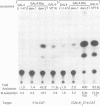
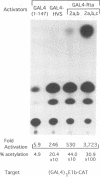
Abstract
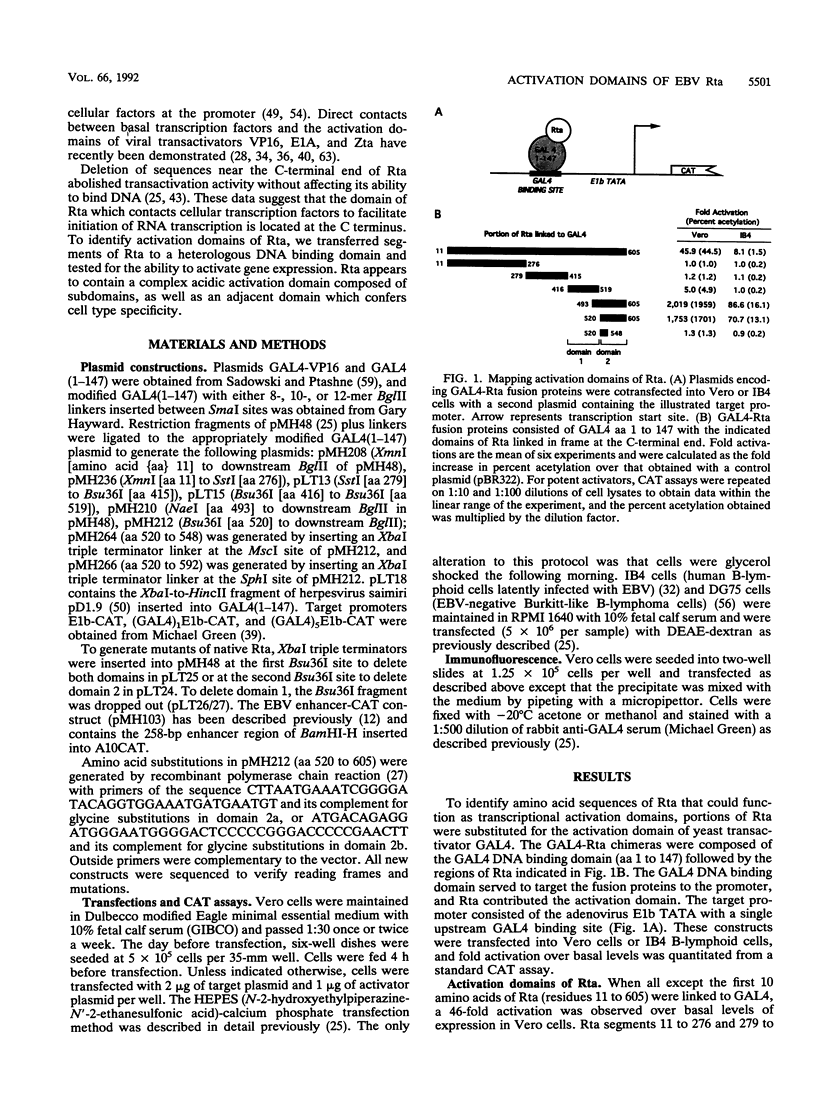
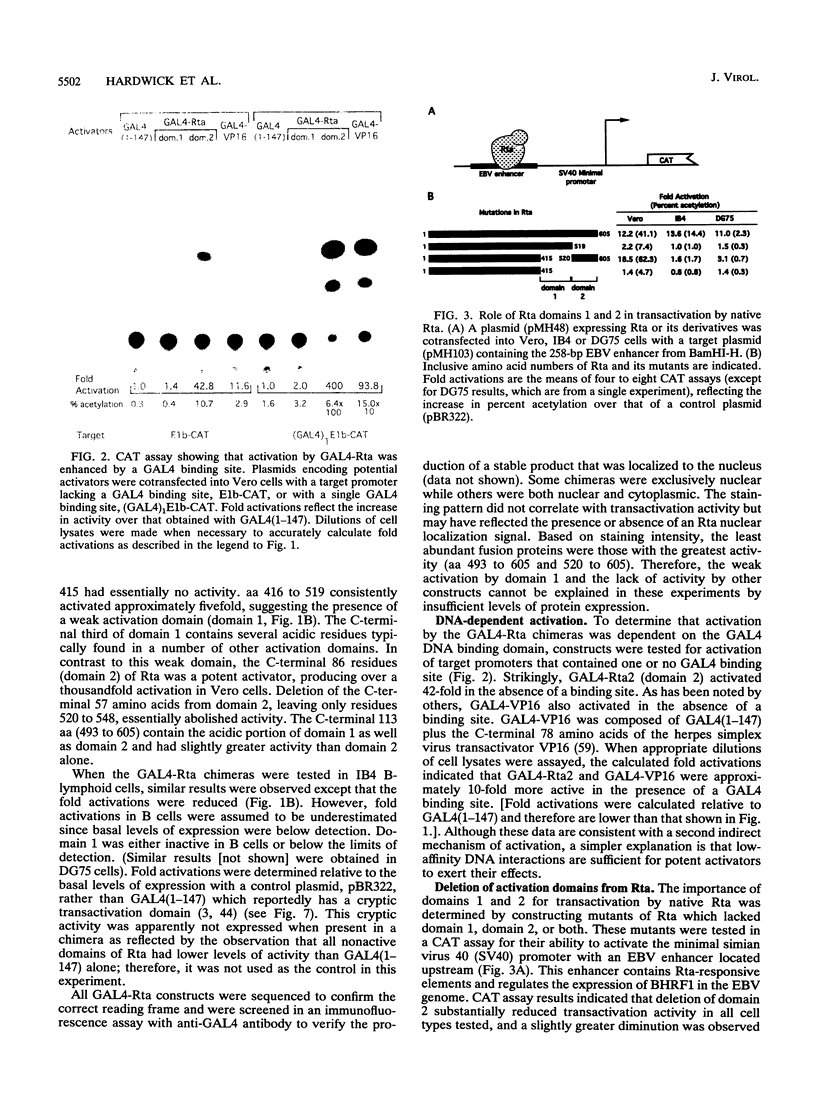
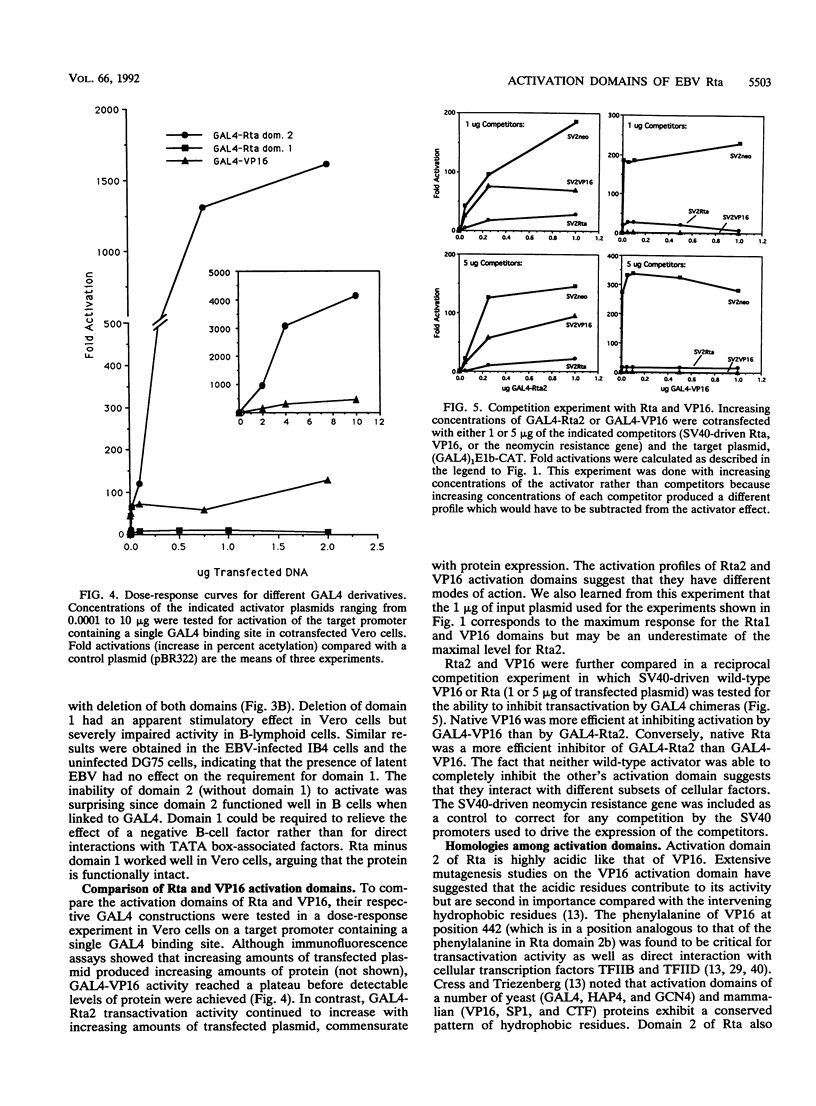
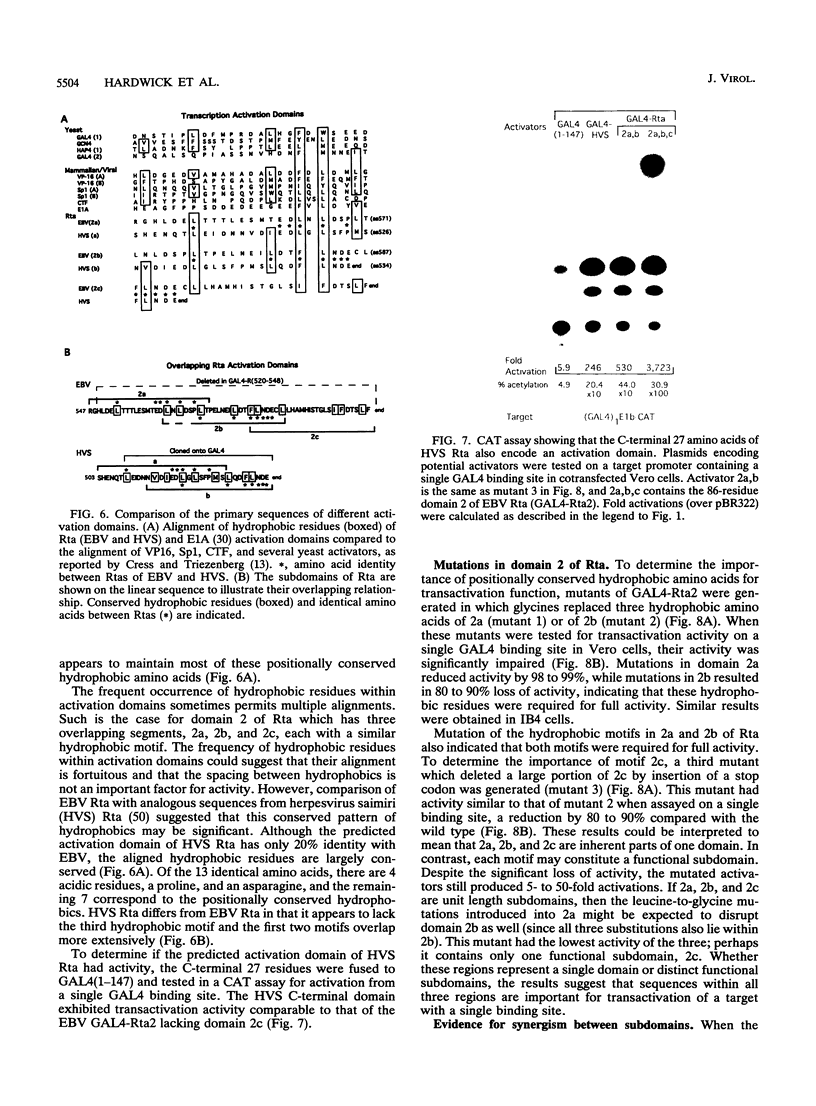
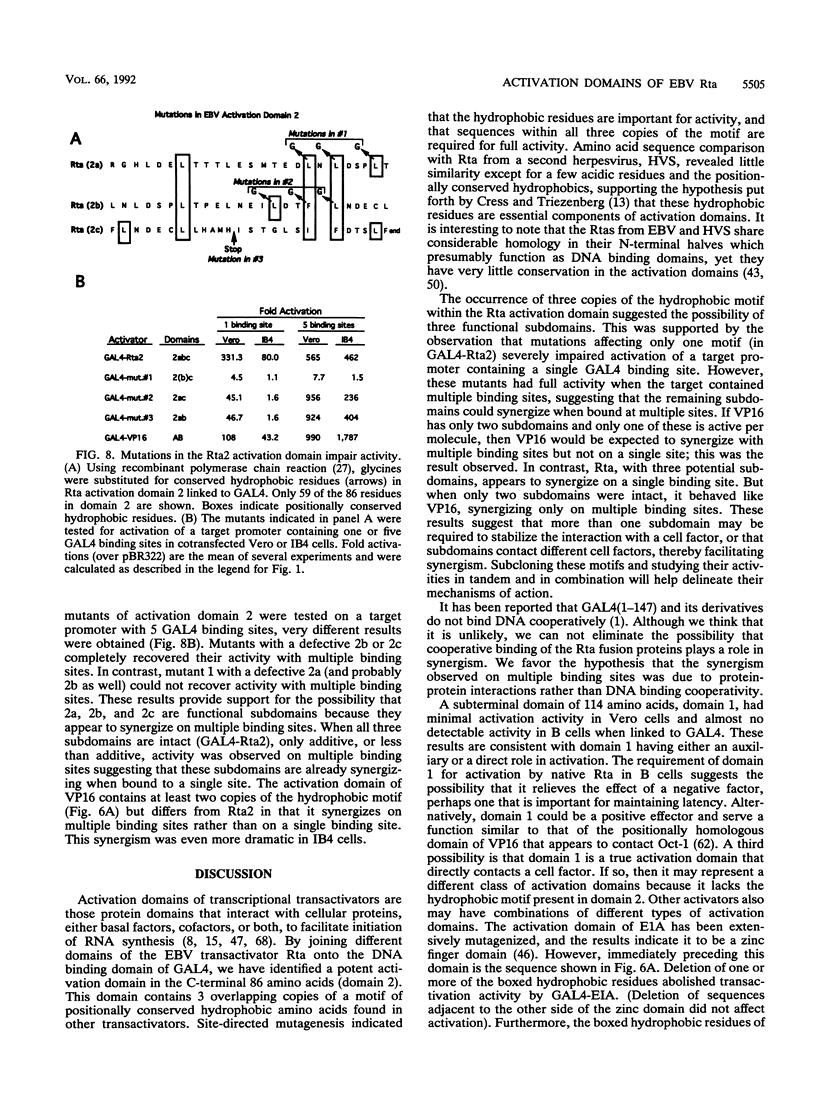
Rta, encoded by Epstein-Barr virus (EBV), is a potent activator of transcription via enhancer sequences located upstream of several viral genes. To identify the domains of Rta that facilitate transcription by interacting with cellular transcription factors, different segments of Rta were linked to the DNA binding domain of yeast transactivator GAL4 (residues 1 to 147). These GAL4-Rta fusion proteins were tested in transfected cells for their ability to activate the adeno E1b promoter with an upstream GAL4 DNA binding site. The acidic C-terminal domain of Rta (amino acids 520 to 605) was a potent activator but behaved differently from VP16 in dose-response and competition experiments. A subterminal domain of Rta (amino acids 416 to 519) linked to GAL4 had weak activation activity. Deletion of these domains from native Rta showed that the C-terminal domain was required for transactivation, but the subterminal domain was required only in B cells. The C-terminal activation domain of Rta contains a pattern of positionally conserved hydrophobic residues shared with VP16 and other transactivators. Substitution of several conserved hydrophobic amino acids in Rta severely impaired transactivation. The improtance of hydrophobic residues was further substantiated by comparing EBV Rta with that of herpesvirus saimiri, which revealed little sequence similarity except for a few acidic residues and the positionally conserved hydrophobic amino acids. The C-terminal domain of EBV Rta contains three partially overlapping copies of this hydrophobic motif. Mutational analysis indicated that all three copies were required for full activity. However, two of the three copies appeared to be sufficient to produce full activity on a target promoter with multiple binding sites, suggesting that these motifs are functional subdomains that can synergize.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniahmad C., Muller M., Altschmied J., Renkawitz R. Co-operative binding of the glucocorticoid receptor DNA binding domain is one of at least two mechanisms for synergism. J Mol Biol. 1991 Nov 20;222(2):155–165. doi: 10.1016/0022-2836(91)90202-h. [DOI] [PubMed] [Google Scholar]
- Biggin M., Bodescot M., Perricaudet M., Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987 Oct;61(10):3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M., Lin Y. S., Green M. R., Ptashne M. A mechanism for synergistic activation of a mammalian gene by GAL4 derivatives. Nature. 1990 May 24;345(6273):361–364. doi: 10.1038/345361a0. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Dong D. L., Hayward G. S., Hayward S. D. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990 Jul;64(7):3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Gruffat H., Manet E., Calender A., Sergeant A. The Epstein-Barr virus (EBV) DR enhancer contains two functionally different domains: domain A is constitutive and cell specific, domain B is transactivated by the EBV early protein R. J Virol. 1989 Feb;63(2):615–623. doi: 10.1128/jvi.63.2.615-623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Comai L., Tanese N., Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992 Mar 6;68(5):965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Countryman J., Jenson H., Seibl R., Wolf H., Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987 Dec;61(12):3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Cox M. A., Leahy J., Hardwick J. M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990 Jan;64(1):313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Donaldson L., Capone J. P. Purification and characterization of the carboxyl-terminal transactivation domain of Vmw65 from herpes simplex virus type 1. J Biol Chem. 1992 Jan 25;267(3):1411–1414. [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Rowe D. T., Rooney C. M., Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989 Jan;8(1):127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E. K., Goldfeld A. E., Speck S. H. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J Virol. 1991 Dec;65(12):7073–7077. doi: 10.1128/jvi.65.12.7073-7077.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990 Mar;64(3):1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Evidence for coiled-coil dimer formation by an Epstein-Barr virus transactivator that lacks a heptad repeat of leucine residues. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9459–9463. doi: 10.1073/pnas.87.23.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan K., Sato H., Rajadurai P., Busson P., Young L., Rickinson A., Tursz T., Raab-Traub N. Novel transcription from the Epstein-Barr virus terminal EcoRI fragment, DIJhet, in a nasopharyngeal carcinoma. J Virol. 1990 Oct;64(10):4948–4956. doi: 10.1128/jvi.64.10.4948-4956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Gruffat H., Duran N., Buisson M., Wild F., Buckland R., Sergeant A. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J Virol. 1992 Jan;66(1):46–52. doi: 10.1128/jvi.66.1.46-52.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruffat H., Manet E., Rigolet A., Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Dec 11;18(23):6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi N., Maguire K., Kralli A., Maldonado E., Reinberg D., Weinmann R. Direct interaction between adenovirus E1A protein and the TATA box binding transcription factor IID. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5124–5128. doi: 10.1073/pnas.88.12.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles C. J., Shales M., Cress W. D., Triezenberg S. J., Greenblatt J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature. 1991 Jun 13;351(6327):588–590. doi: 10.1038/351588a0. [DOI] [PubMed] [Google Scholar]
- Kenney S., Holley-Guthrie E., Mar E. C., Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989 Sep;63(9):3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Holley-Guthrie E., Lin J. C., Mar E. C., Pagano J. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J Virol. 1989 Apr;63(4):1729–1736. doi: 10.1128/jvi.63.4.1729-1736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Thomas-Powell A. L., Raab-Traub N., Hawke M., Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980 Nov;36(2):506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G., Freese U. K., Fischer R., Polack A., Kofler E., Bornkamm G. W. TPA-inducible Epstein-Barr virus genes in Raji cells and their regulation. Virology. 1988 Feb;162(2):503–507. doi: 10.1016/0042-6822(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Lee W. S., Kao C. C., Bryant G. O., Liu X., Berk A. J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991 Oct 18;67(2):365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J Virol. 1990 Jun;64(6):2560–2568. doi: 10.1128/jvi.64.6.2560-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 1991 Dec;5(12B):2441–2454. doi: 10.1101/gad.5.12b.2441. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Hayward S. D. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J Virol. 1989 Jul;63(7):3040–3050. doi: 10.1128/jvi.63.7.3040-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Sample J., Hayward G. S., Hayward S. D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990 Mar;64(3):1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Ha I., Maldonado E., Reinberg D., Green M. R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991 Oct 10;353(6344):569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990 May;64(5):2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon E. M., Glass J. D., Hayward S. D., Mann R. B., Becker P. S., Charache P., McArthur J. C., Ambinder R. F. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991 Oct 19;338(8773):969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- Manet E., Rigolet A., Gruffat H., Giot J. F., Sergeant A. Domains of the Epstein-Barr virus (EBV) transcription factor R required for dimerization, DNA binding and activation. Nucleic Acids Res. 1991 May 25;19(10):2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein R., Carey M., Ptashne M., Harrison S. C. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408–414. doi: 10.1038/356408a0. [DOI] [PubMed] [Google Scholar]
- Marschall M., Schwarzmann F., Leser U., Oker B., Alliger P., Mairhofer H., Wolf H. The BI'LF4 trans-activator of Epstein-Barr virus is modulated by type and differentiation of the host cell. Virology. 1991 Mar;181(1):172–179. doi: 10.1016/0042-6822(91)90482-q. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Lillie J. W., Green M. R. Evidence for interaction of different eukaryotic transcriptional activators with distinct cellular targets. Nature. 1990 Jul 12;346(6280):147–152. doi: 10.1038/346147a0. [DOI] [PubMed] [Google Scholar]
- Meisterernst M., Roy A. L., Lieu H. M., Roeder R. G. Activation of class II gene transcription by regulatory factors is potentiated by a novel activity. Cell. 1991 Sep 6;66(5):981–993. doi: 10.1016/0092-8674(91)90443-3. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Coles L. S., Newman C., Honess R. W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991 May;65(5):2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Williams G. Structural studies of the acidic transactivation domain of the Vmw65 protein of herpes simplex virus using 1H NMR. Biochemistry. 1992 Apr 28;31(16):4150–4156. doi: 10.1021/bi00131a035. [DOI] [PubMed] [Google Scholar]
- Packham G., Economou A., Rooney C. M., Rowe D. T., Farrell P. J. Structure and function of the Epstein-Barr virus BZLF1 protein. J Virol. 1990 May;64(5):2110–2116. doi: 10.1128/jvi.64.5.2110-2116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R., Luka J., Petti L., Sample J., Birkenbach M., Braun D., Kieff E. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology. 1987 Sep;160(1):151–161. doi: 10.1016/0042-6822(87)90055-9. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Ricksten A., Olsson A., Andersson T., Rymo L. The 5' flanking region of the gene for the Epstein-Barr virus-encoded nuclear antigen 2 contains a cell type specific cis-acting regulatory element that activates transcription in transfected B-cells. Nucleic Acids Res. 1988 Sep 12;16(17):8391–8410. doi: 10.1093/nar/16.17.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C. M., Rowe D. T., Ragot T., Farrell P. J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989 Jul;63(7):3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Sakuma S., Ono Y., Takada K. Identification of an enhancer-type sequence that is responsive to Z and R trans-activators of Epstein-Barr virus. Virology. 1989 Oct;172(2):655–658. doi: 10.1016/0042-6822(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Stern S., Herr W. The herpes simplex virus trans-activator VP16 recognizes the Oct-1 homeo domain: evidence for a homeo domain recognition subdomain. Genes Dev. 1991 Dec;5(12B):2555–2566. doi: 10.1101/gad.5.12b.2555. [DOI] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Takada K., Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989 Jan;63(1):445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Pugh B. F., Tjian R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991 Dec;5(12A):2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]
- Urier G., Buisson M., Chambard P., Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989 May;8(5):1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H., Brou C., Wu J., Burton N., Egly J. M., Chambon P. Evidence for a factor required for transcriptional stimulation by the chimeric acidic activator GAL-VP16 in HeLa cell extracts. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7674–7678. doi: 10.1073/pnas.88.17.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]