Abstract
Wnt reporter TOPgal mice carry a β-galactosidase (βgal) gene under the control of the Wnt/β-catenin signaling responsive elements. We found that the intensely immunolabeled βgal+ cells were co-immunolabeled with Nestin and formed a tangentially oriented single-cell layer in the “connecting or docking zone” where the olfactory sensory axons attached to the brain surface during mid-gestation. During early postnatal development, βgal+ cells were located in the inner olfactory nerve layer (ONLi) and co-labeled with olfactory ensheathing cell (OEC) markers S100β and NPY but not with lineage-specific markers for neurons, oligodendrocytes, astrocytes, and microglia, demonstrating that the TOPgal marked a subpopulation of OECs. By confocal microscopy, we found that TOPgal activated processes extended along the developing glomerulus and formed multiple tunnel-like structures that ensheath and bridge olfactory sensory axonal bundles from ONLi to the glomerulus, which may play a key role in glomerulus formation and convergent sorting of the peripheral olfactory axons.
Keywords: olfactory nerve layer (ONL), olfactory ensheathing cells (OECs), olfactory sensory axons, Wnt/β-catenin reporter, TOPgal transgenic mice, glomerulus formation, convergent sorting, NPY, Nestin, S100β
INTRODUCTION
The olfactory system has the unique capability to maintain neuronal regeneration and axonal outgrowth throughout the life of an animal. The unique capability is related to the presence of olfactory ensheathing cells (OECs) (Franssen et al., 2007). The OECs ensheath olfactory sensory neuron (OSN) axons as they exit from the olfactory epithelium, course through the olfactory mucosa, enter the olfactory bulb (OB), and populate the olfactory nerve layer (ONL). Previous studies have demonstrated that several subpopulations of OECs exist in the ONL, which differ by their staining with cell-type specific antibodies (Au et al., 2002). It is thought that different subpopulations of OECs play distinct roles in axonal guidance and targeting in the ONL. In addition, OECs are considered to be important in the initial formation of the olfactory glomeruli, as well as in the maintenance of a rigid framework for newly growing olfactory axons (Valverde et al., 1992).
The existence of several distinct phenotypes of OECs in the ONL has led us to search for the developmental mechanisms that modulate phenotypes of OECs in the ONL. Expression of Wnt signaling molecules was demonstrated in both ONL and glomerular layer of postnatal mouse olfactory bulbs (Shimogori et al., 2004). Wnts are secreted signaling molecules that play essential roles in diverse developmental events and diseases (Logan and Nusse, 2004). However, the role of Wnt signaling in the olfactory system remains little understood. A recent study reported an antagonistic role of the fly Wnt5 and Drl receptor in midline crossing of sensory axon branches and the proper patterning of olfactory glomeruli in Drosophila melanogaster (Yao et al., 2007). However, it remains unknown whether canonical Wnt/β-catenin signaling pathway is involved in this or related processes. The canonical Wnt pathway regulates the ability of β-catenin to activate the transcription of target genes. To examine the role of the canonical Wnt pathway in olfactory development particularly in the OECs, we employed the Wnt reporter mouse line TOPgal (Tcf-optimal promoter β-galactosidase reporter); these mice carry a lacZ reporter gene encoding β-galactosidase (βgal) under the control of a Tcf-optimal promoter that responds to the complex formed by β-catenin and Tcf/Lef1 transcriptional factors (DasGupta and Fuchs, 1999). We discovered TOPgal activities in a small population of putative OECs in the developing ONL and presented our original findings in an international meeting (Molotkov and Zhou, 2007). Recently two groups also reported a small cell population with Wnt reporter activities in the developing olfactory bulb that may play a role in olfactory axonal connections, but the identity of these cells remains unknown (Zaghetto et al., 2007; Booker-Dwyer et al., 2008). Here we demonstrate that these Wnt reporter-activated cells in the developing ONL are a phenotypically unique OEC subgroup that may be directly involved in glomerulus formation and convergent sorting (Mombaerts, 2006) of olfactory sensory axons.
RESULTS
Wnt reporter TOPgal activated cells were found in early embryonic olfactory system
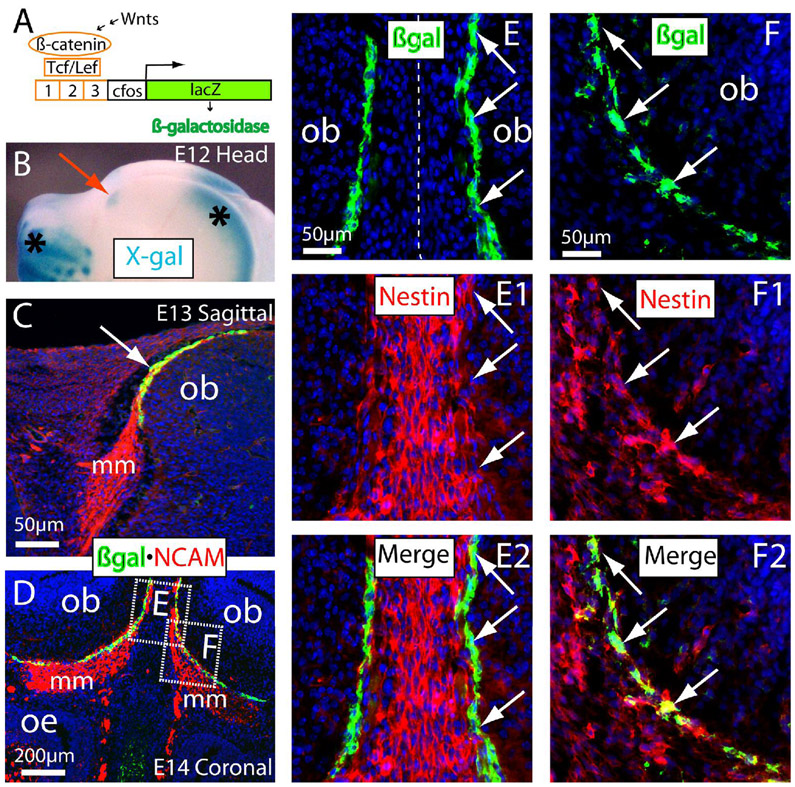
We first observed characteristic X-gal staining for the βgal enzymatic activity at embryonic day (E) 12 in the front tips of rostral brains underneath the skull (red arrow in Fig. 1B). The X-gal stained signals were also reproducibly present in the cortical hem of the telencephalon and the developing facial structures (asterisks in Fig. 1B) in which Wnt signaling plays essential roles in developing neocortex (Zhou et al., 2006), hippocampal formation (Zhou et al., 2004b), and the orofacial primordia (Zhou lab, in preparation). We also found a tangentially oriented single-cell layer of the intensely immunolabeled βgal+ cells along the migratory route on the pia surface of OB anlage (green in Fig. 1C). At this stage, the olfactory axons immunolabeled with the antibodies to NCAM (neural cell adhesion molecule) extend from olfactory epithelium to the pia surface of OB anlage (red in Fig. 1C). NCAM is expressed by olfactory sensory neurons and their axons as well as OECs in the embryonic olfactory system (Aoki et al., 1995; Franceschini and Barnett, 1996). These βgal+ cells were restricted to a “connecting or docking zone” where the axonal bundles extend along the pia for future connections with CNS axons. At E14, we found the intensive βgal+ cells in the connecting zone between OB and the thick migratory mass (which consists of the migrating OECs intermingled with the olfactory axons) (Fig. 1D). All of these βgal+ cells in the connecting zone were co-immunolabeled with NCAM (Fig. 1C,D). In addition, we found that the tangentially oriented βgal+ cells were also co-immunolabeled with Nestin (arrows in Fig. 1E–F2). Nestin is expressed in neural lineage cells including OECs (Wang et al., 2007). We noted many Nestin+ cells in the middle region between two olfactory bulbs (Fig. 1E1,E2) and also inside the migratory mass (Fig. 1F1–F2). The tangentially organized βgal+ cells in these regions were co-immunolabeled with Nestin weakly or intensely (arrows in Fig. 1E–F2). To distinguish the tangentially organized OEC-like TOPgal labeled cells in the connecting zone from the classic OECs inside or surrounding the migratory mass, we arbitrarily propose a temporary name for these OEC-like TOPgal labeled cells in the “connecting or docking zone” between the olfactory migratory mass (the future olfactory nerve layer) and the OB at the early developmental stage as “connecting zone” (CZ) cells.
Figure 1.
Localization and lineage identity of the TOPgal-labeled cells in the olfactory bulb anlage. (A) The Wnt reporter TOPgal construct. (B) Wholemount X-gal staining (blue) of E12 heads of TOPgal mice demonstrated the positively stained (blue) island in the presumably olfactory bulb anlage (red arrow). Note that the dominantly stained X-gal products in the rostral telencephalon and facial regions (asterisks) of the adjacent area faithfully reflected the known activation of Wnt signaling in these sites. (C) Double immunolabeling for β-galactosidase (green) and NCAM (red) on sagittal head sections demonstrated that the Wnt reporter activated cells were single-cell layered and tangentially localized in the connecting zone (white arrow indicates the yellow labeled cell layer with the overlapped red and green signals) where olfactory sensory neuron axons attached to the olfactory bulb anlage at E13. Note that no intense βgal immunolabeling was found in other adjacent tissues including the thick olfactory axon bundles that is a part of migratory mass (mm) on the projecting way from olfactory epithelium to the bulb. (D) Double immunolabeling for βgal and NCAM on coronal sections through olfactory bulb and epithelium at E14. The dash-enclosed areas indicate relevant regions of different tissue sections with a higher magnification in panels E–F2 (single optical sections by confocal microscopy). (E–E2) Double immunolabeling of βgal and Nestin in the middle region between two olfactory bulbs at E14. Most intensely immunolabeled βgal+ cells are co-labeled with weak Nestin-immunoreactivities in the connecting zone (arrows). (F–F2) βgal+/nestin+ cells (arrows) in the connecting zone of the migratory mass. ob, olfactory bulb; oe, olfactory epithelium. The cell nuclei were counterstained by DAPI (blue in C–F2).
The above findings provide evidence that Wnt signaling is active in early embryonic olfactory neural lineage cells, particularly in a distinct cell population that is tangentially located in the connecting zone where olfactory sensory axons attached to the brain surface, and suggest that CZ cells are a unique subpopulation of OECs in which there is intense activation of the Wnt reporter TOPgal.
The TOPgal labeled “connecting zone” cells were found in the inner olfactory nerve layer during glomerulus formation and axonal connections
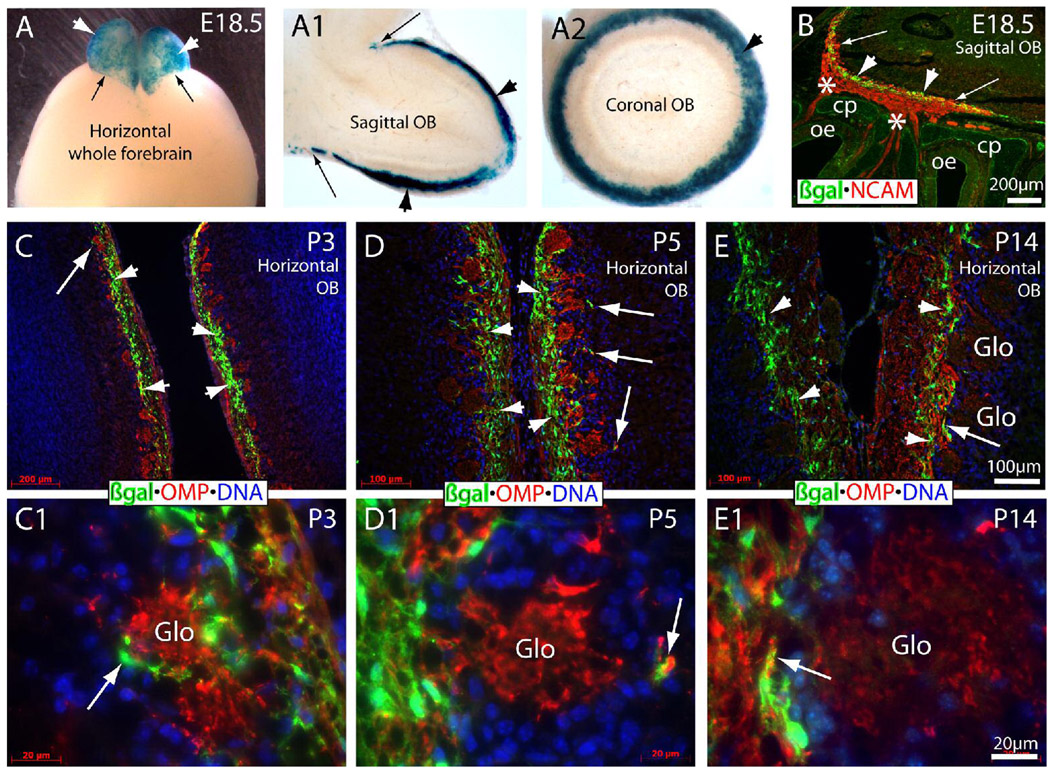
The development of glomeruli starts at E18 in the mouse OB (Valverde et al., 1992), when olfactory axons enter the OB from the ONL. We performed X-gal staining for E18.5 brains of TOPgal mice. X-gal staining that labels Wnt reporter activated cells was found in entire olfactory bulb surface with a sharp ending border between the OB and the major forebrain regions (Fig. 2A). To further assess the localization of X-gal staining, we prepared sagittal and transverse 150 mm vibratome sections of the OB obtained from E18.5 TOPgal embryos and stained them for βgal enzymatic activity. Intense X-gal staining was evident only on the surface layer of the OB on sagittal (Fig. 2A1) and coronal (Fig. 2A2) sections. This demonstrates that the canonical Wnt pathway is consistently active in the forming ONL during prenatal development.
Figure 2.
Wnt reporter activated “connecting zone” cells in developing olfactory nerve layer (ONL) during glomerulus formation from E18.5 to P14 of mice. (A–A2) X-gal staining of whole forebrain (A) and 150-µm-thick sections of sagittal (A1) and coronal (A2) olfactory bulb at E18.5. Arrowheads indicate the intensely stained X-gal products on the bulb surface/olfactory nerve layer. Arrows indicate the sharp border between the stained bulb and non-stained forebrain surface. Note that no positive staining was present inside the bulb in the thick sections (A1,A2). (B) Double immunolabeling of βgal (green) and NCAM (red) on the sagittal section of E18.5 heads. Intensely immunolabeled βgal+ cells formed a multiple-cell layer (arrowheads) in the connecting zone/developing inner ONL where some NCAM+ olfactory axons crossed the connecting zone and entered into the forming glomerular layer (arrows). Asterisks indicate the thick bundles of NCAM+ olfactory axons crossed through the cribriform plate (cp). (C,C1) The βgal+ cells (green) in the ONL of P3 horizontal bulb sections. Arrow (in C,C1) indicates the same region with different magnifications for the βgal+ process as a capsule-like structure surrounding the forming glomerulus immunolabeled by OMP (red) on the bulb side. Arrowheads (in C) indicate the area of significantly expanded βgal+ multiple-cell layer in ONL. (D,D1) The thickness and density of the βgal+ multiple-cell layer was increased further (arrowheads) in P5 ONL. βgal+ processes were still found in the periglomerulus (arrows) on the bulb side. (E,E1) The thickness and density of βgal+ cells were significantly declined and restricted to the inner most ONL at P14 (arrowheads) when most glomeruli are formed. The βgal+ process mixed with OMP+ olfactory axons were found in the olfactory axonal entrance to the glomerulus (arrows). OB, olfactory bulb; Glo, glomerulus (visualized by OMP+ olfactory axons); OMP, olfactory marker protein. The cell nuclei were counterstained by DAPI (blue in C–E1).
From the immunolabeling on sagittal sections at E18.5, we found that the single-cell-layered βgal+ CZ cells expanded as multiple-cell-layer (arrowheads in Fig. 2B,Fig. 3A,3B) in the connecting zone of the migratory mass/forming ONL that was evident by NCAM-immunolabeling. We then examined immunolabeling for βgal on horizontal sections of the OB of P3 ~ P14 TOPgal mice (Fig. 2C–E1) and observed a significant change in the multiple-cell-layered βgal+ CZ cells in the ONL during glomerulus formation. The TOPgal labeled CZ cells were significantly increased in density and numbers with a peak around P3 to P10 but declined by P14 onwards when most glomeruli formed (Fig. 2C–E1). The OMP-immunolabeled olfactory axons were dramatically increased in the outer ONL from P3 to P14. No CZ cells were observed inside of the glomeruli in any of these developmental stages (Fig. 2C1–E1). However, we observed the TOPgal labeled CZ cells forming capsule-like structures around some glomeruli at P3 (Fig. 2C1). The CZ cell-capsules were less apparent at P5 (Fig. 2D1) and were not present in the OB of P14 mice (Fig. 2E1). We also examined the OB of P28 TOPgal mice and found small numbers of TOPgal labeled CZ cells in the ONL (data not shown). These findings suggest that the CZ cells are transiently activated by canonical Wnt signaling in the inner ONL, which may play important roles in related developmental processes.
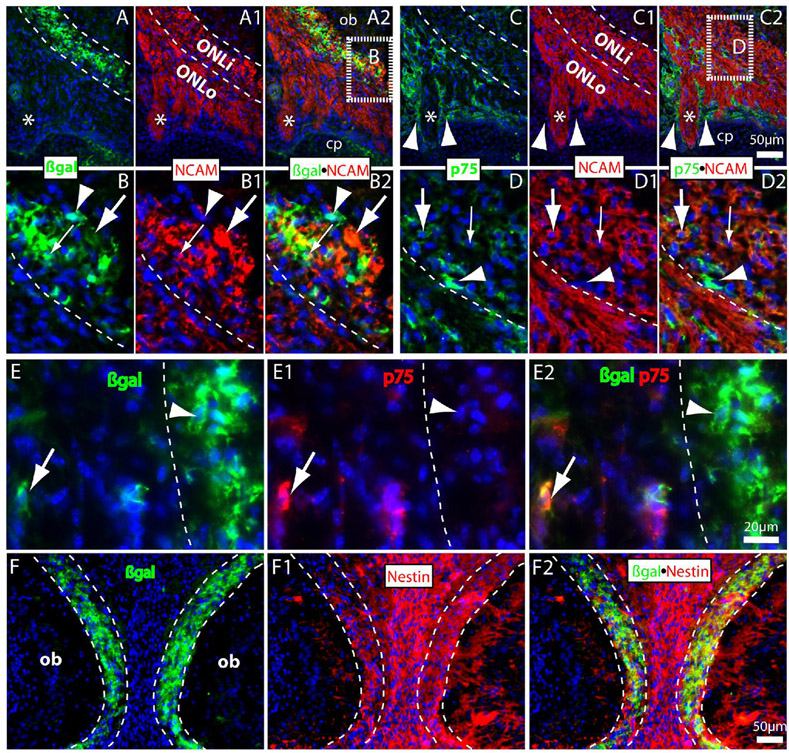
Figure 3.
Double immunolabeling for the TOPgal-labeled “connecting zone” cells with OEC markers on coronal sections of E18.5 olfactory bulb. (A–B2) βgal+ cells (green) consist a multiple-cell layer intermingled with NCAM+ olfactory axons (red) in the inner ONL (dashed lines in parallel). Some of them were co-immunolabeled with NCAM in both perinuclear cytoplasm (small arrows in B–B2) and processes (large arrows in B–B2). Arrowheads (in B–B2) indicate a βgal+/NCAM− cell located in the front line of the connecting zone. (Panels B–B2 were magnified from the dash-lined area indicated in A2). (C–D2) Weakly immunolabeled p75+ OECs (green) were also found in the inner ONL that most of them were co-immunolabeled with NCAM in the perinuclear cytoplasm (arrows). Note that p75+ OECs were dominantly distributed in outer ONL by surrounding the large olfactory axonal bundles (asterisks) but no overlapping (arrowheads) with NCAM immunolabeling. (Panels D–D2 were magnified from the dash-lined area indicated in C2; Arrowheads in D–D2 indicate an intense p75+ cells that was not stained with NCAM in boundary between ONLi and ONLo). (E–E2) A few βgal+ (green) and p75+ (red) double-immunolabeled OEC-like cells were found in the E18.5 ONLo (arrows). Arrowheads indicate an intensely immunolabeled βgal+ cell without p75-immunolabeling in the inner ONL (that divided by a dashed line). (F–F2) Double immunolabeling of βgal and Nestin on a coronal section with left and right bulbs shown at E18.5. Most βgal+ cells (green) in the ONLi were co-immunolabeled with Nestin (red). Note that Nestin immunoreactivities were also found in the intermediate zone between two bulbs and in the developing glomerular layer. cp, cribriform plate. Asterisks (in A–C2) indicate a large olfactory axonal bundle crossed the cribriform plate. The cell nuclei were counterstained by DAPI (blue).
TOPgal labeled “connecting zone” cells are a phenotypically unique subgroup of OECs in postnatal ONL that ensheath olfactory sensory axons connected with glomeruli
To determine the properties of TOPgal labeled CZ cells during late embryonic and early postnatal development, we double-immunolabeled βgal with representative lineage markers at E18.5 to P14 (Fig. 3,Fig. 4). On coronal sections of E18.5 olfactory bulb at higher magnification, we observed the CZ cells also changed in shape, with thick processes and sheet-like structures evident by intense βgal immunolabeling (Fig. 3A,B). Many of the βgal+ cells and processes were co-immunolabeled or tightly associated with NCAM in the forming inner ONL (ONLi) (Fig. 3A–B2). The low affinity neurotrophin receptor p75 has been shown to be specifically expressed in the OECs during development and can be used as a specific marker for the OECs in the olfactory system (Gong et al., 1994; Franceschini and Barnett, 1996). In contrast to that most βgal+ cells were located in the ONLi, the intense p75+ OECs were distributed in the ONLo with particular density surrounding the large axonal bundles (arrowhead and asterisk in Fig. 3C–C2). Some weakly labeled p75+ cells were found in the ONLi that were co-immunolabeled or tightly associated with NCAM (arrows in Fig. 3D–D2). We also detected some scattered βgal+ cells in the outer ONL that were intensely p75+ co-immunolabeled (arrows in Fig. 3E–E2). We further found that most βgal+ cells in the ONLi were co-immunolabeled with Nestin (Fig. 3F–F2). The p75+ OECs were also co-immunolabeled with Nestin (data not shown). These observations suggest that the CZ cells are a distinct subgroup of OECs based on their distributions and antigenic characters at E18.5.
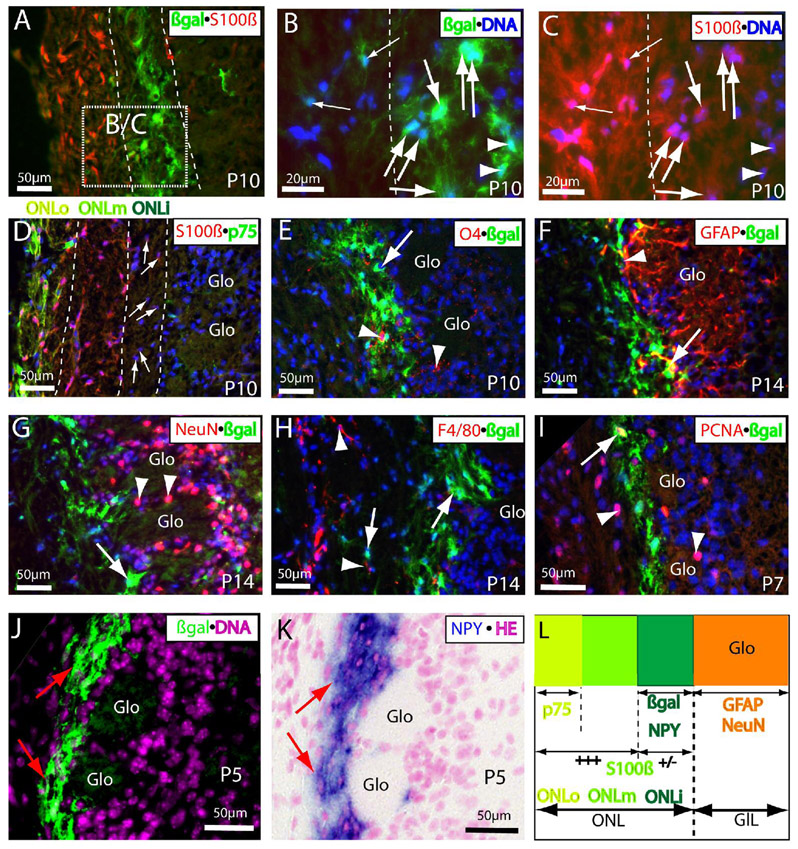
Figure 4.
The Wnt reporter TOPgal marked a distinct subpopulation of OECs in the postnatal ONL. (A–C) βgalintense+/S100βweak+ cells (indicated by large arrows in the same orientations) were detected in the inner ONL at P10. Panels B and C were magnified from the dashed square in panel A. Note that some intense βgal+ cells in the inner ONLi were extremely low immunoreactivities with S100β (arrowheads), and some double immunolabeled βgal+/S100β+ cells (small arrows) were found in the middle or outer ONL. Dashed lines in B and C indicate the border between inner and middle ONL. (D) Three subpopulations of OECs exist in the P10 ONL: S100β+/p75+ in the outer (ONLo), S100β+/p75− in the middle (ONLm), and the weakly S100β immunolabeled OECs (arrows) in the inner (ONLi). (E) O4+ Schwann lineage cells (red indicated by arrowheads) were scattered in the inner ONL where with intense βgal-immunolabeling, but no co-localization in the same cells was found at P10. (F) GFAP+ astrocyte lineage cells and processes (red) were stained intensely in the glomeruli at P14 with a clear border next to the βgal+ inner ONL, but no cells with double immunolabeling were found except that a few processes could be running together tightly in the periglomerular region (arrow). (G) No double-immunolabeling of βgal (green indicated by arrow) with neuronal marker NeuN (red indicated by arrowheads) was found in the periglomerular zone or entire ONL at P14. (H) F4/80+ microglia were found in the outer ONL, and no co-immunolabeling with βgal was found at P14. (I) A small portion of βgal+ cells in the ONL was found co-immunolabeled with PCNA+ (proliferating cell nuclear antigen) at P7 (arrow). Note that PCNA+ cells were found more frequently in adjacent layers, the middle ONL and glomerular layer (arrowheads). (J,K) The distribution pattern of βgal immunolabeling in the ONLi (green indicated by arrows in J) mirrors the NPY expression detected by in situ hybridization (blue indicated by arrows in K) in the same region on an immediately adjacent neighboring section at P5. (L) Schematic summary for the distribution, antigenic characters, and relationship of the Wnt reporter activated or inactivated OECs in the ONL around P5 to P14. The Wnt reporter activated cells in the early postnatal olfactory bulb are a specialized subgroup of OECs with intense βgal and weak S100β immunolabeling as well as NPY expression that are restricted in the ONLi immediately adjacent to the glomerular layer (GlL; In which the cells are CNS origins with characteristic immunolabeling for astrocyte (GFAP) and neuronal (NeuN) markers). Glo, glomerulus. A–J, the cell nuclei were counterstained by DAPI (blue in A–I, pink in J); K, counterstained by hematoxylin and eosin (HE).
S100β is a widely accepted OEC marker particularly in the ONLi (Au et al., 2002). At P10, we found that the βgal+ CZ cells were weakly co-immunolabeled with S100β in the inner ONL (Fig. 4A–C). At this age, we were able to recognize three distinct OEC subgroups in the ONL by combined immunoreactivities of P75 and S100β (Fig. 4D,L). In addition to p75+ OECs restricted in the ONLo, intense S100β+ OECs were distributed widely from the outer (where co-immunolabeled with p75) to middle (where p75 is negative) ONL (Fig. 4D), and very weakly S100β-immunolabled OECs were found in the inner ONL (arrows in Fig. 4D) where they were co-immunolabeled with Wnt reporter TOPgal (arrows in Fig. 4B,C). These data demonstrate that the TOPgal labeled CZ cells are a subgroup of OECs with down-regulated antigenic expression of p75 and S100β in the postnatal ONL. These OECs have extensive processes and sheet-like branches with intense βgal-immunolabeling that form a network in the inner ONL immediately adjacent to the glomerular layer (Fig. 4A,B,E–J). We further excluded the possibility that the majority of CZ cells were other neural lineage cells by co-immunolabeling of βgal with O4 for oligodendrocyte/Schwann lineage cells (Fig. 4E), GFAP for astrocytes (Fig. 4F), NeuN for neuronal lineage cells (Fig. 4G), F4/80 for microglia (Fig. 4H), and saw no co-immunolabeling except that a few CZ cell processes were tightly linked with GFAP+ astrocyte processes in the periglomerular region (arrow in Fig. 4F). In addition, we found that a small population of the TOPgal labeled CZ cells is proliferative by immunolabeling for the proliferating cell nuclear antigen (PCNA) at P7 (arrow in Fig. 4I).
Neuropeptide Y (NPY) is expressed in the ONLi OECs (Ubink and Hokfelt, 2000; Au et al., 2002). Although we could not find a good NPY antibody for co-immunolabeling with βgal in the ONLi, we detected intensive NPY mRNA expression that was overlapped with the βgal+ cells in the ONLi on the adjacent neighboring section (Fig. 4J,K). The distribution and antigenic properties of the βgal+ OECs (or OEC-like cells) and other OEC subgroups in the developing ONL were summarized for a better understanding (Fig. 4L).
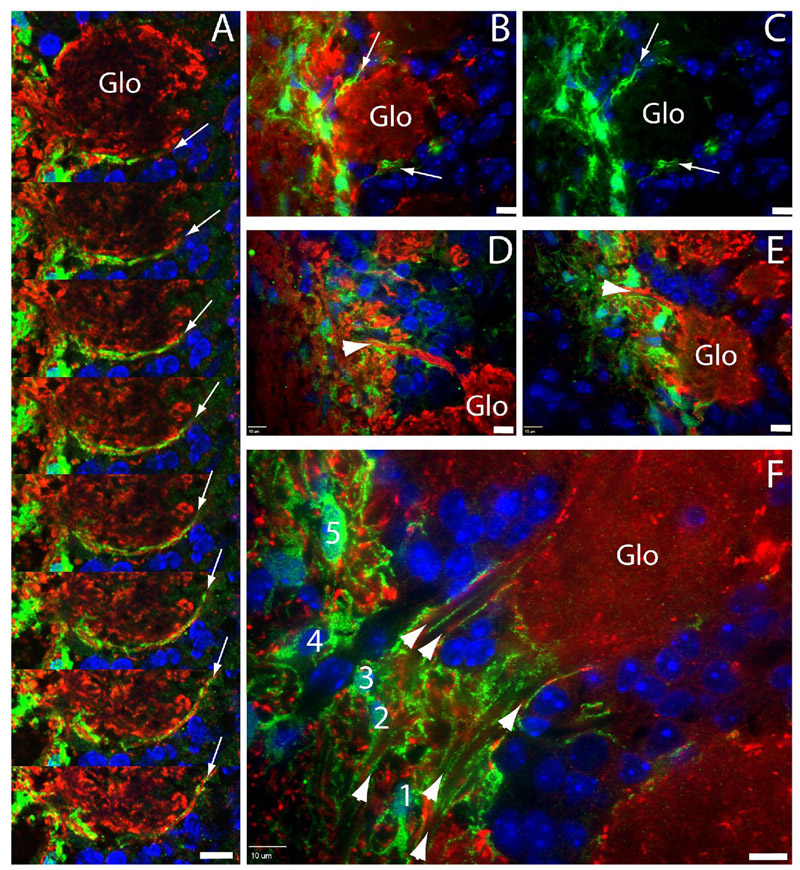
To determine whether CZ cells actually ensheath olfactory sensory axons between ONLi and the forming glomerular layer, we performed confocal microscopy at P3~14 (Fig. 5). At P3, we observed a long extension formed by TOPgal+ CZ cell processes surrounding the edge of a forming glomerulus (green in Fig. 5A indicated by arrows in serial adjacent confocal images). At P5, we found the capsule-like CZ cell processes surrounding a developing glomerulus (arrows in Figs. 5B,C) and a tunnel-like structure wrapping a big olfactory sensory axonal bundle that projected to a glomerulus (arrowheads in Figs. 5D,E). At P14, we found that the CZ cell processes formed multiple tunnel-like structures bridging multiple axonal bundles from ONLi into a single glomerulus (arrowheads in Fig. 5F). In the adjacent area on the same section, we found the net-like processes of a single CZ cell wrapping up multiple axonal bundles that were cut transversely (No. 5 cell in Fig. 5F). Above observations definitively demonstrated that CZ cells are the specialized OECs with intense Wnt/β-catenin reporter activation in the ONLi, and their networked tunnel-like processes ensheath multiple olfactory sensory axonal bundles connected with individual glomeruli.
Figure 5.
Confocal microscopy of TOPgal+ OECs/processes. (A) A long extension (arrows) of TOPgal+ processes along the edge of a glomerulus at P3 in serial adjacent scanning images. (B,C) Capsule-like TOPgal+ processes (arrows) surrounding a glomerulus at P5. (D,E) Two examples of tunnel-like structures (arrowheads) of TOPgal+ processes wrapping axonal bundles towards the glomerulus at P5. (F) Multiple tunnel-like structures (arrowheads) formed by TOPgal+ processes bridging a single glomerulus and ONL at P10. Five TOPgal+ OECs (no. 1~5) on this panel are lining up in the inner most ONL, and some of them (particularly the no. 5 cell) with net-like processes ensheath multiple axonal bundles in the transverse orientation. B–F, single optical sections. Scale bars, 10 µm. Nuclei were counterstained by DAPI (blue).
DISCUSSION
Wnt signaling components in the developing olfactory bulb
It has been reported that Wnt1, Wnt5a, and Wnt7a are expressed in the glomerular and mitral cell layers of postnatal olfactory bulbs of mice (P0~P20) (Shimogori et al., 2004). We have also confirmed the expression of Wnt1, Wnt3, and Wnt5a in the OB of P7 mice and observed their expression in the embryonic OB and OE (Supplement S1). Interestingly, while the Wnt signaling molecules are widely expressed in the OB with their expression domains occupying multiple OB layers, we have only observed the Wnt reporter TOPgal-activated cells in the ONL. Indeed, both Wnt receptor Fzd7 and transcription factor Lef1 have been exclusively expressed in the early postnatal ONL (Shimogori et al., 2004), which correlates well with the βgal immunolabeling in our study. On the other hand, Zaghetto et al. has demonstrated that more than 10 Wnts are detectable by RT-PCR in the embryonic OB and OE, and that the in situ expression of Wnt5b and Fzd7 are closely related to the BATgal labeled cells in the embryonic olfactory bulb (Zaghetto et al., 2007). Further studies will clarify which sets of Wnt signaling molecules are functionally activated in these OECs.
Wnt reporter activated OECs/CZ cells and their possible roles in the developing ONL
In this study we have observed TOPgal-activated cells in the OB primordium beginning around E12. Developing olfactory axons emerge from the OSNs in the OE and are accompanied by OECs, course the olfactory mucosa, and reach the prospective OB by E12 (Aoki et al., 1995). This timing coincides well with the activation of the Wnt signaling in the “connecting zone” (CZ), suggesting that the canonical Wnt signaling and its responsive CZ cells are the key for olfactory axon docking to a designated brain surface at the early developmental stage. The majority of olfactory axons form a dense band, the migratory mass, immediately below the OB around E14. We found that the Wnt reporter labeled CZ cells line up in front of the migratory mass around this age, suggesting that Wnt signaling may be also required to construct a permissive zone for the axons to enter the OB.
The glomeruli in the OB can be identified by P1, with the majority of the glomeruli developed by P14 and P21 (Valverde et al., 1992). We observed intense βgal staining indicative of Wnt signaling in presumably differentiated CZ cells in the ONL, particularly the inner ONL, with a peak around P3 to P10. From P14 onwards, the Wnt reporter labeled cells were decreased and restricted to a narrow zone immediately adjacent to the glomeruli. Olfactory axon sorting is thought to take place in the ONLi (Mombaerts, 2006). OSNs expressing the same odorant receptor are dispersed within a wide area of the olfactory epithelium. Therefore, thousands of axons from OSNs expressing the same odorant receptor are intermingled with many other axons, which do not express this receptor, in the axonal bundles of the olfactory nerve. The convergence of OSN axons into specific glomeruli requires the defasciculation of axonal bundles and their extensive sorting and rearrangement. Subsets of glial cells in the sorting zone and nerve layer of the moth, equivalent to OECs in vertebrates, are thought to be involved in the chemo attraction and repulsion of the OSN axons navigating the ONL (Tucker et al., 2004). Although the expression patterns of many chemoattractive and chemorepulsive molecules have been studied, the navigation and sorting of the OSN axons are still not understood (Nedelec et al., 2005). Recently, canonical Wnt/β-catenin signaling has been demonstrated to act as directional guidance cues for axons in anterior-posterior axis of the vertebrate spinal cord (Zou, 2004) and to control the migration of growth cones and neuronal cell bodies as well as neuronal polarity in anterior-posterior axis of worms (Hilliard and Bargmann, 2006). The Drosophila Wnt5 receptor Drl has been shown to function in glial cells of the olfactory system through its extracellular but not intracellular domain to antagonists Wnt5 to regulate midline crossing of sensory axon branches and the glomerular patterning in flies (Yao et al., 2007). Therefore, Wnts may also play an essential role in olfactory axon guidance in mammals, and the intense activation of canonical Wnt signaling in the ONLi may be directly responsible for OSN axon navigating and sorting into their designated glomeruli. These exciting possibilities are also supported by the fact that the canonical Wnt signaling is activated transiently and dominantly only in the front line of the olfactory axon projections during a critical developmental period but weakly in the normal mature olfactory system. Indeed, two recent studies using the TOPgal or a similar Wnt reporter BATgal transgenic mouse line have found unique Wnt-responsive cells in the embryonic OB that are similar to our CZ cells found in the TOPgal ONL, and have shown that canonical Wnt signaling in these cells may be essential for olfactory axonal connections in a tissue culture system (Zaghetto et al., 2007). This is not a surprise, as we have clearly demonstrated that the Wnt reporter TOPgal activated CZ cells are a specialized subgroup of OECs in the developing ONL. Embryonic CZ cells may contribute to guiding pioneering olfactory axons into the forebrain, and postnatally differentiated CZ cells may contribute to glomerulus formation and convergent sorting of olfactory sensory axons by their richly extended processes forming tunnel-like structures that ensheath and guide olfactory sensory axons into glomeruli. Further studies using genetic approaches will be required to address the role of canonical Wnt signaling in specialization or modulation of these OECs and their unique functions.
Phenotypic subpopulations of OECs in the ONL
Previous studies have demonstrated that several phenotypic subpopulations of the OECs reside in the ONL (Au et al., 2002). The OECs in the ONLi were also intensely immunolabeled by S100β in adult OB (Au et al., 2002). However, we noted that the intensity of S100β immunolabeling in early postnatal ONLi is much weaker than in adult ONLi or postnatal ONLo and ONLm. It has been reported that the OECs in the entire ONL were immunolabeled with the O4 antibody (Franceschini and Barnett, 1996). OEC antigenic phenotypes have been further described in vitro studies by OEC primary cultures derived from the postnatal OBs (Au and Roskams, 2003). However, we detected only a small number of O4+ cells in the ONL that were not co-immunolabeled with βgal+ OECs. Others have also reported that OECs in the ONLi can be immunolabeled with antibodies to GFAP and NPY. We have noted a few processes that may have been co-immunolabeled for βgal and GFAP, but we failed to find a good antibody for NPY to immunolabel OECs in the ONLi. However, we found that the mRNA expression of NPY in embryonic (Ubink and Hokfelt, 2000) and postnatal OB overlaps with the TOPgal activated ONLi cells. We provided partial evidence for the overlapped expression pattern of βgal immunostaining and NPY in situ expression on the adjacent neighboring sections.
Controversial identity of the Wnt reporter activated cells in the ONL
Two recent publications reported no co-immunolabeling of βgal of the Wnt reporter with any of their selected markers including Nestin and NPY in the ONL, and concluded mysteriously unknown identity of these cells (Zaghetto et al., 2007; Booker-Dwyer et al., 2008). Our results challenge these findings. For instance, we clearly demonstrate that βgal+ CZ cells were co-immunolabeled with Nestin at E14 and E18. Zaghetto et al. did not test this marker, but Booker-Dwyer et al. showed a negative result at P16. We suspect they would reach at the same conclusion as we have if they perform double immunolabeling for βgal and Nestin on embryonic OB sections. In addition, Booker-Dwyer et al. reported no co-immunolabeling of βgal with NPY at P16. However, the image from their Figure 2B might tell the positively co-immunolabeled cells with intense βgal but weak NPY immunoreactivities. Indeed, they may obtain a result comparable to ours if they perform NPY in situ hybridization. Furthermore, we have demonstrated the co-immunolabeling of βgal with S100β in the ONLi. Neither groups showed S100β in their papers. Finally, we definitively demonstrated that these βgal+ OEC processes ensheath the olfactory sensory axonal bundles in early postnatal ONL by high power confocal microscopy. We argue that the disagreements between these two groups and our conclusions are due to different selection of OEC markers, sample ages, and ways of detection. As a future plan, we will use a genetic fate mapping approach to demonstrate the lineage origin of these βgal+ CZ cells/OECs in the developing ONL.
In conclusion, using Wnt/β-catenin reporter TOPgal mice we have characterized a significantly specialized and dynamically developing OEC subgroup that may be directly involved in glomerulus formation and convergent sorting of olfactory sensory axons in the ONL.
EXPERIMENTAL PROCEDURES
Animals and Sampling
The Wnt reporter TOPgal transgenic mice were obtained from the Jackson Laboratories (USA) and were housed in the vivarium of the UC Davis Medical School (Sacramento, CA). Pregnant, timed-mated TOPgal mice were euthanized with CO2 gas prior to cesarean section. The embryos were immersion fixed in 4% paraformaldehyde (PFA). Embryos were collected at embryonic day (E) 11.5~18.5; the day of conception is designated E0. Postnatal mice were perfused with 4% PFA in PBS, and the brains with intact olfactory bulbs were collected at postnatal day (P) 3~P28. All research procedures using mice were approved by the UC Davis Animal Care and Use Committee and conformed to NIH guidelines. The embryos and brains were frozen sectioned with a Leica cryostat.
X-gal staining
The TOPgal reporter activity was detected in whole-embryos of E12.5 and brains of E18.5 stained for 18 hours with 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-gal) substrate solution (1 mg/ml X-gal in 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 5 mM MgCl2) for β-galactosidase activity. E18.5 brains were also embedded in 3% agarose and sectioned at 100 µm with a Vibratome (Leica VT1000S, Leica Microsystems, Germany) for X-gal staining.
Antibodies and Immunohistochemistry
The tissue sections were preincubated in a blocking solution of 10% lamb serum in PBS for 2 hours at room temperature to block nonspecific binding. They were then reacted with one or two of the following antibodies: (1) The rabbit antiserum to β-galactosidase (1:1000, Cappel, MP Biomedicals #55976, Solon, Ohio) was produced by hyperimmunizing rabbits with the enzyme β-galactosidase from E. coli, and the antibody was tested on Western blot to ensure specificity; the antibody detected a single band of β-galactosidase with no reaction to other E. coli proteins (manufacturer’s technical information). We actually tested the specificity of this antibody on wild type mouse sections and obtained no any immunolabeling (data not shown). (2) The mouse monoclonal (IgG2a) to β-galactosidase (1:10, Hybridoma Bank #JIE7, Iowa, IA) was prepared against E. coli β-galactosidase. This antibody was developed by Mason T.L. and Partaledis J.A. at the University of Massachusetts. The binding affinity and specificity seem a little less comparable with the Cappel’s rabbit anti-β-galactosidase, and we used it only for double immunolabeling with p75 (as it was a rabbit antibody) and obtained an identical staining pattern. (3) The rabbit antiserum to p75 nerve growth factor receptor (1:100, Chemicon #AB1554, USA) was prepared against extracellular fragment generated from sequences from the third exon of mouse p75 (amino acids 43–161). We obtained an identical immunolabeling pattern in the olfactory system with the published data (Ubink and Hokfelt, 2000; Balmer and LaMantia, 2005). (4) The mouse monoclonal (IgG2a) to S100β (1:100, Abcam #ab16959, Cambridge, MA) was prepared against full length native purified cow protein; the antibody stains a single band of ~10 kDa on Western blot (manufacturer’s technical information). We obtained an identical immunolabeling pattern in the olfactory system with the published data (Ubink and Hokfelt, 2000). (5) The mouse monoclonal (IgG1) to Neural Cell Adhesion Molecule (NCAM) (1:10, Hybridoma Bank #5B8, Iowa, IA) was prepared (by Jessel TM and Dodd J at the Columbia University) against NCAM from rat embryonic spinal cord membranes; the antibody cross-reacts to mouse and rat NCAM (manufacturer’s technical information). We obtained an identical immunolabeling pattern in the olfactory system with the published data (Aoki et al., 1995; LaMantia et al., 2000; Balmer and LaMantia, 2005). (6) The goat antiserum to olfactory marker protein (OMP) (1:1000, Wako Chemicals #544-10001, Richmond, VA) was prepared by multiple immunizations with rodent OMP; this antiserum is highly specific for mature olfactory neurons and their axons in tissue sections of many vertebrate species including rodents (manufacturer’s technical information). We obtained an identical immunolabeling pattern in the olfactory system with published data (Bailey et al., 1999; Treloar et al., 1999; Balmer and LaMantia, 2005). (7) The rabbit antiserum to glial fibrillary acidic protein (GFAP) (1:100, DakoCytomation #Z0334, Denmark) was prepared against GFAP isolated from cow spinal cord; the antibody shows one distinct precipitate (GFAP) with cow brain extract and cross-reacts with GFAP in mouse (manufacturer’s technical information). We obtained an identical immunolabeling pattern with published data (Bailey et al., 1999; Saghatelyan et al., 2004). (8) The mouse monoclonal (IgG1) to neuronal nuclei (NeuN) (1:1000, Chemicon #MAB377, USA) was prepared against purified cell nuclei from mouse brain; the antibody recognizes 2–3 bands (46–48 kDa) on a Western blot (manufacturer’s technical information). (9) The mouse monoclonal (IgG1) to Nestin (1:10, Hybridoma Bank, Rat-401, Iowa, IA; developed by Hockfield S at Yale University) was prepared against homogenized SD rat spinal cord; the antibody detects a single 200 kDa band on a Western blot (manufacturer’s technical information). We obtained an identical immunolabeling pattern in both PNS and CNS (Hockfield and McKay, 1985; Friedman et al., 1990). (10) The rat monoclonal (IgG2a) affinity purified antibody to mouse F4/80 antigen (1:10, eBioscience #14-4801, clone BM8, San Diego, CA,) was prepared against purified mouse F4/80 antigen; the antibody detects 140 kDa band on a Western blot (manufacturer’s technical information). (11) The mouse monoclonal (IgM) antibody to the oligodendrocyte marker O4 was prepared against homogenated of white matter of corpus callosum from bovine brain (1:1000, Chemicon #MAB345). (12) The mouse monoclonal antibody (mAb) to PCNA (proliferating cell nuclear antigen) was produced by immunizing mice with a recombinant protein A-PCNA fusion protein obtained from pC2T (1:1000, Cell Signaling #2586, Beverly, MA, USA). Dilutions of all primary antibodies were made in 1% lamb serum in PBS. After overnight incubation at 4°C with primary antibodies, sections were rinsed 3 times for 10 minutes each with PBS and incubated for 2 hours at room temperature with the secondary antibodies. All secondary antibodies were diluted in 1% lamb serum in PBS and used at dilution of 1:1000. The following secondary antibodies were used: (1) Alexa Fluor 594 goat anti-rabbit IgG, (2) Alexa Fluor 488 goat anti-mouse IgG, (3) Alexa Fluor 594 goat anti-mouse IgG, (4) Alexa Fluor 568 donkey anti-goat IgG, and (5) Alexa Fluor 488 donkey anti-rabbit IgG (all secondary antibodies from Molecular Probes, Inc., Eugene, OR). After three 10-minute washes with PBS, sections were counterstained with DAPI (1 mg/ml) and mounted with Vectashield (Vector Laboratories, CA).
Fluorescence and confocal microscopy
All tissue sections were assessed using a Zeiss Axiophot 2 fluorescent microscope equipped with an AxioCam digital camera (Carl Zeiss, Inc., North America). Confocal microscopy was carried out with a laser-scanning spectral confocal microscope system (Nikon Eclipse TE2000-E2).
In situ hybridization
Nonradioactive in situ hybridization histochemistry was performed using digoxigeninlabeled riboprobes as described previously (Zhou et al., 2004a; Zhou et al., 2004b; Zhou et al., 2006).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Erica Whitney for critical reading of this manuscript, and Lindy Hong, Erica McCauley, and Kai Wang for technical assistance.
Grant sponsor: Shriners Hospitals for Children; Grant number: SHC8610; Grant sponsor: Children’s Miracle Network; Grant number: CMNCZ05; Grant sponsor: American Cancer Society; Grant number: 95-125-07-S-CCZHOU1; Grant sponsor: National Multiple Sclerosis Society; Grant number: pp 1458; Grant sponsor: UC Davis Health System Research Award; Grant number: CZHS.
REFERENCES
- Aoki K, Osumi-Yamashita N, Ninomiya Y, Eto K. Differential expression of NCAM, vimentin and MAP1B during initial pathfinding of olfactory receptor neurons in the mouse embryo. Anat Embryol (Berl) 1995;192:211–220. doi: 10.1007/BF00184745. [DOI] [PubMed] [Google Scholar]
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Au WW, Treloar HB, Greer CA. Sublaminar organization of the mouse olfactory bulb nerve layer. J Comp Neurol. 2002;446:68–80. doi: 10.1002/cne.10182. [DOI] [PubMed] [Google Scholar]
- Bailey MS, Puche AC, Shipley MT. Development of the olfactory bulb: evidence for glia-neuron interactions in glomerular formation. J Comp Neurol. 1999;415:423–448. [PubMed] [Google Scholar]
- Balmer CW, LaMantia AS. Noses and neurons: induction, morphogenesis, and neuronal differentiation in the peripheral olfactory pathway. Dev Dyn. 2005;234:464–481. doi: 10.1002/dvdy.20582. [DOI] [PubMed] [Google Scholar]
- Booker-Dwyer T, Hirsh S, Zhao H. A unique cell population in the mouse olfactory bulb displays nuclear beta-catenin signaling during development and olfactory sensory neuron regeneration. Dev Neurobiol. 2008;68:859–869. doi: 10.1002/dneu.20606. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Franceschini IA, Barnett SC. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev Biol. 1996;173:327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- Franssen EH, de Bree FM, Verhaagen J. Olfactory ensheathing glia: their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Res Rev. 2007;56:236–258. doi: 10.1016/j.brainresrev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Friedman B, Zaremba S, Hockfield S. Monoclonal antibody rat 401 recognizes Schwann cells in mature and developing peripheral nerve. J Comp Neurol. 1990;295:43–51. doi: 10.1002/cne.902950105. [DOI] [PubMed] [Google Scholar]
- Gong Q, Bailey MS, Pixley SK, Ennis M, Liu W, Shipley MT. Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. J Comp Neurol. 1994;344:336–348. doi: 10.1002/cne.903440303. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell. 2006;10:379–390. doi: 10.1016/j.devcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Bhasin N, Rhodes K, Heemskerk J. Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron. 2000;28:411–425. doi: 10.1016/s0896-6273(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Zhou CJ. Canonical Wnt signaling during mouse olfactory development and regeneration. International Conference of Wnt Signaling in Development and Disease; 12–15 September; Berlin, Germany. 2007. p. 152. (Meeting Abstract). [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Nedelec S, Dubacq C, Trembleau A. Morphological and molecular features of the mammalian olfactory sensory neuron axons: What makes these axons so special? J Neurocytol. 2005;34:49–64. doi: 10.1007/s11068-005-5047-7. [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, de Chevigny A, Schachner M, Lledo PM. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 2004;7:347–356. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Purcell AL, Greer CA. Glomerular formation in the developing rat olfactory bulb. J Comp Neurol. 1999;413:289–304. [PubMed] [Google Scholar]
- Tucker ES, Oland LA, Tolbert LP. In vitro analyses of interactions between olfactory receptor growth cones and glial cells that mediate axon sorting and glomerulus formation. J Comp Neurol. 2004;472:478–495. doi: 10.1002/cne.20058. [DOI] [PubMed] [Google Scholar]
- Ubink R, Hokfelt T. Expression of neuropeptide Y in olfactory ensheathing cells during prenatal development. J Comp Neurol. 2000;423:13–25. doi: 10.1002/1096-9861(20000717)423:1<13::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Valverde F, Santacana M, Heredia M. Formation of an olfactory glomerulus: morphological aspects of development and organization. Neuroscience. 1992;49:255–275. doi: 10.1016/0306-4522(92)90094-i. [DOI] [PubMed] [Google Scholar]
- Wang B, Han J, Gao Y, Xiao Z, Chen B, Wang X, Zhao W, Dai J. The differentiation of rat adipose-derived stem cells into OEC-like cells on collagen scaffolds by co-culturing with OECs. Neurosci Lett. 2007;421:191–196. doi: 10.1016/j.neulet.2007.04.081. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wu Y, Yin C, Ozawa R, Aigaki T, Wouda RR, Noordermeer JN, Fradkin LG, Hing H. Antagonistic roles of Wnt5 and the Drl receptor in patterning the Drosophila antennal lobe. Nat Neurosci. 2007;10:1423–1432. doi: 10.1038/nn1993. [DOI] [PubMed] [Google Scholar]
- Zaghetto AA, Paina S, Mantero S, Platonova N, Peretto P, Bovetti S, Puche A, Piccolo S, Merlo GR. Activation of the Wnt-beta catenin pathway in a cell population on the surface of the forebrain is essential for the establishment of olfactory axon connections. J Neurosci. 2007;27:9757–9768. doi: 10.1523/JNEUROSCI.0763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Borello U, Rubenstein JL, Pleasure SJ. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience. 2006;142:1119–1131. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Pinson KI, Pleasure SJ. Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants. J Neurosci. 2004a;24:7632–7639. doi: 10.1523/JNEUROSCI.2123-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004b;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.