Abstract
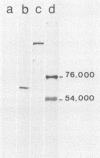
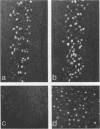
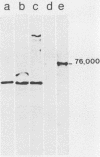
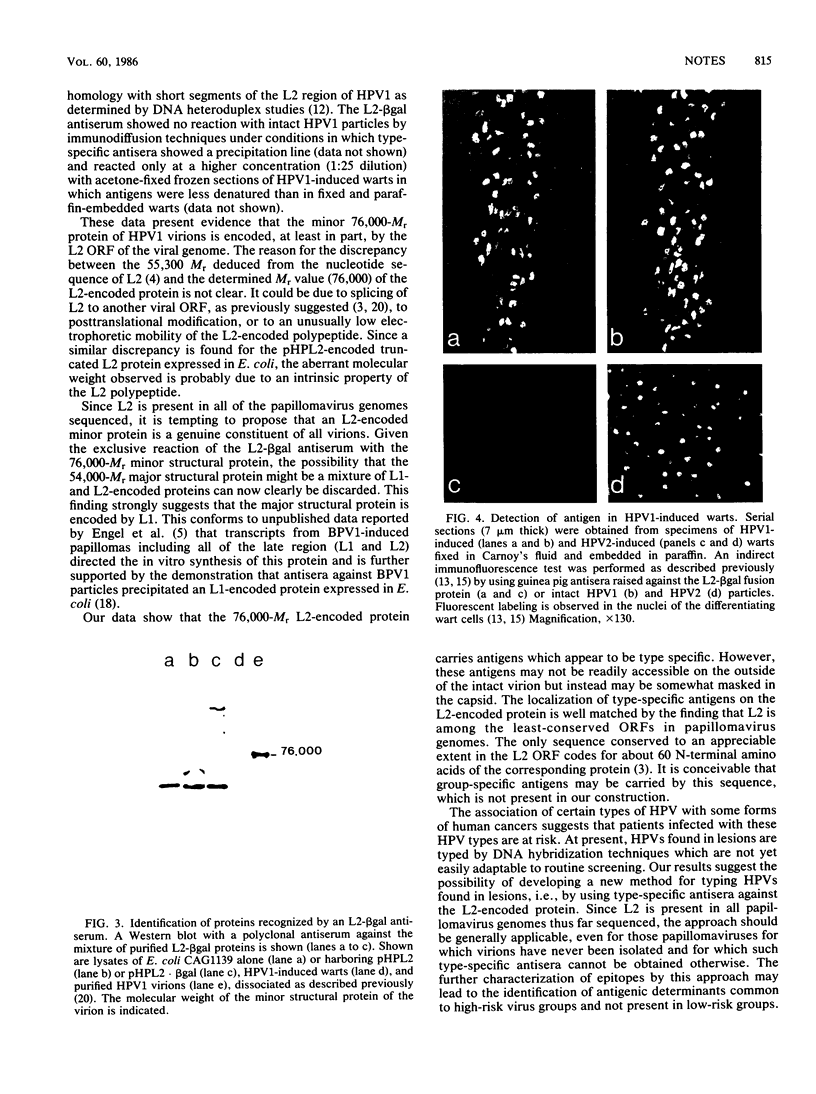
The proteins encoded by the open reading frames of papillomavirus genomes and the minor polypeptides detected in purified virions are still poorly defined. We show here by its expression in Escherichia coli that the open reading frame L2 of human papillomavirus type 1a codes for a minor structural protein of Mr 76,000. Antisera raised against a truncated L2-beta-galactosidase fusion protein in which the conserved N-terminal region of L2 is missing are type specific for human papillomavirus type 1 virions and are reactive at high dilutions. Expression of the L2-encoded type-specific antigens thus provides a powerful new tool for the identification of papillomaviruses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Heilman C. A., Howley P. M. Transcriptional organization of bovine papillomavirus type 1. J Virol. 1983 Sep;47(3):516–528. doi: 10.1128/jvi.47.3.516-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Breitburd F., Croissant O., Orth G. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J Virol. 1977 Mar;21(3):1205–1209. doi: 10.1128/jvi.21.3.1205-1209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Burgess R. R., Walter W., Gross C. A. Mutations in the Ion gene of E. coli K12 phenotypically suppress a mutation in the sigma subunit of RNA polymerase. Cell. 1983 Jan;32(1):151–159. doi: 10.1016/0092-8674(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Orth G., Breitburd F., Favre M. Evidence for antigenic determinants shared by the structural polypeptides of (Shope) rabbit papillomavirus and human papillomavirus type 1. Virology. 1978 Dec;91(2):243–255. doi: 10.1016/0042-6822(78)90373-2. [DOI] [PubMed] [Google Scholar]
- Orth G. Epidermodysplasia verruciformis: a model for understanding the oncogenicity of human papillomaviruses. Ciba Found Symp. 1986;120:157–174. doi: 10.1002/9780470513309.ch11. [DOI] [PubMed] [Google Scholar]
- Orth G., Favre M., Croissant O. Characterization of a new type of human papillomavirus that causes skin warts. J Virol. 1977 Oct;24(1):108–120. doi: 10.1128/jvi.24.1.108-120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Favre M. Human papillomaviruses. Biochemical and biologic properties. Clin Dermatol. 1985 Oct-Dec;3(4):27–42. doi: 10.1016/0738-081x(85)90047-1. [DOI] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Pfister H., Linz U., Gissmann L., Huchthausen B., Hoffmann D., Zur Hausen H. Partial characterization of a new type of bovine papilloma viruses. Virology. 1979 Jul 15;96(1):1–8. doi: 10.1016/0042-6822(79)90166-1. [DOI] [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Ranki A., Kianto U., Kanerva L., Tolvanen E., Johansson E. Immunohistochemical and electron microscopic characterization of the cellular infiltrate in alopecia (areata, totalis, and universalis). J Invest Dermatol. 1984 Jul;83(1):7–11. doi: 10.1111/1523-1747.ep12261627. [DOI] [PubMed] [Google Scholar]
- Roseto A., Pothier P., Guillemin M. C., Peries J., Breitburd F., Bonneaud N., Orth G. Monoclonal antibodies to the major capsid protein of human papillomavirus type 1. J Gen Virol. 1984 Aug;65(Pt 8):1319–1324. doi: 10.1099/0022-1317-65-8-1319. [DOI] [PubMed] [Google Scholar]
- Ullmann A. One-step purification of hybrid proteins which have beta-galactosidase activity. Gene. 1984 Jul-Aug;29(1-2):27–31. doi: 10.1016/0378-1119(84)90162-8. [DOI] [PubMed] [Google Scholar]