Abstract
Autoimmune diseases are thought to result from imbalances in normal immune physiology and regulation. Here, we show that autoimmune disease susceptibility and resistance alleles on mouse chromosome 3 (Idd3) correlate with differential expression of the key immunoregulatory cytokine interleukin-2 (IL-2). In order to test directly that an approximately two-fold reduction in IL-2 underpins the Idd3-linked destabilization of immune homeostasis, we demonstrate that engineered haplodeficiency of IL-2 gene expression not only reduces T cell IL-2 production by two-fold but also mimics the autoimmune dysregulatory effects of the naturally-occurring susceptibility alleles of IL-2. Reduced IL-2 production achieved by both genetic mechanisms correlates with fewer and less functional CD4+CD25+ regulatory T cells, which are critical for maintaining immune homeostasis.
Multifactorial diseases with high population prevalence develop as a result of interactions between multiple genetic and environmental factors. Since the early 1990s, several loci have been mapped by genetic linkage and association analyses in humans and in rodent models of autoimmune disease, including type 1 diabetes (T1D). T1D is caused by the destruction of the insulin-producing pancreatic beta cells by various immune cell types, including CD8+ cytotoxic T-cells. In human T1D, four loci, in addition to the HLA region, have been identified: the genes encoding insulin, the negative immunoregulatory molecules CTLA-4 and LYP, and, most recently, the alpha chain of the interleukin-2 receptor (CD25) 1. All of these common variants or haplotypes support the concept that autoimmunity is a part of normal physiology, and that the balance between immune responses to foreign antigens and to self, in some cases leading to pathology, is dependent on a complex network of negative and positive feedback molecules and mechanisms, shaped during evolution as an effective defence to infections. A better understanding of this homeostasis might help identify, and facilitate the development of, strategies to modulate disease susceptibility based on immune physiology in vivo.
Insulin-dependent diabetes 3 (Idd3), on mouse chromosome 3, has a major effect on T1D development in the nonobese diabetic (NOD) mouse model of T1D, promoting the infiltration of autoreactive lymphocytes into the pancreas 2. Protective alleles at Idd3 reduce T1D frequency approximately 70 and 90% in females and males, respectively 3. The Idd3 region also determines susceptibility to experimental autoimmune encephalomyelitis and autoimmune ovarian dysgenesis (AOD) induced by neonatal thymectomy 4,5, a disease that is highly dependent on regulatory T-cell function 6. Using a positional cloning strategy, we have shown that the Idd3 region spans 780 kb and includes the IL-2 gene, which is a molecule critical for the maintenance of immune homeostatsis 3. Although we have hypothesized that Il2 is Idd3 7, the IL-21 gene is also a positional and functional candidate gene for Idd3 8. The aim of the current study was to test the hypothesis that the IL-2 gene accounts for the effects on T1D mediated by Idd3.
RESULTS
Genetic analyses
To positionally clone the gene accounting for the Idd3 phenotype, we generated congenic NOD strains carrying progressively shorter intervals of B6-derived DNA. The first congenic strain that we developed had approximately 85 Mb of B6 chromosome 3 DNA 2. The region was subsequently narrowed to ~780 kb by studying seven additional B6 Idd3 congenic strains 3. Remarkably, the largest and smallest introgressed intervals conferred equivalent levels of T1D resistance, suggesting that a single gene influences T1D in this region of chromosome 3. In addition, congenic strains of mice with introgressed B6-derived DNA near Idd3 but carrying NOD alleles at Idd3, had a T1D frequency indistinguishable from the NOD parental strain 2,3,9 (and references therein).
Using additional polymorphic markers to define the recombination breakpoints of the NOD.B6 Idd3 congenic strains described by Lyons et al 3, we show here that the Idd3 interval spans ~650 kb and have established that it contains five known genes (Tenr, Il2, Il21, Centrin4 and Fgf2), two predicted genes of unknown function (KIAA1109 and KIAA1371), and three pseudogenes (http://T1DBase.org; http://mouse.ensembl.org; Supplemental Fig. 1a). BAC-based DNA sequence analyses revealed that the NOD and 129 Idd3 intervals are identical by descent (IBD) [0.68 single nucleotide polymorphisms (SNPs) per 10 kb, with nearly all of these few SNPs in intragenic regions] 10 throughout the portions of Idd3 that were sequenced from both (~250 kb). The observation that NOD and 129 are IBD at Idd3 is consistent with the NOD-like T1D frequency of NOD.129 Idd3 congenic mice 7, as well as with the assumption that most genes affecting common disease will be in regions that are not IBD 10. In contrast to the IBD pattern observed for the NOD/129 comparison, the B6 strain has a distinct sequence or haplotype with approximately 100 SNPs per 10 kb throughout the Idd3 region (Supplemental Fig. 1a), which includes amino acid-changing SNPs in exon 1 of the IL-2 gene that alter the glycosylation of IL-2 and could be the molecular basis of Idd3 7.
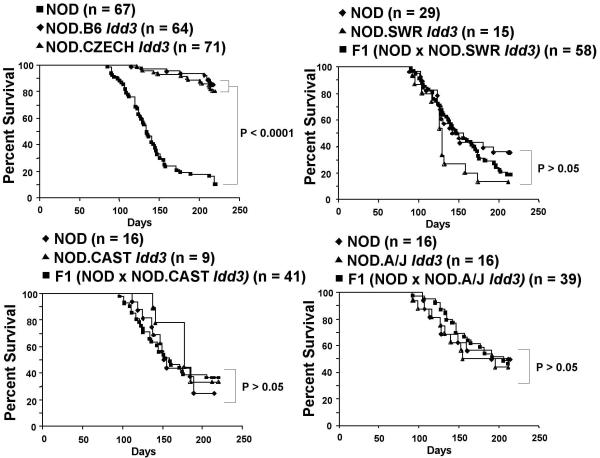
Because comparison of the B6 and NOD/129 SNP maps demonstrated a haplotypic difference throughout the 650 kb Idd3 region, we investigated the correlation between disease susceptibility and Idd3 SNPs by developing, phenotyping and sequencing congenic NOD mice carrying Idd3 DNA from four different inbred mouse strains. Of primary interest was the NOD.CZECH Idd3 congenic strain that was developed from the wild-derived CZECH inbred strain, which has an exon 1 sequence and glycosylation pattern similar to that of NOD 7, and was therefore predicted to be susceptible to T1D. Unexpectedly, we found that NOD.CZECH Idd3 mice are resistant to diabetes, with a T1D frequency identical to that of NOD.B6 Idd3 (Fig. 1), ruling out the possibility that glycosylation differences caused by allelic variation of Il2 exon 1 explain the molecular basis for Idd3. We also developed and phenotyped the NOD.A/J Idd3, NOD.SWR Idd3, and NOD.CAST Idd3 congenic mouse strains. The A/J and SWR Idd3 regions were selected for analysis because the IL-2 molecule encoded by these strains is identical to that of NOD 7, and the Idd3 region of the A/J strain predisposes to autoimmune ovarian dysgenesis 5. The CAST strain from M. musculus castaneous, as well as the CZECH strain from M. musculus musculus, are derived from populations of mice found in the wild, and thus have a greater diversity of sequence variants as compared to inbred laboratory strains, an attribute that could ultimately reduce the number of disease-associated candidate SNPs. In an F2 segregation analysis, mice homozygous for the introgressed Idd3 region from the NOD.SWR Idd3, NOD.CAST Idd3, and NOD.A/J Idd3 incipient congenic strains were found to be as susceptible to T1D as their littermates homozygous for the NOD allele at Idd3 (Fig. 1). We re-sequenced the two main candidate genes, Il2 and Il21 in this panel of T1D susceptible (NOD, NOD.CAST Idd3, NOD.SWR Idd3, NOD.A/J Idd3, and NOD.129 Idd3) and resistant strains (NOD.CZECH Idd3, NOD.B6 Idd3 and NON, in which the resistance phenotype at Idd3 was determined in F2 and backcross segregation analyses 11). However, disease-associated SNPs, defined as those shared by the susceptible strains, but different from those found in the resistant strains, were numerous and found in or near both genes (Supplemental Figs. 1b-e). No nonsynonymous coding or obvious splice site-changing SNPs were identified, and, therefore, we evaluated the hypothesis that Idd3 corresponds to disease-associated SNPs that alter production and/or stability of the IL-2 and/or IL-21 gene transcripts.
Figure 1. Frequency of diabetes in Idd3-congenic NOD strains.
The frequency of the novel NOD.CZECH Idd3 congenic strain was compared with those of the NOD.B6 Idd3 congenic strain (Line 1098, Taconic Farms, NY) and NOD mice. The diabetes phenotypes of the Idd3 alleles of CAST, SWR and A/J were determined by assessing the frequency of diabetes in F2 progeny generated from each strain using heterozygous breeders at the N6, N7 and N8 generations. Each figure includes mice homozygous for the NOD alleles and the non-NOD alleles at Idd3 as well as the heterozygous progeny.
Idd3 regulates Il2 transcription
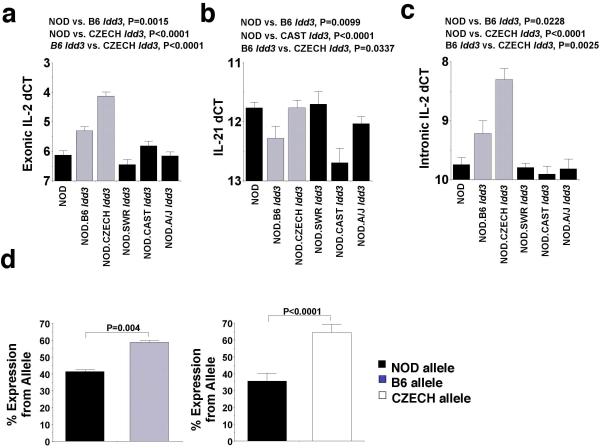
We found a correlation between disease-susceptible versus disease-resistant Idd3 alleles and IL-2 mRNA expression in thymocytes from the five strains that were examined: thymocytes from T1D-susceptible NOD, NOD.129 Idd3 and NOD.CAST Idd3 mice produced approximately 2-fold less IL-2 mRNA than thymocytes from T1D-protected NOD.B6 Idd3 or NOD.CZECH Idd3 mice (Supplemental Fig. 2). Only low levels of IL-2 message were detectable in the splenocytes of these mice ex vivo but a similar difference was observed between resistant and susceptible strains (data not shown). Analysis of splenocytes from mice treated with anti-CD3 mAb in vivo (six strains examined) revealed a strong upregulation of IL-2 mRNA and confirmed the above correlation between IL-2 expression and Idd3-associated susceptibility to diabetes (Fig. 2a): cells from T1D-resistant NOD.B6 Idd3 and NOD.CZECH Idd3 mice had more IL-2 mRNA than those from T1D-susceptible NOD, NOD.A/J Idd3, NOD.SWR Idd3 and NOD.CAST Idd3 mice. In this experimental system, and in contrast to what we saw above with unstimulated thymocytes, splenocytes from anti-CD3 mAb-treated NOD.CZECH Idd3 mice contained higher levels of IL-2 message than splenocytes from anti-CD3 mAb-treated NOD.B6 Idd3 mice (Fig. 2a), possibly reflecting differences in responsiveness of the corresponding Il2 alleles to endogenous stimuli (positive selection in the thymus) versus anti-CD3 mAb. Whatever the underlying mechanisms might be, differential expression of Il2 was seen in both CD4+ and CD8+ T-cells (Supplemental Fig. 3). A correlation between IL-21 mRNA levels and disease susceptibility or resistance was not observed (Fig. 2b). We developed a quantitative PCR assay for intron 2 of Il2 to assess pre-mRNA levels of IL-2 transcripts. We observed similar allele-dependent differences in expression (Fig. 2c), indicating that the above observations reflect differences in the number of cells producing IL-2 mRNA and/or in the rate of IL-2 transcription initiation, rather than differences in mature IL-2 message stability. Allele-specific assays confirmed that the T1D-resistant B6 and CZECH Il2 haplotypes were transcriptionally more active than the NOD haplotype in splenic T-cells from anti-CD3 mAb-treated F1 mice (Fig. 2d). Once again, the CZECH Il2 allele was observed to be transcriptionally more active than the B6 Il2 allele (using the IL-2 message levels corresponding to the NOD allele as a reference point). Analysis of RNA from anti-CD3 mAb-treated B10.BR mice with and without a congenic NOD Idd3 region further indicated that these hypomorphic allelic differences in IL-2 gene expression are Idd3 region-intrinsic (Supplemental Fig. 3b).
Figure 2. Correlation of Idd3-linked disease susceptibility and resistance with Il2 expression.
a and b, RT-QPCR for IL-2 (a) and IL-21 mRNA (b) using RNA isolated from the spleens of mice treated with anti-CD3 mAb (n values for IL-2 and IL-21, respectively: NOD.B6 Idd3=19 and 16; NOD.A/J Idd3=24 and 13; NOD.CZECH Idd3=18 and 18; NOD.SWR Idd3=15 and 11; NOD.CAST Idd3=10 and 10). A dCT of 1 corresponds to a 2-fold increase in message levels, dCT of −1, a 2-fold decrease. Note that whereas “high” and “low” levels of IL-2 mRNA were associated with diabetes resistance and susceptibility, respectively (a), “high” and “low” levels of IL-21 mRNA were not (b). c, Intronic RT-QPCR for IL-2 in the above samples. d, Allele-specific Pyrosequencing® assays were used to measure the relative amounts of RNA from the disease-resistant and disease-susceptible alleles in splenic T-cells from anti-CD3 mAb-treated (NOD x NOD.B6 Idd3) F1 (tested with IL2-5616 forward assay and IL2-6283 reverse assay) and (NOD x NOD.CZECH Idd3) F1 mice (tested with IL2-5616 forward assay).
To confirm these observations, and to elucidate the cellular basis of Idd3, we introduced one or two copies of the B6 Idd3 region into transgenic NOD mice expressing a diabetogenic T-cell receptor (TCR; 8.3-NOD mice) 12. This TCR is representative of a large fraction of islet-associated CD8+ T-cells in NOD mice that use highly homologous TCRα chains 12-14 and recognize the mimotope NRP-A7 in the context of the MHC molecule H-2Kd 15. These T-cells are already a significant component of the earliest NOD islet CD8+ infiltrates 14-16, are pathogenic 12,13, target a peptide from islet-specific glucose 6 phosphatase catalytic subunit-related protein (IGRP206-214, similar to NRP-A7) 17, and are unusually frequent in the periphery (>1/200 CD8+ T-cells) 17,18.
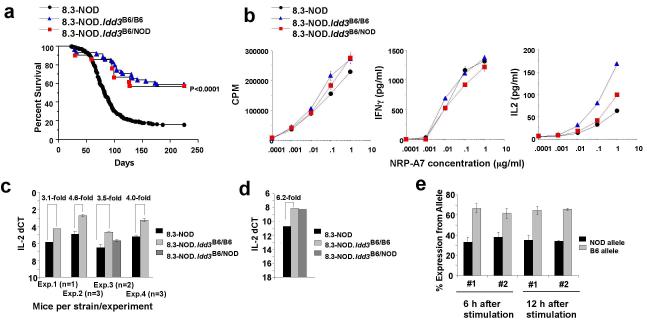
The B6 Idd3 haplotype afforded highly significant protection from T1D in 8.3-NOD mice (Fig. 3a). Cytofluorometric studies of thymii and spleens from 8.3-NOD and 8.3-NOD.B6 Idd3 mice (referred to as 8.3-NOD.Idd3B6/B6 herein) indicated that the B6 Idd3 haplotype does not interfere with the development of 8.3-CD8+ T-cells (Supplemental Fig. 4a). At the functional level, the splenic CD8+ T-cells of both types of mice proliferated equally well and secreted similar levels of interferon gamma (IFN-γ) in response to antigenic stimulation (Fig. 3b). Notably, however, 8.3-CD8+ T cells purified from the spleens of 8.3-NOD.Idd3B6/B6 mice consistently secreted higher levels of IL-2, and produced higher levels of IL-2 message in response to peptide-stimulation than 8.3-CD8+ T cells purified from 8.3-NOD mice (Figs. 3b and 3c). These differences were seen with both NRP-A7 and IGRP206-214 peptides, over a wide range of concentrations, and at different times after activation (Fig. 3b and data not shown). Similar results were obtained when the T-cells were stimulated with peptide/MHC monomers (Supplemental Fig. 4b) or with anti-CD3 plus anti-CD28 mAbs (Supplemental Fig. 4c). Intracellular IL-2/IFN-γ staining of 8.3-CD8+ T-cells stimulated with peptide-pulsed DCs suggested that the Idd3B6/B6 genotype promotes IL-2 (but not IFN-γ) expression in a higher fraction of cells than the Idd3NOD/NOD genotype (Supplemental Fig. 5).
Figure 3. Development and function of IGRP206-214-reactive CD8+ T-cells in the presence or absence of the B6 Idd3 region.
a, Frequency of diabetes in female 8.3-NOD (n=598), 8.3-NOD.Idd3B6/B6 (n=58) and 8.3-NOD.Idd3B6/NOD mice (n=21). b, Proliferation and cytokine secretion of 8.3-CD8+ T-cells from Idd3-congenic and non-congenic 8.3-NOD mice. Data are representative of at least 3 different experiments. c, Exonic RT-QPCR for IL-2 in in vitro-stimulated 8.3-CD8+ T-cells. 8.3-CD8+ T-cells were stimulated with NRP-A7-pulsed, irradiated NOD splenocytes for 12 hours prior to RNA extraction. d, Intronic RT-QPCR for IL-2 in in vitro-stimulated 8.3-CD8+ T-cells (at 12 hours after stimulation). e, Allele-specific assays in antigen-stimulated 8.3-CD8+ T-cells from 8.3-NOD.Idd3NOD/B6 mice (tested with the IL2-6283 reverse assay).
Quantitative PCR assays for intron 2 of IL-2 pre-mRNA yielded similar allele-dependent differences in expression between 8.3-NOD and 8.3-NOD.Idd3B6/B6 mice (Fig. 3d), again suggesting differences in IL-2 transcription rather than in IL-2 mRNA stability. Allele-specific assays using purified 8.3-CD8+ T-cells from TCR-transgenic (NOD x NOD.B6 Idd3) F1 mice confirmed that the T1D-resistant B6 Il2 haplotype is transcriptionally more active than the NOD haplotype (Fig. 3e). Thus, differential production of IL-2 pre-mRNA from the protective and susceptible IL-2 alleles is caused by one or more cis-acting SNPs. No differences in IL-21 gene transcription were observed (Supplemental Fig. 4d).
Il2 haplodeficiency recapitulates Idd3 susceptibility
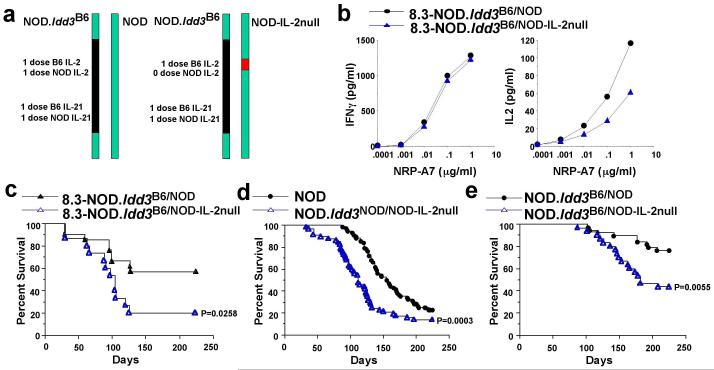
Notwithstanding the fact that the above differences in Il2 transcription are 2-fold and close to the technical resolution of the expression assays, many human diseases are due to haploinsufficiency/2-fold differences in gene expression. Recently, for example, it has been shown that a 1.5-fold increase in dosage of two critical genes on chromosome 21 destabilize a regulatory circuit that accounts for many of the phenotypes of Down’s syndrome 19. When we considered the implications of the IL-2 gene sequencing and expression data, it was clear that although the data were correlated, another gene in the Idd3 region could still be the gene causing disease. An obvious experimental strategy would be to replace a susceptible IL-2 allele with a resistant IL-2 allele, or vice versa. However, consideration of the following two possibilities prompted us to choose an alternative, more informative approach: i) that the expression differences observed could be caused by a combination of multiple disease-causing SNPs affecting chromatin accessibility; and ii) that the disease-associated SNPs could even extend beyond the ~16 kb region for which we have defined disease-associated SNPs. Therefore, in order to prove that Idd3 is Il2, we took advantage of the fact that we could achieve a 50% reduction in IL-2 production without altering any of the other genes in the Idd3 haplotype, based on our findings that the NOD and 129 strain Idd3 haplotypes are IBD (Supplemental Fig 1a) and afford similar T1D susceptibilities 7. We introduced one dose of a susceptible haplotype carrying a targeted mutation of Il2 (produced in 129 embryonic stem cells 20 with the resulting targeted strain backcrossed to the NOD parental strain) into 8.3-NOD mice carrying one copy of the protective B6 Idd3 haplotype (Fig. 4a). As expected, 8.3-CD8+ T-cells isolated from Il2-hemizygous 8.3-NOD.Idd3B6/NOD-IL-2null mice produced ~50% less IL-2 than T-cells from 8.3-NOD.Idd3B6/NOD mice (Fig. 4b). Importantly, 8.3-NOD.Idd3B6/NOD-IL-2null mice developed a significantly higher frequency of T1D than 8.3-NOD.Idd3B6/NOD mice (Fig. 4c). We also showed that a ~50% decrease in IL-2 production in non-transgenic NOD mice accelerated T1D and abrogated the diabetes resistance afforded by one copy of the B6 Idd3 haplotype (Figs. 4d and 4e). We, therefore, conclude that Idd3 is Il2.
Figure 4. IL-2 production competency and diabetes susceptibility of Il2-hemizygous mice.
a, Schematic representation of chromosome 3 pairs in NOD.Idd3B6/NOD vs. NOD.Idd3B6/NOD-IL-2null mice. b, Production of IFN-γ (left) and IL-2 (right) by 8.3-CD8+ T-cells from 8.3-NOD.Idd3B6/NOD and 8.3-NOD.Idd3B6/NOD-IL-2null mice in response to NRP-A7-pulsed, irradiated NOD splenocytes. c, Diabetes frequency in Il2 hemizygous 8.3-NOD.Idd3B6/NOD-IL-2null (n=15) versus 8.3-NOD.Idd3B6/NOD mice (n=21). d, Diabetes frequency in Il2-hemizygous NOD.Idd3NOD/NOD-IL-2null mice (n=57) versus Il2-homozygous NOD mice (n=68). e, Diabetes frequency in NOD.Idd3B6/NOD-IL-2null mice (n=30) versus NOD.Idd3B6/NOD mice (n=38).
Idd3 and the function of autoreactive CD8+ T-cells
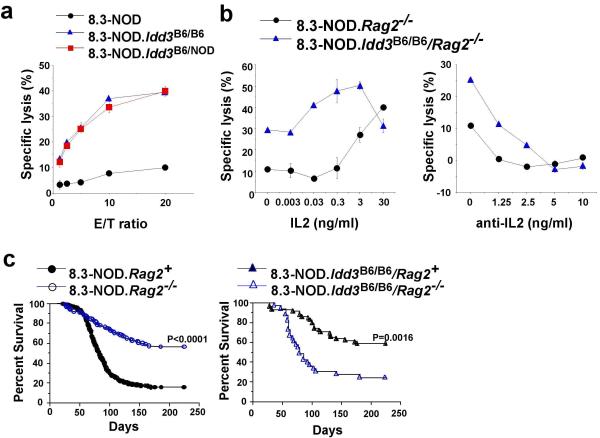
We next set out to investigate the possible mechanisms through which differential expression of IL-2 might afford autoimmune disease susceptibility or resistance. Enhanced production of IL-2 from the B6 Idd3 allele had a dramatic effect on the ability of 8.3-CD8+ T-cells to differentiate into cytotoxic effectors in vitro and in vivo. Naïve CD8+ T-cells purified from the spleens of 8.3-NOD.Idd3B6/B6 and 8.3-NOD.Idd3B6/NOD mice differentiated into cytotoxic T-lymphocytes (CTLs) within 3 days of antigenic stimulation in vitro, whereas 8.3-CD8+ T-cells purified from 8.3-NOD mice did not (Fig. 5a). Similar differences in cytotoxic activity were seen between CD8+ T-cells from recombination activating gene (Rag2)-deficient (Rag2−/−) 8.3-NOD and Rag2−/− 8.3-NOD.Idd3B6/B6 mice, which have a monoclonal T-cell repertoire devoid of CD4+ T-cells and B lymphocytes (Fig. 5b). These differences in cytolytic potential were likely a direct consequence of differences in IL-2 secretion by the CTL precursors for two reasons: 8.3-NOD.Rag2−/−–derived CD8+ T-cells stimulated in the presence of recombinant mouse IL-2 (rmIL-2) acquired cytolytic activity in an IL-2 dose-dependent manner (Fig. 5b, left), and addition of a blocking anti-IL-2 mAb to cultures of 8.3-NOD.Idd3B6/B6/Rag2−/−–derived CD8+ T-cells inhibited the generation of effectors, also in a dose-dependent manner (Fig. 5b, right). Thus, Idd3 regulates the differentiation of naïve CD8+ T-cells into CTL via differential expression of IL-2.
Figure 5. Enhanced effector function of 8.3-CD8+ CTL in 8.3-NOD.Idd3B6/B6 mice.
a, Cytolytic activity of NRP-A7 (1 μM)-stimulated 8.3-CD8+ T-cells from transgenic mice carrying 0, 1 or 2 copies of the B6 Idd3 interval. Purified 8.3-CD8+ T-cells were stimulated with NRP-A7-pulsed NOD splenocytes for 3 days and then tested in 51-Cr-release assays using NRP-A7- or TUM-pulsed RMA-S-Kd cells as targets. Background responses against the negative control peptide TUM were subtracted. b, Addition of exogenous rmIL-2 allows antigen-stimulated 8.3-CD8+ T-cells from 8.3-NOD mice to acquire cytolytic activity (left). Addition of a blocking anti-IL-2 mAb abrogates acquisition of cytolytic activity by 8.3-CD8+ cells of 8.3-NOD.Idd3B6/B6 mice (right). Data in a and b are representative of at least 3 different experiments per condition. c, Frequency of diabetes in female 8.3-NOD.Rag2−/− (n=106) versus 8.3-NOD mice (n=598) (left) and in female 8.3-NOD.Idd3B6/B6/Rag2−/− mice (n=33) versus female 8.3-NOD.Idd3B6/B6 (n=58) mice (right).
Although naïve 8.3-CD8+ T-cells can trigger T1D in the absence of CD4+ T-cells, they are more diabetogenic in their presence 12,21. As a result, 8.3-NOD.Rag2−/− mice develop T1D less frequently than 8.3-NOD.Rag2+ mice (Fig. 5c, left). Surprisingly, introduction of a Rag2 deficiency into 8.3-NOD.Idd3B6/B6 mice dramatically increased the frequency of T1D as compared with that seen in both 8.3-NOD.Rag2−/− and Rag2+ 8.3-NOD.Idd3B6/B6 mice (Fig. 5c, left and right, respectively). Because IL-2 plays a key role in the development and function of CD4+CD25+ T regulatory cells (Tregs), one interpretation of these data is that increased production of IL-2 from the B6 haplotype, in the absence of the ability to generate Treg control in a Rag2-deficient setting, allows the CD8+ T-cells to be even more pathogenic, fuelled by their own IL-2. We, therefore, investigated the role of Tregs in a possible regulatory feedback loop initiated by IL-2-producing CTLs.
Idd3 controls Treg function and recruitment
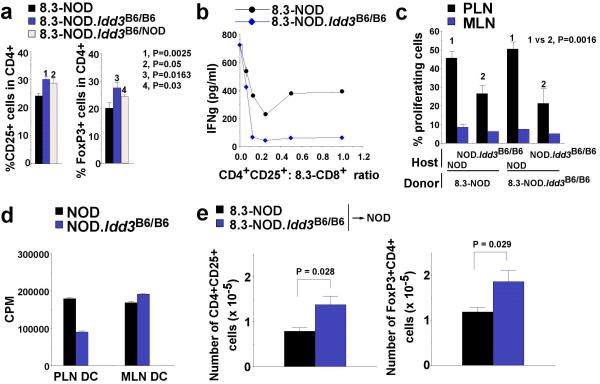
To determine whether Idd3 controlled the size and/or function of the CD4+CD25+ Treg pool, mesenteric (MLN) and pancreatic lymph nodes (PLN) of 8.3-NOD.Idd3B6/B6 mice were examined and found to have higher percentages of CD4+CD25+ and FoxP3+CD4+ T-cells than those of 8.3-NOD mice (Fig. 6a, and data not shown). These relatively small differences in the peripheral frequency of Tregs were not a peculiarity of TCR-transgenic mice, because they were also seen between NOD and NOD.B6 Idd3 or NOD.CZECH Idd3 mice (Supplemental Figs 6a and 6b). These CD4+CD25+ T-cells had the hallmarks of Tregs 22 (Supplemental Fig. 7) and suppressed 8.3-CD8+ T-cell responses induced by peptide-pulsed APCs in vitro (Fig. 6b and Supplemental Figs 6c and 6d). Most importantly, the CD4+CD25+ T-cells of 8.3-NOD.Idd3B6/B6 or NOD.Idd3B6/B6 and NOD.Idd3CZECH/CZECH mice had more regulatory activity than the CD4+CD25+ T-cells of 8.3-NOD or NOD mice, respectively (Fig. 6b and Supplemental Figs 6c and 6d). Similar observations were made in vivo: a single injection of a small number (2×105 cells) of CD4+CD25+ T-cells from NOD.B6 Idd3 (but not NOD) mice suppressed diabetes in 8.3-NOD.Rag2−/− mice (Supplemental Fig. 6e). Since Tregs inhibit the activation of 8.3-CD8+ T-cells [via dendritic cells (DCs)] in a CTLA-4-dependent manner 23, we also compared the effects of CTLA-4 blockade on diabetogenesis in 8.3-NOD and 8.3-NOD.Idd3B6/B6 mice. Whereas CTLA-4 blockade in vivo did not affect the frequency of diabetes in 8.3-NOD mice, it abrogated the diabetes resistance of 8.3-NOD.Idd3B6/B6 mice (Supplemental Fig. 8), suggesting that Tregs play an essential role in the B6 Idd3-associated resistance to T1D.
Figure 6. Enhanced function of CD4+CD25+ Tregs in 8.3-NOD.Idd3B6/B6 and 8.3-NOD.Idd3B6/NOD mice.
a, Percentages of CD4+CD25+ or FoxP3+CD4+ T-cells in PLNs and MLNs of congenic and non-congenic 8.3-NOD mice (8 to 11 weeks-old). The percentages of CD4+CD25+ T-cells were comparable to those of FoxP3+CD4+ T-cells, as expected. Data (average + S.E.) correspond to 5-10 mice per group. b, Secretion of IFN-γ by 8.3-CD8+ T-cells in response to NRP-A7-pulsed splenocytes in the presence of in vitro-activated CD4+CD25+ T-cells from 8.3-NOD and 8.3-NOD.Idd3B6/B6 mice at different CD4+:CD8+ ratios. c, Proliferation of CFSE-labelled 8.3-CD8+ T-cells in the PLNs of NOD and NOD.Idd3B6/B6 hosts. Labelled 8.3-CD8+ T-cells from 8.3-NOD or 8.3-NOD.Idd3B6/B6 mice were injected into 17-20 week-old NOD and NOD.Idd3B6/B6 hosts. The PLNs and MLNs of the hosts were analyzed for presence of proliferating CD8+ T-cells 6 days later (n=5-6 mice per group). d, Proliferation of 8.3-CD8+ T-cells in response to DCs isolated from the PLNs or MLNs of 18-20 week-old NOD and NOD.Idd3B6/B6 mice after pulsing with NRP-A7. e, Absolute numbers of CD4+CD25+ (left) or FoxP3+CD4+ T-cells (right) in the PLNs of NOD mice 5 days after receiving a transfusion of 8.3-CD8+ T-cells from 8.3-NOD or 8.3-NOD.Idd3B6/B6 mice (107 cells, i.v.) and a single injection of NRP-A7 in the foot-pad (100 μg in PBS). Data correspond to average ± S.E. of five 9 wk-old mice/group.
Activation and recruitment of 8.3-like CD8+ T-cells to the pancreas is preceded by cross-presentation of beta cell autoantigen by DCs in the PLNs 24. We have previously shown that CD4+CD25+ Tregs inhibit 8.3-like CD8+ T-cell responses indirectly, by suppressing the maturation of DCs and their ability to cross-prime T-cells 23. To investigate whether the enhanced regulatory activity of NOD.B6 Idd3 mouse-derived CD4+CD25+ T-cells resulted in inefficient cross-presentation of IGRP206-214 in the PLNs, we compared the proliferative activity of CFSE-labelled 8.3-CD8+ T-cells from 8.3-NOD and 8.3-NOD.Idd3B6/B6 mice in the PLNs and MLNs (as negative controls) of NOD and NOD.B6 Idd3 hosts. Regardless of the source of the donor strain, 8.3-CD8+ T-cells proliferated significantly less in the PLNs of NOD.B6 Idd3 hosts than in the PLNs of NOD hosts (Fig. 6c). These differences in proliferation were not due to differences in the size of the autoantigen-load carried by the DCs, because they persisted upon pulsing with NRP-A7 peptide ex vivo (Fig. 6d). Hence, the Idd3-encoded increase in Treg function in NOD.B6 Idd3 mice hinders cross-presentation of beta cell autoantigens to autoreactive CD8+ T-cells in the PLNs.
IL-2 production in response to antigenic stimulation is known to promote the recruitment and activation of CD4+CD25+ Tregs 25,26. The above data suggested that, by producing higher levels of IL-2, autoantigen-activated autoreactive CD8+ T-cells from NOD.B6 Idd3 mice might be able to more efficiently induce or recruit CD4+CD25+ Tregs to the PLNs. To test this possibility, we transferred naïve splenic 8.3-CD8+ T-cells from 8.3-NOD or 8.3-NOD.Idd3B6/B6 mice into NOD hosts. One day after transfer, we injected the hosts in the footpads with soluble NRP-A7 peptide in the absence of adjuvant to synchronize antigen-induced activation of the donor cells in the host lymph nodes. The absolute number of CD4+CD25+ or FoxP3+CD4+ T cells contained in the PLNs of the mice transfused with CD8+ T-cells from 8.3-NOD.Idd3B6/B6 mice was significantly higher than that seen in the hosts transfused with T-cells from 8.3-NOD mice (Fig. 6e). Hence, the amount of IL-2 produced by autoreactive CD8+ T-cells in response to antigen appears to control the size of the local Treg pool.
Il2 haplodeficiency decreases Treg function
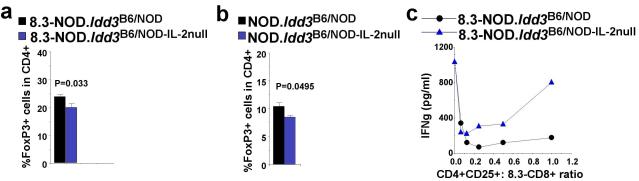
These results begged the question of whether the Idd3-encoded control of Treg function and recruitment is a consequence of Idd3-encoded differences in IL-2 production. In agreement with this idea, the PLNs and MLNs of 8.3-NOD.Idd3B6/NOD-IL-2null mice and non-transgenic NOD.Idd3B6/NOD-IL-2null mice harboured significantly fewer FoxP3+CD4+ T-cells (Figs. 7a and b, and data not shown), and less suppressive CD4+CD25+ T-cells (Fig. 7c) than those of 8.3-NOD.Idd3B6/NOD and NOD.Idd3B6/NOD mice, respectively. These data are consistent with the observation above that both 8.3-NOD.Idd3B6/NOD-IL-2null mice and NOD.Idd3B6/NOD-IL-2null mice develop a significantly higher frequency of T1D than 8.3-NOD.Idd3B6/NOD mice and NOD.Idd3B6/NOD mice, respectively (Figs 4c and 4e).
Figure 7. Enhanced function of CD4+CD25+ Tregs in 8.3-NOD.Idd3B6/B6 and 8.3-NOD.Idd3B6/NOD is IL-2 production-dependent.
a and b, FoxP3+CD4+ T-cells in the PLNs of 8 to 11 weeks-old 8.3-NOD.Idd3B6/NOD and 8.3-NOD.Idd3B6/NOD-IL-2null (a) or NOD.Idd3B6/NOD and NOD.Idd3B6/NOD-IL-2null mice (b). Data (average + S.E.) correspond to 4-9 mice per group. c, Secretion of IFN-γ by 8.3-CD8+ T-cells in response to NRP-A7-pulsed splenocytes in the presence of in vitro-activated CD4+CD25+ T-cells from 8.3-NOD.Idd3B6/NOD and 8.3-NOD.Idd3B6/NOD-IL-2null mice.
DISCUSSION
Our data indicate that the NOD haplotype of Il2 SNPs predisposes to organ-specific autoimmune disease by reducing IL-2 production from antigen-specific T-cells. This, in turn, impairs a feedback mechanism initiated by activated, IL-2-producing autoreactive T cells in the PLNs that increases CD4+CD25+ Treg activity. Activated CD4+CD25+ Treg cells interact with DCs 27, suppress DC maturation 23, and inhibit the cross-presentation of beta cell autoantigens to autoreactive T cells 23, thereby maintaining homeostatic control of autoreactive T-cells and preventing disease progression. Although we demonstrate that higher IL-2 production by a resistant Il2 haplotype paradoxically enhances the pathogenic potential of autoreactive CD8+ T-cells when they are examined on a RAG-deficient background, in the presence of a normal immune repertoire, this increase is not evident because the feed-back mechanism is dominant.
King et al recently suggested that the IL-21 gene could be Idd3. They presented data showing that NOD mice have reduced T cell numbers and that their autoimmune susceptibility may be the result of exaggerated homeostatic-type proliferation driven by IL-21 8, a cytokine produced by activated T cells. We think it is unlikely that such a mechanism contributes to the Idd3-linked susceptibility and resistance to autoimmunity for two reasons: i) our studies of Il2 hemizygous mice have shown that Idd3 is Il2; and ii) we do not find disease-associated differences in IL-21 expression between T1D-prone and T1D-resistant Idd3-congenic NOD mice. Our observations suggest that the reported variation of expression of the adjacent IL-21 gene 8 is either secondary to the genetic alterations in IL-2 production described here or is intrinsic to the IL-21 gene but does not influence susceptibility to T1D.
Although we cannot rule out the possibility that the etiological Idd3 ‘mutation’ occurs in only one of the 46 disease-associated SNPs, we favour the hypothesis that it corresponds to at least several SNPs and that different IL-2 alleles conferring the same T1D-susceptibility phenotype may have additional unique SNPs that affect the competency to produce IL-2 following activation and/or the rate of transcription. In support of this hypothesis, 33 of the 46 disease-associated SNPs are located in a segment of DNA that extends as far as 10 kb upstream of the known Il2 promoter (see DNaseI track on Supplementary Fig. 1). This 5′ region of Il2 has locus control region-like activity that determines the competency of a cell to express IL-2 mRNA if properly activated 28-30. A large portion of the 10 kb upstream region is also critical for the production of physiological levels of IL-2 in the thymus 28. The competency to produce IL-2 if appropriately activated and active IL-2 gene transcription have overlapping as well as unique features and are associated with presence of dimethylated histone H3/K4 and DNaseI hypersensitive sites in the former situation, and acetylated histones H3 and H4 and additional DNaseI hypersensitive sites in the latter case 29,30. Since RNA pol II association has only been observed immediately adjacent to the transcription start site, the epigenetic events described above likely mark regions that require proper histone modification to enable the recruitment of the transcription machinery. Accordingly, it is possible that the SNPs present in this extended region determine the IL-2 production competency of individual T cells depending on their T cell receptor and the events surrounding its positive selection in the thymus. This hypothesis, however, does not exclude the possibility that disease-associated SNPs elsewhere in Il2 contribute to disease phenotype by affecting locus accessibility or other functions. The above scenario would also help explain why the B6 and CZECH alleles produce different amounts of IL-2 mRNA upon T cell activation; accumulation of additional SNPs between these two alleles near the key SNP(s) could further influence the quality and/or quantity of the histone modifications that define IL-2 production competency.
The mechanisms leading to increased production of IL-2 by T cells carrying at least one copy of the B6 Idd3 allele remain to be determined. However, since there is intrinsic variability in IL-2 commitment within clonal T cell populations, and since prolonged interactions with mature DCs are necessary, but not sufficient, for initiation of IL-2 transcription 31, we suspect that increased production of IL-2 by T cells carrying at least one copy of the B6 Idd3 allele is due to: i) faster transcription of Il2 on a per cell basis owing to improved accessibility of transcription factors to the resistance allele; and/or ii) an increase in the fraction of cells that actively produce IL-2 upon making stable contacts with DCs, owing to a reduction in the threshold of remodelling required by the resistance allele to enable transcription. Intracellular IL-2 staining of antigen-activated 8.3-CD8+ T-cells from both types of mice suggest that the latter mechanism is at play. Whether the former mechanism also plays a role cannot be excluded however, and remains a matter of future investigation.
Whatever the contribution of each SNP to the overall phenotype, it is clear that the anti-diabetogenic activity of increased IL-2 production requires the presence of CD4+CD25+ Tregs. In fact, in the absence of endogenous TCR expression (hence, absence of CD4+CD25+ T-cells), 8.3-CD8+ T cells carrying the B6 Idd3 allele are exquisitely more pathogenic than those carrying two copies of the NOD Idd3 allele. Experiments in vitro indicated that this is because the former produce sufficient IL-2 in response to antigenic stimulation to foster their own differentiation into effector cells. Although IL-2 is not necessary for initiation of CD8+ T cell cycling, cells that express IL-2 in primary responses have enhanced sensitivity to antigen and increased expression of effector cytokines in secondary responses as compared with cells that cannot make IL-2 32. Furthermore, IL-2 signaling during priming has been shown to program CD8+ memory T cells for efficient expansion during subsequent encounters with antigen 33.
Overall, our observations indicate that Idd3 is the IL-2 gene and link Idd3 activity with the activity of Tregs bearing the CD25 (alpha chain of the IL-2 receptor) and CD4 receptors. In human T1D, susceptibility has been mapped to the gene encoding the IL-2Rα chain, CD25/IL-2RA 34. The differentiation, activation and homeostasis of CD4+CD25+ Tregs is highly dependent on CD25 function and IL-2 signalling 25,26,35-40. Targeted disruption of the IL-2 or IL-2 receptor genes in mice causes autoimmune disease 41-43, as does mutation of the IL-2 receptor in humans 44. The direct relevance of our results to human T1D is also supported indirectly by reports of reduced Treg number and/or function in cases with T1D 45-47. Based on all of these observations, it is evident that spontaneous development of organ-specific autoimmunity is critically dependent on the physiological regulation of T-cell activation and expansion, and that in genetically susceptible subjects with a lower set point of immune homeostasis, a modest increase in IL-2-receptor signalling may reduce the risk of disease.
METHODS
Mice
Idd3-congenic mice were produced by introgressing Idd3 alleles onto the NOD background as described 2,3 using the CZECH, SWR, A/J, and CASTANEOUS inbred strains (obtained from The Jackson Laboratory). The NOD.CZECH Idd3 congenic strain was developed using a ‘speed congenic’ strategy in which evenly-spaced NOD/CZECH polymorphisms from throughout the genome were tested to select breeders having the greatest level of NOD homozygosity, except at the Idd3 region (selected congenic interval: D3Mit329/23.0 Mb to D3Mit274/51.4 Mb), for the subsequent backcross generation. Following an intercross at N6 to obtain NOD.CZECH Idd3 mice homozygous for the CZECH allele, a T1D frequency study was performed. To develop the NOD strains congenic for the Idd3 regions of SWR, A/J, and CASTANEOUS, mice were backcrossed to the NOD strain and at the N6 generation all three strains were confirmed to be NOD homozygous at Idd1, 4, 5, 6, 9, 10, 17, and 18 1. A disease frequency study was then initiated using Idd3 heterozygous males and females to produce an F2 cohort for each of the three Idd congenic strains. All progeny (NOD at Idd3, heterozygous at Idd3, and homozygous non-NOD at Idd3) were monitored for disease frequency. 8.3-NOD and 8.3-NOD.Rag2−/− mice have been described 12. NOD.Il2+/− mice were provided by D. Serreze (The Jackson Laboratory, Bar Harbor, ME). All mice were housed in specific pathogen-free conditions.
SNP identification
NOD, 129 and B6 BAC sequences were aligned and SNPs identified using SSAHA 48. The SNPs were entered into TIDBase 49 to generate graphic displays of the Idd3 region. Using these polymorphisms, new markers were developed by designing primers with Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3) and synthesized by Sigma Genosys (St. Louis, MO), with the forward primer labelled with the fluorescent dye fam. For the haplotype analysis of the IL-2 and IL-21 genes, nested PCR primers were designed to span the region to be sequenced. Outer primers and inner primers generated 1500 and 500 bp products, respectively, each overlapping by 50 bp to generate a continuous sequence. Big Dye Terminator 3 (Applied Biosystems, Foster City, CA) was used to sequence the products. All sequences generated were entered into T1DBase for SNP identification and disease SNP haplotype mapping.
Anti-CD3 mAb treatment
Mice were injected with 1 μg anti-CD3 mAb (clone 2C11, BioExpress, VT) i.v. and killed 90 min later. Spleens were immediately homogenised in 1.5 ml TRIzol® (Invitrogen, Carlsberg, CA) using a polytron, or used in cell purification procedures.
Anti-CTLA-4 mAb treatment
Mice were injected i.p. with 100 μg of anti-CTLA-4 mAb 3 times/week from 3 to 6 weeks of age, and then followed for diabetes for 15 weeks.
Reverse Transcription followed by quantitative PCR (RT-QPCR)
Intronic and exonic RT-QPCR assays were performed in an ABI Prism 7700 using 1 μg of RNA and Taqman one-step RT-PCR mastermix (Applied Biosystems). For the exonic-based IL-2 QPCR assay, we used a Taqman kit from Applied Biosystems. QPCR for B2M (control) and IL-21 were done using primers and probes designed in house and synthesised by Sigma Genosys. For intron-based assays, the RNA was treated with DNase. The effectiveness of DNase treatment was verified by analyzing samples in parallel that were not treated with reverse transcriptase (RT). For RT-negative samples, successful DNase treatment yielded Ct values equal to those obtained with water. B2M forward: GCTATCCAGAAAACCCCTCAAAT; B2M reverse: CTGTGTTACGTAGCAGTTCAGTATGTTC; B2M probe: Fam-AGTATACTCACGCCACCCACCGGAGAAT-Tam; IL-21 forward: CTATGAAAATGACTTGGATCCTGAAC; IL-21 reverse: ACAGGCAAAAGCTGCATGCT; IL-21 probe: Fam-AGCTCCACAAGATGTAAAGGGGCACTGT-Tam; IL-2 intron forward: GGTGACAAGCTGAGAAGGAAA; IL-2 intron reverse: TGCCTCATTTGACTTACAAGGA; and IL-2 intron probe: Fam-TGACAAATTTATGGGATGGCTTTTCCA-Tam.
Pyrosequencing® - Quantification of allelic expression ratios
Total RNA from cells heterozygous at the IL-2 locus was purified using TRIzol®. The RNA was then DNase-treated using the RNeasy and RNase-Free DNase kits (Qiagen, West Sussex, UK) following the manufacturer’s instructions. DNase-treated RNA was used as a template for cDNA synthesis using Supercript II™RT (Invitrogen). Pyrosequencing® (Biotage, Uppsala, Sweden) utilises a sequencing method coupled to a luciferase enzyme/substrate reaction, which produces light each time a nucleotide is added in the sequencing reaction. The PSQ96 system (Biotage) calculates, from the amount of light generated, the relative abundance of that nucleotide within the sample. Previously identified SNPs within intron 2 of Il2 were used to design pyrosequencing assays. Forward and reverse PCR primers were designed to conserved sequences near a SNP to amplify a 150-200 bp PCR product. A pyrosequencing primer was designed using the SNP primer design web site at http://techsupport.pyrosequencing.com. The assays were performed according to the manufacturer’s instructions. Genomic DNA standard curves generated from each strain represented in the F1 were used to normalise the cDNA results. Primers used in IL2-5616 forward assay (measures NOD versus B6 and NOD versus CZECH alleles): forward: CCTGTGTATTTATGTGTTGGG; reverse: biotin-AAATGAGACCCTCTCAAGTCA; pyrosequencing: TGCTGGGATAATTTAAAG. Primers used in IL2-6283 reverse assay (measures NOD versus B6 alleles): forward: biotin-GTCCCAAGTAAATCCAAGCC; reverse: GTGACTGTAATTAAGCTGG; pyrosequencing: CCCTGACTCAATAGGAATG.
Diabetes
Diabetes was monitored by measuring urine glucose with Diastix (Miles, Ontario, Canada). Animals were considered diabetic after two consecutive readings ≥3. The frequency of diabetes was compared between strains with the Kaplan-Meier log-rank test using Prism software (GraphPad, San Diego, CA).
Cell Lines and Antibodies
All monoclonal antibodies (mAbs) were from PharMingen (San Diego, CA), unless indicated otherwise. The 9H10 (anti-CTLA-4) hybridoma was from C. Chambers (University of Massachusetts). Anti-mouse GITR goat IgG and recombinant mouse IL-2 (rmIL-2) were from R&D Systems (Minneapolis, MN). Anti-FoxP3 Ab (FJK-16s) was from eBioscience (San Diego, CA). Streptavidin-PerCP was from Becton-Dickinson (San Jose, CA). FITC-labeled donkey anti-goat IgG, anti-hamster IgG biotin and streptavidin-Cy3 were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Peptides and Peptide/MHC Monomers
Peptides and peptide/MHC monomers and tetramers were produced as described 16,17.
Preparation of DCs
DCs were purified directly from the PLNs or MLNs using CD11c mAb-coated beads (Miltenyi Biotec, Auburn, CA) 23.
8.3-CD8+ and CD4+CD25+ T-cell isolation and adoptive T-cell transfers
Purified splenic CD8+ T-cells (see below) were labelled with CFSE and 107 transfused i.v. Hosts were killed 6 days later and CFSE+ T-cells in their PLNs and MLNs were quantified. To purify CD4+CD25+ T-cells, lymph node and/or splenic cells were depleted of CD8+ T and B-cells, incubated with anti-CD25-PE, and separated using anti-PE mAb-coated beads (Miltenyi Biotec). The purity was >90% for CD4+CD25− and >85% for CD4+CD25+ T-cells. In another set of experiments, 8.3-NOD.Rag2−/− mice were injected i.v. with naïve CD4+CD25+ T-cells from NOD or NOD.Idd3B6/B6 donors (2×105/mouse) and monitored for diabetes for 15 weeks.
8.3-CD8+ T cell-induced recruitment of CD4+CD25+ T-cells
Mice were injected i.v. with 107 purified 8.3-CD8+ T-cells from 8.3-NOD or 8.3-NOD.Idd3B6/B6 mice. Twenty-four hours later, the hosts received a single injection of NRP-A7 in the foot-pad (100 μg in PBS). The numbers of CD4+CD25+ or FoxP3+CD4+ T-cells in the PLNs were determined 5 days later.
Generation of 8.3-CD8+ CTL and cytotoxicity assays
8.3-CD8+ splenocytes were isolated to >90% purity with anti-CD8-coated beads (Miltenyi Biotec), adjusted to 2 × 104 cells/well, and stimulated with NRP-A7-pulsed (0.0001 to 1 μM) APCs (105 irradiated splenocytes/well) for 3 days in the presence or absence of a blocking anti-IL-2 mAb (0-10 μg/ml; clone S4B6) or rmIL-2 (R&D systems, Minneapolis, MN; 0-30 ng/ml). The cytolytic activity of these cells was measured using NRP-A7- or TUM-pulsed (1 μM) RMA-SKd cells 12.
Proliferation and cytokine assays
Purified splenic CD8+ T-cells (2 × 104/well) were incubated with peptide-pulsed (0.0001-1 μM) APCs (105 irradiated splenocytes/well) or with plate-bound anti-CD3 mAb (3 μg/ml) and soluble anti-CD28 mAb (3 μg/ml) for 2 or 3 days (for cytokine and proliferation assays, respectively) at 37°C in 5% CO2. The cytokine contents in the supernatants (IL-2, IL-4, IL-10, IL-21 and IFN-γ) were measured by ELISA (R&D systems). The 3-day cultures were pulsed with 1 μCi [3H] thymidine during the last 18 hours of culture and harvested. In other experiments, CD8+ T-cells were cultured in the presence of peptide/MHC monomers (0.08–2.2 μg/ml) without APCs. The regulatory activity of CD4+CD25+ T-cells was measured by adding 0.125–2 × 104 CD4+CD25+ T-cells (pre-activated with anti-CD3 mAb and rIL-2) to CD8+ cell:APCs co-cultures.
Intracellular cytokine staining
Purified splenic 8.3-CD8+ T-cells (105) were stimulated with NRP-A7 (1 μM)-pulsed bone marrow-derived DCs (104) for 14 h, incubated in Golgi Stop (BD Biosciences) for 6 h and stained with anti-IL-2, anti-IFN-γ and isotype control mAbs using the CytoFix/CytoPerm kit (BD Biosciences) as per the manufacturer’s instructions.
In vitro responsiveness of CD4+CD25+ T-cells
Purified CD4+CD25+ T-cells (105) were incubated, in duplicate, with plate-bound anti-CD3 mAb (3 μg/ml) and with or without rIL-2 for 2 or 3 days (to collect supernatants or measure proliferative activity, respectively). In other assays these T-cells were cultured with irradiated NOD splenocytes (105) with or without rIL-2 and anti-GITR antibodies (0.1-1 μg/ml) for 3 days (to measure their proliferative activity).
Statistical analyses
Data were compared by Logrank, Student’s t, Mann-Whitney U or χ2 tests.
Supplementary Material
Acknowledgments
We thank S. Bou, M. Deuma, S. Thiessen and H. Vandermeer for excellent technical assistance with mouse breeding, genotyping and animal care, D. Serreze for providing NOD.Il2+/− mice, L. Kennedy and L. Robertson for flow cytometry and A. Shameli, P. Taylor, S. Tsai, U. Walter and Y. Yang for reading the manuscript. This work was supported by grants from the Canadian Institutes of Health Research, the Juvenile Diabetes Research Foundation (JDRF) and the Wellcome Trust. J. Y. was supported by fellowships from the Canadian Diabetes Association and JDRF. P. Serra was supported by a studentship from the Alberta Heritage Foundation from Medical Research (AHFMR). P. Santamaria is a Scientist of AHFMR. The JMDRC is supported by the Diabetes Association (Foothills). NOD.B6 Idd3 and NOD.CZECH Idd3 are available from Taconic through the Emerging Models Program (Lines 1098 and 1590, respectively). The availability of NOD congenic mice through the Taconic Farms Emerging Models Program has been supported by grants from the Merck Genome Research Institute, NIAID, and the JDRF.
Footnotes
Competing interests statement. The authors declare that they have no competing financial interests.
Supplementary Information is available on the Nature Genetics website.
REFERENCES
- 1.Maier L, Wicker L. Genetic susceptibility to type 1 diabetes. Curr. Opin. Immunol. 2005;17:601–608. doi: 10.1016/j.coi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Wicker L, et al. Resistance alleles at two non-major histocompatibility complex-linked insulin-dependent diabetes loci on chromosome 3, Idd3 and Idd10, protect nonobese diabetic mice from diabetes. J. Exp. Med. 1994;180:1705–1713. doi: 10.1084/jem.180.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons PA, et al. Congenic mapping of the type 1 diabetes locus, Idd3, to a 780-kb region of mouse chromosome 3: identification of a candidate segment of ancestral DNA by haplotype mapping. Genome Res. 2000;10:446–453. doi: 10.1101/gr.10.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Encinas J, et al. QTL influencing autoimmune diabetes and encephalomyelitis map to a 0.15-cM region containing Il2. Nat. Gen. 1999;21:158–160. doi: 10.1038/5941. [DOI] [PubMed] [Google Scholar]
- 5.Teuscher C, Wardell B, Lunceford J, Michael S, Tung K. Aod2, the locus controlling development of atrophy in neonatal thymectomy-induced autoimmune ovarian dysgenesis, co-localizes with Il2, Fgfb, and Idd3. J. Exp. Med. 1996;183:631–637. doi: 10.1084/jem.183.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samy E, Parker L, Sharp C, Tung K. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J. Exp. Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podolin PL, et al. Differential glycosylation of interleukin 2, the molecular basis for the NOD Idd3 type 1 diabetes gene? Cytokine. 2000;12:477–482. doi: 10.1006/cyto.1999.0609. [DOI] [PubMed] [Google Scholar]
- 8.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 9.Lyons PA, et al. Mapping by genetic interaction: high-resolution congenic mapping of the type 1 diabetes loci Idd10 and Idd18 in the NOD mouse. Diabetes. 2001;50:2633–7. doi: 10.2337/diabetes.50.11.2633. [DOI] [PubMed] [Google Scholar]
- 10.Cervino A, et al. A comprehensive mouse IBD database for the efficient localization of quantitative trait loci. Mamm Genome. 2006;17:565–574. doi: 10.1007/s00335-005-0170-4. [DOI] [PubMed] [Google Scholar]
- 11.McAleer MA, et al. Crosses of NOD mice with the related NON strain. A polygenic model for IDDM. Diabetes. 1995;44:1186–1195. doi: 10.2337/diab.44.10.1186. [DOI] [PubMed] [Google Scholar]
- 12.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdaguer J, et al. Acceleration of spontaneous diabetes in TCR-β-transgenic nonobese diabetic mice by β-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-α chains. J. Immunol. 1996;157:4726–4735. [PubMed] [Google Scholar]
- 14.DiLorenzo T, et al. MHC class I-restricted T-cells are required for all but end stages of diabetes development and utilize a prevalent T cell receptor α chain gene rearrangement. Proc Natl Acad Sci USA. 1998;95:12538–12542. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P. Prevalent CD8+ T cell response against one peptide/MHC complex in autoimmune diabetes. Proc. Natl. Acad. Sci. U S A. 1999;96:9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amrani A, et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman S, et al. Identity of the antigen targeted by prevalent pathogenic T cells in diabetes. Proc. Natl. Acad. Sci. USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trudeau JD, et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J. Clin. Invest. 2003;111:217–223. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arron J, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 20.Schorle H, Holtsche T, Honig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–623. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 21.Amrani A, et al. CD154-dependent priming of diabetogenic CD4+ T cells dissociated from activation of antigen-presenting cells. Immunity. 2002;16:719–732. doi: 10.1016/s1074-7613(02)00315-1. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, et al. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity and transplantation tolerance. Immunoloigcal Reviews. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 23.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. In situ β-cell death promotes priming of diabetogenic CD8 T lymphocytes. J. Immunol. 2002;168:1466–1472. doi: 10.4049/jimmunol.168.3.1466. [DOI] [PubMed] [Google Scholar]
- 25.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yui MA, Hernandez-Hoyos G, Rothenberg EV. A new regulatory region of the IL-2 locus that confers position-independent transgene expression. J Immunol. 2001;166:1730–1739. doi: 10.4049/jimmunol.166.3.1730. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Kametani Y, Katano I, Habu S. T-ell specific enhancement of histone H3 acetylation in 5′ flanking region of the IL-2 gene. Biochem. Biophys Res. Comun. 2005;331:589–594. doi: 10.1016/j.bbrc.2005.03.216. [DOI] [PubMed] [Google Scholar]
- 30.Adachi A, Rothenberg E. Cell-type-specific epigenetic marking of the IL2 gene at a distal cis-regulatory region in competent, nontranscribing T-cells. Nucleic Acids Res. 2005;33:3200–3210. doi: 10.1093/nar/gki637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurez V, et al. Restricted clonal expression of IL-2 by naive T cells reflects differential dynamic interactions with dendritic cells. J Exp Med. 2003;198:123–132. doi: 10.1084/jem.20022230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saparov A, et al. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 1999;11:271–280. doi: 10.1016/s1074-7613(00)80102-8. [DOI] [PubMed] [Google Scholar]
- 33.Williams M, Tyznik A, Bevan M. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vella A, et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am. J. Hum. Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf M, Schimpl A, Hunig T. Control of T cell hyperactivation in IL-2-deficient mice by CD4+CD25− and CD4+CD25+ T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001;31:1637–1345. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 36.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 37.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 39.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 40.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadlack B, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 42.Willerford DM, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharfe N, Dadi H, Shahar M, Roifman C. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc. Natl. Acad. Sci. USA. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kukreja A, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindley S, et al. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 47.Brusko T, Wasserfall C, Clare-Salzler M, Schatz D, Atkinson M. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 48.Ning Z, Cox A, Mullikin J. SSAHA: a fast search method for large DNA databases. Genome Res. 2001;11:1725–1729. doi: 10.1101/gr.194201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smink L, et al. T1DBase, a community web-based resource for type 1 diabetes research. Nucleic Acids Res. 2005;33:D544–D549. doi: 10.1093/nar/gki095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Korn L, Gamero A, Leonard W. Calcium-dependent activation of interleukin-21 gene expression in T cells. J. Biol. Chem. 2005;280:25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.