Abstract
Integrin-mediated cell attachment to the extracellular matrix is an established regulator of the cell cycle, with the best characterized targets being cyclin D1 and the cip/kip family of cdk inhibitors. Manipulation of intracellular tension affects the same targets, supporting the close relationship between integrin activation and intracellular tension. Several signaling cascades, including FAK, Rho GTPases, and ERK, transmit the integrin and tensional signals to the cell cycle. However, the experimental approaches that have generated these results alter cell adhesion and tension in ways that do not reflect the subtlety of those occurring in vivo. Increasing emphasis is therefore being placed on approaches which use micropatterning to control cell spreading and deformable substrata to model the compliance of biological tissue.
Introduction
The extracellular microenvironment has profound effects on a number of cellular functions including differentiation, apoptosis and proliferation. While many studies have shown that localized release of soluble factors affects cellular function, the mixture of matrix proteins, proteoglycans, and glucosaminoglycans that comprise the extracellular matrix (ECM) provide equally important cues that direct cellular decisions. Perhaps the newest idea about cellular control by the microenvironment is that the stiffness of the ECM (also referred to as its “compliance”) itself provides information to the cells. Several recent reviews have discussed the effects of ECM stiffness on cell differentiation [1,2]. Here, we will discuss data supporting the importance of the ECM, intracellular tension, cell shape, and ECM stiffness as regulators of integrin-dependent cell proliferation. Though our focus will be on studies showing that the ECM regulates progression through G1 phase, we note a recent study indicating that integrin-mediated adhesion can also regulate M phase [3].
Regulation of cyclin D1 expression by the ECM
Cyclin D1 has been termed a “mitogen sensor” because its expression is induced by many mitogenic factors including growth factors, cytokines, and hormones. However, our early studies showed that the induction of cyclin D1 is blocked when mitogen-stimulated cells are deprived of adhesion to substratum (reviewed in [4]). Some ECM components, such as fibronectin, synergize with growth factors to induce cyclin D1 mRNA while others, such as hyaluronan [5] and SPARC [6], antagonize growth factor signaling and inhibit the induction of cyclin D1. Thus, it is more accurate to say that cyclin D1 induction is a sensor of the mitogenic microenvironment. Although cyclin D1 induction is typically regulated transcriptionally as cells enter the cycle from quiescence, the ECM is also thought to regulate cyclin D1 translation, at least in endothelial cells (reviewed in [4]). In cycling cells, the levels of cyclin D1 mRNA are constant and the levels of cyclin D1 protein are mainly controlled by ubiquitin-mediated proteolysis [7]. PI3K-dependent activation of GSK-37beta; leads to phosphorylation of T286, thereby marking cyclin D1 for proteolysis [7], but little is known about the role of the ECM in this process. Once induced, cyclin D1 binds to cdk4/6, and the active cyclin D1-cdk4/6 holoenzyme phosphorylates the retinoblastoma protein (Rb). Rb phosphorylation and release of bound E2F are thought to signal progression through the restriction point and entry into the cell autonomous portion of the cycle.
Cyclin D1 gene expression requires ECM-mediated sustained ERK activity
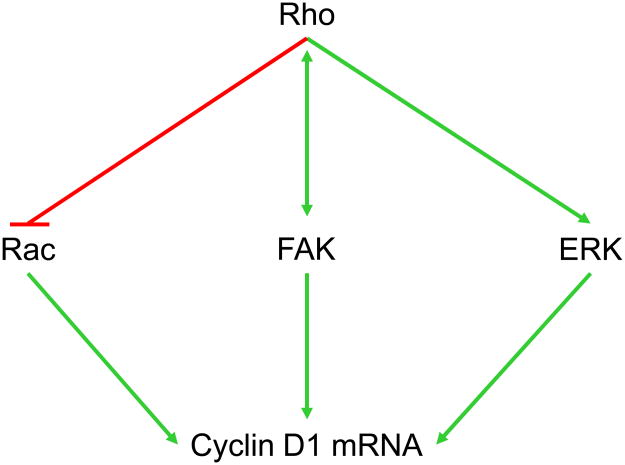
Studies using pharmacological MEK inhibitors and dominant negative/constitutively active mutants of the ERK cascade indicate that ERK activity (probably ERK5 as well as the prototypical ERK1 and ERK2 [8]) stimulates cyclin D1 expression in mid-G1 phase in many cell types (Fig. 1; reviewed in [9]). ERK activity stimulates the cyclin D1 promoter whereas ERK inhibition represses the cyclin D1 promoter; corresponding changes occur in cyclin D1 mRNA. Several studies have shown that the induction of cyclin D1 mRNA seen after mitogenic stimulation of quiescent MEFs requires a sustained activation of ERKs for 5–6 h. Within this period, the first 3 hours of ERK activity are dispensable while the period between 3–6 h is essential [10]. Both growth factor activation of receptor tyrosine kinases and integrin-mediated adhesion to the ECM can stimulate transient ERK activity, but the sustained activity needed for cyclin D1 gene induction requires a cooperative interaction between the two signaling pathways (reviewed in [9]). Since cultured mesenchymal cells require cyclin D1 for S phase entry, the synergistic effects of growth factors and the ECM on ERK signal duration can explain, at least in part, why cell adhesion to the ECM is required for cell proliferation.
Figure 1. Integrin-mediated signaling pathways to the cyclin D1 gene.
Well characterized integrin-mediated pathways that regulate cyclin D1 gene expression. The figure diagrams the primary pathways discussed in this review.
FAK and cyclin D1 gene expression
Focal Adhesion Kinase (FAK) is the canonical mediator of integrin signals [11], and several reports have linked FAK to the induction of cyclin D1 (Fig. 1). FAK is recruited to focal adhesions via its FAT domain and is autophosphorylated at Y397 upon ECM-induced integrin clustering. Y397 phosphorylation creates a binding site for Src and results in the Src-dependent tyrosine phosphorylation of FAK at several other sites including Y576 and Y577 (which are thought to increase FAK kinase activity). FAK and Src phosphorylate p130Cas which activates Jun kinase and promotes G1 phase progression [12]. Jun kinase activity has been linked to cyclin D1 gene expression [13]. FAK is also thought to stimulate cyclin D1 gene transcription by regulating two transcription factors (an Ets-like factor and KLF8) which, in turn, activate the cyclin D1 promoter [14,15]. Ets-like factors are regulated by ERK activity, and provide a potential link between the reported effects of FAK on ERK activity and cyclin D1. However, the role of FAK in regulating ERK activity remains controversial: some studies indicate an important role for FAK in the cooperative effects of growth factors and the ECM on ERK activity, while other studies support a role for Src-family kinases (SFKs) independent of FAK (reviewed in [16]). It appears that the effect of FAK on ERK activity may be dependent on cell type or the cellular context.
If FAK is not required for ERK activation, a second pathway must exist by which FAK can regulate cyclin D1 expression. As described above, FAK can upregulate the transcription factor KLF8, and KLF8 can bind to and upregulate the cyclin D1 promoter [15]. KLF8 binds to a GT box motif (GTGGG) in the cyclin D1 promoter and is negatively regulated by sumoylation by PIAS family SUMO E3 ligases [17]. Desumoylation is a positive regulator of cyclin D1 expression in LNCaP prostate cancer cells [18]. KLF8 is also required for v-Src-induced transformation of NIH-3T3 fibroblasts and overexpressed in several types of human cancer cells and primary tumor tissues [19].
It is important to emphasize that the effect of FAK deletion on proliferation has been variable. Tissue specific knock-out of FAK inhibits proliferation of mammary epithelial cells [20] and cardiomyocytes [21] but not keratinocytes [22] or endothelial cells. [23]. These different results may be due to the recent finding that unphosphorylated FAK at integrins can act as a proliferation inhibitor [24]. Alternatively, tissue-specific compensation by Pyk2 [22,25] may explain the different proliferative phenotypes seen after FAK knock-out. Many signaling pathways can induce cyclin D1 mRNA [26]; some of these may be FAK-independent and support cell type-specific proliferation in the absence of FAK.
ECM binding to integrins downregulates cip/kip cdk inhibitors
In addition to cyclin D1, the ECM regulates the expression of the cip/kip cdk inhibitor family, including p21cip1 and p27kip1, which regulates G1 phase cdk2 activity and S phase entry (reviewed in [4,16]). When integrin signaling is blocked by incubating cells in suspension, the levels of p21cip1 and p27kip1 increase. Skp2 is the substrate-targeting component of the E3 ligase that degrades p27kip1, and cell adhesion to ECM regulates p27kip1 levels by controlling the levels of Skp2 mRNA and protein [27]. FAK also regulates Skp2 post-translationally by preventing its proteosomal degradation [28]. The mechanism responsible for the elevation of p21cip1 is not yet resolved. This increase in cip/kip cdk inhibitors results in the inhibition of cyclin E-cdk2 and Rb phosphorylation. Though cip/kip proteins also facilitate the assembly of cyclin D-cdk4/6 complexes, the low levels of cyclin D1 in nonadherent cells (see above) implies that the assembly function of cip/kips is not utilized when integrin signaling is blocked.
Effects of intracellular tension, the actin cytoskeleton, and Rho family GTPases on adhesion-dependent signaling to the cell cycle
The actin cytoskeleton plays a major role in determining the tensional state of a cell, and a high tensional state is characterized by the appearance of actin stress fibers. Studies using actin depolymerizing drugs have shown that sustained ERK activity and ERK-dependent cyclin D1 induction correlate with actin stress fibers and intracellular tension (reviewed in [4]). The Rho-Rho kinase pathway stimulates actin stress fiber formation by increasing actin polymerization and cellular contractility [29–31], and Rho-dependent contractility leads to the clustering of integrins [32]. Like actin depolymerizing drugs, inhibition of the Rho-Rho kinase pathway blocks sustained ERK activity and ERK-dependent cyclin D1 induction [33–35]. Thus, Rho activity may stimulate cyclin D1 by regulating integrin-dependent ERK signal duration (Fig. 1). Cip/kip levels are upregulated upon inhibition of Rho (reviewed in [4]). Rho and FAK signaling appear to be in a dynamic equilibrium (Fig. 1) because the high tensional state associated with active Rho signaling and stress fiber formation is linked to the activation of FAK [36,37]. Conversely, FAK can negatively regulate Rho activity [22,38].
Like sustained ERK activity, Rac plays an important role in G1 phase cell cycle progression and cyclin D1 induction (Fig. 1). In addition to its ability to regulate ERK activity through PAK [39], Rac1 stimulates ERK-independent transcription of the cyclin D1 promoter, induces cyclin D1 mRNA in fibroblasts, and stimulates the translation of cyclin D1 mRNA in endothelial cells (reviewed in [16]). Some studies indicate that Rac regulates cyclin D1 through activation of NF-κB[40], but we find that Rac and NF-κB work in parallel pathways to regulate the cyclin D1 promoter in fibroblasts [41]. Integrin-mediated adhesion to the ECM stimulates the GTP-loading of Rac and also allow for the coupling between activated Rac and its effectors [42]. The Rac pathway results in an early G1 phase induction of cyclin D1, but this is rarely seen in fibroblasts because Rac signaling to cyclin D1 is inhibited by the Rho-Rho kinase pathway downstream of Rac GTP-loading (Fig. 1; [35]). Deliberate inhibition of Rho-Rho kinase signaling inhibits stress fiber formation, sustained ERK activity, and ERK-dependent cyclin D1 induction but does not prevent Rac signaling to cyclin D1 [35]. Thus, Rac can signal to cyclin D1 in a low tensional environment where ERK signaling to cyclin D1 is precluded. Rac signaling to cyclin D1 is readily detectable in MCF10A mammary epithelial cells [43], perhaps because these cells do not have the high intracellular tensional environment characteristic of fibroblasts.
Others have reported that depolymerization of f-actin does not prevent cycling in N2 neuroblastoma and Chinese hamster ovary cells after release from an M phase synchronization [44]. In this study, cell cycling occurred despite loss of focal adhesions and inhibition of FAK phosphorylation, ERK activity, and cyclin D1 expression. It will be important to determine whether the tensional requirements for cycling also differ in nontransformed cells entering G1 phase from G0 or the previous M phase.
Effects of cell spreading on integrin signaling and cell proliferation
While the majority of studies of intracellular tension use pharmacological inhibitors of actin polymerization or dominant-negative Rho GTPases to disrupt intracellular tension, an alternative approach is to control cell spreading. Cell spreading can be controlled by plating cells on different densities of matrix protein or by plating cells onto micropatterned, matrix protein-coated “islands” of defined size; to date most of the substrates have been rigid. Micropatterning allows for careful control of cell spreading without the confounding effects of altering ligand density [45]. Using these approaches, the Ingber lab showed that cyclin D1 protein (but not mRNA) levels, Rb phosphorylation, and S phase entry were directly proportional to spreading of capillary endothelial cells while the levels of p27kip1 were inversely proportional to spreading [46]. Subsequent studies showed that the spreading-dependent changes in p27kip1 were regulated by a Rho-dependent induction of Skp2 involving the Rho effectors mDia and Rho kinase [47]. The mechanism underlying this effect has not been described, but exogenous expression of constitutively active Rho rescued p27kip1 degradation and S phase entry.
Cell spreading directly correlates with FAK activity and S phase entry when endothelial cells are cultured on fibronectin-coated micropatterned islands [45]. More recent studies indicate that FAK can be both pro- and anti-proliferative depending on the degree of cell spreading [24]. In spread cells with a developed actin cytoskeleton, FAK is phosphorylated leading to pro-proliferative downstream signaling. However, FAK is anti-proliferative in unspread cells because, in addition to loss of pro-proliferative FAK-mediated signaling, unphosphorylated FAK actively inhibits Rho signaling and causes a decrease in intracellular tension [24]. FAK inhibits intracellular tension by activating p190RhoGAP at focal adhesions, as well as by directing focal adhesion localization of PKL-PIX-PAK (reviewed in [48]) to repress Myosin Light Chain Kinase activity [22].
Modeling the effects of tissue and ECM compliance on integrin signaling and proliferation
Although actin depolymerizing drugs and deliberate Rho-Rho kinase inhibition (e.g. with C3 toxin, dominant negative constructs, RNAi, or Y27632) have been widely used to study effects of integrin signaling on cellular function, these approaches likely result in much more severe changes in f-actin and Rho-Rho kinase activity than occur in vivo. Additionally, a pervasive shortcoming of these approaches is that the cells are cultured on nondeformable 2D substrata (culture dishes or glass coverslips) which have little relationship to the compliant ECM that cells encounter in vivo (Table 1). The Grinnell lab among others has championed the use of 3D collagen gels to study the effect of a more physiological matrix and tensional setting on the cell cycle in fibroblasts. They reported that human foreskin fibroblasts in free-floating (tensionally relaxed) collagen gels have high levels of p27kip1, do not phosphorylate ERK, and do not induce cyclin D1 [49,50]. Similarly, cyclin D1 expression is reduced when hepatocytes are cultured on collagen gels, relative to rigid collagen films [33]. Rho and FAK activities also decrease on floating collagen gels [50,51], thereby regulating tension-dependent cyclin D1 expression (see above). Others have concluded that increased cdk inhibitor expression (p27kip1, p21cip1, or p15INK4B depending upon cell type) is responsible for G1 phase arrest that occurs when cells are plated on collagen gels [49,52,53].
Table 1. Compliance of biological tissues and culture models.
The table summarizes the compliances of several biological tissues and compares them to the compliances of traditional culture substrata (polystyrene and glass) or hydrogels.
Collectively, these data suggest that changes in intracellular tension resulting from differences in ECM stiffness can regulate the cell cycle even when integrin-mediated adhesion persists. However, the compliance of collagen gels is much greater than that of many physiological tissues (10–100 Pa vs. 200–10,000 Pa) [54]. Moreover, changing the compliance of collagen gels by increasing the collagen concentration inherently increases the integrin ligand concentration, so observed effects can not be attributed solely to changes in matrix stiffness. The same complication exists when cells are cultured in Matrigel, which is also less well-defined chemically.
Careful control of substratum compliance, as might be seen during physiological or pathological matrix remodeling, is perhaps best achieved by seeding cells on ECM-coated hydrogels [55]. Hydrogels prepared from polyethylene glycol have elastic moduli of 20–400 Pa [56] making them particularly useful when studying very compliant tissue (Table I). Hydrogels based on polyacrylamide have elastic moduli of ~100–50,000 Pa [55] which encompasses the compliance range of most physiological tissues (Table I). Major advantages of ECM-coated hydrogels include the facts that i) the gels are inert as adhesive surfaces and only the proteins covalently bound to their surface can act as cellular ligands; ii) the elastic modulus (compliance) can adjusted in a quantifiable and reproducible way; iii) cell recovery is straightforward. Polyacrylamide-based substrata have become increasingly used to study the role of substratum compliance on integrin signaling complexes, [57], the actin cytoskeleton [58,59], neural growth [60], and cell differentiation [61]. By remodeling their actin cytoskeleton, cells adapt their internal stiffness to match the compliance of their ECM [62].
Early studies showed that normal rat kidney cells were unable to fully spread or form focal adhesions at their periphery when plated on soft collagen-coated polyacrylamide gels [63]. Focal adhesions were irregularly shaped and much more dynamic on the soft substrate, whereas phosphopaxillin and overall phosphotyrosine were more abundant in cells on stiff substrates [63]. Others have plated Fyn-reconstituted Src/Yes/Fyn knockout cells on fibronectin-coated polyacrylamide substrata to show that Fyn recruitment to focal complexes at the leading edge of spreading cells is inversely related to ECM compliance [57]. This led to a rigidity-dependent recruitment and phosphorylation of p130Cas [57]. Keratinocytes and fibroblasts induce expression of h2-calponin when cultured on a low compliance substrate [59]. h2-calponin binds to lamellipodial protrusions and the ends of actin stress fibers, conferring increased resistance of the actin cytoskeleton to depolymerizing drugs when cells are cultured on a nondeformable (glass) substratum [59,64]. Thus, the rigidity-dependent induction of h2-calponin may stabilize the actin cytoskeleton and thereby regulate compliance-dependent integrin signaling.
Recently, matrix protein-coated hydrogels have been used to show that S phase entry and cell proliferation are inversely related to ECM compliance ([37,65,66] and our unpublished results). ECM rigidity positively regulates Rho activity, FAK autophosphorylation, and ERK activity in mammary epithelial cells and NIH-3T3 fibroblasts [37]. As Rho and ERK activities are increased, intracellular contractility is enhanced, stress fibers form, and focal complexes mature into vinculin-containing focal adhesions. The rising levels of endogenous forces within the cell positively feedback to promote further focal adhesion formation, Rho, and ERK activation [37].
Two recent studies have examined the effect of ECM compliance on transformed colon and breast cancer cells [37,67]. As opposed to nontransformed cells, spreading and FAK autophosphorylation in highly transformed cells were relatively insensitive to ECM compliance. Transformed mammary epithelial cells also had active Rho and ERK on highly compliant substratum, and deliberate inhibition of intracellular tension reduced FAK and ERK activities as well as cell spreading. The tension-inhibited cells also assumed a more normal cellular morphology in 3D culture. One of these studies proposed that normalization of mechanosignaling by pharmacological inhibition of Rho kinase, ERK activity, or integrin activation has the potential to reduce the malignant phenotype of tumors [37].
A working model for cell cycle control by intracellular tension and extracellular stiffness
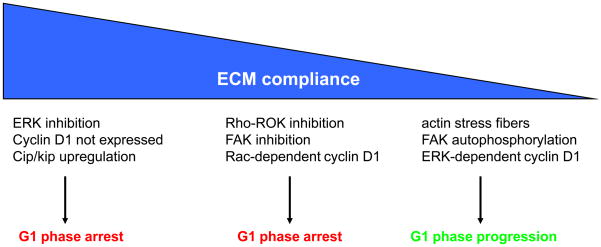
Collectively, the results discussed above indicate that integrin-regulated cell cycle progression is not a binary process of “off” or “on” but rather a collection of signaling events with different compliance/tensional thresholds (Fig. 2). For example, at highest ECM compliance, FAK autophosphorylation is reduced, ERK activity is not sustained, cyclin D1 is not expressed, and cdk inhibitor expression is upregulated. This molecular signature, which results in G1 phase arrest, is seen upon actin depolymerization, Rho inhibition, or complete blockade of integrin signaling. A very similar phenotype is observed when cells are cultured in relaxed collagen gels. At intermediate compliances where Rho-Rho kinase activity is still low, Rac signaling to cyclin D1 results in partial, but still incomplete, progression through G1 phase. This phenotype has been seen in mammary epithelial cells cultured in on deformable substrata of near physiological compliance (our unpublished results). Further decreases in compliance restore normal Rho and FAK signaling and are associated with ERK activation, ERK-dependent induction of cyclin D1 mRNA, and efficient S phase entry. It is unlikely that the partial G1 phase progression seen at intermediate compliance is biologically important for proliferation since these cells do not enter S phase. However, the distinct molecular signatures seen at high, intermediate, and low compliance do indicate that complete progression through G1 phase reflects the existence of graded mechanical controls on the cell cycle.
Figure 2. Tensional sensing in cell proliferation.
The figure shows a working model of how changes in ECM compliance affect signal transduction and G1 phase cell cycle events. Highly compliant substrata such as relaxed collagen gels (left) inhibit cyclin D1 expression and also upregulate the cdk inhibitors that prevent cdk2 activation. Intermediate compliances (middle) allow for Rac-dependent cyclin D1 induction, but this is not sufficient for Rb phosphorylation and S phase entry (our unpublished results). Low ECM compliances (right) as seen when cells are plated on non-deformable substrata or relatively stiff deformable substrata lead to FAK autophosphorylation, ERK-dependent cyclin D1 gene expression, Rho activity, the appearance of actin stress fibers and cell cycling.
Conclusions and Future Questions
The extracellular matrix is remodeled physiologically and also pathologically in diseases as diverse as fibrosis, cancer, and atherosclerosis. The results to date indicate that changes in ECM stiffness affect cell morphology, integrin signaling, and the actin cytoskeleton to control the cell cycle. Since different integrin-dependent signaling events have distinct compliance thresholds, the molecular composition of integrin signaling complexes at high and low tissue stiffness, as well as the compliance-regulated targets within the cell cycle, will be important matters to resolve. Such information should help us to understand how ECM remodeling, tissue compliance, and intracellular tension regulate cell proliferation in physiological and pathological tissues.
Acknowledgments
Work in our lab is supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Discher DE, et al. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. Am Physiological Soc. 2005;98(4):1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 3.Reverte CG, et al. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J Cell Biol. 2006;174(4):491–497. doi: 10.1083/jcb.200603069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11(1):48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 5.Kothapalli D, et al. Hyaluronan and CD44 antagonize mitogen-dependent cyclin D1 expression in mesenchymal cells. J Cell Biol. 2007;176(4):535–544. doi: 10.1083/jcb.200611058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francki A, et al. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J Cell Biochem. 2003;88(4):802–811. doi: 10.1002/jcb.10424. [DOI] [PubMed] [Google Scholar]
- 7.Diehl JA, et al. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulloy R, et al. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene. 2003;22(35):5387–5398. doi: 10.1038/sj.onc.1206839. [DOI] [PubMed] [Google Scholar]
- 9.Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22(9):818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Villanueva J, et al. ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets. Mol Biol Cell. 2007;18(4):1457–1463. doi: 10.1091/mbc.E06-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(8):1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 12.Oktay M, et al. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145(7):1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Bergami P, et al. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11(5):447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J, et al. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12(12):4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, et al. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11(6):1503–1515. doi: 10.1016/s1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114(14):2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 17.Wei H, et al. Sumoylation delimits KLF8 transcriptional activity associated with the cell cycle regulation. J Biol Chem. 2006;281(24):16664–16671. doi: 10.1074/jbc.M513135200. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, et al. Role of desumoylation in the development of prostate cancer. Neoplasia. 2006;8(8):667–676. doi: 10.1593/neo.06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26(3):456–461. doi: 10.1038/sj.onc.1209796. [DOI] [PubMed] [Google Scholar]
- 20.Nagy T, et al. Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J Biol Chem. 2007;282(43):31766–31776. doi: 10.1074/jbc.M705403200. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Wu X, Druso JE, Wei H, Park AYJ, Kraus MS, Alcaraz A, Chen J, Chien S, Cerione RA, Guan JL. Cardiac development defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci U S A. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schober M, et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol. 2007;176(5):667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braren R, et al. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172(1):151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirone DM, et al. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol. 2006;174(2):277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weis SM, et al. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol. 2008;181(1):43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu M, et al. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145(12):5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 27.Carrano AC, Pagano M. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J Cell Biol. 2001;153(7):1381–1390. doi: 10.1083/jcb.153.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bond M, et al. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. J Biol Chem. 2004;279(36):37304–37310. doi: 10.1074/jbc.M404307200. [DOI] [PubMed] [Google Scholar]
- 29.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 30.Kaibuchi K, et al. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 31.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 32.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133(6):1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen LK, Albrecht JH. Regulation of the hepatocyte cell cycle by type I collagen matrix: role of cyclin D1. J Cell Sci. 1999;112(17):2971–2981. doi: 10.1242/jcs.112.17.2971. [DOI] [PubMed] [Google Scholar]
- 34.Swant JD, et al. Rho GTPase-dependent signaling is required for macrophage migration inhibitory factor-mediated expression of cyclin D1. J Biol Chem. 2005;280(24):23066–23072. doi: 10.1074/jbc.M500636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welsh CF, et al. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3(11):950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 36.Sinnett-Smith J, et al. Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130(CAS) Exp Cell Res. 2001;266(2):292–302. doi: 10.1006/excr.2001.5219. [DOI] [PubMed] [Google Scholar]
- 37.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Ren XD, et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113 (Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 39.Eblen ST, et al. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22(17):6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joyce D, et al. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274(36):25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 41.Klein EA, et al. NFkappaB-independent signaling to the cyclin D1 gene by Rac. Cell Cycle. 2007;6(9):1115–1121. doi: 10.4161/cc.6.9.4147. [DOI] [PubMed] [Google Scholar]
- 42.del Pozo MA, et al. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. Embo J. 2000;19(9):2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fournier AK, et al. Rac-dependent cyclin D1 gene expression regulated by cadherin-and integrin-mediated adhesion. J Cell Sci. 2008;121(2):226–233. doi: 10.1242/jcs.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margadant C, et al. Focal adhesion signaling and actin stress fibers are dispensable for progression through the ongoing cell cycle. J Cell Sci. 2007;120(1):66–76. doi: 10.1242/jcs.03301. [DOI] [PubMed] [Google Scholar]
- 45.Chen CS, et al. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 46.Huang S, et al. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9(11):3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mammoto A, et al. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279(25):26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 48.Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 49.Fringer J, Grinnell F. Fibroblast quiescence in floating or released collagen matrices: contribution of the ERK signaling pathway and actin cytoskeletal organization. J Biol Chem. 2001;276(33):31047–31052. doi: 10.1074/jbc.M101898200. [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeldt H, Grinnell F. Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J Biol Chem. 2000;275(5):3088–3092. doi: 10.1074/jbc.275.5.3088. [DOI] [PubMed] [Google Scholar]
- 51.Wozniak MA, et al. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163(3):583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koyama H, et al. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87(6):1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 53.Wall SJ, et al. The cyclin-dependent kinase inhibitors p15INK4B and p21CIP1 are critical regulators of fibrillar collagen-induced tumor cell cycle arrest. J Biol Chem. 2007;282(33):24471–24476. doi: 10.1074/jbc.M702697200. [DOI] [PubMed] [Google Scholar]
- 54.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. Journal of Biomaterials Science, Polymer Edition. 2004;15(12):1521–1531. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 55.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 56.Peyton SR, et al. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27(28):4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Kostic A, Sheetz MP. Fibronectin Rigidity Response through Fyn and p130Cas Recruitment to the Leading Edge. Molecular Biology of the Cell. 2006;17(6):2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goffin JM, et al. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172(2):259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hossain MM, et al. h2-Calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J Biol Chem. 2005;280(51):42442–42453. doi: 10.1074/jbc.M509952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Georges PC, et al. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90(8):3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 62.Solon J, et al. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danninger C, Gimona M. Live dynamics of GFP-calponin: isoform-specific modulation of the actin cytoskeleton and autoregulation by C-terminal sequences. J Cell Sci. 2000;113(21):3725–3736. doi: 10.1242/jcs.113.21.3725. [DOI] [PubMed] [Google Scholar]
- 65.Khatiwala CB, et al. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am J Physiol Cell Physiol. 2006;290(6):C1640–1650. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]
- 66.Kong HJ, et al. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci U S A. 2006;103(49):18534–18539. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.von Wichert G, et al. Focal adhesion kinase mediates defects in the force-dependent reinforcement of initial integrin-cytoskeleton linkages in metastatic colon cancer cell lines. Eur J Cell Biol. 2008;87(1):1–16. doi: 10.1016/j.ejcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Samani A, et al. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 2007;52(6):1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- 69.Kellner AL, et al. Simulation of mechanical compression of breast tissue. IEEE Trans Biomed Eng. 2007;54(10):1885–1891. doi: 10.1109/TBME.2007.893493. [DOI] [PubMed] [Google Scholar]
- 70.Georges PC, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1147–1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 71.Rai US, Singh RK. Synthesis and mechanical characterization of polymer-matrix composites containing calcium carbonate/white cement filler. Materials Letters. 2004;58(1–2):235–240. [Google Scholar]
- 72.Bauccio M, editor. ASM engineered materials reference book. ASM International; 1994. [Google Scholar]