Abstract
Because camptothecins are effective against previously resistant tumors and are the only class of topoisomerase I (Top1) inhibitors approved for cancer treatment, we developed the indenoisoquinolines. Like camptothecins, the indenoisoquinolines selectively trap Top1-DNA cleavage complexes and have been co-crystallized with the Top1-DNA cleavage complexes. Indenoisoquinolines show antitumor activity in animal models. They have several advantages over the camptothecins: 1) They are synthetic and chemically stable (unlike camptothecins); 2) The Top1 cleavage sites trapped by the indenoisoquinolines have different genomic locations than camptothecins, implying differential targeting of cancer cell genomes; 3) The Top1 cleavage complexes trapped by indenoisoquinolines are more stable than for camptothecins, indicative of prolonged drug action; and 4) They are less or not substrates for the multidrug resistance efflux pumps (ABCG2 and MDR-1). Among the more than 400 indenoisoquinolines synthesized and evaluated, three have been retained as leads for clinical development by the NCI: NSC 706744, NSC 725776 (Indimitecan) and NSC 724998 (Indotecan). The trapping of Top1 cleavage complexes by indenoisoquinolines in cells results in the rapid and sustained phosphorylation of histone H2AX (referred to as γ-H2AX). We discuss the use of γ–H2AX as a pharmacodynamic biomarker for the clinical development of the indenoisoquinolines.
Keywords: topoisomerase, camptothecin, pharmacodynamic, histone, DNA repair
Rationale for non-camptothecin Top1 inhibitors and historical development of the indenoisoquinolines
Camptothecins are presently the only class of Top1 inhibitor approved for cancer treatment. Topotecan is prescribed for the treatment of ovarian and lung cancers and irinotecan for the treatment of colorectal cancers. Both drugs are water-soluble derivatives of camptothecin and act solely and selectively by inhibiting Top1 (1, 2).
Because of the potent anticancer activity of topotecan and irinotecan, Top1 is empirically validated as an anticancer target. The rationale for developing non-camptothecin Top1 inhibitors stems from two considerations. First, it is empirically established that drugs with a common molecular target can have very different therapeutic activities. For instance, colchicines and vinca alkaloids both act by blocking tubulin polymerization. However, only the Vinca alkaloids are used as anticancer agents whereas colchicine is used for the treatment of gout. Even more striking is the differential clinical activities of topotecan vs. irinotecan in spite of their common chemical scaffold. Another consideration for developing non-camptothecin Top1 inhibitors is that camptothecins have pharmacological and clinical limitations that restrict the dose of active drug that can reach the tumor while sparing normal tissues. That limitation was initially overseen in mice models (3) because mouse bone marrow progenitors are markedly resistant to camptothecins compared to humans (4). Interestingly, that difference is minimal for the indenoisoquinolines, suggesting that animal models should be more reliable predictors of tumor response and dose-limiting toxicity1.
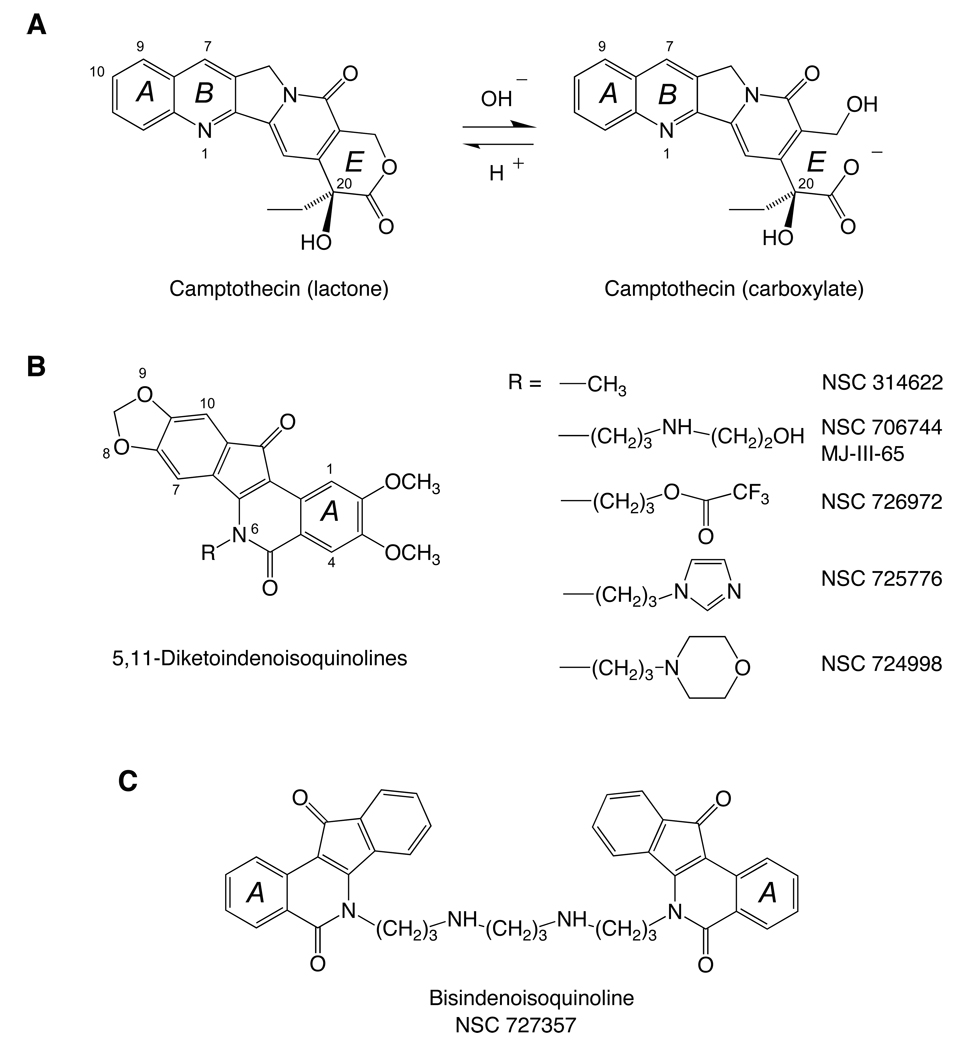
In 1978, we described the synthesis of the first of our indenoisoquinolines (NSC 314622) (5). This compound was found to be moderately cytotoxic in cancer cell cultures with a median growth inhibition 50% concentration of 20 µM in the NCI in vitro screen. Approximately 20 years after its initial discovery, interest in NSC 314622 spiked with the discovery that a COMPARE analysis (6) of its cytotoxicity profile (7) paralleled that of camptothecin, which led to a detailed investigation of the effect of NSC 314622 against Top1 (7). The results showed clearly that micromolar concentrations of NSC 314622 induced Top1-mediated DNA cleavage, and that the cleavage site specificity was different from camptothecin (7). Comparison of the properties of NSC 314622 with those of the camptothecins revealed a number of differences that are advantageous for the indenoisoquinolines in general. First, in contrast to the camptothecins, which are inactivated by lactone hydrolysis at physiological pH (Fig. 1A), the indenoisoquinolines are chemically stable (Fig. 1B). Second, the enzyme-DNA cleavage complexes stabilized by indenoisoquinolines are more persistent than those induced by camptothecins (7–10). Long camptothecin infusion times are necessary to compensate for the reversibility of the camptothecin cleavage complexes during chemotherapy in order to achieve maximal activity. Third, the indenoisoquinolines induce unique patterns of DNA cleavage sites relative to the camptothecins, indicating that they target the human genome differently and therefore may potentially exhibit a different spectrum of anticancer activity from the camptothecins (7–10). And Fourth, the indenoisoquinolines are not or less substrates for the ABCG2 multidrug efflux pump that confers resistance to irinotecan and topotecan (10).
Figure 1.
Chemical structures of camptothecin and selected indenoisoquinolines.
There were therefore good reasons to pursue the development of novel non-camptothecin Top1 inhibitors based on the use of NSC 314622 as a lead compound. However, the potential clinical utility of the lead compound NSC 314622 itself was limited by its moderate activity, both as a cytotoxic agent in cancer cell cultures and as a Top1 poison. Following the discovery of NSC 314622 as Top1 inhibitor, approximately 400 derivatives have been synthesized and evaluated for Top1 inhibition using recombinant enzyme and purified DNA substrates and cellular assays in the NCI-60 cell line panel (7–34). Out of these hundreds of derivatives, the most promising compounds were developed based on the following criteria: 1) potent induction of Top1 cleavage complexes at low or submicromolar concentrations; 2) lack of significant DNA intercalation; 3) DNA cleavage pattern different from camptothecins; 4) potent growth inhibitory activity across the 60 cell lines of the NCI anticancer screen; 5) Top1-dependent antiproliferative activity in cells with genetic Top1 deficiencies (9, 35) and in cells with Top1 mutations that confer camptothecin resistance (9); 6) Lack of cross-resistance in cells overexpressing the ABCG2 (MXR/BCRP) plasma membrane drug efflux transporter (10), which confers resistance to topotecan and irinotecan (36, 37).
Several additional aspects of a second lead compound NSC 706744 (MJ-III-65) (Fig. 1B) are of interest (9, 12): 1) MJ-III-65 does not unwind DNA in the absence of Top1, indicating that it is not a DNA intercalator in the absence of enzyme; 2) MJ-III-65 remains active against the camptothecin-resistant Asn722Ser and Arg364His mutant Top1 and human leukemia cells bearing the Asn722Ser mutation (38, 39); 3) MJ-III-65 inhibits Top1-mediated DNA relaxation, thus demonstrating inhibition of Top1 catalytic activity; 4) the Top1-associated DNA nicks induced by MJ-III-65 are reversed by salt treatment, which is consistent with reversible trapping of the Top1 cleavage complexes; 5) MJ-III-65 induces protein-linked single-strand breaks in human breast carcinoma MCF7 cells, thus demonstrating intracellular activity in a cellular system; 6) the Top1 cleavage complexes induced by MJ-III-65 are more persistent upon drug removal than those induced by camptothecin, demonstrating favorable pharmacodynamic properties; and 7) MJ-III-65 produced antitumor activity in animal models (12). However, the poor solubility of MJ-III-65 impaired its further development.
Currently two indenoisoquinolines have been selected for clinical development by the NCI: NSC 725776 and NSC 724998 (10). Our selection criteria were based on potent activity against Top1 in vitro and in cells, potent antiproliferative activity against a range of cancer cell lines from the NCI screen, Top1-dependent antiproliferative activity (demonstrating selective targeting of Top1 in cells), and antitumor activity in mouse models. The two indenoisoquinolines NSC 725776 and NSC 724998 are endowed of three additional favorable characteristics: 1) Top1 cleavage produced at nanomolar concentrations in cancer cells; 2) persistence of Top1 cleavage complexes after drug removal for longer time than camptothecin; 3) activity in multidrug-resistant cells overexpressing the drug efflux transporter ABCG2 and MDR-1; 4) concentration-dependent γ-H2AX foci formation at pharmacologically relevant doses for up to 24 hours (10).
A series of bisindenoisoquinolines have also been synthesized (18, 28, 29). One of them, the dimeric indenoisoquinoline NSC 727357 (Fig. 1C), shows potent antiproliferative activity in the NCI-60 cell line panel, promising hollow fiber activity (score = 32) and activity against xenografts. Submicromolar concentrations of the bisindenoisoquinoline NSC 727357 induce Top1 cleavage complexes at specific sites in biochemical assays. At higher concentrations an inhibition of Top1 catalytic activity and DNA intercalation are observed. NSC 727357 also induces a limited number of topoisomerase II (Top2)-DNA cleavage complexes. In contrast to the effect of other Top1 inhibitors, cells treated with the bisindenoisoquinoline NSC 727357 show an arrest of cell cycle progression in G1 with no significant inhibition of DNA synthesis following a short exposure to the drug. Moreover, unlike camptothecin and the indenoisoquinoline MJ-III-65 (NSC 706744), the cytotoxicity of bisindenoisoquinoline NSC 727357 is only partially dependent on Top1 and p53, indicating that this drug has additional targets besides Top1 and Top2.
Interfacial inhibition: a common molecular mechanism of action of Top1 inhibitors
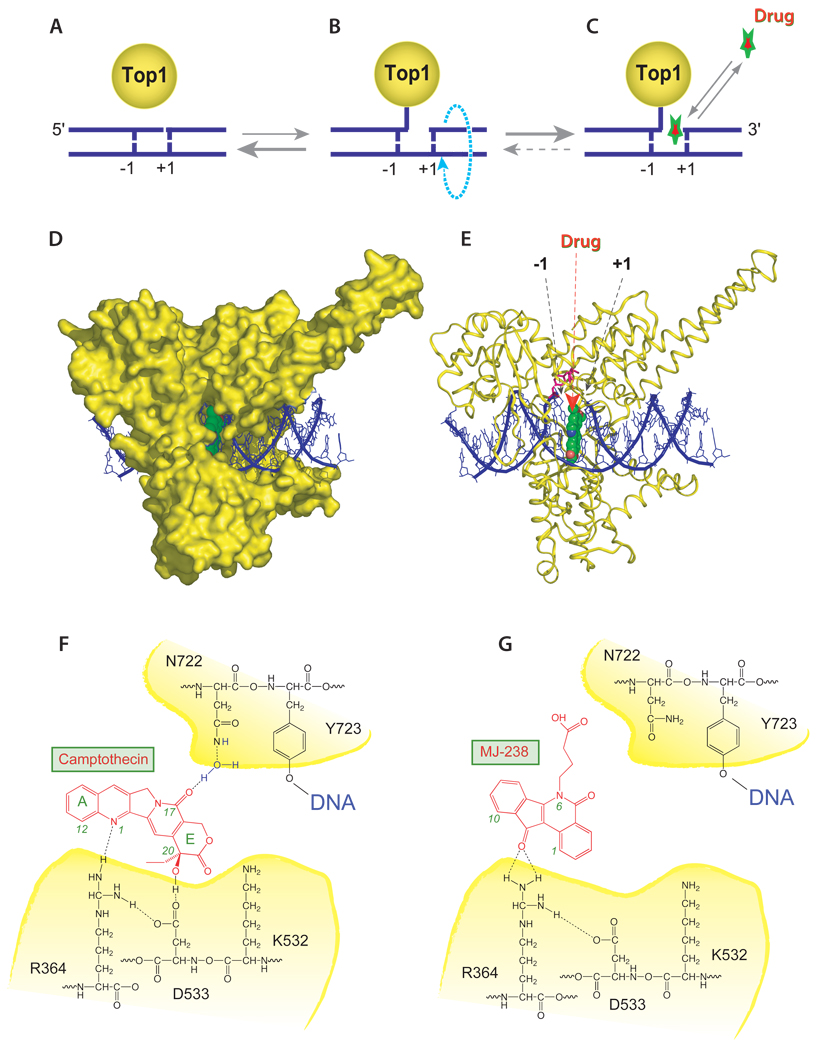
All Top1 inhibitors (camptothecins, indenoisoquinolines and indolocarbazoles) act as “interfacial inhibitors” (Fig. 2) (1, 24, 40, 41). Under normal conditions, cleavage complexes are the catalytic intermediates that enable DNA relaxation by Top1 (Fig. 2A–C). Most of Top1 is non-covalently associated with chromatin (Fig. 2A). The cleavage complexes (Fig. 2B) form only transiently, and the Top1-mediated religation step is highly favored over the cleavage step (1). That equilibrium can be shifted in the presence of a Top1 inhibitor as the drug selectively intercalates between the base pairs that flank the nick made by Top1 (Fig. 2C–E). We first proposed this mechanism of action based on our finding that the Top1 cleavage sites trapped by camptothecins exhibited a strong bias for thymine at position −1 (see scheme in Fig. 1C) and guanine at position +1 (42). Direct evidence for this model was later provided by co-crystal structures; first of topotecan bound in a ternary complex with Top1 and its DNA substrate (43), and later of the natural camptothecin alkaloid (19), an indolocarbazole derivative (19), and two indenoisoquinoline derivatives (14, 19, 24) (Fig. 2D–E).
Figure 2.
(A) Topoisomerase I (Top1) is an abundant nuclear enzyme. It is mostly associated non-covalently with chromatin. (B) Top1 relaxes DNA by making single-strand breaks that are generated by the covalent linkage of Top1 to the 3'-end of DNA. (C) Camptothecin or indenoisoquinolines bind reversibly to the Top1-DNA cleavage complex and slow down DNA religation. (D) Ternary complex including Top1 (yellow), DNA (dark blue ribbons), and indenoisoquinolines or camptothecins (green and red in the middle). (E) Same structure except Top1 is in ribbon representation. (F) Hydrogen bond network between camptothecin and Top1 amino acid residues. (G). Hydrogen bond network between the indenoisoquinoline derivative MJ-238 and Top1. Note that mutation of asparagine 722 to serine (N722S), which confers resistance to camptothecin and only partially to indenoisoquinolines, is also present in camptothecin-producing plants.
Two main components are responsible for the drug binding interactions and for the highly selective fit of Top1 inhibitors within Top1 cleavage complexes: 1) stacking π–π interactions with the DNA, and 2) hydrogen bonds with Top1 amino acid residues. The coplanar ring system of Top1 inhibitors (see Fig. 1) is approximately the size of a base pair. Thus it can form extensive π–π stacking interactions with the base pairs (positions −1 and +1) flanking the DNA cleavage site produced by Top1 (see Fig. 2C–E). Hydrogen bond interactions are also critical for drug binding. The key hydrogen bonds are shown in Figure 2F and 2G for camptothecin and the indenoisoquinoline MJ-238, respectively. In the case of camptothecin, three hydrogen bonds are formed between camptothecin residues 1, 17 and 20 and amino acid residues Arg364, Asn722 and Asp533, respectively (14, 19, 24) (Fig. 2F). Mutation of any one of these three Top1 amino acid residues is sufficient to confer high resistance to camptothecins (38, 39, 44–47). On the other hand, the binding geometries, binding site selectivities, and structure-activity relationships of the indenoisoquinolines and camptothecins can be predicted by ab initio quantum mechanics calculations involving only the Top1 inhibitors and the neighboring DNA base pairs in the absence of protein structure, emphasizing the importance of π–π stacking interactions (48–50). Noticeably, single mutations do not prevent the binding of camptothecin to the Top1-DNA complex (51), which demonstrates the dynamic nature of the drug-DNA-Top1 interactions and the importance of all those interactions together for selective drug binding. Also noticeable is the recent discovery that camptothecin-producing plants (such as Camptotheca acuminata) have a TOP1 gene bearing the same mutation Asn722Ser (52) that had been found in human leukemia cells selected for resistance to camptothecin (38).
The drug polycyclic ring system and the hydrogen bond network are different for the indenoisoquinolines and camptothecins (see Fig. 1 and Fig. 2). Those differences probably account for: 1) the trapping of Top1 cleavage complexes at different genomic sequences (8–10, 12), 2) the slower reversibility of the indenoisoquinoline-Top1 cleavage complexes (9, 10, 12), as the indenoisoquinolines are probably better “retained” at the Top1-DNA interface than camptothecins, and 3) the ability of the indenoisoquinolines to remain active against Top1 mutations that otherwise confer resistance to camptothecins (9, 12).
Histone γ-H2AX as a pharmacodynamic biomarker for the indenoisoquinolines
As Top1 inhibitors bind reversibly to Top1 cleavage complexes (43), the drugs do not directly damage DNA. However, upon collision with a replication fork or the transcription machinery those reversible drug-DNA-Top1 cleavage complexes are converted into irreversible Top1 covalent complexes and subsequently DNA damage (double-strand breaks), which if not repaired lead to cell death (1). Thus, replication-fork collision is the primary cytotoxic mechanism of Top1 poisons in dividing cells. One of the best characterized molecular responses to replication double-strand breaks is the phosphorylation of the H2AX histone variant (53). Histone H2AX constitutes 5–20% of the cellular pool of histone H2A and is randomly distributed throughout nucleosomes (54). The phosphorylated form of H2AX, termed γ-H2AX, is observed within minutes after the formation of camptothecin-induced replication double-strand breaks (55, 56). γ-H2AX can be detected by immunofluorescence (57) or immunostaining (58), as it accumulates and forms a nuclear focus around each double-strand break. γ-H2AX is an extremely sensitive marker for double-strand breaks that are not only produced by DNA-damaging agents (59), but also by genomic instability (58) and apoptosis (60).
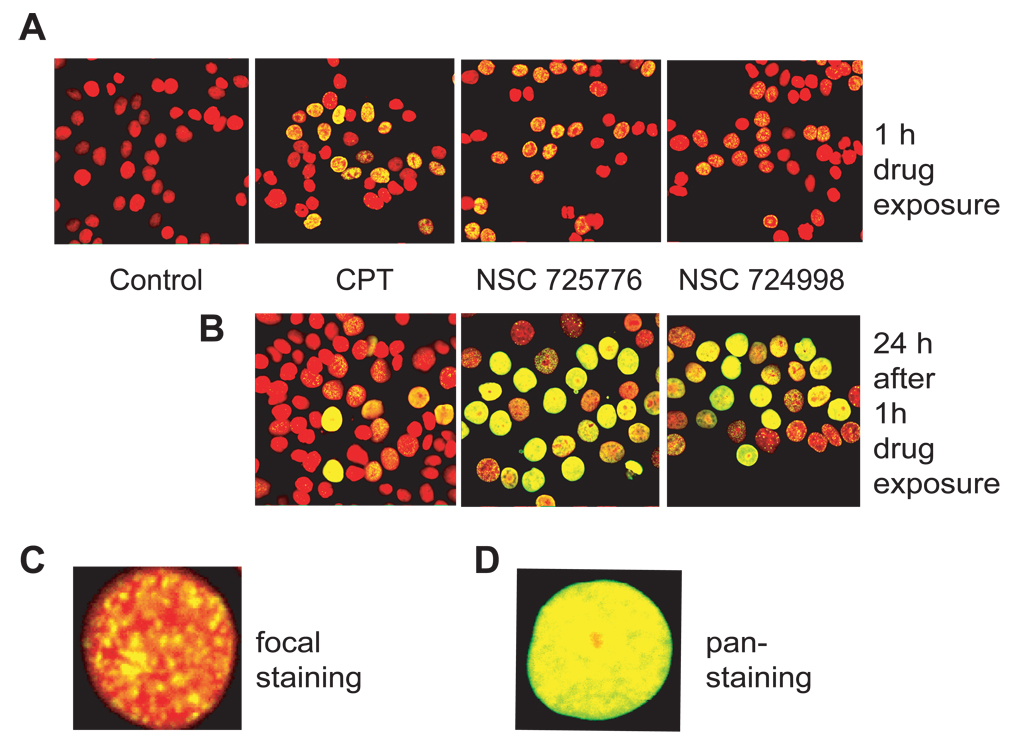
We recently found γ-H2AX formation in various cell lines treated with NSC 725776 and NSC 724998 (10). γ-H2AX foci formed within one hour upon NSC 725776 and NSC 724998 treatment at concentrations as low as 0.1 µM, which are well within the pharmacological concentration range. Figure 3 shows the rapid induction of γ-H2AX by the indenoisoquinolines within one hour of drug treatment. It also shows the changes in γ-H2AX signal at different times following drug removal. Noticeably, γ-H2AX staining, which starts as well-defined DNA damage foci (Fig. 3C) tends to be converted into pan-nuclear staining (Fig. 3D), which might be indicative of irreversible DNA damage preceding apoptosis. In either case, γ-H2AX will be scored as positive and indicative of drug activity. Thus, γ-H2AX may be a useful clinical marker for monitoring the efficacy of NSC 725776 and NSC 724998 in tumor therapies.
Figure 3.
γ-H2AX induction by NSC 725776 and NSC 724998 in human colon cancer HT29 cells. A) Rapid induction of γ-H2AX foci after 1 h treatment with 1 µM of CPT, NSC 725776 or NSC 724998. B) Persistent γ-H2AX signal induced by indenoisoquinolines. After 1 h drug treatments, HT29 cells were further incubated in drug-free medium for 24 h. C) Representative image of a single cell with typical γ-H2AX damage foci. D) Representative image of a single cell with diffuse pan-nuclear staining, which typically develops several hours after drug exposure. Fixed cells were stained with mouse anti-γ-H2AX antibody and goat anti-mouse antibody conjugated with AlexaFluor 488 (green). Nuclei were stained with PI (red).
Conclusions
Ten years after the identification of the first indenoisoquinoline as a Top1 inhibitor, two derivatives NSC 724776 and 725998 are poised for clinical trials. Based on the differential genomic targeting of Top1 cleavage complexes, the differences in chemical structure and chemical stability of the indenoisoquinolines compared to camptothecins, and the low cross-resistance to camptothecins based on drug efflux and Top1 point mutations, it is likely that the indenoisoquinolines will exhibit unique clinical properties that will set them apart from the camptothecins. Because indenoisoquinoline derivatives can be synthesized relatively easily and detailed knowledge of the Top1-DNA-indenoisoquinoline interaction is now available, it is plausible that, if needed, additional optimization (61–64) will lead to novel drugs selectively targeted to Top1 with significant activity against cancers (65).
Acknowledgements
We wish to thank the both the Intramural (Center for Cancer Research) and the Extramural programs of the National Cancer Institute for sustained support during the course of these studies through NIH Research Grant UO1 CA89566. We also wish to thank our coworkers, graduate students and post-doctoral fellows for their dedication, and the members of the Division of Cancer Treatment and Diagnosis (DCTD), NCI for supporting the development of the indenoisoquinolines and of histone γ-H2AX as a biomarker for the indenoisoquinolines. Among the members of the DCTD team, special thanks to Dr. Ven Narayanan, Dr. Joseph Tomaszwewski, Dr. Melinda Hollingshead, Dr. Ralph Parchment, and Dr. James Doroshow for their support.
Abbreviations
- CPT
camptothecin
- Top1
DNA topoisomerase I
Footnotes
Parchment, R.E and Tomaszewski, J.: personal communication.
References
- 1.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 2.Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–1726. [PubMed] [Google Scholar]
- 3.Giovanella BC, Stehlin JS, Wall ME, et al. DNA topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science. 1989;246:1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- 4.Erickson-Miller CL, May RD, Tomaszewski J, et al. Differential toxicity of camptothecin, topotecan and 9-aminocamptothecin to human, canine, and murine myeloid progenitors (CFU-GM) in vitro. Cancer Chemother Pharmacol. 1997;39:467–472. doi: 10.1007/s002800050600. [DOI] [PubMed] [Google Scholar]
- 5.Cushman M, Cheng L. Stereoselective oxidation by thionyl chloride leading to the indeno[1,2-c]isoquinoline system. J Org Chem. 1978;43:3781–3783. [Google Scholar]
- 6.Paull KD, Shoemaker RH, Hodes L, et al. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of a mean graph and COMPARE algorithm. J Natl Cancer Inst. 1989;81:1088–1092. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 7.Kohlhagen G, Paull K, Cushman M, Nagafufuji P, Pommier Y. Protein-linked DNA strand breaks induced by NSC 314622, a non-camptothecin topoisomerase I poison. Mol Pharmacol. 1998;54:50–58. doi: 10.1124/mol.54.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Strumberg D, Pommier Y, Paull K, Jayaraman M, Nagafuji P, Cushman M. Synthesis of cytotoxic indenoisoquinoline topoisomerase I poisons. J Med Chem. 1999;42:446–457. doi: 10.1021/jm9803323. [DOI] [PubMed] [Google Scholar]
- 9.Antony S, Jayaraman M, Laco G, et al. Differential induction of topoisomerase I-DNA cleavage complexes by the indenoisoquinoline MJ-III-65 (NSC 706744) and camptothecin: base sequence analysis and activity against camptothecin-resistant topoisomerases I. Cancer Res. 2003;63:7428–7435. [PubMed] [Google Scholar]
- 10.Antony S, Agama KK, Miao ZH, et al. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topoisomerase I cleavage complexes and overcome multidrug resistance. Cancer Res. 2007;67:10397–10405. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M, Jayaraman M, Vroman JA, et al. Synthesis of new Indeno[1,2-c]isoquinolines: cytotoxic non-camptothecin topoisomerase I inhibitors. J Med Chem. 2000;43:3688–3698. doi: 10.1021/jm000029d. [DOI] [PubMed] [Google Scholar]
- 12.Antony S, Kohlhagen G, Agama K, et al. Cellular Topoisomerase I Inhibition and Antiproliferative Activity by MJ-III-65 (NSC 706744), an Indenoisoquinoline Topoisomerase I Poison. Mol Pharmacol. 2005;67:523–530. doi: 10.1124/mol.104.003889. [DOI] [PubMed] [Google Scholar]
- 13.Fox BM, Xiao X, Antony S, et al. Design, synthesis, and biological evaluation of cytotoxic 11-alkenylindenoisoquinoline-camptothecin hybrids. J Med Chem. 2003;46:3275–3282. doi: 10.1021/jm0300476. [DOI] [PubMed] [Google Scholar]
- 14.Ioanoviciu A, Antony S, Pommier Y, Staker BL, Stewart L, Cushman M. Synthesis and Mechanism of Action Studies of a Series of Norindenoisoquinoline Topoisomerase I Poisons Reveal an Inhibitor with a Flipped Orientation in the Ternary DNA-Enzyme-Inhibitor Complex As Determined by X-ray Crystallographic Analysis. J Med Chem. 2005;48:4803–4814. doi: 10.1021/jm050076b. [DOI] [PubMed] [Google Scholar]
- 15.Jayaraman M, Fox BM, Hollingshead M, Kohlhagen G, Pommier Y, Cushman M. Synthesis of new dihydroindeno[1,2-c]isoquinoline and indenoisoquinolinium chloride topoisomerase I inhibitors having high in vivo anticancer activity in the hollow fiber animal model. J Med Chem. 2002;45:242–249. doi: 10.1021/jm000498f. [DOI] [PubMed] [Google Scholar]
- 16.Morrell A, Antony S, Kohlhagen G, Pommier Y, Cushman M. Synthesis of nitrated indenoisoquinolines as topoisomerase I inhibitors. Bioorg Med Chem Lett. 2004;14:3659–3663. doi: 10.1016/j.bmcl.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Nagarajan M, Morrell A, Fort BC, et al. Synthesis and Anticancer Activity of Simplified Indenoisoquinoline Topoisomerase I Inhibitors Lacking Substituents on the Aromatic Rings. J Med Chem. 2004;47:5651–5661. doi: 10.1021/jm040025z. [DOI] [PubMed] [Google Scholar]
- 18.Nagarajan M, Xiao X, Antony S, Kohlhagen G, Pommier Y, Cushman M. Design, synthesis, and biological evaluation of indenoisoquinoline topoisomerase I inhibitors featuring polyamine side chains on the lactam nitrogen. J Med Chem. 2003;46:5712–5724. doi: 10.1021/jm030313f. [DOI] [PubMed] [Google Scholar]
- 19.Staker BL, Feese MD, Cushman M, et al. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J Med Chem. 2005;48:2336–2345. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 20.Xiao X, Antony S, Kohlhagen G, Pommier Y, Cushman M. Design, synthesis, and biological evaluation of cytotoxic 11-aminoalkenylindenoisoquinoline and 11-diaminoalkenylindenoisoquinoline topoisomerase I inhibitors. Bioorg Med Chem. 2004;12:5147–5160. doi: 10.1016/j.bmc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Antony S, Kohlhagen G, Pommier Y, Cushman M. Novel Autoxidative Cleavage Reaction of 9-Fluoredenes Discovered during Synthesis of a Potential DNA-Threading Indenoisoquinoline. J Org Chem. 2004;69:7495–7501. doi: 10.1021/jo048808f. [DOI] [PubMed] [Google Scholar]
- 22.Xiao X, Antony S, Pommier Y, Cushman M. On the binding of indeno[1,2-c]isoquinolines in the DNA-topoisomerase I cleavage complex. J Med Chem. 2005;48:3231–3238. doi: 10.1021/jm050017y. [DOI] [PubMed] [Google Scholar]
- 23.Xiao X, Miao ZH, Antony S, Pommier Y, Cushman M. Dihydroindenoisoquinolines function as prodrugs of indenoisoquinolines. Bioorg Med Chem Lett. 2005;15:2795–2798. doi: 10.1016/j.bmcl.2005.03.101. [DOI] [PubMed] [Google Scholar]
- 24.Marchand C, Antony S, Kohn KW, et al. A novel norindenoisoquinoline structure reveals a common interfacial inhibitor paradigm for ternary trapping of topoisomerase I-DNA covalent complexes. Mol Cancer Ther. 2006;5:287–295. doi: 10.1158/1535-7163.MCT-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrell A, Antony S, Kohlhagen G, Pommier Y, Cushman M. Synthesis of benz[d]indeno[1,2-b]pyran-5,11-diones: versatile intermediates for the design and synthesis of topoisomerase I inhibitors. Bioorg Med Chem Lett. 2006;16:1846–1849. doi: 10.1016/j.bmcl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Antony S, Pommier Y, Cushman M. Total synthesis and biological evaluation of 22-hydroxyacuminatine. J Med Chem. 2006;49:1408–1412. doi: 10.1021/jm051116e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrell A, Jayaraman M, Nagarajan M, et al. Evaluation of indenoisoquinoline topoisomerase I inhibitors using a hollow fiber assay. Bioorg Med Chem Lett. 2006;16:4395–4399. doi: 10.1016/j.bmcl.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Antony S, Agama KK, Miao ZH, et al. Bisindenoisoquinoline Bis-1,3-{(5,6-dihydro-5,11-diketo-11H-indeno[1,2-c]isoquinoline)-6-propyla mino}propane bis(trifluoroacetate) (NSC 727357), a DNA Intercalator and Topoisomerase Inhibitor with Antitumor Activity. Mol Pharmacol. 2006;70:1109–1120. doi: 10.1124/mol.106.024372. [DOI] [PubMed] [Google Scholar]
- 29.Nagarajan M, Morrell A, Antony S, et al. Synthesis and Biological Evaluation of Bisindenoisoquinolines as Topoisomerase I Inhibitors. J Med Chem. 2006;49:5129–5140. doi: 10.1021/jm060046o. [DOI] [PubMed] [Google Scholar]
- 30.Nagarajan M, Morrell A, Ioanoviciu A, et al. Synthesis and evaluation of indenoisoquinoline topoisomerase I inhibitors substituted with nitrogen heterocycles. J Med Chem. 2006;49:6283–6289. doi: 10.1021/jm060564z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell A, Antony S, Kohlhagen G, Pommier Y, Cushman M. A systematic study of nitrated indenoisoquinolines reveals a potent topoisomerase I inhibitor. J Med Chem. 2006;49:7740–7753. doi: 10.1021/jm060974n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrell A, Placzek MS, Steffen JD, et al. Investigation of the Lactam Side Chain Length Necessary for Optimal Indenoisoquinoline Topoisomerase I Inhibition and Cytotoxicity in Human Cancer Cell Cultures. J Med Chem. 2007;50:2040–2048. doi: 10.1021/jm0613119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrell A, Placzek M, Parmley S, et al. Nitrated Indenoisoquinolines as Topoisomerase I Inhibitors: A Systematic Study and Optimization. J Med Chem. 2007;50:4419–4430. doi: 10.1021/jm070361q. [DOI] [PubMed] [Google Scholar]
- 34.Morrell A, Placzek M, Parmley S, et al. Optimization of the Indenone Ring of Indenoisoquinoline Topoisomerase I Inhibitors. J Med Chem. 2007;50:4388–4404. doi: 10.1021/jm070307+. [DOI] [PubMed] [Google Scholar]
- 35.Miao ZH, Player A, Shankavaram U, et al. Nonclassic Functions of Human Topoisomerase I: Genome-Wide and Pharmacologic Analyses. Cancer Res. 2007;67:8752–8761. doi: 10.1158/0008-5472.CAN-06-4554. [DOI] [PubMed] [Google Scholar]
- 36.Brangi M, Litman T, Ciotti M, et al. Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res. 1999;59:5938–5946. [PubMed] [Google Scholar]
- 37.Takagi K, Dexheimer TS, Redon C, et al. Novel E-ring camptothecin keto analogues ( S38809 and S39625) are stable, potent, and selective topoisomerase I inhibitors without being substrates of drug efflux transporters. Mol Cancer Ther. 2007;6:3229–3238. doi: 10.1158/1535-7163.MCT-07-0441. [DOI] [PubMed] [Google Scholar]
- 38.Fujimori A, Harker WG, Kohlhagen G, Hoki Y, Pommier Y. Mutation at the catalytic site of topoisomerase I in CEM/C2, a human leukemia cell resistant to camptothecin. Cancer Res. 1995;55:1339–1346. [PubMed] [Google Scholar]
- 39.Fujimori A, Hoki Y, Popescu N, Pommier Y. Silencing and selective methylation of the normal topoisomerase I gene in camptothecin-resistant CEM/C2 human leukemia cells. Oncol Res. 1996:295–301. [PubMed] [Google Scholar]
- 40.Pommier Y. Camptothecins and topoisomerase I: a foot in the door. Targeting the genome beyond topoisomerase I with camptothecins and novel anticancer drugs: importance of DNA replication, repair and cell cycle checkpoints. Curr Med Chem Anti-Canc Agents. 2004;4:429–434. doi: 10.2174/1568011043352777. [DOI] [PubMed] [Google Scholar]
- 41.Pommier Y, Cherfils J. Interfacial protein inhibition: a nature's paradigm for drug discovery. Trends Pharmacol Sci. 2005;28:136–145. doi: 10.1016/j.tips.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Jaxel C, Capranico G, Kerrigan D, Kohn KW, Pommier Y. Effect of local DNA sequence on topoisomerase I cleavage in the presence or absence of camptothecin. J Biol Chem. 1991;266:20418–20423. [PubMed] [Google Scholar]
- 43.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr., Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002;99:15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urasaki Y, Laco G, Pourquier P, et al. Characterization of a novel topoisomerase I mutation from a camptothecin-resistant human prostate cancer cell line. Cancer Res. 2001;61:1964–1969. [PubMed] [Google Scholar]
- 45.Andoh T, Ishii K, Suzuki Y, et al. Characterization of a mammalian mutant with a camptothecin resistant DNA topoisomerase I. Proc Natl Acad Sci USA. 1987;84:5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura H, Kohchi C, Yamada R, et al. Molecular cloning of a cDNA of a camptothecin-resistant human DNA topoisomerase I and identification of mutation sites. Nucleic Acids Res. 1991;19:69–75. doi: 10.1093/nar/19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pommier Y, Pourquier P, Urasaki Y, Wu J, Laco G. Topoisomerase I inhibitors: selectivity and cellular resistance. Drug Resistance Update. 1999;2:307–318. doi: 10.1054/drup.1999.0102. [DOI] [PubMed] [Google Scholar]
- 48.Xiao X, Cushman M. An ab initio quantum mechanics calculation that correlates with ligand orientation and DNA cleavage site selectivity in camptothecin-DNA-topoisomerase I ternary cleavage complexes. J Am Chem Soc. 2005;127:9960–9961. doi: 10.1021/ja042485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao X, Cushman M. Effect of E-ring modifications in camptothecin on topoisomerase I inhibition: a quantum mechanics treatment. J Org Chem. 2005;70:9584–9587. doi: 10.1021/jo0513360. [DOI] [PubMed] [Google Scholar]
- 50.Song Y, Cushman M. The Binding Orientation of a Norindenoisoquinoline in the Topoisomerase I-DNA Cleavage Complex Is Primarily Governed by pi-pi Stacking Interactions. J Phys Chem B. 2008;112:9484–9489. doi: 10.1021/jp8005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chrencik JE, Staker BL, Burgin AB, et al. Mechanisms of camptothecin resistance by human topoisomerase I mutations. J Mol Biol. 2004;339:773–784. doi: 10.1016/j.jmb.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 52.Sirikantaramas S, Yamazaki M, Saito K. Mutations in topoisomerase I as a self-resistance mechanism coevolved with the production of the anticancer alkaloid camptothecin in plants. Proc Natl Acad Sci U S A. 2008;105:6782–6786. doi: 10.1073/pnas.0801038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furuta T, Takemura H, Liao ZY, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA-double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 54.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 55.Huang X, Traganos F, Darzynkiewicz Z. DNA damage induced by DNA topoisomerase I-and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle. 2003;2:614–619. [PubMed] [Google Scholar]
- 56.Furuta T, Takemura H, Liao ZY, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 57.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 58.Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka T, Kurose A, Huang X, Dai W, Darzynkiewicz Z. ATM activation and histone H2AX phosphorylation as indicators of DNA damage by DNA topoisomerase I inhibitor topotecan and during apoptosis. Cell Prolif. 2006;39:49–60. doi: 10.1111/j.1365-2184.2006.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 61.Cheng Y, An LK, Wu N, et al. Synthesis, cytotoxic activities and structure-activity relationships of topoisomerase I inhibitors: indolizinoquinoline-5,12-dione derivatives. Bioorg Med Chem. 2008;16:4617–4625. doi: 10.1016/j.bmc.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 62.Cho WJ, Le QM, My Van HT, et al. Design, docking, and synthesis of novel indeno[1,2-c]isoquinolines for the development of antitumor agents as topoisomerase I inhibitors. Bioorg Med Chem Lett. 2007;17:3531–3534. doi: 10.1016/j.bmcl.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 63.Van HT, Le QM, Lee KY, et al. Convenient synthesis of indeno[1,2-c]isoquinolines as constrained forms of 3-arylisoquinolines and docking study of a topoisomerase I inhibitor into DNA-topoisomerase I complex. Bioorg Med Chem Lett. 2007;17:5763–5767. doi: 10.1016/j.bmcl.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 64.Tseng CH, Chen YL, Lu PJ, Yang CN, Tzeng CC. Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. Bioorg Med Chem. 2008;16:3153–3162. doi: 10.1016/j.bmc.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 65.Teicher BA. Next generation topoisomerase I inhibitors: Rationale and biomarker strategies. Biochem Pharmacol. 2008;75:1262–1271. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]