j.cell.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annual Review of Biochemistry. 2009;78:857. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 13.Zerial M, McBride H. Rab proteins as membrane organizers. Nature Review Molecular Cell Biology. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 14.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nature Cell Biology. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 15.Storrie B, White J, Rottger S, Stelzer EHK, Suganuma T, Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. Journal of Cell Biology. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcon-Perez JM, Nazarian R, Sabatti C, Dell'Angelica EC. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. Journal of Cell Science. 2005;118:5243–5255. doi: 10.1242/jcs.02633. [DOI] [PubMed] [Google Scholar]
- 17.Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. Journal of Biological Chemistry. 1989;264:21522–21528. [PubMed] [Google Scholar]
- 18.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating Mitochondrial Redox Potential with Redox-sensitive Green Fluorescent Protein Indicators. Journal of Biological Chemistry. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 19.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. The European Molecular Biology Organization Journal. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straight AF, Marshall WF, Sedat JW, Murray AW. Mitosis in Living Budding Yeast: Anaphase A But No Metaphase Plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- 21.Sahay G, Gautam V, Luxenhofer R, Kabanov A. The utilization of pathogen-like cellular trafficking by single chain block copolymer. Biomaterials. 2009 Dec 4; doi: 10.1016/j.biomaterials.2009.11.020. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nature Biotechnology. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasir JK, Labhasetwar V. Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials. 2008;29:4244–4252. doi: 10.1016/j.biomaterials.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercer J, Helenius A. Virus entry by macropinocytosis. Nature Cell Biology. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 25.Ivanov AI. Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful? Methods in Molecular Biology. 2008 doi: 10.1007/978-1-59745-178-9_2. Review. [DOI] [PubMed] [Google Scholar]
- 26.Sahay G, Kim JO, Kabanov AV, Bronich TK. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials. 2010 Feb;31(5):923–933. doi: 10.1016/j.biomaterials.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuli A, Sharma M, Capek HL, Naslavsky N, Caplan S, Solheim JC. Mechanism for amyloid precursor-like protein 2 enhancement of major histocompatibility complex class I molecule degradation. Journal of Biological Chemistry. 2009 Dec 4;284(49):34296–34307. doi: 10.1074/jbc.M109.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damm E-M, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. Journal of Cell Biology. 2005;168:477–488. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumbleton M, Stephens DJ. Coming out of the dark: the evolving role of fluorescence imaging in drug delivery research. Advanced Drug Delivery Reviews. 2005;57:5–15. doi: 10.1016/j.addr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Lai SK, Hanes J. Real-Time Multiple Particle Tracking of Gene Nanocarriers in Complex Biological Environments. Methods in Molecular Biology. 2008;434:81–97. doi: 10.1007/978-1-60327-248-3_6. [DOI] [PubMed] [Google Scholar]
- 31.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cellular and Molecular Life Sciences. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual Review of Immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 33.Rabinovitch M. Professional and nonprofessional phagocytes-an introduction. Trends in Cell Biology. 1995;5:85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 34.Cannon G, Swanson J. The macrophage capacity for phagocytosis. Journal of Cell Sciences. 1992;101:907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- 35.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proceedings of the National Academy of Sciences. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pucadyil TJ, Schmid SL. Conserved Functions of Membrane Active GTPases in Coated Vesicle Formation. Science. 2009;325:1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nature Review Neuroscience. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 39.Ford MGJ, Mills IG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 40.Rappoport JZ. Focusing on clathrin-mediated endocytosis. Biochemical Journal. 2008;412:415–423. doi: 10.1042/BJ20080474. [DOI] [PubMed] [Google Scholar]
- 41.Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochemical and Biophysical Research Communications. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 42.Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface Charge of Nanoparticles Determines Their Endocytic and Transcytotic Pathway in Polarized MDCK Cells. Biomacromolecules. 2008;9:435–443. doi: 10.1021/bm700535p. [DOI] [PubMed] [Google Scholar]
- 43.Lahtinen U, Honsho M, Parton RG, Simons K, Verkade P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. Federation of the Societies of Biochemistry and Molecular Biology Letters. 2003;538:85–88. doi: 10.1016/s0014-5793(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 44.Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. Journal of Cell Science. 1998;111:825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- 45.Lü J-M, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Review of Molecular Diagnostics. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panyam J, Labhasetwar V. Dynamics of Endocytosis and Exocytosis of Poly(D,L-Lactide-co-Glycolide) Nanoparticles in Vascular Smooth Muscle Cells. Pharmaceutical Research. 2003;20:212–220. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- 47.Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharmaceutical Research. 2004;21:641–648. doi: 10.1023/b:pham.0000022411.47059.76. [DOI] [PubMed] [Google Scholar]
- 48.Nan A, Bai X, Son SJ, Lee SB, Ghandehari H. Cellular Uptake and Cytotoxicity of Silica Nanotubes. Nano Letters. 2008;8:2150–2154. doi: 10.1021/nl0802741. [DOI] [PubMed] [Google Scholar]
- 49.Chung T-H, Wu S-H, Yao M, Lu C-W, Lin Y-S, Hung Y, Mou C-Y, Chen Y-C, Huang D-M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Ma Z, Lim L-Y. Uptake of Chitosan and Associated Insulin in Caco-2 Cell Monolayers: A Comparison Between Chitosan Molecules and Chitosan Nanoparticles. Pharmaceutical Research. 2003;20:1812–1819. doi: 10.1023/b:pham.0000003379.76417.3e. [DOI] [PubMed] [Google Scholar]
- 51.Huang M, Ma Z, Khor E, Lim L-Y. Uptake of FITC-Chitosan Nanoparticles by A549 Cells. Pharmaceutical Research. 2002;19:1488–1494. doi: 10.1023/a:1020404615898. [DOI] [PubMed] [Google Scholar]
- 52.Nam HY, Kwon SM, Chung H, Lee S-Y, Kwon S-H, Jeon H, Kim Y, Park JH, Kim J, Her S, Oh Y-K, Kwon IC, Kim K, Jeong SY. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. Journal of Controlled Release. 2009;135:259–267. doi: 10.1016/j.jconrel.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Advanced Drug Delivery Reviews. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tekle C, Deurs Bv, Sandvig K, Iversen T-G. Cellular Trafficking of Quantum Dot-Ligand Bioconjugates and Their Induction of Changes in Normal Routing of Unconjugated Ligands. Nano Letters. 2008;8:1858–1865. doi: 10.1021/nl0803848. [DOI] [PubMed] [Google Scholar]
- 55.Sahay G, Batrakova EV, Kabanov AV. Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjugate Chemistry. 2008;19:2023–2029. doi: 10.1021/bc8002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Review Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 57.Nabi IR. Cavin fever: regulating caveolae. Nature Cell Biology. 2009;11:789–791. doi: 10.1038/ncb0709-789. [DOI] [PubMed] [Google Scholar]
- 58.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. Journal of Biological Chemistry. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 59.Parton RG, Simons K. The multiple faces of caveolae. Nature Review Molecular Cell Biology. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 60.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nature Review Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 61.Rejman J, Conese M, Hoekstra D. Gene Transfer by Means of Lipo- and Polyplexes: Role of Clathrin and Caveolae-Mediated Endocytosis. Journal of Liposome Research. 2006;16:237–247. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proceedings of National Academy of Science U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV. Polymer Micelle with Cross-Linked Ionic Core. Journal of the American Chemical Society. 2005;127:8236–8237. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- 64.Nishikawa T, Iwakiri N, Kaneko Y, Taguchi A, Fukushima K, Mori H, Morone N, Kadokawa J-i. Nitric Oxide Release in Human Aortic Endothelial Cells Mediated by Delivery of Amphiphilic Polysiloxane Nanoparticles to Caveolae. Biomacromolecules. 2009;10:2074–2085. doi: 10.1021/bm900128x. [DOI] [PubMed] [Google Scholar]
- 65.Zhang LW, Monteiro-Riviere NA. Mechanisms of Quantum Dot Nanoparticle Cellular Uptake. Toxicological Sciences. 2009;110:138–155. doi: 10.1093/toxsci/kfp087. [DOI] [PubMed] [Google Scholar]
- 66.Moreno-Aspitia A, Perez EA. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncology. 2005;1:755–762. doi: 10.2217/14796694.1.6.755. [DOI] [PubMed] [Google Scholar]
- 67.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opinion on Pharmacotherapy. 2006;7:1041. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 68.Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. American Journal of Physiology, Heart and Circulatory Physiology. 1992;262:H246–H254. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- 69.Desai NP, Trieu V, Hwang LY, Wu RJ, Soon-Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anti-Cancer Drugs. 2008;19:899–909. doi: 10.1097/CAD.0b013e32830f9046. [DOI] [PubMed] [Google Scholar]
- 70.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opinion on Pharmacotherapy. 2006;7:1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 71.Oba M, Aoyagi K, Miyata K, Matsumoto Y, Itaka K, Nishiyama N, Yamasaki Y, Koyama H, Kataoka K. Polyplex Micelles with Cyclic RGD Peptide Ligands and Disulfide Cross-Links Directing to the Enhanced Transfection via Controlled Intracellular Trafficking. Molecular Pharmaceutics. 2008;5:1080–1092. doi: 10.1021/mp800070s. [DOI] [PubMed] [Google Scholar]
- 72.Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW. Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. Journal of Cell Biology. 2005;171:527–536. doi: 10.1083/jcb.200503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Developmental Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 74.Tuli A, Sharma M, McIlhaney MM, Talmadge JE, Naslavsky N, Caplan S, Solheim JC. Amyloid Precursor-Like Protein 2 Increases the Endocytosis, Instability, and Turnover of the H2-Kd MHC Class I Molecule. Journal of Immunology. 2008;181:1978–1987. doi: 10.4049/jimmunol.181.3.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Advanced Drug Delivery Reviews. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 76.Rijnboutt S, Jansen G, Posthuma G, Hynes J, Schornagel J, Strous G. Endocytosis of GPI-linked membrane folate receptor-alpha. Journal of Cell Biology. 1996;132:35–47. doi: 10.1083/jcb.132.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerr MC, Teasdale RD. Defining Macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 78.Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. Journal of Cellular and Molecular Medicine. 2007;11:670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nature Review Molecular Cell Biology. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gratton SEA, Williams SS, Napier ME, Pohlhaus PD, Zhou Z, Wiles KB, Maynor BW, Shen C, Olafsen T, Samulski ET, DeSimone JM. The Pursuit of a Scalable Nanofabrication Platform for Use in Material and Life Science Applications. Accounts of Chemical Research. 2008;41:1685–1695. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proceedings of the National Academy of Sciences. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. Journal of Controlled Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai SK, Hida K, Chen C, Hanes J. Characterization of the intracellular dynamics of a non-degradative pathway accessed by polymer nanoparticles. Journal of Controlled Release. 2008;125:107–111. doi: 10.1016/j.jconrel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discovery Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 85.Kitchens K, Foraker A, Kolhatkar R, Swaan P, Ghandehari H. Endocytosis and Interaction of Poly (Amidoamine) Dendrimers with Caco-2 Cells. Pharmaceutical Research. 2007;24:2138–2145. doi: 10.1007/s11095-007-9415-0. [DOI] [PubMed] [Google Scholar]
- 86.Seib FP, Jones AT, Duncan R. Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells. Journal of Controlled Release. 2007;117:291–300. doi: 10.1016/j.jconrel.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 87.Kitchens KM, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis Inhibitors Prevent Poly(amidoamine) Dendrimer Internalization and Permeability across Caco-2 Cells. Molecular Pharmaceutics. 2008;5:364–369. doi: 10.1021/mp700089s. [DOI] [PubMed] [Google Scholar]
- 88.Kitchens K, Kolhatkar R, Swaan P, Eddington N, Ghandehari H. Transport of Poly(Amidoamine) Dendrimers across Caco-2 Cell Monolayers: Influence of Size. Charge and Fluorescent Labeling, Pharmaceutical Research. 2006;23:2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 89.Kabanov AV, Kabanov VA. DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconjugate Chemistry. 1995;6:7–20. doi: 10.1021/bc00031a002. [DOI] [PubMed] [Google Scholar]
- 90.von Gersdorff K, Sanders NN, Vandenbroucke R, De Smedt SC, Wagner E, Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Molecular Therapy. 2006;14:745–753. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 91.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. The Journal of Gene Medicine. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 92.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Molecular Therapy. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 93.Yang Z, Sahay G, Sriadibhatla S, Kabanov AV. Amphiphilic Block Copolymers Enhance Cellular Uptake and Nuclear Entry of Polyplex-Delivered DNA. Bioconjugate Chemistry. 2008;19:1987–1994. doi: 10.1021/bc800144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lühmann T, Rimann M, Bittermann AG, Hall H. Cellular Uptake and Intracellular Pathways of PLL-g-PEG-DNA Nanoparticles. Bioconjugate Chemistry. 2008;19:1907–1916. doi: 10.1021/bc800206r. [DOI] [PubMed] [Google Scholar]
- 95.Wong AW, Scales SJ, Reilly DE. DNA Internalized via Caveolae Requires Microtubule-dependent, Rab7-independent Transport to the Late Endocytic Pathway for Delivery to the Nucleus. Journal of Biological Chemistry. 2007;282:22953–22963. doi: 10.1074/jbc.M611015200. [DOI] [PubMed] [Google Scholar]
- 96.Bausinger R, Gersdorff Kv, Braeckmans K, Ogris M, Wagner E, Bräuchle C, Zumbusch A. The Transport of Nanosized Gene Carriers Unraveled by Live-Cell Imaging13. Angewandte Chemie International Edition. 2006;45:1568–1572. doi: 10.1002/anie.200503021. [DOI] [PubMed] [Google Scholar]
- 97.Suh J, Wirtz D, Hanes J. Efficient active transport of gene nanocarriers to the cell nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3878–3882. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Advanced Drug Delivery Reviews. 2009;61:603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 100.Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional Activation of Gene Expression by Pluronic Block Copolymers in Stably and Transiently Transfected Cells. Molecular Therapy. 2006;13:804–813. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- 101.Gaymalov ZZ, Yang Z, Pisarev VM, Alakhov VY, Kabanov AV. The effect of the nonionic block copolymer pluronic P85 on gene expression in mouse muscle and antigen-presenting cells. Biomaterials. 2009;30:1232–1245. doi: 10.1016/j.biomaterials.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fumoto S, Nishi J, Ishii H, Wang X, Miyamoto H, Yoshikawa N, Nakashima M, Nakamura J, Nishida K. Rac-Mediated Macropinocytosis Is a Critical Route for Naked Plasmid DNA Transfer in Mice. Molecular Pharmaceutics. 2009;6:1170–1179. doi: 10.1021/mp900042p. [DOI] [PubMed] [Google Scholar]
- 103.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Advanced Drug Delivery Reviews. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Advanced Drug Delivery Reviews. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. Journal of Controlled Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 106.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nature Medicine. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 107.Patel L, Zaro J, Shen W-C. Cell Penetrating Peptides: Intracellular Pathways and Pharmaceutical Perspectives. Pharmaceutical Research. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 108.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. "SMART" drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjugate Chemistry. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar P, Wu H, McBride JL, Jung K-E, Hee Kim M, Davidson BL, Kyung Lee S, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, Lou J, Jiang C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–4202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 111.Liu J, Kopečková P, Bühler P, Wolf P, Pan H, Bauer H, Elsässer-Beile U, Kopeček Ji. Biorecognition and Subcellular Trafficking of HPMA Copolymer-Anti -PSMA Antibody Conjugates by Prostate Cancer Cells. Molecular Pharmaceutics. 2009;6:959–970. doi: 10.1021/mp8002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of Endothelial Targeting and Intracellular Delivery of Therapeutic Enzymes by Modulating the Size and Shape of ICAM-1-targeted Carriers. Molecular Therapy. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Champion J, Mitragotri S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharmaceutical Research. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kabanov Alexander V, Vinogradov Serguei V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angewandte Chemie International Edition. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proceedings of the National Academy of Sciences. 2009;106:7957–7961. doi: 10.1073/pnas.0902857106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alakhova DY, Rapoport NY, Batrakova EV, Timoshin AA, Li S, Nicholls D, Alakhov VY, Kabanov AV. Differential metabolic responses to pluronic in MDR and non-MDR cells: A novel pathway for chemosensitization of drug resistant cancers. Journal of Controlled Release. doi: 10.1016/j.jconrel.2009.09.026. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Capitani M, Sallese M. The KDEL receptor: New functions for an old protein. Federation of the Societies of Biochemistry and Molecular Biology Letters. In Press, Corrected Proof. [Google Scholar]
- 118.Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Advanced Drug Delivery Reviews. 2004;56:1099–1109. doi: 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 119.Arima Y, Toda M, Iwata H. Complement activation on surfaces modified with ethylene glycol units. Biomaterials. 2008;29:551–560. doi: 10.1016/j.biomaterials.2007.10.015. [DOI] [PubMed] [Google Scholar]