Summary
Alzheimer's disease (AD) is a neurodegenerative disorder in which vascular pathology plays an important role. Since the β-amyloid peptide (Aβ) is a critical factor in this disease, we examined its relationship to fibrin clot formation in AD. In vitro and in vivo experiments showed that fibrin clots formed in the presence of Aβ are structurally abnormal and resistant to degradation. Fibrin(ogen) was observed in blood vessels positive for amyloid in mouse and human AD samples, and intravital brain imaging of clot formation and dissolution revealed abnormal thrombosis and fibrinolysis in AD mice. Moreover, depletion of fibrinogen lessened cerebral amyloid angiopathy pathology and reduced cognitive impairment in AD mice. These experiments suggest that one important contribution of Aβ to AD is via its effects on fibrin clots, implicating fibrin(ogen) as a potential critical factor in this disease.
Introduction
One common pathology in Alzheimer's disease (AD) patients is the deposition of the β-amyloid peptide (Aβ) in the walls of capillaries, arteries, and arterioles, known as cerebral amyloid angiopathy (CAA) (Vinters, 1987). CAA is an important factor in the severity of AD pathology (Nicoll et al., 2004), as it provokes the degeneration of vessel wall components, affects cerebral blood flow (Thal et al., 2008b), and worsens cognitive decline (Esiri et al., 1999; Greenberg et al., 2004).
In addition to CAA being a contributing factor to the vascular pathology in AD, there is other evidence suggesting that AD has a strong vascular component. Epidemiology links vascular diseases such as stroke (Honig et al., 2003; Kalaria and Ballard, 2001), atherosclerosis (Hofman et al., 1997; Roher et al., 2003), atrial fibrillation (Mielke et al., 2007; Ott et al., 1997), and hypertension (Mielke et al., 2007; Skoog et al., 1996) with an increased risk for dementia and AD (Breteler et al., 1998; de la Torre, 2002). The combined presence of these vascular risk factors further increases the risk for AD (Luchsinger et al., 2005). In addition, cerebrovascular dysfunction takes place in AD (Farkas and Luiten, 2001; Iadecola, 2004; Niwa et al., 2002); there is decreased and altered cerebral blood flow in AD patients (Johnson et al., 2005; Staffen et al., 2009), and chronic brain hypoperfusion is associated with the development of AD (de la Torre, 2006). Compromised blood flow can lead to pathological synaptic changes typical of AD (Wen et al., 2004), and thus circulatory deficiencies could play an important role in its pathogenesis, with neuronal loss and memory deficits being secondary to vascular problems (de la Torre, 2004; Farkas and Luiten, 2001; Iadecola, 2004). A mechanism for how Aβ could alter thrombosis and hemostasis is not known, although several characteristics of this peptide suggest that it may be involved in blood flow (Hardy, 2007) and blood vessel function (Smith and Greenberg, 2009).
Fibrinogen is a large glycoprotein that circulates in the blood at micromolar concentrations and can be converted to insoluble fibrin, which is essential for coagulation (Weisel, 2005). Elevated fibrinogen levels are correlated with increased risk for AD (van Oijen et al., 2005; Xu et al., 2008), and the level of fibrinogen-γ-A chain precursor in cerebral spinal fluid has been proposed as a biomarker for AD (Lee et al., 2007). Fibrinogen is normally excluded from the brain, but it has been found to accumulate in the extravascular space in AD (Fiala et al., 2002; Lipinski and Sajdel-Sulkowska, 2006; Paul et al., 2007; Ryu and McLarnon, 2009). The inhibition of tissue plasminogen activator (tPA)/plasmin fibrinolytic activity (Ledesma et al., 2000; Melchor et al., 2003), the increased levels of pro-thrombotic molecules (Grammas et al., 2006; Johnson et al., 1997; Zipser et al., 2007), and the blood brain barrier (BBB) disorder found in AD brains (Bowman et al., 2007; Kalaria, 1999; Paul et al., 2007; Ujiie et al., 2003; Zipser et al., 2007) could contribute to the accumulation of fibrinogen or fibrin (designated fibrin(ogen)). Furthermore, previous studies demonstrated that reducing fibrinogen levels decreased BBB permeability in AD mouse models (Paul et al., 2007). Therefore, since fibrin(ogen) may play a critical role in AD (Cortes-Canteli and Strickland, 2009), we analyzed its participation in this disease.
Here, we report that Aβ induces the formation of abnormal, degradation-resistant blood clots. We also demonstrate that AD mice with decreased levels of fibrinogen in their blood present less CAA burden and perform better in memory tasks. We suggest that the association between Aβ and fibrinogen causes altered fibrin clotting, and this aberrant hemostasis could lead to compromised blood flow and increased inflammation, thereby contributing to cognitive decline in AD.
Results
Fibrin(ogen) clearance is delayed in the brain of TgCRND8 mice
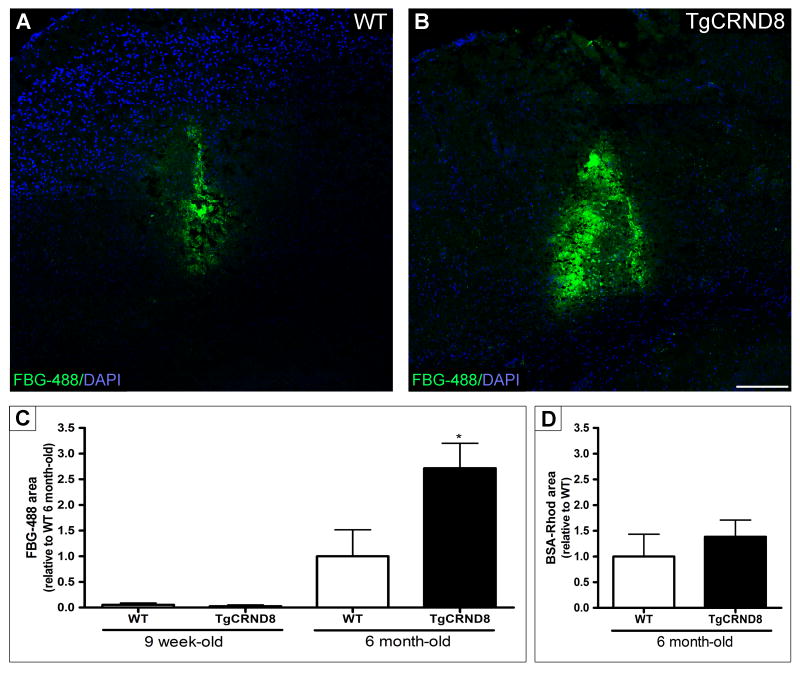
Fibrin(ogen) accumulates in the brains of AD patients (Fiala et al., 2002; Ryu and McLarnon, 2009) and TgCRND8 mice transgenic for human amyloid precursor protein (APP) (Paul et al., 2007). Since one explanation for fibrin(ogen) accumulation in mice is a higher level of fibrinogen in the blood, we measured fibrinogen levels in wild type (WT) and TgCRND8 mice and found no difference (Figure S1A). A second possibility was that blood is hypercoagulable in TgCRND8 mice, but the coagulation times for WT and TgCRND8 mice were the same (Figure S1B). A third option is increased persistence of fibrin(ogen) in AD mice. Therefore, we investigated whether these mice had increased stability of fibrinogen injected into the brain. Nine-week- and 6-month-old TgCRND8 mice and their littermates were injected with fluorescently-labelled human fibrinogen into the hippocampus and sacrificed the following day (Figure 1A-1C). Using two different ages of this transgenic line allowed us to determine whether any differences in fibrinogen clearance correlated with the degree of pathology. At 9 weeks-of-age, TgCRND8 mice have not yet fully developed AD pathology and present very low levels of Aβ, while at 6 months they have abundant amyloid plaques and CAA (Chishti et al., 2001). Both 9-week-old TgCRND8 mice and their WT littermates showed almost complete clearance of the injected fibrinogen compared to the older mice (Figure 1C). However, the injected fibrin(ogen) persisted longer in the 6-month-old mice, and its clearance was delayed almost three-fold in TgCRND8 mice compared to WT (Figure 1A-1C). In contrast to fibrin(ogen), the rate of clearance of bovine serum albumin (BSA) was similar in 6-month-old TgCRND8 and WT mice (Figure 1D), demonstrating that the impaired clearance and increased persistence of fibrin(ogen) in the AD mouse brain were specific for fibrinogen.
Figure 1. AD mouse brains do not clear fibrin(ogen) efficiently.
TgCRND8 and their non-transgenic littermates (WT) were stereotaxically injected with fluorescently-labelled fibrinogen (FBG-488). Representative confocal tile scan images of FBG-488 (green) at the site of the injection in 6-month-old WT (A) and TgCRND8 (B) mice one day after injection. Nuclei were counterstained with DAPI (blue). Scale bar, 200 μm. Quantification of FBG-488 area in 9-week and 6-month-old injected mice (C) showed the amyloid burden and degree of pathology affect fibrin(ogen) clearance. A control molecule, tetramethylrhodamine-BSA (BSA-Rhod), was also injected and quantified in another set of 6-month-old mice (D). Values represent the mean ± SEM from 3-4 mice/group. *p<0.05; TgCRND8 vs WT.
To examine if the fluorescent labelling of fibrinogen interfered with the clearance process, purified unlabelled human fibrinogen was injected into 6-month-old TgCRND8 and WT mice and detected by immunostaining. The unlabeled fibrin(ogen) also persisted longer in TgCRND8 than in WT mice (Figure S1C-S1E). Conversion of injected fibrinogen to fibrin was demonstrated by detection of D-dimer in brain homogenates (Figure S1F), indicating that the injected fibrinogen was polymerized and cross-linked by transglutaminase prior to proteolytic cleavage. Therefore, injected fibrinogen was converted to fibrin in the TgCRND8 hippocampus, and this fibrin persisted longer in TgCRND8 than in WT.
These results indicate that there is an impairment in fibrin(ogen) clearance that is dependent on the degree of pathology and amyloid burden.
Aβ42 affects clot formation and degradation in vitro
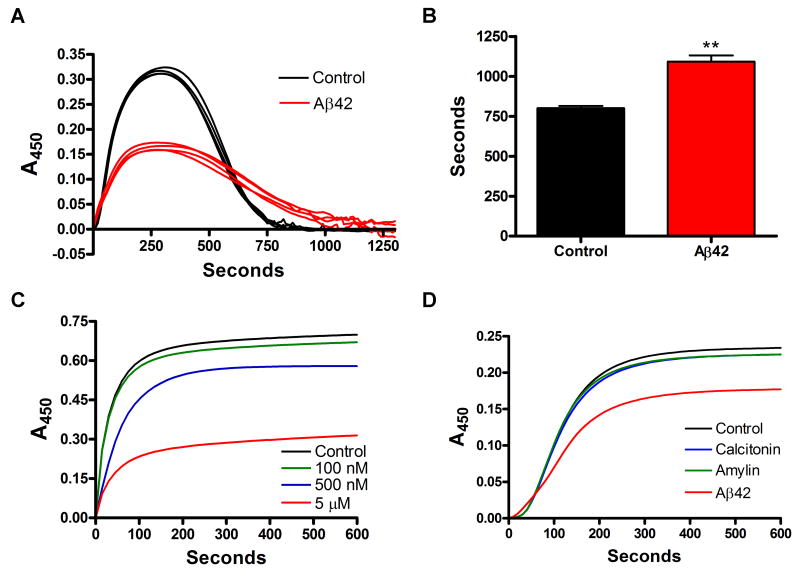
Elevated levels of fibrin(ogen) could be due to increased formation or decreased clearance. To examine these possibilities, we performed clot formation/degradation experiments with purified human fibrinogen, thrombin, tPA, and plasminogen in the presence or absence of Aβ42 (Merkle et al., 1996). As thrombin converts fibrinogen to fibrin, the newly formed fibers scatter light and the solution increases in turbidity. As fibrin fibers are formed, they become substrates for tPA-activated plasmin, and the equilibrium shifts from formation to dissolution, reducing the turbidity as the clot dissolves. Aβ42 was incubated overnight at room temperature to promote nucleation and growth of oligomers (Chauhan et al., 2001) and form a heterogeneous mixture of Aβ fibrils and soluble prefibrillar amyloid assemblies (Figure S2A). When the clot experiments were performed in the presence of Aβ42, the dissolution of the fibrin clot was delayed (Figure 2A and 2B). To examine if Aβ42 affects tPA and plasminogen interactions (Hoylaerts et al., 1982), the experiment was repeated using streptokinase, and the purified fibrin clot lysis was also delayed in the presence of Aβ (data not shown). It was possible that Aβ promoted transglutaminase activity, since increased cross-linking could strengthen the fibrin clot and reduce lysis speed. However, there was no difference in D-dimer in the presence or absence of Aβ as determined by the analysis of fibrin degradation products (data not shown). To test this effect in the presence of all components involved in hemostasis, we clotted recalcified human plasma in the presence of tPA and, similar to pure fibrin clots, fibrinolysis was delayed in the presence of Aβ42 (data not shown).
Figure 2. Aβ alters the development of fibrin clot turbidity and slows degradation.
(A) Combined fibrin formation/degradation assays. Clotting of purified fibrinogen was initiated with thrombin in the presence of tPA and plasminogen and either 10 μM of Aβ42 or vehicle (control) (n=4/group). (B) Total formation/degradation time for pure fibrin clots in (A) determined from initiation to complete dissolution; **p <0.01. (C) Dose response of the effect of Aβ42 on clot formation by adding human thrombin to fibrinogen at time 0. Control (vehicle); Aβ42 100 nM; Aβ42 500 nM; Aβ42 5 μM. (D) The addition of other β-pleated sheet amyloids (calcitonin and amylin; 5 μM) does not affect the turbidity of the clot like Aβ42 (5 μM). The lower general turbidity obtained in (D) is due to DMSO added in each curve, as explained in Experimental Procedures. Curves in C & D are representative of 4 experiments.
Previous results have shown that Aβ can be a scaffold for efficient conversion of plasminogen to plasmin by tPA (Kranenburg et al., 2002), although Aβ42 had no direct enhancement of tPA proteolytic activity (Figure S2B). Increased plasmin generation is inconsistent with delayed fibrinolysis. Therefore, Aβ must play a role in clot lysis that counteracts its effect on increasing plasmin generation. Additionally, there was no effect of Aβ42 on thrombin or plasmin activity (Figure S2C and S2D).
To separate possible consequences of Aβ on clot formation and dissolution, we tested the effect of Aβ42 on fibrin during the formation phase only. The presence of Aβ42 during thrombin-induced clot formation from pure fibrinogen produced a dose-dependent decrease in the normal rise in turbidity (Figure 2C), which could reflect incomplete clotting. However, in both the presence and absence of Aβ42, all fibrinogen was removed from solution and incorporated into the clot (data not shown), indicating complete clotting in both cases. Therefore, the lower turbidity suggested that the fibrin clot formed in the presence of Aβ42 was structurally abnormal. Structurally altered clots can be resistant to fibrinolysis (Collet et al., 2000), which may explain the persistence of fibrin in the presence of Aβ42. The experiment was repeated in the presence of other types of amyloid peptides known to be associated with other human diseases. Amylin (Clark et al., 1987) and calcitonin (Arvinte et al., 1993) did not affect the formation of the fibrin clot as the turbidity was not different from the control (Figure 2D). This result showed that the effect of Aβ42 on fibrin clot formation is specific for this peptide.
Clot structure is affected in vitro by Aβ42
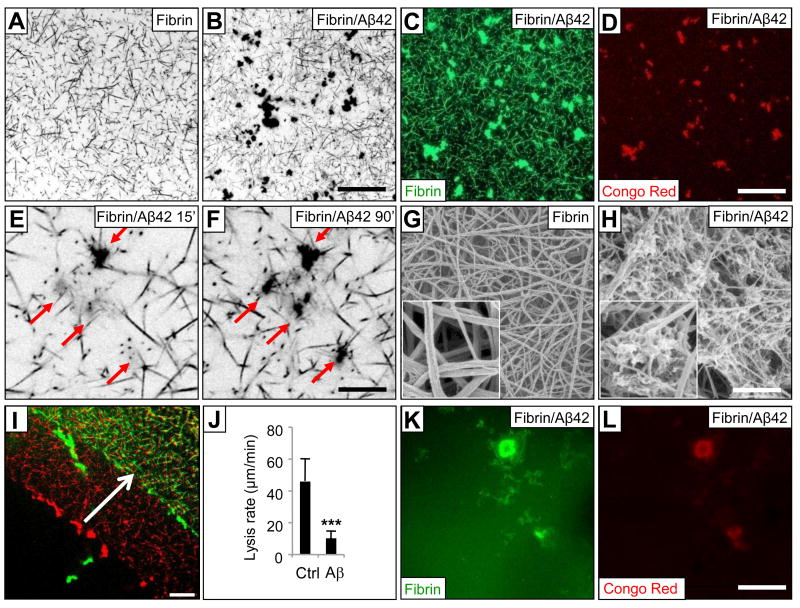
The decreased turbidity during clot formation in the presence of Aβ42 (Figure 2C) prompted examination of the clot structure. To visualize the fibrin network, we clotted human plasma in the presence of fluorescent fibrinogen. Confocal microscopy showed that clots were structurally altered in the presence of Aβ42, with fibrils arranged in a non-homogeneous network. Areas of normal clotting were interrupted by irregular regions of clustering in Aβ42-influenced fibrin (Figure 3A and 3B). Immunostaining of fibrin in the presence of Aβ42 without the use of labelled fibrinogen produced identical aggregates (data not shown). Fibrin aggregates stained positive with Congo Red (Figure 3C and 3D), suggesting they contained both fibrin and fibrillar forms of Aβ. Neither fibrin fluorescence nor Congo Red-positive Aβ aggregates were observed when thrombin was omitted from the reaction mix (data not shown). To further investigate the co-localization of fibrin with aggregated Aβ, we used biotinylated Aβ42 in the clotting reaction and then stained with fluorophore-conjugated streptavidin. Staining for Aβ was only observed in the aggregates, confirming that the peptide was confined to these areas (data not shown). Aβ incubated without fibrinogen did not produce any cluster formation (data not shown).
Figure 3. Aβ alters clot structure.
Confocal images (inverted gray levels) at low magnification of a control fibrin clot (A) or an Aβ42-influenced clot (B) using platelet-deficient human plasma and thrombin in the presence of fluorescent-conjugated fibrinogen. Scale bar in A & B, 36.5 μm. (C) Fluorescent fibrin forms a network with aggregates in the presence of Aβ42. (D) Congo Red fluorescence in the same field as in (C). Scale bar in C & D, 36.5 μm. Confocal image of fibrin clot showing aggregates acquired 15 (E) and 90 (F) min after thrombin addition. Scale bar in E & F, 8.75 μm. SEM image obtained from control fibrin clot formed from pure fibrinogen and thrombin (G) or in the presence of Aβ42 (H). Scale bar in G & H, 1.25 μm. Inset, 1 μm × 1 μm. (I) Red and green show the edge of a clot formed from plasma in the presence of Aβ42 before and 5 min after the addition of tPA, respectively. Scale bar, 36.5 μm. (J) The lysis front retreat rate (μm/min) was determined from 5 min time-lapse confocal acquisitions (n=4); ***p <0.001. Aggregates remaining after fibrinolysis contain fibrin(ogen) (K) and Aβ42 (L).
The size of these aggregates ranged from 2-40 μm in diameter, and the average size and number increased over time (Figure 3E and 3F). Adding equal amounts of Aβ42 to collagen did not introduce any irregularities into the network of collagen fibrils (Figure S3A and S3B), indicating specificity in the fibrin-Aβ interaction. Scanning electron microscopy (SEM) images of purified fibrin clots also showed aggregate formation in the presence of Aβ42 (Figure 3G and 3H). Since SEM images were obtained from clots that were fixed and post-processed, fibrils appeared tangled and up to 10 times thinner than in normal hydrated clots. Aβ can induce platelet aggregation (Kowalska and Badellino, 1994), but the aggregates observed in our experiments were not platelets since we used platelet-deficient plasma (Figure 3A-3F) as well as purified fibrinogen (Figure 3G, 3H and Figure S3C, S3D).
To observe the degradation process alone, we added tPA to a clot previously formed from plasma and observed fibrin(ogen) degradation using confocal time-lapse image acquisition (Collet et al., 2000) (Figure 3I). The lysis front retreated as the clot was degraded by plasmin, and the retreat rate was calculated. In the presence of Aβ42, lysis was delayed and retreat of the lysis front was slowed (Figure 3J). During clot lysis, aggregates often detached and dissolved slowly (Movie S1). Degradation-resistant aggregates that were Congo Red-positive remained after the surrounding fibrin had been degraded (Figure 3K and 3L). Aggregate formation and delayed lysis were not detected in control clots or those formed in the presence of scrambled Aβ42 peptide (data not shown). All these results indicate that Aβ42 has an effect on fibrin clot structure and on its formation and degradation in vitro.
Fibrin(ogen) is deposited in CAA-positive vessels
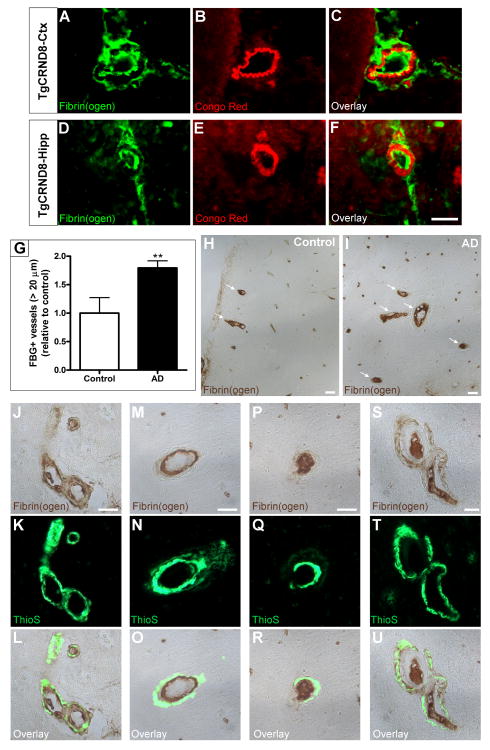
We next analyzed whether fibrin(ogen) and Aβ were interacting in brain blood vessels, since excess brain-derived Aβ is actively drained through the vasculature (Shibata et al., 2000). We examined fibrin(ogen) deposition in CAA-positive vessels in the cortex and hippocampus of 6-month-old TgCRND8 mice. Congo Red staining and fibrin(ogen) immunohistochemistry revealed amyloid-laden vessels containing fibrin(ogen) deposited intra- and extra-vascularly (Figure 4A-4F). To investigate the clinical significance of this finding, we analyzed fibrin(ogen) deposition in human post-mortem brain sections from control and AD patients. We found that AD patients presented significantly more large parenchymal vessels (>20 μm) affected with fibrin(ogen) deposits than non-demented controls (Figure 4G-4I). We used Thioflavin S staining to detect CAA and found vessels where fibrin(ogen) co-localized with Aβ deposits in the vessel wall and the tunica media (Figure 4J-4L), as well as CAA-positive vessels where fibrin(ogen) lined the interior (Figure 4M-4O) or occupied the entire lumen of the vessel (Figure 4P-4R). We also found CAA-positive vessels completely occluded by fibrin(ogen) deposits with additional fibrin(ogen) accumulation in the vessel wall (Figure 4S-4U).
Figure 4. Fibrin(ogen) deposition in the CAA-positive vessels of TgCRND8 mice and human AD patients.
Perfused TgCRND8 mice at 6 months-of-age have fibrin(ogen) deposits at sites of CAA, detected by Congo Red fluorescence, in the cortex (A-C) and hippocampus (D-F). Fibrin(ogen) immunohistochemistry was performed on human post-mortem sections from the frontal cortex of 5 control and 9 AD patients. The number of parenchymal vessels >20 μm that contained fibrin(ogen) was quantified (G), and patients diagnosed with AD presented nearly twice the number of fibrin(ogen)-positive vessels compared to control subjects; **p<0.01. Representative 20× images showing fibrin(ogen)-positive vessels (arrows) in controls (H) and AD patients (I). High power magnification showed that fibrin(ogen) in AD patients co-localized with CAA (green, via Thioflavin S staining; J-L), lined the interior of the vessel wall (M-O), and filled the lumen (P-R) in addition to being deposited in the tunica media of CAA-positive vessels (S-U). Scale bars, 20 μm.
Decreasing fibrin(ogen) levels lessens CAA pathology
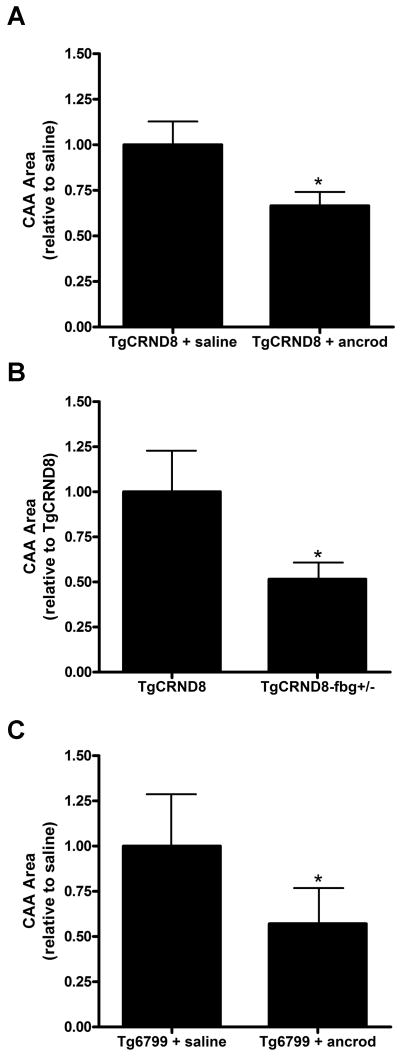
One proposed hypothesis for the formation of CAA is that Aβ clearance is impaired throughout the vasculature, leading to its accumulation in the vessel wall (Nicoll et al., 2004; Thal et al., 2008b). Since we detected fibrin(ogen) deposition in mouse and human CAA-positive vessels, we analyzed whether this deposition could be playing a direct role in CAA pathogenesis. We depleted fibrin(ogen) from the blood of 3-month-old TgCRND8 mice by administering ancrod, a serine protease purified from the venom of the Malayan pit viper Agkistrodon rhodostoma, which has been used to alleviate fibrin-mediated pathology (Akassoglou et al., 2004; Busso et al., 1998). We implanted mice with pumps to deliver ancrod or saline for 4 weeks, and we quantified the total area of CAA via Thioflavin S staining. We found that treatment with ancrod produced a significant decrease in vascular amyloid relative to saline treatment (Figure 5A). As previously described (Paul et al., 2007), fibrinogen levels in the plasma of ancrod-treated mice decreased by ∼50%, and ancrod treatment did not have a significant effect on the total amount of Aβ42 in the brains of the implanted mice (see below for more detail). These data suggest that the effect of ancrod on CAA levels is not due to a direct effect on Aβ production but to a decrease in fibrin(ogen) levels. To complement this pharmacological experiment, we crossed TgCRND8 mice with mice deficient in one copy of the fibrinogen Aα chain gene (TgCRND8-fbg+/- mice) and determined if CAA was also decreased. We found that genetic reduction of fibrinogen also lessens CAA pathology (Figure 5B). These results demonstrate that Aβ deposition as CAA is affected by the amount of fibrinogen in blood, since depleting fibrinogen levels, either pharmacologically or genetically, decreases CAA pathology.
Figure 5. Decrease in fibrinogen levels reduces CAA in AD mice.
Fibrinogen levels were pharmacologically (A) or genetically (B) reduced in TgCRND8 mice and Tg6799 (C) AD mice. The distribution of amyloidosis in the brain was analyzed after treatment. The decrease in fibrinogen levels provoked a significant decrease in the total amount of CAA in both transgenic AD mouse lines. CAA was determined by Thioflavin S staining in 2-4 sections from 4-7 mice/group. Bars represent mean ± SEM. *p<0.05.
Given the importance that reducing CAA could have on the progression of AD, we examined whether decreasing fibrinogen levels would have the same effect in a different AD transgenic mouse model. Tg6799 mice carry five different familial AD mutations, present amyloid deposition as early as 2 months-of-age, and exhibit neuronal death, memory deficits, and decreased levels of synaptic markers (Oakley et al., 2006). Since no studies had been performed on their CAA pathology, we analyzed the brains of 2.5-, 4.5-, and 7.5-month-old Tg6799 mice. Because Thioflavin S staining showed CAA-positive vessels starting at 4.5 months (data not shown), we implanted pumps filled with ancrod or saline in Tg6799 mice of that age. We analyzed the total amount of CAA after 4 weeks of treatment and, as with the TgCRND8 mice, found a significant reduction of total CAA area in ancrod-treated Tg6799 mice compared to the saline-treated mice (Figure 5C). Given the involvement of CAA in AD pathogenesis (Nicoll et al., 2004; Thal et al., 2008b), the finding that decreasing fibrin(ogen) levels lessens CAA pathology in two different transgenic AD mouse lines implicates fibrin(ogen) as a contributing factor to CAA pathogenesis and therefore to AD.
TgCRND8 mice present an increased tendency of thrombosis
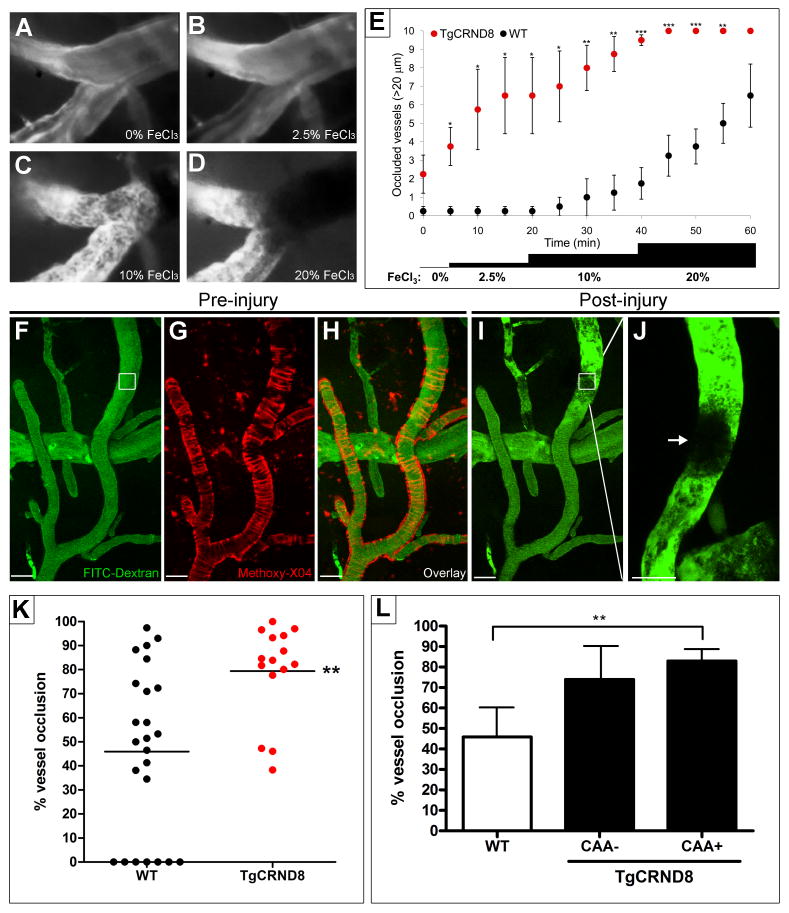
Since fibrin clot formation was affected by Aβ42 in vitro (Figures 2 and 3) and fibrin(ogen) was found deposited in CAA-positive vessels (Figure 4), we investigated clot formation in vivo. We used an intravital microscopy system for inducing and monitoring thrombosis in real time in 6-month-old TgCRND8 mice and WT littermates. A cranial window was created, and blood flow was monitored via injected fluorescent-conjugated dextran. We used two different yet complementary procedures to induce clot formation in different sets of mice. The first method for inducing thrombosis was the topical application of FeCl3, which provokes oxidative injury, endothelial damage, and subsequent formation of occluding thrombi rich in platelets. It has been used broadly to induce arterial thrombosis in rodents (Westrick et al., 2007). The addition of increasing concentrations of FeCl3 to the brain surface caused clot formation as revealed by the appearance of an enlarging shadow superimposed over normal blood flow (Figure 6A-6D & Movie S2). We counted the number of visibly occluded large vessels (>20 μm) over time for both TgCRND8 and WT mice and found that TgCRND8 mice often thrombosed spontaneously, even before the addition of FeCl3, and lower doses were needed to occlude vessels of similar size (Figure 6E).
Figure 6. Altered thrombosis in TgCRND8 mice.
A cranial window was opened in 6-month-old TgCRND8 and WT littermates, and in vivo imaging and clot formation were carried out. Representative images of time series of clot formation before (A) and after the addition of 2.5% (B), 10% (C) and 20% (D) FeCl3. Fluorescent-labeled blood flow (gray) is interrupted with dark zones representing clot formation (black). (E) The number of occluded vessels after FeCl3 treatment at incremental doses was recorded in TgCRND8 mice and WT littermates over time. TgCRND8 mice show occlusion earlier and with lower doses of FeCl3. Clot formation was also induced using the near-infrared laser of a two-photon laser scanning fluorescence microscope. Representative images of maximum projections (Z-stack) from the same area taken before (F-H) and after (I, J) the laser-induced injury in a TgCRND8 mouse. Methoxy-X04 was injected to label Aβ deposits and identify CAA-positive vessels (G, pseudocolored red). The white boxes show the region of interest where the laser was focused to form a clot (arrow in J). Scale bars, 40 μm. (K) Percent vessel occlusion after injury was quantified. TgCRND8 vessels showed a significant increase in the degree of occlusion compared to WT vessels. Note that under the same conditions as TgCRND8 mice, some of the vessels in WT mice did not occlude. (L) The same vessels quantified in (K) were classified and plotted as CAA-positive and CAA-negative based on the Methoxy-X04 staining. *p<0.05, **p<0.01, ***p<0.001; TgCRND8 vs WT mice.
As a second method to induce clot formation, we used a laser-induced thrombosis procedure with a two-photon laser scanning microscope (Nishimura et al., 2006). In contrast with the generalized damage that occurs after the addition of FeCl3, this method allows for the induction of clot formation in individual vessels (Movie S3 and Figure 6F-6J), and the content of amyloid in these specific vessels can be visualized using the fluorescent dye Methoxy-X04 (Klunk et al., 2002) (Figure 6G and 6H). The quantification of the area occupied by the clot in each of the targeted vessels showed that WT mice presented an average of 46 ± 7% vessel occlusion while TgCRND8 had 79 ± 5% blockage (Figure 6K). The laser conditions used in these experiments were chosen after testing pulses of different wavelengths, laser intensities, and number of repetitions adequate to induce reproducible damage in the TgCRND8 mice. However, the same conditions were not enough to induce any occlusion in some of the vessels of the non-transgenic WT littermates (Figure 6K). This finding, combined with the increased number of occluded vessels after FeCl3 application (Figure 6E) and the higher degree of occlusion after laser-induced thrombosis in TgCRND8 mice compared to control (Figure 6K), indicate that the AD vasculature is more prone to clot.
To determine whether the amyloid content in the vessel wall was affecting clot propensity in the vessels of the TgCRND8 mice, we used Methoxy-X04 staining (Klunk et al., 2002) in vivo to visualize cerebrovascular amyloid as ring-like structures surrounding the vessels (Figure 6G and 6H). Re-plotting the injured AD vessels from Figure 6K as positive or negative for CAA showed that the laser-targeted CAA-positive vessels occluded almost completely (83 ± 5%), while the degree of occlusion in CAA-negative vessels (74 ± 10%) did not reach a statistically significant difference (p=0.075) compared to WT mice (Figure 6L). This result indicates that the deposition of Aβ in the vessels as CAA promotes a higher degree of occlusion after a thrombotic event. However, it should be noted that vessels lacking CAA also tend to present an elevated degree of occlusion when compared to WT vessels. Therefore, it is possible that factors other than CAA could affect clotting in the AD brain.
Laser-induced thrombosis experiments were also carried out in 9-week-old pre-depositing mice. We found no difference between the average percentage of vessel occlusion in 9-week-old TgCRND8 (60 ± 23%) and WT mice (57 ± 15%), suggesting that the increased tendency to clot in TgCRND8 mice is dependent on the degree of AD progression.
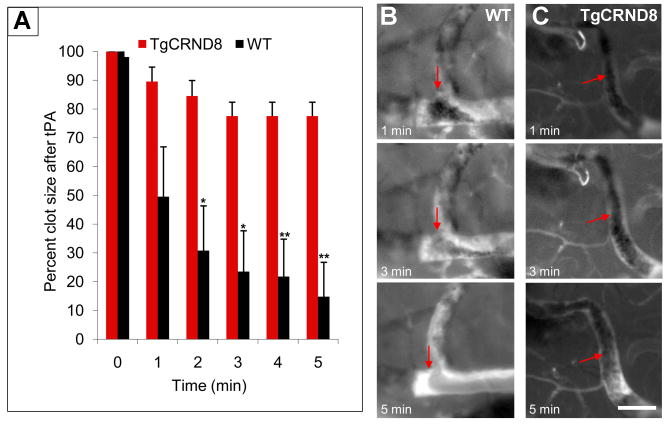
Clot degradation is inhibited in TgCRND8 mice
Because the clot degradation process was affected in vitro (Figures 2 and 3), we examined this process in vivo. The rate of fibrinolysis was determined by topically administering tPA to clots formed with 10% FeCl3 as described in Figure 6. Clot lysis was observed as the shrinking and eventual disappearance of the shadow over the fluorescently labelled blood flow (Movie S4). The size of this shadow was measured at 1-min time intervals. Clots formed in TgCRND8 mice maintained similar size for more than 5 min, while WT clots lysed quickly, often dissolving within 1 min (Figure 7A-7C). The lack of clot lysis in TgCRND8 animals is not due to the increased levels of plasminogen activator inhibitor in the AD brain (Melchor et al., 2003), since the activity of the added tPA was comparable between TgCRND8 mice and WT littermates (data not shown). These experiments show that clot degradation is inhibited in the brain of a living AD mouse.
Figure 7. Delayed fibrinolysis in TgCRND8 mice.
(A) Clots formed after topical application of 10% FeCl3 in TgCRND8 and WT mice were treated with tPA, and clot size was followed and determined over time. Representative images of the time series of clot dissolution after tPA treatment of a pre-formed clot in WT (B) and TgCRND8 mice (C). Blood flow is interrupted with dark zones representing clot formation (arrows). Scale bar, 50 μm. *p<0.05, **p<0.01, ***p<0.001; TgCRND8 vs WT mice.
Fibrin(ogen) levels affect the memory and cognitive performance of mice
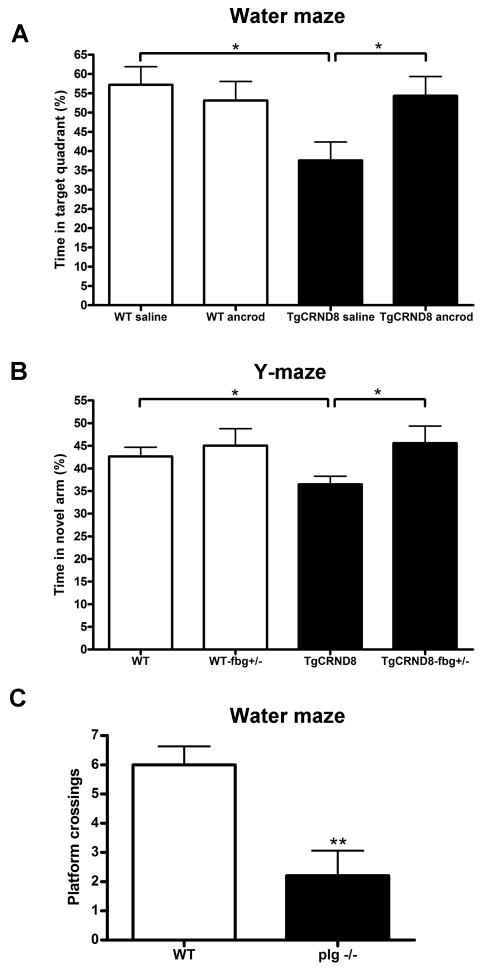
To analyze the physiological significance of fibrin(ogen) deposition in brain vessels (Figure 4) and the increased clotting propensity in the TgCRND8 mice (Figure 6), we examined the cognitive performance of TgCRND8 mice with reduced fibrinogen levels. WT and TgCRND8 mice were infused for 4 weeks with ancrod to decrease fibrinogen levels and tested in the Morris water maze, a learning and spatial memory task. Mice infused with saline were used as controls. A decrease in escape latency times during the hidden platform test showed that the four groups of mice learned to locate the platform (data not shown). TgCRND8 mice had profound memory impairment; they spent significantly less time in the target quadrant than WT littermates (Figures 8A and S4A). However, TgCRND8 mice infused with ancrod showed better performance, as they spent significantly more time in the target quadrant than TgCRND8 infused with saline (Figures 8A and S4A), demonstrating a higher retention of spatial memory than TgCRND8 controls.
Figure 8. The modulation of fibrinogen levels in mice affects cognitive performance.
(A) TgCRND8 mice were implanted with pumps delivering ancrod or saline for 4 weeks and tested in the Morris water maze. TgCRND8 mice with reduced fibrinogen levels spent significantly more time in the target quadrant than TgCRND8 controls, indicating that the pharmacological reduction of fibrinogen improves spatial memory retention. (B) TgCRND8 mice heterozygous for fibrinogen (TgCRND8-fbg+/-) and their littermates were tested in the Y-maze. TgCRND8-fbg+/- mice spent significantly more time exploring the novel arm than TgCRND8 control mice, indicating that the genetic reduction in fibrinogen improves working memory. (C) In contrast, mice deficient in plasminogen (plg-/-), which are predisposed to severe thrombosis and present fibrinogen deposits in different organs, showed memory impairment compared to WT mice in the Morris water maze. Bars represent the mean ± SEM. *p<0.05, **p<0.01.
Fibrin(ogen) levels in plasma were measured at three different times (before, during, and end of ancrod treatment), with an average 46% reduction in fibrin(ogen) (*p=0.0112 compared to saline-infused). Mice that did not reach a significant decrease in fibrin(ogen) levels at any point were excluded from the analysis. To determine if ancrod affects Aβ levels, we measured the total Aβ42 content in brain homogenates of the TgCRND8 mice implanted with saline or ancrod pumps and found that there was no significant difference in Aβ42 content (25.9 ± 5.2 μg/g of tissue TgCRND8-saline vs 21.6 ± 8.2 μg/g of tissue TgCRND8-ancrod; p=0.668). These results indicate that the effect of ancrod treatment in reducing memory impairment and cognitive deficits of TgCRND8 mice is due to a reduction in fibrin(ogen) levels and not the total amyloid burden.
To complement the results obtained with the pharmacological reduction in fibrin(ogen) levels, we performed water maze experiments with TgCRND8-fbg+/- mice and their littermates. TgCRND8-fbg+/- mice presented a tendency to spend more time in the target quadrant than TgCRND8, although the results were not significant (time in target quadrant: 38.8% ± 4.2 TgCRND8 vs 46.6% ± 3.05 TgCRND8-fbg+/-; p=0.169). The improvement in spatial memory of the TgCRND8-fbg+/- mice was not as robust as TgCRND8 mice treated with ancrod (Figure 8A). This difference can be explained by the level of fibrinogen in the animals, as treatment with ancrod provokes ∼50% decrease in fibrin(ogen) levels, while genetic depletion of one copy of the fibrinogen gene only produces ∼35% reduction compared to the mice bearing two copies of the gene (Suh et al., 1995). Also, we found that the background of fbg+/- mice interferes with the water maze analysis, since the difference between WT and TgCRND8 mice on this background was reduced. Therefore, we tested the mice in the novel arm version of the Y-maze, another memory task. We found that the TgCRND8-fbg+/- mice spent significantly more time in the novel arm compared to TgCRND8 mice (Figure 8B). These data complement our previous results showing that reducing fibrinogen levels pharmacologically improves memory of TgCRND8 mice.
If fibrin(ogen) deposition and the increased tendency to clot in the AD brain are factors contributing to poor cognitive function in AD, then mice with an increased rate of thrombosis will have memory deficits. Plasminogen-deficient mice (plg-/-) present fibrin(ogen) deposits in different tissues and are predisposed to thrombosis (Bugge et al., 1995; Ploplis et al., 1995). We therefore tested 3-month-old plg-/- mice in the water maze. Although these mice present progressive wasting during their lives (Bugge et al., 1995; Bugge et al., 1996), their weight gain is normal for the first 3 months (Bugge et al., 1996) and their swim speed was similar to that of WT mice during the probe trial (20.1 ± 0.3 cm/s WT vs 18.4 ±1.0 cm/s plg-/-; p=0.12). In addition, decrease of escape latency times during the hidden platform test showed that plg-/- mice were not impaired in the training session (data not shown), indicating no motor or visual problems at this age. However, during the probe trial, plg-/- mice showed profound memory impairment compared to WT mice (Figures 8C and S4B). The removal of fibrinogen from plg-/- mice corrects many of their abnormalities, indicating that the inability to degrade fibrin accounts for the major pathology (Bugge et al., 1996; Degen et al., 2001). Therefore, the memory deficits observed in plg-/- mice are likely due to impaired fibrinolysis, demonstrating a role for fibrin(ogen) in this process.
Discussion
The work presented here is especially relevant to two critical features of AD: 1) the slow, progressive loss of nervous system function and cognitive decline, and 2) a role for Aβ. We will discuss our results with these features in mind.
Altered hemostasis in Alzheimer's disease
One ubiquitous cause of cell deterioration in animals is a compromised vascular system, since all cells require oxygen and nutrients for survival. This requirement is especially acute in the brain, which is highly vascularized, metabolically active, and consumes 20% of oxygen used by mammals (Squire et al., 2003). Our work shows that the hemostatic system is abnormal in AD mouse models. Clots are formed more rapidly (Figure 6) and are more difficult to lyse (Figures 3 and 7) in the brains of AD mice. This situation would lead to accumulation of blood clots in the vessels that could compromise cerebral blood flow and hence neuronal function and survival.
The AD brain is known to have altered blood flow (Farkas and Luiten, 2001), impaired vascular function (Smith and Greenberg, 2009), and to constitute a pro-thrombotic environment. There are increased levels of prothrombin (Zipser et al., 2007), thrombin (Grammas et al., 2006), and transglutaminase (Johnson et al., 1997) and decreased fibrinolytic activity (Ledesma et al., 2000; Melchor et al., 2003). In fact, subcortical infarcts play a role in the cognitive impairment present in AD (Schneider et al., 2007); there are white matter lesions in AD brains resembling those observed after ischemia (Brun and Englund, 1986), and ischemia has been shown to induce tauopathy (Wen et al., 2004). Furthermore, cerebral emboli have been detected in patients with AD and associated with cognitive decline (Purandare and Burns, 2009).
Circulatory deficits are notoriously insidious and can cause progressive pathology over many years. Therefore, even a slightly increased propensity to clot, coupled with the clots being harder to clear, could lead to a slow decline in neuronal function.
A possible new role for Aβ in Alzheimer's disease
Aβ has been strongly implicated in AD due to analysis of early onset cases, which are genetic in nature. All mutations known to increase risk of AD influence the amount of Aβ that can be generated, and this peptide may also play a critical role in sporadic forms of the disease (Dawbarn and Allen, 2007). Since Aβ is a component of plaques that are often associated with AD, the deposited material has been hypothesized to be a pathogenic factor (Thal et al., 2008a). However, the lack of correlation between plaque burden and cognitive impairment (Arriagada et al., 1992) suggests that soluble Aβ (Shankar et al., 2008) or its deposition in vessels (Greenberg et al., 2004) may be more important.
Our work identifies a new role for Aβ that ties it to potential circulatory deficiencies. The association of Aβ with fibrinogen leads to abnormal clots that are more resistant to lysis, which could explain the observations that plaques accumulate fibrin(ogen) (Paul et al., 2007; Ryu and McLarnon, 2009). The persistence of fibrin could have multiple deleterious consequences: first, it could lead to occlusion of blood vessels and impede blood flow, with ensuing neuronal death; second, fibrin is a pro-inflammatory protein and could promote chronic inflammation in the brain (Adams et al., 2004; Paul et al., 2007), resulting in neuronal dysfunction. In fact, fibrinogen is closely associated with the inflammatory response in AD (Ryu and McLarnon, 2009), and intriguingly, microgliosis is reduced when fibrinogen is depleted (Paul et al., 2007; Ryu and McLarnon, 2009).
A possible role for fibrinogen in Alzheimer's disease
There is evidence for a role of fibrinogen in AD pathology. Fibrin(ogen) deposition is observed in vessels (Figure 4) but also in the parenchyma of AD mice (Paul et al., 2007) and AD patients (Fiala et al., 2002; Ryu and McLarnon, 2009). However, other molecules do not cross the BBB in AD (DeMattos et al., 2001; Sagare et al., 2007), suggesting that fibrinogen leakage into the brain parenchyma might be specific for this molecule. In fact, high levels of fibrinogen have been associated with an increased risk for AD (van Oijen et al., 2005) and with an elevated risk for dementia conversion in patients with mild cognitive impairment (Xu et al., 2008). A causative role for fibrinogen is indicated by our experiments that show that decreasing fibrin(ogen) levels lessens CAA pathology and cognitive decline in transgenic AD mouse models (Figures 5 and 8).
CAA has been defined as a risk factor for ischemic cerebral infarction (Cadavid et al., 2000) and cognitive dysfunction (Greenberg et al., 2004). It is considered responsible for many of the abnormalities present in the vessel wall, alteration of blood flow, and impairment of vascular function in AD (Smith and Greenberg, 2009). Increasing evidence suggests that impaired Aβ clearance through the vasculature is one of the main mechanisms of Aβ accumulation in the cerebral vessels (Bell and Zlokovic, 2009). Our results provide a connection between the vascular deposition of amyloid, first described over 100 years ago, and the hemostatic system.
Additional evidence supporting an important role for fibrin(ogen) and clot formation in AD are the positive results obtained with anticoagulant therapy for this disease (Ratner et al., 1972; Walsh et al., 1978). Although those studies were small, the anticoagulant treatment either stopped the deterioration or provoked an improvement of the disease (Walsh, 1996). More recently, a study carried out in patients with atrial fibrillation, a known vascular risk factor for AD (Mielke et al., 2007; Ott et al., 1997), showed that subjects on anticoagulant treatment present less cognitive impairment than those who were not treated (Barber et al., 2004). Also, an association between AD and hypercoagulability disorders such as hyperhomocysteinemia (Seshadri et al., 2002) and Factor V Leiden (Bots et al., 1998) has been postulated. Combined, these studies support a critical role for fibrinogen and clot formation in the development of AD.
In general terms, the deleterious effects of persistent fibrin have been dramatically shown in mice. Plasmin generated from plasminogen is the primary fibrinolytic enzyme, and plg-/- mice have multi-organ failure and die around 6 months-of-age (Bugge et al., 1995). This wasting is due to accumulation of fibrin, since genetic reduction of fibrinogen rescues plg-/- mice (Bugge et al., 1996). This result demonstrates that fibrin accumulation has multitudinous effects that compromise normal physiology. Specific examples of this concept include arthritis (Busso et al., 1998), in which fibrin persistence worsens joint pathology, and peripheral nerve regeneration (Akassoglou et al., 2002), where fibrin inhibits the process.
Hypothetical model of Alzheimer's disease pathogenesis
Putting our results together with previous findings, we propose a model that could explain many aspects of AD pathology: Aβ accumulates in the brain where it could associate with fibrinogen in the parenchyma, around blood vessels, or inside vessels. The prothrombotic environment would lead to clot formation, and the presence of Aβ would make these clots abnormal and lysis-resistant. Fibrin persistence could obstruct blood flow or provoke inflammation that would damage neurons and lead to their dysfunction. It is also possible that fibrinogen in blood vessels of AD patients entraps Aβ, impeding its clearance through the vasculature and potentiating the formation of CAA, decreasing blood circulation, and eventually leading to cognitive decline.
A more complete understanding of amyloid-dependent vasculopathology will help the identification of candidate treatments for this disease (Greenberg et al., 2004). One aspect of our proposed mechanism is that it could be specifically targeted therapeutically. A drug that could interfere with the effects of Aβ on fibrin clot formation would in theory normalize any blood clots formed in the brain and increase their lysis, hence improving cerebral blood flow and neuronal function and survival. Such a drug would have little effect on general clotting in other locations where Aβ levels are low. Therefore, this approach, perhaps in conjunction with other strategies, could have significant therapeutic benefit for the treatment of AD.
Experimental Procedures
Animals
TgCRND8 transgenic mice (Chishti et al., 2001) express a double mutant form of APP695 (K670N/M671L + V717F) driven by the human prion protein promoter, are on a mixed background (C57XC3H/C57), develop Aβ-associated pathology, and exhibit defects in memory as early as 3 months-of-age (provided by A. Chishti and D. Westaway, University of Toronto, Canada). Tg6799 mice (Jackson Laboratory) are double transgenic for APP/Presenilin 1 and co-express five early onset familial AD mutations and rapidly accumulate Aβ42 by 2 months-of-age (Oakley et al., 2006). Mice heterozygous for the fibrinogen Aα chain (fbg+/-) (Suh et al., 1995) were crossed with TgCRND8 and used for CAA determination and behavioral experiments. Non-transgenic (WT) littermates were used as controls in all experiments. Plasminogen-deficient mice (plg-/-) (Bugge et al., 1995; Ploplis et al., 1995), backcrossed onto a C57/BL6 background, and age-matched WT C57/BL6 mice (Charles River) were used in this study. Genotypes were double-checked by taking a tail tissue sample the day of sacrifice. Both genders were used in all experiments, and the proportion of females to males was kept constant when comparing groups. Mice were maintained in The Rockefeller University's Comparative Biosciences Center and treated in accordance with IACUC-approved protocols.
Stereotactic injections
A 500 nl solution containing 500 ng of fluorescently labelled human fibrinogen (Alexa Fluor 488-fibrinogen, Molecular Probes) was stereotactically injected into the right hemisphere (posterior -2.0 mm, lateral 1.8 mm, depth 1.2 mm (Franklin and Paxinos, 2008)) of 6-month and 9-week-old (pre-depositing) TgCRND8 and WT mice. As control, the same amount of tetramethylrhodamine-BSA (Molecular Probes) was injected in another set of 6-month-old mice. Mice were perfused with a saline/heparin solution after 24 hr, and 20 μm-thick coronal brain sections from the injected hemisphere were fixed in ethanol, washed, and mounted with Vectashield containing DAPI (Vector Labs, Burlingame, CA). Ten sections/mouse (n=3-4 mice/group) were examined for the fluorescent compounds using an inverted LSM 510 laser scanning confocal microscope (Zeiss) equipped with a motorized stage. To quantify the area where fluorescence was present, a tile-scan (4×4) was obtained using a Plan-NeoFluor 25×/0.80 objective and thresholded using Image J (NIH). The results are normalized to the area obtained in WT mice.
Clot formation/degradation and turbidity experiments
Fibrin clots were formed by mixing purified human fibrinogen (10 μM; Calbiochem) or citrated normal human plasma (New York Blood Center, centrifuged at 10,000 × g for 15 min to obtain platelet-deficient plasma), with human thrombin (1 U/ml; Sigma) in the presence of Aβ42 peptide (100 nM - 10 μM; Anaspec) or vehicle (PBS). CaCl2 was adjusted to 5 mM. Absorbance was measured for 10 min at 450 nm. Clots were also formed in the presence of the amyloid peptides calcitonin and amylin (5 μM; Anaspec). Human calcitonin was reconstituted in dH2O, and human amylin (1-37) in DMSO. To allow comparison among all three amyloid peptides, clots formed in the presence of Aβ42, amylin, or calcitonin were controlled for the final concentration of vehicle present. For formation/degradation curves, clots were formed under the same conditions with tPA (14 nM; Genentech) and purified human plasminogen (100 nM; Sigma). Amyloid solutions were shaken for 24-48 hr at room temperature before use.
Confocal image analysis and lysis front retreat rates
Clots were formed as described above on a glass-bottomed dish with Alexa Fluor 488-fibrinogen (50 μg/ml; Molecular Probes) in the presence or absence of 500 nM Aβ42. Some clots contained Congo Red (10 mM; Sigma). For lysis experiments, tPA was injected into the center of the pre-formed clot (20 min after mixing), and time-lapse image stacks were recorded at 15 sec intervals for 5 min as the lysis front retreated from the center. Initial and final images were overlayed, and the distance between lysis fronts was divided by the 5 min collection period (n=3-4 random lysis fronts in 4 separate experiments). Images were obtained with an inverted Axiovert 200 microscope, acquired with LSM 510 v. 3.2 confocal software (Carl Zeiss, Mannheim, Germany), and analyzed with MetaMorph software (Universal Imaging).
Electron microscopy
Fibrin clots were formed from purified fibrinogen on glass coverslips. After 20 min, clots were washed with sodium cacodylate buffer, fixed with 2% glutaraldehyde, dehydrated, critical point dried, and sputter-coated with gold palladium. Images were obtained using a LEO 1550 scanning electron microscope.
Fibrin(ogen) immunohistochemistry
Six-month-old TgCRND8 mice were saline/heparin-perfused, and 20 μm coronal brain sections were prepared, ethanol-fixed, and stained with FITC-conjugated fibrin(ogen) antibody (Dako). Tissue was counterstained for 30 min at room temperature with 0.2 % Congo Red (Sigma) in 70% isopropanol to detect CAA. Immunofluorescence images were acquired using an inverted Zeiss Axiovert 200 microscope.
Human brain immunohistochemistry
Human post-mortem brain tissue was provided by the Harvard Brain Tissue Resource Center and the Washington University AD Research Center. Paraffin sections (7 μm) from the frontal cortex of 5 control (52-90 years-of-age) and 9 AD (77-91 years-of-age) cases were deparaffinized, treated with proteinase K (Dako), and immersed in methanol/H2O2 to inactivate endogenous peroxidases. Immunohistochemistry was carried out using a rabbit polyclonal anti-fibrin(ogen) antibody (Dako) and the Tyramide Signal Amplification™ system (Perkin Elmer) according to manufacturer's instructions. Sections were developed using diaminobenzidine, co-stained for 30 min with 1% Thioflavin S (Sigma) in 70% ethanol, dehydrated, and mounted. Twenty to 25 fields per section were acquired using an inverted Zeiss Axiovert 200 microscope, and vessels > 20 μm that contained fibrin(ogen) were quantified.
Ancrod treatment and CAA determination
Tg6799, TgCRND8, and their WT littermates were treated with ancrod as described (Paul et al., 2007). To calculate total amyloidosis, 3-month-old TgCRND8 and 4.5-month-old Tg6799 mice implanted with pumps delivering saline or ancrod, and 7-11 month-old TgCRND8-fbg+/- mice were used. Mice were saline/heparin-perfused, and 20 μm coronal brain cryostat sections were fixed with ethanol and stained for 30 min with 0.5% Thioflavin S (Sigma) in 70% ethanol. Pictures of all the areas with CAA were acquired, thresholded using Image J, and the total CAA area per section was calculated. The average of 2-4 different sections from 4-7 mice per group was determined and plotted relative to the corresponding control group.
Intravital imaging of thrombosis
To observe blood circulation and to induce thrombosis, a cranial window was prepared over the parietal cortex of 6-month and 9-week-old TgCRND8 mice and WT littermates following the surgical procedure described (Mostany and Portera-Cailliau, 2008) with some modifications (see Supplemental Experimental Procedures). Two different methods were used to induce clot formation:
Topical application of FeCl3
Increasing concentrations of FeCl3 (2.5%-20%) were added directly to the brain surface with an interval of ∼15 min, and thrombosis was recorded using realtime video acquisition with a video camera fitted to an upright Zeiss Axiovert 200 epi-fluorescence microscope (Metavue software). The whole procedure lasted approximately 60 min per mouse (n=2-4 mice/group and up to 10 vessels > 20 μm were thrombosed per animal). After treatment with 10% FeCl3, some mice were topically administered with recombinant tPA (140 nM; Genentech) to activate fibrinolysis. After imaging, mice were perfused and brains were processed for in situ zymography as described (Melchor et al., 2003). Clot formation and dissolution were observed using time-stamped image stacks. Clot size was traced by hand using Metamorph software to calculate the area of the dark zone representing the clot,.
Laser-induced thrombosis was provoked and imaged using a Fluoview 1000MPE two-photon laser scanning fluorescence microscope (Olympus) equipped with a SpectraPhysics MaiTai DeepSee laser (with a tuneable range of 690-1040 nm) and a 25×/1.05 NA objective. To identify CAA-positive vessels, Methoxy-X04 (Klunk et al., 2002) (Neuroptix Corporation) was administered via tail vein injection 1 hr prior to imaging (3 mg/kg dissolved as described (Garcia-Alloza et al., 2009)). The procedure to induce localized laser thrombosis was adjusted from the one described (Nishimura et al., 2006). See Supplemental Experimental Procedures for details. We targeted 3-4 vessels/mouse with a diameter greater than 20 μm (n=5-6 at 6 months-of-age; n=2 at 9 weeks-of-age TgCRND8 and WT). A 2×-zoom Z-stack of the area where the clot was formed was acquired for vessel occlusion quantification using Image J (diameter of the clot vs diameter of the vessel in all the planes).
Behavioral analysis
Morris water maze
TgCRND8 and WT mice infused with saline or ancrod (n=7, 3 to 5-month-old), TgCRND8-fbg+/- mice and their littermates (n=5-10, ∼4-month-old), and plg-/- and C57/BL6 mice (n=5-6, 3-month-old) were tested in the Morris water maze to evaluate cognitive function (Chishti et al., 2001) with some minor modifications (see Supplemental Experimental Procedures for details). Spatial memory was measured by quantifying the percent time spent in the target quadrant and the number of platform crossings during the probe trial test. The experiment was recorded and analyzed using Ethovision video tracking system (Noldus).
Y-maze
TgCRND8-fbg+/- mice and their littermates (n=8-16, 7-11-month-old) were tested in the Y-maze. Experiments were performed in a sound-attenuated room under soft illumination, and visual clues were placed on walls of the testing room. Each trial consisted of two 5 min periods, separated by a 2 min inter-trial interval in which the mouse was placed in its home cage. During the first 5 min period, one of the three arms was blocked by an opaque plexiglass insert; this arm acts as the novel arm in the subsequent 5 min testing period. The entire experiment was recorded, and the first 2 min of the testing period were analyzed using Ethovision (Noldus). Time spent in the novel arm was averaged and compared between groups.
Aβ42 and fibrinogen ELISA
To calculate the total Aβ42 brain content, tissue was weighed and homogenized in 5 M guanidine HCl/50 mM Tris-HCl pH 8 buffer, agitated for 4 hr at room temperature, and centrifuged for 20 min at 16,000 × g to extract total Aβ (Chishti et al., 2001). Aβ42 concentration was determined by the BetaMark™ x-42 ELISA kit according to manufacturer's instructions (Covance). Tail prick blood samples were taken before, during, and after pump implantation in mice treated with ancrod, and the decrease in fibrinogen levels in plasma was measured by ELISA (GenWay Biotech).
Statistical analysis
All numerical values presented in graphs are mean ± SEM. Statistical significance was determined using two-tailed t-test analysis comparing control to experimental groups.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS50537 and GM66699); Institute for the Study of Aging (261104); Alzheimer's Drug Discovery Foundation (281203); Alzheimer's Association (IIRG-04-1356); Woodbourne Foundation; Blanchette Hooker Rockefeller Fund; Rudin Family; and NIH Medical Scientist Training Program (GM07739). M.C.-C. was supported by The Rockefeller University Women & Science Fellowship Program and by the American Health Assistance Foundation. We thank the Alzheimer's Disease Research Center at Washington University (P50 AG05681 grant) and the Harvard Brain Tissue Resource Center (PHS grant R24-MH 068855) for providing human samples, and the support of the two-photon Olympus microscope by the Empire State Stem Cell Fund through NYSDOH Contract #C023046. We thank Anita Ramnarain for assistance in mouse genotyping, Alexander Bounoutas, Maxime Kinet, Barry Coller, Marketa Jirouskova, and members of the Strickland laboratory for helpful discussions, and Alison J. North and Kunihiro Uryu at The Rockefeller University's Bio-Imaging and Electron Microscopy Resource Centers. We greatly appreciate assistance from Sarah Bhagat and the Bruce McEwen laboratory with behavioral experiments. We thank M. Azhar Chishti and David Westaway for the TgCRND8 mice, Genentech for recombinant tPA, the New York Blood Center for human plasma, and David E. Levy, Neurobiological Technologies, for ancrod. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RA, Passino M, Sachs BD, Nuriel T, Akassoglou K. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4:163–176. doi: 10.1124/mi.4.3.6. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Yu WM, Akpinar P, Strickland S. Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron. 2002;33:861–875. doi: 10.1016/s0896-6273(02)00617-7. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Arvinte T, Cudd A, Drake AF. The structure and mechanism of formation of human calcitonin fibrils. J Biol Chem. 1993;268:6415–6422. [PubMed] [Google Scholar]
- Barber M, Tait RC, Scott J, Rumley A, Lowe GD, Stott DJ. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. J Thromb Haemost. 2004;2:1873–1878. doi: 10.1111/j.1538-7836.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots ML, van Kooten F, Breteler MM, Slagboom PE, Hofman A, Haverkate F, Meijer P, Koudstaal PJ, Grobbee DE, Kluft C. Response to activated protein C in subjects with and without dementia. The Dutch Vascular Factors in Dementia Study. Haemostasis. 1998;28:209–215. doi: 10.1159/000022432. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, Bots ML, Ott A, Hofman A. Risk factors for vascular disease and dementia. Haemostasis. 1998;28:167–173. doi: 10.1159/000022428. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- Busso N, Peclat V, Van Ness K, Kolodziesczyk E, Degen J, Bugge T, So A. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998;102:41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Mena H, Koeller K, Frommelt RA. Cerebral beta amyloid angiopathy is a risk factor for cerebral ischemic infarction. A case control study in human brain biopsies. J Neuropathol Exp Neurol. 2000;59:768–773. doi: 10.1093/jnen/59.9.768. [DOI] [PubMed] [Google Scholar]
- Clark A, Cooper GJ, Lewis CE, Morris JF, Willis AC, Reid KB, Turner RC. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;2:231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Strickland S. Fibrinogen, a possible key player in Alzheimer's disease. J Thromb Haemost. 2009;7 1:146–150. doi: 10.1111/j.1538-7836.2009.03376.x. [DOI] [PubMed] [Google Scholar]
- Chauhan VP, Chauhan A, Wegiel J. Fibrillar amyloid beta-protein forms a membrane-like hydrophobic domain. Neuroreport. 2001;12:587–590. doi: 10.1097/00001756-200103050-00031. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Dawbarn D, Allen SJ. Neurobiology of Alzheimer's disease: (molecular and cellular neurobiology) 3rd. Oxford: New York, Oxford University Press; 2007. [Google Scholar]
- de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer's disease? Neurol Res. 2006;28:637–644. doi: 10.1179/016164106X130362. [DOI] [PubMed] [Google Scholar]
- Degen JL, Drew AF, Palumbo JS, Kombrinck KW, Bezerra JA, Danton MJ, Holmback K, Suh TT. Genetic manipulation of fibrinogen and fibrinolysis in mice. Ann N Y Acad Sci. 2001;936:276–290. doi: 10.1111/j.1749-6632.2001.tb03515.x. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd. Amsterdam: London, Elsevier; 2008. [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Hyman BT, Bacskai BJ. Antioxidants have a rapid and long-lasting effect on neuritic abnormalities in APP:PS1 mice. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.11.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer's disease microvessels: implications for disease pathogenesis. J Alzheimers Dis. 2006;9:51–58. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- Hardy J. Does Abeta 42 have a function related to blood homeostasis? Neurochem Res. 2007;32:833–835. doi: 10.1007/s11064-006-9221-9. [DOI] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Cox TM, Lockhart JP, Zinnerman MD, Miller ML, Powers RE. Transglutaminase activity is increased in Alzheimer's disease brain. Brain Res. 1997;751:323–329. doi: 10.1016/s0006-8993(96)01431-x. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN. The blood-brain barrier and cerebrovascular pathology in Alzheimer's disease. Ann N Y Acad Sci. 1999;893:113–125. doi: 10.1111/j.1749-6632.1999.tb07821.x. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Ballard C. Stroke and cognition. Curr Atheroscler Rep. 2001;3:334–339. doi: 10.1007/s11883-001-0028-5. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- Kowalska MA, Badellino K. beta-Amyloid protein induces platelet aggregation and supports platelet adhesion. Biochem Biophys Res Commun. 1994;205:1829–1835. doi: 10.1006/bbrc.1994.2883. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Bouma B, Kroon-Batenburg LM, Reijerkerk A, Wu YP, Voest EE, Gebbink MF. Tissue-type plasminogen activator is a multiligand cross-beta structure receptor. Curr Biol. 2002;12:1833–1839. doi: 10.1016/s0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG. Brain plasmin enhances APP alpha-cleavage and Abeta degradation and is reduced in Alzheimer's disease brains. EMBO Rep. 2000;1:530–535. doi: 10.1093/embo-reports/kvd107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Namkoong H, Kim HK, Kim S, Hwang DW, Na HR, Ha SA, Kim JR, Kim JW. Fibrinogen gamma-A chain precursor in CSF: a candidate biomarker for Alzheimer's disease. BMC Neurol. 2007;7:14. doi: 10.1186/1471-2377-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski B, Sajdel-Sulkowska EM. New insight into Alzheimer disease: demonstration of fibrin(ogen)-serum albumin insoluble deposits in brain tissue. Alzheimer Dis Assoc Disord. 2006;20:323–326. doi: 10.1097/01.wad.0000213844.21001.a2. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor JP, Pawlak R, Strickland S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle DL, Cheng CH, Castellino FJ, Chibber BA. Modulation of fibrin assembly and polymerization by the beta-amyloid of Alzheimer's disease. Blood Coagul Fibrinolysis. 1996;7:650–658. doi: 10.1097/00001721-199609000-00011. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- Mostany R, Portera-Cailliau C. A craniotomy surgery procedure for chronic brain imaging. J Vis Exp. 2008;15 doi: 10.3791/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Yamada M, Frackowiak J, Mazur-Kolecka B, Weller RO. Cerebral amyloid angiopathy plays a direct role in the pathogenesis of Alzheimer's disease. Pro-CAA position statement. Neurobiol Aging. 2004;25:589–597. doi: 10.1016/j.neurobiolaging.2004.02.003. discussion 603-584. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–2593. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- Purandare N, Burns A. Cerebral emboli in the genesis of dementia. J Neurol Sci. 2009;283:17–20. doi: 10.1016/j.jns.2009.02.306. [DOI] [PubMed] [Google Scholar]
- Ratner J, Rosenberg G, Kral VA, Engelsmann F. Anticoagulant therapy for senile dementia. J Am Geriatr Soc. 1972;20:556–559. doi: 10.1111/j.1532-5415.1972.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke. 2009;40:2601–2606. doi: 10.1161/STROKEAHA.108.536839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ. Fundamental neuroscience. 2nd. Amsterdam: Boston, Academic Press; 2003. [Google Scholar]
- Staffen W, Bergmann J, Schonauer U, Zauner H, Kronbichler M, Golaszewski S, Ladurner G. Cerebral perfusion (HMPAO-SPECT) in patients with depression with cognitive impairment versus those with mild cognitive impairment and dementia of Alzheimer's type: a semiquantitative and automated evaluation. Eur J Nucl Med Mol Imaging. 2009;36:801–810. doi: 10.1007/s00259-008-1028-2. [DOI] [PubMed] [Google Scholar]
- Suh TT, Holmback K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, Braak H. Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease. J Cell Mol Med. 2008a;12:1848–1862. doi: 10.1111/j.1582-4934.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 2008b;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Walsh AC. Anticoagulant therapy for Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1996;8:361–362. doi: 10.1176/jnp.8.3.361. [DOI] [PubMed] [Google Scholar]
- Walsh AC, Walsh BH, Melaney C. Senile-presenile dementia: follow-up data on an effective psychotherapy-anticoagulant regimen. J Am Geriatr Soc. 1978;26:467–470. doi: 10.1111/j.1532-5415.1978.tb03326.x. [DOI] [PubMed] [Google Scholar]
- Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004;1022:30–38. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series) Arterioscler Thromb Vasc Biol. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang H, Zhang S, Fan X, Liu X. Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int J Clin Pract. 2008;62:1070–1075. doi: 10.1111/j.1742-1241.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.