Abstract
The ability of the adaptive immune system to restrict Mycobacterium tuberculosis (Mtb) is impeded by activated Foxp3+ regulatory T (T reg) cells. The importance of pathogen-specific T reg cells in this process has not been addressed. We show that T reg cell expansion after aerosol Mtb infection does not occur until Mtb is transported to the pulmonary lymph node (pLN), and Mtb-specific T reg cells have an increased propensity to proliferate. Even small numbers of Mtb-specific T reg cells are capable of delaying the priming of effector CD4+ and CD8+ T cells in the pLN and their subsequent accumulation in the lung, the primary site of infection. This delay likely prolongs the initial phase of bacterial expansion and explains the higher bacterial burden observed in these mice. Thus, T reg cells recognizing Mtb-derived antigens specifically and potently restrict protective immune responses during tuberculosis.
T reg cells that express the transcription factor Foxp3 are critical for preventing autoimmunity by dampening self-reactive immune responses (Sakaguchi et al., 2008). Their role in infections, however, is complex. On the one hand, activated T reg cells protect the host by curbing inflammation and limiting collateral tissue damage, but on the other hand by limiting host immunity, they can also inhibit pathogen clearance. T reg cells dampen immune responses in a wide range of infections caused by bacteria, fungi, parasites, and viruses, especially those that persist in the host (Belkaid and Tarbell, 2009).
Mycobacterium tuberculosis (Mtb) is one of the most formidable persistent pathogens and a major cause of morbidity and mortality worldwide (WHO, 2008). The role of T reg cells in facilitating Mtb persistence is still unclear. Initially, after inhalation of infectious bacilli, T reg numbers are reduced in the peripheral blood of asymptomatic individuals, and this decrease has been attributed to their accumulation at sites of infection in the lung (Burl et al., 2007). Later, during active tuberculosis (TB), T reg cell numbers are increased in the peripheral blood and infected tissues (Guyot-Revol et al., 2006; Ribeiro-Rodrigues et al., 2006), and T reg cells isolated from infected individuals are capable of suppressing T cell responses to Mtb antigens in vitro (Chen et al., 2007; Hougardy et al., 2007). Recent studies in the mouse model have further clarified the role of T reg cells in TB, especially during the first 4 wk of infection, a critical period in which containment of Mtb by the immune system is established. We have shown that rigorous depletion of T reg cells during early infection results in enhanced bacterial clearance (Scott-Browne et al., 2007), whereas Kursar et al. (2007) reported similar results when they transferred T reg–depleted CD4+ T cells into lymphopenic recipients. Although these studies reveal that T reg cells deter the clearance of Mtb during early infection, the basic rules governing T reg cell activity during this period, including their specificity and mode of immunosuppression, remain unknown.
With respect to their specificity in TB, as in other infections, it is unclear whether T reg cells need to recognize pathogen-derived antigens to proliferate and suppress immunity. Because T reg cells are believed to have a relatively high affinity for self-antigens (Hsieh et al., 2004; Bautista et al., 2009; DiPaolo and Shevach, 2009), recognition of self-antigens may be sufficient to drive their expansion and function during the inflammatory milieu of TB. Conversely, T reg cells that recognize Mtb antigens may play a predominant role in TB. Although pathogen-specific T reg cells have been convincingly demonstrated during Leishmania major infection (Suffia et al., 2006), and implicated in several other infections (Belkaid and Tarbell, 2009), the unique functional properties of pathogen-specific T reg cells are not well understood.
Furthermore, the mechanisms by which T reg cells interfere with immune protection against infection remain unclear. In TB, infection occurs by inhalation of respiratory droplets containing only a few bacilli, which undergo robust replication within lung macrophages before the onset of adaptive immunity (Cooper, 2009). The adaptive immune response is initiated only when Mtb is transported to the pulmonary LN (pLN) between days 8 and 11 after infection (Chackerian et al., 2002; Wolf et al., 2007; Reiley et al., 2008). Mtb-specific effector T cells are primed in the LN, expand their numbers, and traffic to the lung, where they recognize their specific antigens presented by infected macrophages and dendritic cells (Cooper, 2009). These activated T cells produce protective cytokines, including IFN-γ, that promote macrophage activation and curtail mycobacterial growth, but they fail to achieve Mtb eradication. Instead, bacterial numbers are held constant during persistent infection, though this plateau is not established until approximately day 21 after infection. Thus, adaptive immunity develops much slower during Mtb infection than in other infections, allowing the bacteria to establish a foothold in the lung (Cooper, 2009). There is a tight correlation between the rapidity of the appearance of Mtb-specific T cells in the lung and the ability to establish immune control. Early arrival of Mtb-specific T cells, including after immunization, is associated with enhanced immune control and lower bacterial burdens (Jung et al., 2005, 2008; Gallegos et al., 2008), whereas delayed arrival is associated with a higher infection plateau (Caruso et al., 1999; Chackerian et al., 2001, 2002). Mtb-specific Th17 cells have been shown to facilitate the rapid recruitment of Mtb-specific T cells to the lungs of immunized mice, thereby promoting immune protection and lowering bacterial burdens (Khader et al., 2007).
In this study, we hypothesized that T reg cells may have the opposite effect of Th17 cells and impede the effector T cell response by delaying their arrival. Furthermore, we hypothesized that T reg cells recognizing Mtb antigens would have an increased capacity to mediate this effect. To address these questions, we performed transfer experiments using Mtb-specific TCR transgenic T cells to examine T reg cell origin, specificity, and influence on effector T cell recruitment during early Mtb infection. Our data indicate that T reg cells expand from the preexisting population and that pathogen-specific T reg cells proliferate preferentially. Furthermore, even low numbers of Mtb-specific T reg cells can impair immune protection by delaying the appearance of Mtb-specific effector T cells in the lung. Our data support a model in which the timeliness of effector T cell recruitment to the lung is critical for establishing immune control of Mtb infection, and Mtb-specific T reg cells slow the rate at which this recruitment occurs.
RESULTS
T reg cells responding during early Mtb infection are derived from preexisting T reg cells
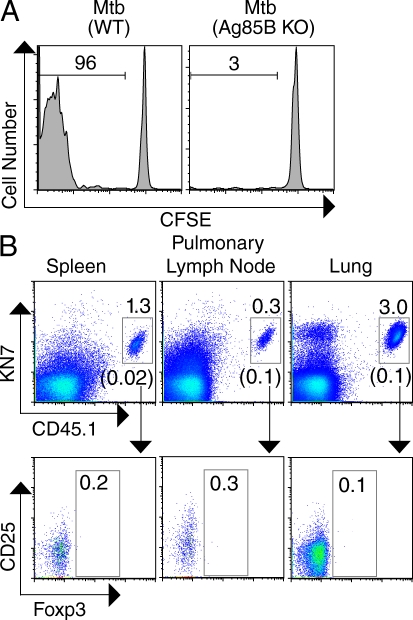
To investigate whether Mtb-specific, conventional CD4+ T cells are induced to express Foxp3 during the early immune response to Mtb, we obtained P25 TCR transgenic mice containing CD4+ T cells specific for an immunodominant Mtb antigen, Ag85B240–254:I-Ab (Tamura et al., 2004; Ariga et al., 2007). To verify that P25 T cells undergo antigen-specific proliferation in Mtb-infected mice, we transferred CFSE-labeled P25 T cells into mice recently infected with either Mtb (WT) or a recombinant strain that lacks expression of Ag85B, Mtb (Ag85B KO). In agreement with a recent report (Wolf et al., 2008), we observed that P25 T cells proliferated only in mice infected with Mtb expressing Ag85B (Fig. 1 A), confirming that P25 T cells undergo robust antigen-driven expansion during TB. To address whether T reg cells are generated de novo during the expansion of Mtb-specific CD4 T cells, we backcrossed P25 mice to Foxp3-GFP reporter mice (CD45.1 expressing) so that T reg cells could be readily identified by virtue of their green fluorescence (Fontenot et al., 2005). Foxp3-GFPneg CD4+ cells from P25 mice were transferred into B6 mice recently infected with Mtb or into uninfected controls. At day 26 after infection, transferred CD4+ P25 cells that expressed the transgenic TCR recognized by KN7, an anticlonotypic antibody, were analyzed for their expression of Foxp3. As expected, we observed dramatically higher numbers of KN7+ P25 cells in the spleens, pLNs, and lungs of infected mice compared with tissues from uninfected controls. Despite this expansion, induction of Foxp3 within these Mtb-specific CD4+ T cells was not observed (Fig. 1 B).
Figure 1.
Foxp3 expression is not induced in Mtb-specific, transgenic CD4+ T cells during early Mtb infection. (A) Proliferation of CFSE-labeled CD4+ T cells (105 cells transferred) from CD45.1 P25 TCR transgenic mice (analyzed on day 18 after infection) 6 d after transfer into Mtb (WT)– or Mtb (Ag85B KO)–infected B6 mice. Numbers represent the percentage of proliferating CD45.1 CD4+ T cells in the spleen as measured by CFSE dilution. (B, top) Transgenic TCR (detected by KN7 anticlonotypic antibody for the P25 transgenic TCR) and CD45.1 expression on gated CD4+ cells from the spleen, pLN, and lung of Mtb-infected B6 mice, transferred on day 11 and analyzed on day 26 after infection. Numbers shown above the gray gates represent the percentage of transferred KN7+ CD45.1 cells within the CD4+ cells in each tissue, and the numbers shown in parenthesis below the gates represent the corresponding percentages in uninfected controls. (bottom) The expression of CD25 and Foxp3 by cells within the KN7+ CD45.1 population; numbers represent the percentage of Foxp3+ cells within this gate. Each experiment was performed three times with three Mtb-infected mice per group. Results from representative mice are shown.
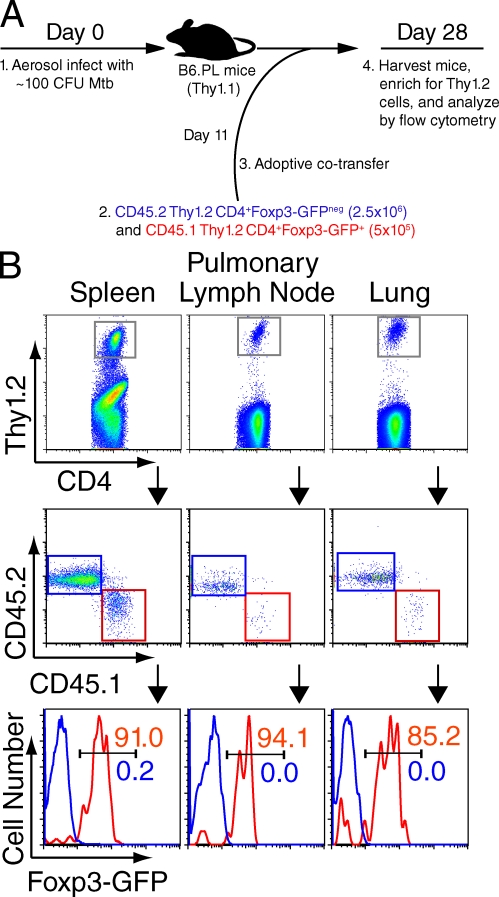
To confirm that these results using TCR transgenic T cells were true for endogenous CD4+ T cells, we also transferred polyclonal T cell populations from nontransgenic mice into Mtb-infected mice. 11 d after aerosol infection, conventional CD4+ T cells (Thy1.2 CD45.2 CD4+Foxp3-GFPneg cells) and T reg cells (Thy1.2 CD45.1 CD4+Foxp3-GFP+ cells) from uninfected Foxp3-GFP mice were cotransferred into Mtb-infected, Thy1.1 recipients (Fig. 2 A). On day 28 after infection, spleen cells were analyzed after magnetic bead enrichment of transferred Thy1.2 cells, whereas pLN and lung cells were analyzed directly. Thy1.2 CD4+ cells from each organ were examined to determine the percentage of CD45.2 (blue gates and histograms; transferred as Foxp3− cells) and CD45.1 (red gates and histograms; transferred as Foxp3+ cells) cells that expressed Foxp3 (Fig. 2 B). Although most of the transferred Foxp3+ cells retained their Foxp3 expression, induction of Foxp3 in conventional CD4+ T cells was not observed. Together with our results using TCR transgenic T cells (Fig. 1), our data indicate that T reg cells responding during the early stages of Mtb infection are not generated de novo but expand from preexisting T reg cells.
Figure 2.
Foxp3 expression is not induced in endogenous CD4+ T cells during early Mtb infection. (A) Schematic of experimental design. 2.5 × 106 conventional CD4+ T cells (blue) and 5 × 105 T reg cells (red) were transferred on day 11 and analyzed on day 28 after infection. (B, top) Gated CD4+ T cells; the gray gate represents the transferred Thy1.2 cells within these populations in the enriched spleen, pLN, and lung. (middle) CD45.2 (blue gate) and CD45.1 cells (red gate) within the Thy1.2 populations. (bottom) Foxp3-GFP expression within CD45.2 (blue histograms, representing cells sorted and transferred as Foxp3-GFPneg cells) and CD45.1 cells (red histograms, representing cells sorted and transferred as Foxp3-GFP+ cells). Percentages of cells are shown. The experiment was performed three times with three mice per group in each experiment. Results from representative mice are shown.
Expansion of T reg cells after aerosol Mtb infection is delayed
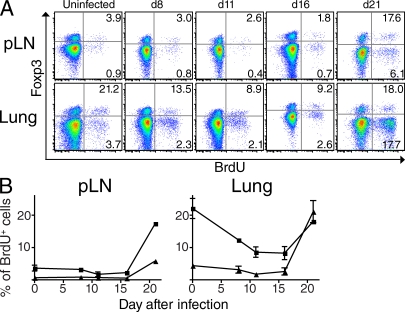
We previously showed that T reg cells display an increased rate of proliferation between days 21 and 35 after infection (Scott-Browne et al., 2007). However, when T reg cell proliferation is initiated has not been addressed. Expansion of effector T cells does not occur until after Mtb is transported to the pLN, which begins to occur on days 8–11 after infection (Chackerian et al., 2002; Wolf et al., 2007; Reiley et al., 2008). It is unknown whether T reg cell expansion is also delayed or whether the inflammatory milieu in the lung drives T reg cell proliferation at earlier time points. To address this question, we administered the nucleotide analogue BrdU intraperitoneally to Mtb-infected mice at various time points after aerosol infection. Mice were analyzed 20 h later, and the percentages of CD4+Foxp3+ and CD4+Foxp3− cells that incorporated BrdU in the pLN and lung were determined (Fig. 3). Before infection, T reg cells had a higher level of basal proliferation than conventional CD4+Foxp3− T cells, especially in the lung. Surprisingly, T reg cells in the lung actually exhibited decreased proliferation for the first 16 d after infection but then dramatically increased proliferation on day 21. Overall, T reg cells displayed a delay in proliferation in both the pLN and lung that was very similar to that observed for conventional CD4+ T cells. The fact that T reg cell expansion is not initiated until after presentation of Mtb antigens in the pLN begins to occur raises the possibility that T reg cells responding during early TB are Mtb specific.
Figure 3.
Proliferation of endogenous T reg cells is delayed similarly to effector T cell proliferation. (A) Representative BrdU incorporation of CD4+-gated T cells in the pLNs (top) or lungs (bottom) of uninfected or Mtb-infected mice at various time points after aerosol infection. Mice were administered 1 mg BrdU intraperitoneally 20 h before analysis. Numerical values represent the percentage of Foxp3+ (top right quadrant) and Foxp3− cells (bottom right quadrant) that incorporated BrdU. (B) Cumulative data showing the frequency of Foxp3-expressing CD4+ T cells (squares) and Foxp3− CD4+ T cells (triangles) that incorporated BrdU in the pLNs or lungs of mice at various time points after Mtb infection. Means and SEM of three mice per group at each time point are shown. Data are representative of two independently performed experiments.
P25 mice contain a population of Mtb-specific T reg cells
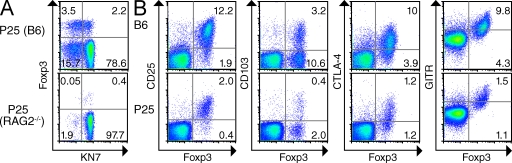
We next sought to directly test whether Mtb-specific T reg cells have a greater capacity to proliferate and affect protective immunity than polyclonal T reg cells during the early response to Mtb infection. The specificity of T reg cells in P25 mice was examined to determine if these cells could be used to address this question. As shown in Fig. 4 A, ∼40% of the CD4+Foxp3+ cells in spleens of P25 mice, comprising ∼2% of the total CD4+ T cells, also expressed the transgenic TCR conferring specificity for Ag85B240–254/I-Ab. Because T reg cell development is thought to result from relatively high affinity TCR interactions with self-antigen–MHC complexes in the thymus (Hsieh et al., 2004; Bautista et al., 2009; DiPaolo and Shevach, 2009), we suspected that expression of endogenous TCRs was required to direct this process in P25 mice, as has been shown in other TCR transgenic mice (Thornton and Shevach, 2000; Hori et al., 2002; Eggena et al., 2004; Chen et al., 2005; Schenk et al., 2005). Consistent with this idea, we found that P25 mice lacking the ability to rearrange their endogenous TCR loci (i.e., on a RAG2−/− background) possessed very few CD4+Foxp3+ T cells (Fig. 4 A). These results support the idea that T reg cells in P25 mice are not only Mtb-specific by virtue of their expression of the transgenic TCR, but represent a polyclonal population of T reg cells that also express endogenous TCRs that drive the expression of Foxp3 during thymic development. Furthermore, we found that P25 T reg cells expressed levels of CD25, CD103, CTLA-4, and GITR that were similar to patterns observed in the polyclonal population of T reg cells in B6 mice. In particular, as in B6 mice, CD25 was noted to be a good marker for identifying T reg cells in P25 mice; ∼80% of Foxp3+ cells in P25 mice expressed CD25, and >95% of CD25-expressing CD4 cells were Foxp3+ (Fig. 4 B). Collectively, we reasoned that the dual specificity of P25 T reg cells (i.e., specificity for an Mtb antigen and self-ligand) could be exploited to determine whether T reg cells bearing a pathogen-specific TCR have unique functional properties, or whether all CD4+Foxp3+ cells are capable of responding equally during TB.
Figure 4.
P25 mice contain a population of pathogen-specific T reg cells. (A) Expression of Foxp3 and transgenic TCR (detected by KN7 antibody) on CD4-gated spleen cells from P25 mice on WT B6 or RAG2−/− (bottom) backgrounds. The percentage of cells within each quadrant is shown. (B) Expression of the indicated markers by CD4-gated spleen cells from B6 mice (top) or KN7+CD4+ spleen cells from P25 mice (bottom). Numbers represent the frequency of cells in the indicated quadrants. The experiment was performed twice with three mice per group in each experiment. Results from representative mice are shown.
Pathogen-specific T reg cells exhibit preferential proliferation during TB
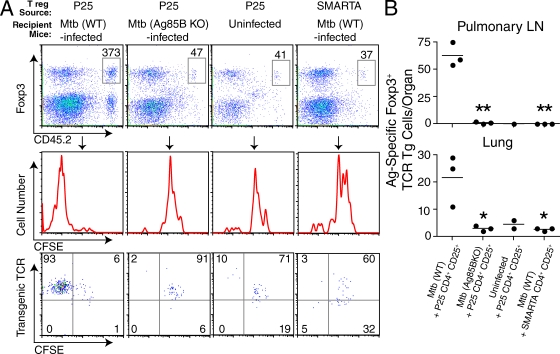
Having previously shown that T reg cell expansion is delayed until presentation of Mtb antigens has occurred in the pLN (Fig. 3), we wanted to determine if T reg cells specific for Mtb-derived antigens showed preferential expansion. Alternatively, because T reg cells express TCRs with relatively high affinities for self-antigens (Hsieh et al., 2004; Bautista et al., 2009; DiPaolo and Shevach, 2009) and exhibit an activated phenotype (Sakaguchi et al., 2008), it is possible that all T reg cells are driven to proliferate by the inflammatory cytokine milieu in TB. To test these possibilities, CD4+CD25+ T cells from CD45.2 P25 mice were labeled with CFSE and transferred into CD45.1 recipients infected with either Mtb (WT) or Mtb (Ag85B KO), which is equally virulent (Wolf et al., 2008). As additional controls, CD4+CD25+ T cells from P25 mice were transferred into uninfected mice, and CD4+CD25+ T cells from TCR transgenic mice with an irrelevant antigen specificity (i.e., SMARTA mice with CD4+ T cells recognizing an epitope of lymphocytic choriomeningitis virus glycoprotein presented by I-Ab; Oxenius et al., 1998) were transferred into Mtb-infected mice. To examine the period in which endogenous T reg cells were observed to initiate proliferation (Fig. 3), we transferred T reg cells on day 15 after infection and assessed proliferation on day 20. Because we transferred small numbers of T reg cells, we used magnetic bead enrichment to analyze most of the transferred CD45.2 T cells in an entire spleen (Hataye et al., 2006). As shown in Fig. 5 A, we found that T reg cells from P25 mice did not proliferate during TB unless the infecting strain of Mtb expressed Ag85B. Eightfold more transferred cells expressing Foxp3 were found in the spleens of WT-infected mice than in Ag85B KO–infected mice (Fig. 5 A). In Mtb (WT)–infected mice, 93% of these T reg cells had fully diluted their CFSE and continued to express high levels of the transgenic TCR. In contrast, very few P25 T reg cells diluted their CFSE in uninfected or Ag85B KO–infected mice, and very few SMARTA T reg cells diluted their CFSE in Mtb (WT)–infected mice. Similar results were observed in the pLNs and lungs; increased numbers of the Ag85B-specific T reg cells were seen in Mtb (WT)– but not Mtb (Ag85B KO)–infected or uninfected mice. Likewise, very few SMARTA T reg cells were recovered in the pLN and lung (Fig. 5 B). We conclude that inflammation induced by Mtb infection is not sufficient to drive the nonspecific proliferation of all CD4+Foxp3+ cells, but T reg cells recognizing Mtb antigens undergo preferential expansion.
Figure 5.
Preferential expansion of T reg cells recognizing Mtb-derived antigens. (A, top) Expression of Foxp3 and CD45.2 on gated CD4+ CD45.2-enriched spleen cells from Mtb (WT)–infected, Mtb (Ag85B KO)–infected, or uninfected mice receiving 105 CFSE-labeled CD4+CD25+ T cells from P25 or SMARTA TCR transgenic mice, as indicated. T reg cells were transferred on day 15 and analyzed on day 20 after infection. Numerical values represent the absolute number of CD45.2 CD4+ Foxp3+ cells recovered from the spleen of a representative mouse from each group. (middle) The degree of proliferation (CFSE dilution) exhibited by these cells. (bottom) Expression of transgenic TCR (i.e., KN7 for P25 and Vα2 for SMARTA T reg cells) and CFSE. Numbers represent the frequency of cells in each quadrant. (B) Circles represent the absolute number of antigen-specific P25 or SMARTA T reg cells recovered from the pLN (top) or lung (bottom) from individual mice, and bars indicate the mean of each group. The experiment was performed three times with three mice per group in each experiment. Results from representative mice are shown in A. An unpaired Student’s t test was performed, and p-values for groups significantly different from the Mtb (WT) + P25 CD4+CD25+ group are indicated (*, P < 0.05; **, P < 0.001).
Pathogen-specific T reg cells impair protective immunity
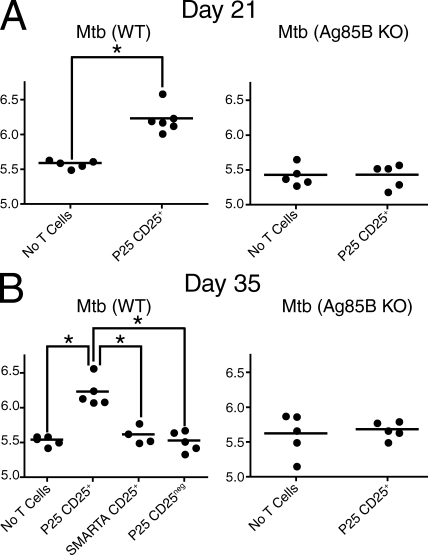
We next asked whether pathogen specificity enhanced the ability of T reg cells to suppress protective immunity during TB. Using a T reg cell transfer strategy similar to the one used to examine proliferation (Fig. 5), CD4+CD25+ T cells from P25 mice were transferred into B6 mice infected with Mtb (WT) or Mtb (Ag85B KO), as controls. We chose day 11 after infection as the day of transfer because it represents a time point at which Mtb has recently been transported from the lung to the pLN (Wolf et al., 2007; Chackerian et al., 2002; Reiley et al., 2008) but T cell expansion has not been initiated (Fig. 3). The lung bacterial burdens were assessed at two time points: day 21 after infection was examined because it is the critical time when the adaptive immune response begins to control Mtb in the lung (Cooper, 2009), whereas day 35 represents a later time that is often used to assess protective immunity in vaccine studies (Orme, 2005). Two additional controls were included at the later time point: Mtb (WT)–infected mice received CD4+CD25neg cells from P25 mice or CD4+CD25+ T cells from SMARTA mice. At both time points examined, Mtb (WT)–infected mice receiving P25 T reg cells had an increased lung bacterial burden (∼0.7 log) compared with controls receiving no transferred T reg cells (Fig. 6). In contrast, P25 T reg cells had no effect on the bacterial burden in the lungs of Mtb (Ag85B KO)–infected mice. Likewise, at day 35 after infection, conventional T cells (CD4+CD25neg) from P25 mice and T reg cells (CD4+CD25+) from SMARTA mice had no effect on the bacterial burden (Fig. 6 B). These results indicate that Mtb-specific T reg cells but not T reg cells with irrelevant specificities can impair immunity and increase the lung bacterial load in TB. The potency of Mtb-specific T reg cells is highlighted by the fact that when identical numbers of either Mtb-specific T reg cells or Mtb-specific conventional CD4+ T cells were transferred into infected mice, only T reg cells had an impact on the bacterial load (Fig. 6 B).
Figure 6.
Pathogen-specific T reg cells suppress protective immunity in TB. (A) Day 21 lung CFU analysis of Mtb (WT)– or Mtb (Ag85B KO)–infected mice that received either no T cells or CD4+CD25+ T cells from P25 mice intravenously on day 11 after infection (4 × 104 cells/mouse). (B) Day 35 lung CFU analysis of Mtb (WT)– or Mtb (Ag85B KO)–infected mice that received either no T cells or P25 CD4+CD25+, SMARTA CD4+CD25+, or P25 CD4+CD25neg T cells intravenously on day 11 after infection (105 cells/mouse), as indicated. Circles represent individual mice and bars represent the mean of each group (*, P < 0.01, unpaired Student’s t test). The experiment was performed three times at day 21 and once at day 35 with five mice per group in each experiment.
Pathogen-specific T reg cells delay the appearance of IFN-γ–producing effector T cells in the lung
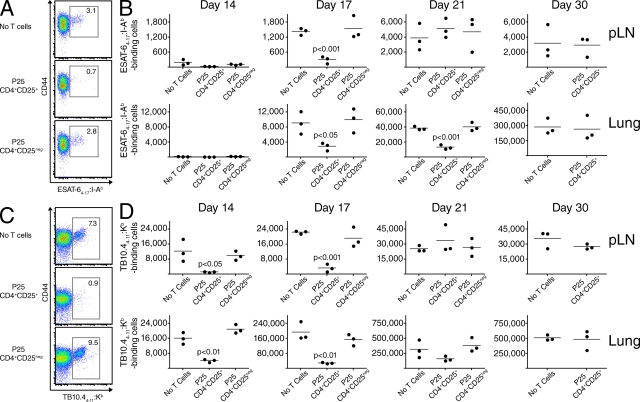
Our data indicate that pathogen-specific T reg cells interfere with the initial immune control of Mtb infection, but we wondered how even small numbers of cells (i.e., 4–10 × 104 cells transferred, of which only 40% expressed KN7 and were Mtb specific) could mediate such profound effects on the mycobacterial burden. Because establishing immune control is tightly correlated with the rapid appearance of Mtb-specific effector T cells in the lung (Cooper, 2009), we hypothesized that pathogen-specific T reg cells may compromise the protective immune response by delaying the arrival of these cells. To investigate this idea, we used MHC class II and I tetramers to track CD4+ and CD8+ T cells recognizing immunodominant MHC class II (i.e., ESAT-64–17:I-Ab) and I (i.e., TB10.44–11:Kb)–restricted epitopes in Mtb-infected mice. T reg cells (CD4+CD25+ cells) or conventional CD4+ T cells (CD4+CD25neg cells) from P25 mice were transferred into Mtb (WT)–infected Foxp3-GFP mice, and an additional group of controls received no transferred cells. Multiple time points during early Mtb infection were examined to assess the rate of effector T cell expansion and accumulation in the pLNs and lungs. Mice receiving P25 T reg cells had delayed expansion of both ESAT-6–specific CD4+ (Fig. 7, A and B) and TB10.4-specific CD8+ T cells (Fig. 7, C and D) in the pLN and lung. On average, mice receiving P25 T reg cells contained approximately fourfold fewer ESAT-6–specific CD4+ T cells in the pLN on day 17 (Fig. 7 B and Fig. S1 A), and threefold fewer cells in the lungs on days 17 and 21 after infection (Fig. 7 B). Importantly, the ESAT-6–specific CD4+ T cells were not T reg cells because they did not express Foxp3 (Fig. S2 and not depicted). In preliminary data, we found that transfer of fivefold higher numbers of Mtb-specific T reg cells caused an even greater reduction in the number of ESAT-6–specific CD4+ T cells recovered from the lungs of day 17 Mtb-infected mice (Fig. S3), suggesting that the ability of Mtb-specific T cells to suppress expansion of effector T cells is dose dependent. Interestingly, Mtb-specific CD8+ T cell responses occurred earlier than CD4+ responses, and T reg cell–mediated reductions in CD8+ effector T cell numbers were more marked. Mice receiving P25 T reg cells contained ∼7–10-fold fewer TB10.4-specific CD8+ T cells in the pLN (Fig. 7 D and Fig. S1 B) and 4–6-fold fewer cells in the lungs on days 14 and 17 after infection (Fig. 7 D). In preliminary data, effector CD4+ and CD8+ T cells eventually increased to levels seen in control groups; no differences in either ESAT-6– or TB10.4-specific T cells were seen in the pLN by day 21, or in the lung by day 30 after infection.
Figure 7.
Mtb-specific T reg cells delay effector T cell expansion. (A) Representative expression of CD44 and binding to ESAT-64–17:I-Ab tetramers by CD3+CD4+-gated T cells in the lungs of Mtb (WT)–infected B6 mice on day 21 after infection. As indicated, mice received no T cells or P25 CD4+CD25+ or P25 CD4+CD25neg T cells (105 cells/mouse) intravenously on day 11 after infection. Numerical values represent the percentage of cells binding the tetramer. (B) Cumulative data showing the absolute numbers of ESAT-64–17:I-Ab tetramer–binding cells in the pLNs and lungs at different time points after Mtb infection. Circles represent individual mice and bars represent the mean of each group. (C) Representative expression of CD44 and binding to TB10.44–11:Kb tetramers by CD3+CD8+-gated T cells in the lungs of Mtb (WT)–infected B6 mice on day 17 after infection in the same experiment depicted in A and B. Numerical values represent the percentage of cells binding the tetramer. (D) Cumulative data showing the absolute numbers of TB10.44–11:Kb tetramer–binding cells in the pLNs and lungs at different time points after Mtb infection. Circles represent individual mice and bars represent the mean of each group. The unpaired Student’s t test was performed, and p-values for groups significantly different from the group receiving no T cells are shown. The experiments were performed twice at day 17 and once at other time points.
In addition to tetramer analysis, we performed intracellular cytokine staining to assess the ability of ESAT-6– and TB10.4-specific T cells to produce IFN-γ after in vitro stimulation. We observed a delay in IFN-γ–producing T cells at early time points that was similar to our observations using tetramer staining (Fig. S4). Thus, transferred Mtb-specific T reg cells primarily interfered with the expansion of effector T cells and had minimal effect on IFN-γ production by existing effector T cells. Collectively, our data show that the capacity of small numbers of pathogen-specific T reg cells to deter immune control of Mtb infection is associated with delayed expansion of Mtb-specific, CD4+ and CD8+ T cells in the pLN, and delayed arrival of these effector T cells at the primary site of infection in the lung.
DISCUSSION
Our results show that small numbers of Mtb-specific T reg cells, even in the midst of an abundance of endogenous polyclonal T reg cells, are uniquely capable of impairing protective immunity by delaying the arrival of effector T cells to the primary site of infection. These findings may help explain an important paradox in the field: despite the central importance of IFN-γ–producing T cells in immunity to Mtb, multiple studies have now demonstrated that the frequency of these cells in the periphery of immunized individuals correlates poorly with the degree of protection conferred (Leal et al., 2001; Elias et al., 2005; Fletcher, 2007; Mittrücker et al., 2007). Instead of the size of the peripheral population, a growing body of literature suggests that a cardinal feature of protective immunity is the rate at which IFN-γ–producing CD4+ T cells reach the lung (Cooper, 2009). The factors that regulate this recruitment, however, are only beginning to be elucidated. Recently, Mtb-specific Th17 cells were shown to be important for the rapid recruitment of Th1 effector cells to the lungs of immunized mice (Khader et al., 2007). Our data reveal that Mtb-specific T reg cells have an opposite effect, retarding this recruitment.
Previous studies have investigated T reg cell function during infection by globally depleting T reg cells using antibodies (Scott-Browne et al., 2007), transferring naive T cells depleted of T reg cells into lymphopenic mice (Kursar et al., 2007), or administering diphtheria toxin to conditional knockin mice expressing diphtheria toxin receptor in T reg cells (Lund et al., 2008). These experiments can sometimes be difficult to interpret because of the confounding influence of autoimmune activation that is inherently induced using these approaches (Kim et al., 2007). In this study, we circumvented this problem by transferring small numbers of T reg cells from TCR transgenic mice into recipients with normal numbers of endogenous T reg cells, allowing the effects of antigen-specific T reg cells to be studied in the absence of autoimmunity. It is noteworthy that our work finds an opposite role for T reg cells to a recent report that concluded that T reg cells help facilitate effector T cell recruitment to tissue sites of infection (Lund et al., 2008). These contrasting results could be explained by the differences in T reg cell function in viral versus mycobacterial infection, or because of the presence versus absence of autoimmune cytokine release in LNs, an occurrence that may disrupt the chemokine gradient required to recruit effector T cells to tissue sites of inflammation.
We found that T reg cells specific for Mtb Ag85B240–254 delay the priming of effector T cells recognizing different MHC class I (TB10.44–11) and II (ESAT-64–17)–restricted Mtb epitopes in the pLN and their subsequent accumulation in the lung (Fig. 7 and Fig. S1). Previous studies investigating the effects of T reg cells on dendritic cell function may provide a framework for understanding how relatively small numbers of T reg cells can restrict the expansion of effector T cells, including T cells recognizing epitopes that are distinct from the epitope recognized by the T reg cells. Interaction between activated T reg cells and dendritic cells results in the down-regulation of CD80/86 expression on dendritic cells, impairing their capacities to deliver co-stimulatory signals and to establish lasting interactions with naive T cells. These effects depend not only on CTLA-4 and LFA-1 but also on TCR-mediated signals (Onishi et al., 2008; Wing et al., 2008). Thus, it is likely that T reg cells recognizing antigens presented by the dendritic cells would preferentially mediate this activity. If T reg cells recognizing Mtb antigens are among the first T cells to specifically interact with Mtb-infected dendritic cells in the pLN, the ability of these dendritic cells to subsequently prime effector T cells may be compromised.
Although we found no evidence for early induction of Foxp3 by non–T reg cells in B6 mice infected with the H37Rv strain of Mtb, in the future it will be important to look at later time points during persistent infection, as it is possible that chronic antigenic stimulation could induce Foxp3 expression in effector CD4+ T cells. In addition, it is possible that genetic differences in the infecting strain or the host could influence this outcome. A recent report indicates that a hypervirulent strain of Mtb (HN878) induces 10-fold higher numbers of T reg cells in the lung (Ordway et al., 2007). It is not known whether this strain is more efficient in expanding preexisting T reg cells or is uniquely capable of inducing T reg cells de novo. Conversely, some individuals may possess genetic polymorphisms that promote the expansion or generation of T reg cells in the periphery. For example, some humans infected with malaria were found to exhibit higher TGF-β and T reg responses in their peripheral blood, and these individuals also had a faster parasitic growth rate (Walther et al., 2005). Further studies are needed to determine if bacterial and host genetic factors governing expansion or de novo generation of T reg cells are correlated with impaired immune control of Mtb and rapid progression of TB.
Consistent with previous findings during Leishmania infection (Belkaid et al., 2002; Suffia et al., 2006), we found that T reg cells responding to early Mtb infection arise from the population of preexisting T reg cells that expressed Foxp3 before infection. In addition, we observed that T reg cells in the pLN and lung do not undergo enhanced proliferation until at least 16 d after infection. This delay parallels the previously described delay in proliferation of conventional CD4 T cells that occurs only after Mtb is transported to the pLN and presentation of Mtb antigens begins to occur at this site (Cooper, 2009). In the case of T reg cells located in the lung, we find that not only is proliferation delayed until after day 16, but in comparison to lung T reg cells in uninfected mice, proliferation is actually diminished before this time. We speculate that this diminished proliferation is mediated by Toll-like receptor–mediated signals that have previously been shown to restrict T reg cell activation during inflammatory responses (Pasare and Medzhitov, 2003, 2004; Yang et al., 2004; Peng et al., 2005). Furthermore, we hypothesize that high affinity interactions with Mtb antigens presented in the pLN enable clonal populations of Mtb-specific T reg cells to overcome this restriction. In support of this idea, our data clearly show that despite being a highly activated population of T cells expressing the high affinity IL-2R (CD25; Sakaguchi et al., 2008), not all T reg cells are equally capable of responding during the inflammatory milieu of TB. Mtb-specific T reg cells, compared with a polyclonal population of T reg cells, displayed a greatly enhanced ability to proliferate and suppress protective immunity. These results, however, do not exclude the possibility that relatively rare clonal populations of self-reactive T reg cells might also undergo expansion and contribute to immunoregulation.
The fact that we did not detect Foxp3+ cells specific for ESAT-64–17:I-Ab, the immunodominant Mtb epitope for effector CD4+ T cells in B6 mice, further supports our finding that Mtb-specific effector T cells do not convert into T reg cells, and also indicates that T reg cells expanding from the preexisting T reg cell population do not recognize this epitope. These results are also consistent with other reports in which MHC class II tetramers containing epitopes recognized by effector T cells failed to bind Foxp3+ cells (Antunes et al., 2008; Burchill et al., 2008). These findings, however, do not exclude the possibility that T reg cells recognize Mtb epitopes that do not overlap, or only partially overlap, with epitopes recognized by effector T cells. Conventional CD4+ T cells and T reg cells are both selected in the thymus via interactions with self-antigens (Piccirillo and Shevach, 2004; Fontenot and Rudensky, 2005), and conventional CD4+ T cells possess the ability to respond to foreign antigens in the periphery. The degree to which T reg cells respond to foreign antigens has not been well established, but T reg cells possess a TCR repertoire that is at least as diverse as that of conventional CD4+ T cells (Hsieh et al., 2006; Pacholczyk et al., 2007). Therefore, it seems likely that individuals possess populations of T reg cells recognizing most, if not all, microbes. Indeed, pathogen-specific T reg cells have been reported in a variety of infections (Belkaid and Tarbell, 2009). Because T reg cells are selected in the thymus by higher affinity interactions with self-antigens than conventional CD4+ T cells (Hsieh et al., 2004; Bautista et al., 2009; DiPaolo and Shevach, 2009), it is possible that the foreign antigens that each T cell type recognizes will not be identical. In support of this idea, Suffia et al. (2006) have shown that T reg cells responding to L. major infection proliferate specifically to Leishmania-infected bone marrow–derived dendritic cells. However, despite being Leishmania specific, these T reg cells do not proliferate in response to immunodominant L. major epitopes recognized by effector T cells, suggesting that they recognize distinct Leishmania-specific epitopes (Belkaid, Y., personal communication).
An important implication of our data is that expanded populations of preexisting T reg cells recognizing Mtb antigens may adversely affect an individual’s ability to mount a rapid and effective immune response to Mtb. Preexisting Foxp3+ T cells are frequently considered to be “natural” T reg cells that develop intrathymically via interactions with self-antigens (Hsieh et al., 2004; Sakaguchi et al., 2008), though they could also represent CD4+ T cells induced to express Foxp3 extrathymically after environmental exposures or even by immunization (Belkaid, 2008). Induction of T reg cells in the periphery has been shown to occur most efficiently in the gut, a highly regulated immunological site where the immune system must tolerate constant exposure to commensal bacteria and food antigens without mounting harmful inflammatory responses, and is facilitated by a population of tolerogenic dendritic cells in the gut-associated lymphoid tissue that releases bioactive TGF-β (Coombes et al., 2007; Sun et al., 2007). Although this process may not be important to immune responses to Mtb in mice reared in a specific pathogen–free facility receiving sterilized food and water, it may be critical in human populations with frequent oral exposure to non-TB mycobacteria (NTM) sharing many antigenic determinants with Mtb (Andersen and Doherty, 2005).
Bacillus Calmette-Guérin (BCG; an attenuated strain of M. bovis), the currently used TB vaccine, has demonstrated widely disparate degrees of efficacy in different studies, from 0 to 80% (Andersen and Doherty, 2005). Interestingly, some epidemiological studies have concluded that BCG performs relatively well in industrialized countries with lower levels of exposure to NTM in the environment but performs poorly, if at all, in the developing world where individuals have higher levels of exposure (Fine, 1995; Black et al., 2002; Andersen and Doherty, 2005; Weir et al., 2006). More studies are needed to verify this correlation, but it is tempting to speculate that frequent, high level oral exposure to environmental NTM induces populations of mycobacteria-specific T reg cells in the gut. Our results suggest that expanded numbers of mycobacteria-specific T reg cells that cross react with BCG and/or Mtb antigens would impede vaccine-induced immunity against Mtb, and may help explain the failure of BCG in many parts of the world. Two recent mouse studies have shown that inactivation of T reg cells before immunization enhances BCG-induced T cell responses but only marginally improves, or does not improve, protection against Mtb (Quinn et al., 2006; Jaron et al., 2008). These studies, however, were performed in mice reared in relatively sterile environments that most likely would not have expanded populations of mycobacteria-specific T reg cells. Future studies are needed to determine if oral exposure to NTM induce or expand populations of T reg cells that cross react with Mtb. If so, inactivation of T reg cells before booster immunizations may provide a greater benefit for protective immunity to TB in a natural setting in which individuals may have increased numbers of mycobacteria-specific T reg cells. We propose that immunization and environmental exposures may expand not only protective T cell subsets, including Mtb-specific Th1 and Th17 cells, but also T cell subsets, such as Mtb-specific T reg cells, that impede protection. An individual’s overall level of immune protection may depend on their relative ratio of protective versus suppressive T cell subsets.
MATERIALS AND METHODS
Mice.
C57BL/6, C57BL/6.PL (CD90.1), and RAG2−/− mice were purchased from the Jackson Laboratory. B6.SJL-Ptprca/BoyAiTac (CD45.1) mice were purchased from Taconic. P25 TCR transgenic mice have been previously described (Tamura et al., 2004). SMARTA TCR transgenic mice and Foxp3-GFP mice (backcrossed 12 times to C57BL/6 mice) were provided by M. Bevan (University of Washington, Seattle, WA) and A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY), respectively. All mice were housed and bred under specific pathogen–free conditions at the University of Washington. All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Bacteria and aerosol infections.
A stock of Mtb strain H37Rv or a strain of Mtb engineered to lack the expression of Ag85B (Mtb Ag85B KO; provided by J. Ernst, New York University, New York, NY) was sonicated before use, and mice were infected in an aerosol infection chamber (Glas-Col) with ∼50–100 CFU deposited in the lungs of each mouse. Two mice were sacrificed on day 1 after infection to determine the infectious dose in each experiment. To determine viable numbers of CFUs, the left lung of each mouse was homogenized in PBS with 0.05% Tween 80. 10-fold serial dilutions were made in PBS with 0.05% Tween 80 and plated on Mitchison 7H10 plates. Colonies were counted after 21 d of incubation at 37°C, and CFUs per organ were determined.
Cell enrichment and sorting.
For adoptive transfer experiments, CD4+ T cells were negatively enriched to >95% purity from freshly isolated spleen and LN cells using magnetic microbeads and subsequent column purification according to the manufacturer’s protocol (Miltenyi Biotec). In some experiments the cells were stained by PE-conjugated anti-CD25 antibody and then sorted for the CD25hi or CD25neg fraction, whereas in other experiments Foxp3-GFP+ and Foxp3-GFPneg cells were sorted on a cell sorter (FACSAria or FACSVantage; BD). Sorted cells were analyzed for purity and adoptively transferred into mice.
Cell-surface staining.
Single-cell suspensions of intraparenchymal lung lymphocytes were prepared by Liberase Blendzyme 3 (Roche) digestion of perfused lungs in the presence of the inducible nitric oxide synthase inhibitor aminoguanidine (Sigma-Aldrich), as previously described (Urdahl et al., 2003). Cells from spleens and LNs were prepared as previously described (Urdahl et al., 2003). Fc receptors were blocked with anti-CD16/32 (2.4G2). Cells were suspended in PBS containing 0.1% NaN3 and 2.5% fetal bovine serum (i.e., sorter buffer) and stained at saturating conditions using antibodies specific for CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD25 (PC61), GITR (DTA-1), CD103 (M290), CTLA-4 (UC10-4F10-11), and Vα2 (B20.1) obtained from BD, or F4/80 (BM8), CD11b (M1/70), CD11c (N418), CD19 (eBio1D3), CD44 (1M7), Thy1.1 (HIS51), Thy1.2 (53-2.1), CD45.1 (A20), and CD45.2 (104) obtained from eBioscience. For the detection of P25 transgenic cells, we used the rat anti–mouse clonotypic TCR mAb KN7 that recognizes TCR expressed on the Ag85B240–254–reactive Th1 clone of B6 mice. Samples were fixed in a 2% paraformaldehyde solution in PBS and analyzed using a FACSCanto or LSR II (both from BD) and FlowJo software (Tree Star, Inc.).
Detection of transferred cells by magnetic bead enrichment.
For detection of small numbers of transferred cells in an entire spleen, we used a magnetic bead enrichment technique recently described by Hataye et al. (2006). In brief, spleen cells from the entire spleen of each individual mouse were labeled with biotin-labeled anti-CD90.2 or anti-CD45.2 antibody, washed, labeled with streptavidin beads under nonsaturating conditions (Miltenyi Biotec), and passed over magnetized LS columns (Miltenyi Biotec). After washing the columns to remove unlabeled cells, the columns were removed from the magnets and the bound cells were eluted. This enriched cell population was labeled with fluorochrome-conjugated streptavidin, and also for cell-surface and intracellular molecules, as outlined. The entire stained sample was analyzed as described in Cell-surface staining.
Intracellular staining.
Intracellular IFN-γ staining was performed using a kit as instructed by the manufacturer (BD) with a few modifications. In brief, lung cells were stimulated with ESAT-64–17 peptide (5 µg/ml final concentration) or TB10.44–11 (0.5 µg/ml final concentration) for 4 h in complete RPMI (RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 10 mM Hepes, 0.5 µM 2-ME, 100 U/ml penicillin, and 100 µg/ml streptomycin) in the presence of monensin. Cells were washed, stained with antibodies against surface markers, and fixed in 2% paraformaldehyde. Cells were subsequently permeabilized in permeabilization buffer (BD) and stained with anti–IFN-γ–allophycocyanin (APC; XMG1.2; BD). In some experiments, cells were permeabilized and stained for intracellular Foxp3 using anti-Foxp3–APC (FJK-16s) and an intracellular staining kit according to the instructions provided by the manufacturer (eBioscience). Stained cells were acquired and analyzed as described in Cell-surface staining.
Analysis of cell proliferation.
To track the division of adoptively transferred cells, isolated CD4+CD25+ T cells were stained in vitro with 5 µM CFSE (Invitrogen) followed by incubation for 10 min at 37°C with CFSE. The labeling reaction was quenched by washing in ice-cold RPMI 1640 supplemented with 10% FCS. CFSE-labeled cells were injected intravenously into recipient mice. 5–7 d later, CD4+ T cells were isolated from lungs, spleen, and draining LN as described, and cell division as a measure of decrease in CFSE intensity was analyzed by flow cytometry. To detect the proliferation of endogenous T cells after Mtb infection, mice were administered 1 mg of the thymidine analogue BrdU intraperitoneally and harvested 20 h later. After preparation of single-cell suspensions from pLNs and lungs, cells were stained for cell-surface markers. The BrdU flow kit (BD) protocol was followed for intracellular BrdU staining with slight modifications to allow for Foxp3 costaining.
Detection of ESAT-64–17– and TB10.44–11–specific cells.
PE-labeled MHC class II tetramers (I-Ab) containing the stimulatory residues 4 to 17 (QQWNFAGIEAAASA) of the early secreted antigenic target 6 kD (ESAT-6) of Mtb were provided by M. Jenkins (University of Minnesota, Minneapolis, MN; Moon et al., 2007). APC-labeled MHC class I tetramers (Kb) containing the stimulatory residues 4 to 11 (IMYNYPAM) of the low molecular weight protein antigen TB10.4 of Mtb were obtained from the National Institutes of Health Tetramer Core Facility. Single-cell preparations from lungs and pLNs were incubated for 1 h at room temperature with 5–10 nM of ESAT-64–17:I-Ab tetramers in 1:1 Fc Block/sorter buffer, washed with sorter buffer, and incubated for 30 min on ice with TB10.44–11:Kb tetramers in 100 µl of sorter buffer at a dilution of 1:400. Lung cells were washed in sorter buffer and stained with PerCP-Cy5.5–labeled anti-CD3, Pacific orange–labeled anti-CD4 (Invitrogen), Alexa Fluor 700–labeled anti-CD44, APC–eFluor 780–labeled anti-CD8, and a Pacific blue–labeled non–T cell cocktail containing anti-F4/80, anti-CD19, anti-CD11c, and anti-CD11b. ESAT-64–17–specific T cells were identified as CD3+, non–T cell cocktail–negative, CD4+, CD8neg, tetramer+, CD44hi events. Likewise, TB10.44–11–specific cells were identified as CD3+, non–T cell cocktail–negative, CD4neg, CD8+, tetramer+, CD44hi events. Because of the lower frequency of antigen-specific T cells in the LNs, after the tetramer staining steps, the pLNs were coenriched for ESAT-64–17– and TB10.44–11:Kb–specific cells by incubation with sorter buffer containing anti-PE– and anti-APC–conjugated magnetic microbeads (Miltenyi Biotec) on ice for 30 min and passing over a magnetized LS column. The column was washed with sorter buffer and removed from the magnetic field. The bound fraction was obtained by pushing 5 ml of sorter buffer through the column with a plunger. The enriched fractions were stained with other surface markers as described for lungs.
Online supplemental material.
Fig. S1 shows representative flow cytometric plots demonstrating that Mtb-specific T reg cells restrict the LN priming of Mtb-specific CD4+ and CD8+ T cells, as defined by MHC class II and I tetramers, respectively, during early Mtb infection. Fig. S2 shows that tetramer-binding Mtb-specific CD4+ T cells do not express Foxp3 during early Mtb infection. Fig. S3 shows that transfer of high numbers of Mtb-specific T reg cells mediates an increased reduction in the appearance of effector T cells in the lungs of recipient mice. Fig. S4 shows that Mtb-specific T reg cells delay the appearance of IFN-γ–producing CD4+ and CD8+ effector T cells in the lungs of recently Mtb-infected mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091885/DC1.
Acknowledgments
We thank M. Dao, K. Stohr, N. Tapias, and K. Ishida-Tsubota for technical assistance; Dr. M. Jenkins and H. Chu for technical advice regarding tetramer staining; and Drs. M. Bevan, D. Campbell, L. Ramakrishnan, and M. Williams for comments on the manuscript.
This work was funded by a grant to K.B. Urdahl from the National Institutes of Health (AI 076327-01), a Burroughs-Wellcome Fund Career Award in the Biological Sciences, and by funds from the Department of Pediatrics, University of Washington.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BCG
- bacillus Calmette-Guérin
- Mtb
- Mycobacterium tuberculosis
- NTM
- non-TB mycobacteria
- pLN
- pulmonary LN
- TB
- tuberculosis
References
- Andersen P., Doherty T.M. 2005. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3:656–662 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- Antunes I., Tolaini M., Kissenpfennig A., Iwashiro M., Kuribayashi K., Malissen B., Hasenkrug K., Kassiotis G. 2008. Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology. Immunity. 29:782–794 10.1016/j.immuni.2008.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H., Shimohakamada Y., Nakada M., Tokunaga T., Kikuchi T., Kariyone A., Tamura T., Takatsu K. 2007. Instruction of naive CD4+ T-cell fate to T-bet expression and T helper 1 development: roles of T-cell receptor-mediated signals. Immunology. 122:210–221 10.1111/j.1365-2567.2007.02630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista J.L., Lio C.W., Lathrop S.K., Forbush K., Liang Y., Luo J., Rudensky A.Y., Hsieh C.S. 2009. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10:610–617 10.1038/ni.1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y. 2008. Role of Foxp3-positive regulatory T cells during infection. Eur. J. Immunol. 38:918–921 10.1002/eji.200738120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Tarbell K. 2009. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 27:551–589 10.1146/annurev.immunol.021908.132723 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507 10.1038/nature01152 [DOI] [PubMed] [Google Scholar]
- Black G.F., Weir R.E., Floyd S., Bliss L., Warndorff D.K., Crampin A.C., Ngwira B., Sichali L., Nazareth B., Blackwell J.M., et al. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 359:1393–1401 10.1016/S0140-6736(02)08353-8 [DOI] [PubMed] [Google Scholar]
- Burchill M.A., Yang J., Vang K.B., Moon J.J., Chu H.H., Lio C.W., Vegoe A.L., Hsieh C.S., Jenkins M.K., Farrar M.A. 2008. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 28:112–121 10.1016/j.immuni.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burl S., Hill P.C., Jeffries D.J., Holland M.J., Fox A., Lugos M.D., Adegbola R.A., Rook G.A., Zumla A., McAdam K.P., Brookes R.H. 2007. FOXP3 gene expression in a tuberculosis case contact study. Clin. Exp. Immunol. 149:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso A.M., Serbina N., Klein E., Triebold K., Bloom B.R., Flynn J.L. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407–5416 [PubMed] [Google Scholar]
- Chackerian A.A., Perera T.V., Behar S.M. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666–2674 10.1128/IAI.69.4.2666-2674.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian A.A., Alt J.M., Perera T.V., Dascher C.C., Behar S.M. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501–4509 10.1128/IAI.70.8.4501-4509.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Herman A.E., Matos M., Mathis D., Benoist C. 2005. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202:1387–1397 10.1084/jem.20051409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhou B., Li M., Deng Q., Wu X., Le X., Wu C., Larmonier N., Zhang W., Zhang H., et al. 2007. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 123:50–59 10.1016/j.clim.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27:393–422 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo R.J., Shevach E.M. 2009. CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur. J. Immunol. 39:234–240 10.1002/eji.200838772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggena M.P., Walker L.S., Nagabhushanam V., Barron L., Chodos A., Abbas A.K. 2004. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J. Exp. Med. 199:1725–1730 10.1084/jem.20040124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Akuffo H., Britton S. 2005. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 99:363–368 10.1016/j.trstmh.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Fine P.E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 346:1339–1345 10.1016/S0140-6736(95)92348-9 [DOI] [PubMed] [Google Scholar]
- Fletcher H.A. 2007. Correlates of immune protection from tuberculosis. Curr. Mol. Med. 7:319–325 10.2174/156652407780598520 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rudensky A.Y. 2005. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6:331–337 10.1038/ni1179 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., Pamer E.G., Glickman M.S. 2008. Delayed protection by ESAT-6–specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 205:2359–2368 10.1084/jem.20080353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot-Revol V., Innes J.A., Hackforth S., Hinks T., Lalvani A. 2006. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 173:803–810 10.1164/rccm.200508-1294OC [DOI] [PubMed] [Google Scholar]
- Hataye J., Moon J.J., Khoruts A., Reilly C., Jenkins M.K. 2006. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 312:114–116 10.1126/science.1124228 [DOI] [PubMed] [Google Scholar]
- Hori S., Haury M., Coutinho A., Demengeot J. 2002. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 99:8213–8218 10.1073/pnas.122224799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougardy J.M., Place S., Hildebrand M., Drowart A., Debrie A.S., Locht C., Mascart F. 2007. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am. J. Respir. Crit. Care Med. 176:409–416 10.1164/rccm.200701-084OC [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Liang Y., Tyznik A.J., Self S.G., Liggitt D., Rudensky A.Y. 2004. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 21:267–277 10.1016/j.immuni.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Zheng Y., Liang Y., Fontenot J.D., Rudensky A.Y. 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7:401–410 10.1038/ni1318 [DOI] [PubMed] [Google Scholar]
- Jaron B., Maranghi E., Leclerc C., Majlessi L. 2008. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PLoS One. 3:e2833 10.1371/journal.pone.0002833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.J., Ryan L., LaCourse R., North R.J. 2005. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 201:1915–1924 10.1084/jem.20050265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.J., LaCourse R., Ryan L., North R.J. 2008. ‘Immunization’ against airborne tuberculosis by an earlier primary response to a concurrent intravenous infection. Immunology. 124:514–521 10.1111/j.1365-2567.2007.02803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S.A., Bell G.K., Pearl J.E., Fountain J.J., Rangel-Moreno J., Cilley G.E., Shen F., Eaton S.M., Gaffen S.L., Swain S.L., et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Kursar M., Koch M., Mittrücker H.W., Nouailles G., Bonhagen K., Kamradt T., Kaufmann S.H. 2007. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178:2661–2665 [DOI] [PubMed] [Google Scholar]
- Leal I.S., Smedegård B., Andersen P., Appelberg R. 2001. Failure to induce enhanced protection against tuberculosis by increasing T-cell-dependent interferon-gamma generation. Immunology. 104:157–161 10.1046/j.1365-2567.2001.01305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J.M., Hsing L., Pham T.T., Rudensky A.Y. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 320:1220–1224 10.1126/science.1155209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrücker H.W., Steinhoff U., Köhler A., Krause M., Lazar D., Mex P., Miekley D., Kaufmann S.H. 2007. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. USA. 104:12434–12439 10.1073/pnas.0703510104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. 2007. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 27:203–213 10.1016/j.immuni.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA. 105:10113–10118 10.1073/pnas.0711106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway D., Henao-Tamayo M., Harton M., Palanisamy G., Troudt J., Shanley C., Basaraba R.J., Orme I.M. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179:522–531 [DOI] [PubMed] [Google Scholar]
- Orme I.M. 2005. Mouse and guinea pig models for testing new tuberculosis vaccines. Tuberculosis (Edinb.). 85:13–17 10.1016/j.tube.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Oxenius A., Bachmann M.F., Zinkernagel R.M., Hengartner H. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390–400 [DOI] [PubMed] [Google Scholar]
- Pacholczyk R., Kern J., Singh N., Iwashima M., Kraj P., Ignatowicz L. 2007. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 27:493–504 10.1016/j.immuni.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C., Medzhitov R. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036 10.1126/science.1078231 [DOI] [PubMed] [Google Scholar]
- Pasare C., Medzhitov R. 2004. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 21:733–741 10.1016/j.immuni.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Peng G., Guo Z., Kiniwa Y., Voo K.S., Peng W., Fu T., Wang D.Y., Li Y., Wang H.Y., Wang R.F. 2005. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 309:1380–1384 10.1126/science.1113401 [DOI] [PubMed] [Google Scholar]
- Piccirillo C.A., Shevach E.M. 2004. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin. Immunol. 16:81–88 10.1016/j.smim.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Quinn K.M., McHugh R.S., Rich F.J., Goldsack L.M., de Lisle G.W., Buddle B.M., Delahunt B., Kirman J.R. 2006. Inactivation of CD4+CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 84:467–474 [DOI] [PubMed] [Google Scholar]
- Reiley W.W., Calayag M.D., Wittmer S.T., Huntington J.L., Pearl J.E., Fountain J.J., Martino C.A., Roberts A.D., Cooper A.M., Winslow G.M., Woodland D.L. 2008. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Natl. Acad. Sci. USA. 105:10961–10966 10.1073/pnas.0801496105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Rodrigues R., Resende Co T., Rojas R., Toossi Z., Dietze R., Boom W.H., Maciel E., Hirsch C.S. 2006. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin. Exp. Immunol. 144:25–34 10.1111/j.1365-2249.2006.03027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance. Cell. 133:775–787 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Schenk S., Kish D.D., He C., El-Sawy T., Chiffoleau E., Chen C., Chen C., Wu Z., Sandner S., Gorbachev A.V., et al. 2005. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J. Immunol. 174:3741–3748 [DOI] [PubMed] [Google Scholar]
- Scott-Browne J.P., Shafiani S., Tucker-Heard G., Ishida-Tsubota K., Fontenot J.D., Rudensky A.Y., Bevan M.J., Urdahl K.B. 2007. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204:2159–2169 10.1084/jem.20062105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffia I.J., Reckling S.K., Piccirillo C.A., Goldszmid R.S., Belkaid Y. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777–788 10.1084/jem.20052056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Ariga H., Kinashi T., Uehara S., Kikuchi T., Nakada M., Tokunaga T., Xu W., Kariyone A., Saito T., et al. 2004. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int. Immunol. 16:1691–1699 10.1093/intimm/dxh170 [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190 [DOI] [PubMed] [Google Scholar]
- Urdahl K.B., Liggitt D., Bevan M.J. 2003. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/−Db−/− mice, but provide minimal protection. J. Immunol. 170:1987–1994 [DOI] [PubMed] [Google Scholar]
- Walther M., Tongren J.E., Andrews L., Korbel D., King E., Fletcher H., Andersen R.F., Bejon P., Thompson F., Dunachie S.J., et al. 2005. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 23:287–296 10.1016/j.immuni.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Weir R.E., Black G.F., Nazareth B., Floyd S., Stenson S., Stanley C., Branson K., Sichali L., Chaguluka S.D., Donovan L., et al. 2006. The influence of previous exposure to environmental mycobacteria on the interferon-gamma response to bacille Calmette-Guérin vaccination in southern England and northern Malawi. Clin. Exp. Immunol. 146:390–399 10.1111/j.1365-2249.2006.03222.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2008. Global tuberculosis control: surveillance, planning, financing. WHO Report 2008 Geneva, World Health Organization [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- Wolf A.J., Linas B., Trevejo-Nuñez G.J., Kincaid E., Tamura T., Takatsu K., Ernst J.D. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179:2509–2519 [DOI] [PubMed] [Google Scholar]
- Wolf A.J., Desvignes L., Linas B., Banaiee N., Tamura T., Takatsu K., Ernst J.D. 2008. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205:105–115 10.1084/jem.20071367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Huang C.T., Huang X., Pardoll D.M. 2004. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol. 5:508–515 10.1038/ni1059 [DOI] [PubMed] [Google Scholar]