Summary
Type IB DNA topoisomerases (TopIB) are enzymes that relax supercoils by cleaving and resealing one strand of duplex DNA within a protein clamp that embraces a DNA segment. A longstanding conundrum concerns the capacity of TopIB enzymes to stabilize intramolecular duplex DNA crossovers and, in the case of poxvirus TopIB, form protein-DNA synaptic filaments. Here we report a structure of D. radiodurans TopIB in complex with a 12-bp duplex DNA that demonstrates a secondary DNA binding site located on the C-terminal domain. It comprises a distinctive interface with one strand of the DNA duplex and is conserved in all TopIB enzymes. Modeling of a TopIB with both DNA sites suggests that the secondary site could account for DNA crossover binding, nucleation of DNA synapsis, and generation of a filamentous plectoneme. In support of this, mutations of the secondary site eliminate synaptic plectoneme formation without affecting DNA cleavage or supercoil relaxation.
Introduction
Type IB DNA topoisomerases (TopIB) are encoded in the genomes of all eukarya, several eukaryal viruses (poxviruses and mimivirus; Benarroch et al., 2006), many bacteria (Krogh and Shuman, 2002), and several archaea (Forterre et al., 2007). They play important roles in relaxing supercoils generated during DNA replication and transcription. TopIB enzymes accomplish this task by repeatedly breaking and rejoining one strand of the DNA duplex through a covalent DNA-(3′-phosphotyrosyl)-enzyme intermediate (Corbett and Berger, 2004). Within the covalent TopIB–DNA complex, the noncovalently held 5′-OH DNA segment swivels about the protein-DNA nick before being religated to the 3′-phosphate of the covalently held strand. The number of supercoils removed by TopIB per cleavage-religation cycle follows an exponential distribution that depends on the torque stored in the supercoiled DNA and friction at the protein-DNA interface during the swivel (Koster et al., 2005).
Crystal structures of DNA-bound cellular and poxvirus TopIB enzymes captured at sequential steps along the reaction pathway (pre-cleavage, transition-state, and post-cleavage covalent complex) have fully illuminated the DNA-protein interactions and reaction chemistry (Davies et al., 2006; Perry et al., 2006, 2010; Redinbo et al., 1998). These structures, together with biochemical studies (Krogh and Shuman, 2000; Tian et al., 2005), revealed how nucleophilic attack of the active-site tyrosine hydroxyl on the DNA phosphodiester bond is catalyzed by two arginines, a lysine and a histidine that stabilize the pentacoordinate transition-state and expel the 5′-OH leaving strand. The crystal structures also underscored how all TopIB enzymes envelop the duplex DNA cleavage site by forming a C-shaped protein clamp, wherein a C-terminal catalytic domain engages the DNA minor groove at and surrounding the scissile phosphodiester while an N-terminal domain module engages the DNA major groove on the face of the duplex opposite the cleavage site.
Comparison of the structures of DNA-bound poxvirus TopIB (Perry et al., 2006) and the free apoenzyme (Cheng et al., 1998) highlighted that the active site is not preassembled prior to DNA binding. Indeed, in the poxvirus apoenzyme, three of the five catalytic residues (the lysine and arginine general acids and the tyrosine nucleophile) are either disordered or out of position to perform transesterification chemistry. The formation of a catalytically competent active site entails multiple conformational switches and disordered-to-ordered transitions within the catalytic domain that are triggered by recognition of specific nucleobases and backbone phosphates of the consensus 5′-CCCTT↓/3′-GGGAA cleavage site for poxvirus TopIB (Perry et al., 2006; Tian et al., 2004a; Tian et al., 2004b; Yakovleva et al., 2006).
Many bacterial species encode a TopIB that resembles the poxvirus and mimivirus enzymes with respect to their small size, primary structures, and bipartite domain organization (Krogh and Shuman, 2002). It is speculated that horizontal transfer of TopIB genes among bacteria and eukaryal viruses occurred during their cohabitation in a unicellular eukaryal host, e.g., amoebae (Benarroch et al., 2006). The bacterial TopIB clade is exemplified by Deinococcus radiodurans TopIB (DraTopIB), the only member that has been characterized biochemically and structurally (Krogh and Shuman, 2002; Patel et al., 2006). Although the crystal structures and active sites of poxvirus TopIB and DraTopIB are quite similar (more so to each other than to the much larger eukaryal cellular TopIB enzymes), three features of DraTopIB stand out in comparison to the poxvirus TopIB: (i) DraTopIB does not transesterify at the poxvirus 5′-CCCTT↓ cleavage site, (ii) the five catalytic amino acids of DraTopIB are pre-assembled in the apoenzyme crystal structure, and (iii) a segment of the DraTopIB catalytic domain flanking a catalytic arginine that is disordered in the apoenzyme crystal structure corresponds to the “specificity helix” of poxvirus TopIB that is critical for DNA site recognition and cleavage (Patel et al., 2006; Perry et al., 2006; Yakovleva et al., 2008).
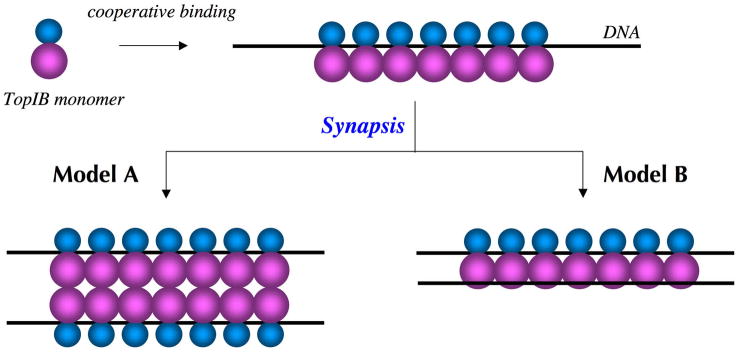
It has long been suspected that the catalytic DNA binding mode seen in the available TopIB-DNA crystal structures might not be the only means by which TopIB interacts with DNA. In a pioneering study, Zechiedrich and Osheroff observed by electron microscopy that mammalian TopIB prefers to bind to relaxed circular and linear plasmid DNA molecules at the nodes created by the crossing of two duplex helices (Zechiedrich and Osheroff, 1990). They suggested that TopIB might initially bind to one DNA segment and then capture a second DNA segment at a distant site in the same plasmid molecule. Later, Madden et al. reported that the Y723F active site mutant of human TopIB binds preferentially to positively or negatively supercoiled plasmid DNA compared to relaxed DNA molecules (Madden et al., 1995). Crossover recognition provides a “topology sensor” and a potential means to direct TopIB action to plectonemic DNAs. Electron microscopy has also been used to visualize complexes formed by poxvirus TopIB on plasmid DNAs (Shuman et al., 1997). The poxvirus TopIB formed intramolecular loop structures in which non-contiguous DNA segments were synapsed at protein-containing nodes or within filamentous protein stems. The formation of filaments along the DNA suggested that poxvirus TopIB binds DNA cooperatively. At high TopIB concentrations, the DNA appeared to be “zipped up” within the protein filaments such that the duplex was folded back on itself. Formation of loops and filaments was also observed with an active site mutant, TopIB-Phe274. The zipped-up poxvirus TopIB-DNA complexes formed on relaxed DNA were shown to be plectonemic supercoils, in which the two duplexes encompassed by the protein filaments are interwound in a right-handed helix (Shuman et al., 1997). Thus, TopIB binding to DNA directly imposes a higher order DNA structure. Further insights to the DNA binding properties of poxvirus TopIB were gained by applying atomic force microscopy (AFM) to the problem (Moreno-Herrero et al., 2005). AFM verified that poxvirus TopIB formed nodes and filaments on linear or nicked-circular DNAs by intramolecular synapsis of two distant DNA segments. Measuring the filament length as a function of TopIB concentration showed that synapsis is a highly cooperative process. The congruence of the EM and AFM studies suggested that TopIB-mediated DNA synapsis might contribute to organization of the 200-kbp vaccinia genome into a higher order structure conducive to transcription within virus cores.
A key question is how TopIB bridges distant DNA sites. Is it via protein-protein interactions between two DNA-bound TopIB molecules? Or can a single molecule of TopIB bind simultaneously to two DNA segments? These two simple physical mechanisms to account for synapsis by the poxvirus TopIB are depicted in Fig. 1 as models A and B, respectively. In model A, protein-protein interactions between TopIB molecules provide the “glue” for synapsis of two TopIB-DNA filaments. (The model arbitrarily depicts the interaction between the C-terminal catalytic domains of DNA-bound protomers; it could just as well entail N-domain/N-domain interactions or N-domain/C-domain contacts.) In model B, the synapsed DNA duplex is captured at a putative secondary DNA binding site on the TopIB protomer. Similarly, the binding of TopIB to DNA crossovers can be explained by either TopIB-TopIB interactions or simultaneous occupancy of two DNA binding sites on TopIB.
Figure 1. Models for TopIB-mediated DNA synapsis.
Single molecule imaging by EM and AFM has shown that poxvirus TopIB binds cooperatively to linear plasmid DNA and forms protein-DNA filaments in which distant segments in the same DNA molecule are synapsed. The TopIB protomer consists of a small N-terminal domain (depicted as a cyan sphere) and a larger C-terminal domain (magenta sphere) that contains the active site. The primary DNA binding site resides within a protein clamp formed by the N and C domains. Two possible mechanisms to account for synapsis are depicted as models A and B. In model A, TopIB-TopIB interactions promote synapsis of two TopIB-DNA filaments. In model B, the synapsed DNA duplex is captured at a distinct secondary DNA binding site on the TopIB protomer.
Here we report the crystal structure of the bacterial DraTopIB enzyme in a complex with DNA. The structure demonstrates a secondary DNA binding site located on the surface of the C-terminal domain. The secondary DNA site is ~30 Å from the catalytic DNA site and comprises an extensive network of direct and water-mediated hydrogen bonds from the enzyme to one strand of the DNA duplex. The secondary site appears to be conserved in the poxvirus and eukaryal cellular TopIB enzymes. A model of the poxvirus TopIB enzyme with both DNA sites filled suggests how second site capture might account for DNA crossover binding, nucleation of DNA synapsis, and plectonemic supercoiling within the synaptic filament. We provide biochemical evidence in support of this model by showing that mutations in the putative secondary DNA binding site of poxvirus TopIB affect the generation of plectonemic supercoils, but not supercoil relaxation.
Results and Discussion
Structure of a DraTopIB-DNA complex
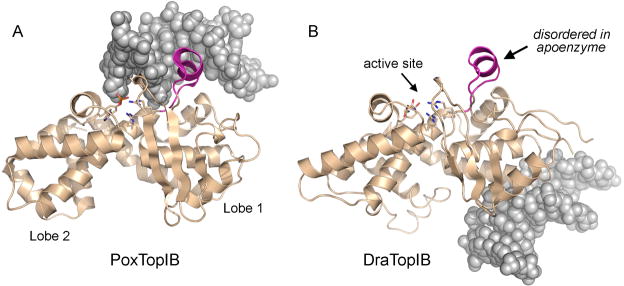
Crystals of DraTopIB were grown in the presence of a 12-bp DNA duplex (see Materials and Methods) that is not a substrate for cleavage by the enzyme. The crystals belong to space group C2, unlike the DraTopIB apoenzyme (Patel et al., 2006), which crystallized in space group P21. The structure of the DraTopIB-DNA complex was solved by Molecular Replacement using the structure of the DraTopIB apoenzyme catalytic domain as a search molecule and refined at 1.65 Å resolution to Rwork/Rfree of 0.193/0.222 (Table I). In the crystal lattice, the short DNA duplexes are stacked end-to-end to form a pseudo-continuous helix to which DraTopIB is bound. In prior TopIB-DNA structures, the DNA fits into an interdomain cleft, the domains form a circumferential clamp around the duplex, and the active site residues in the C-domain directly coordinate the scissile phosphodiester (Fig. 2A). By contrast, in the DraTopIB-DNA complex, the N- and C-domains are splayed apart (not shown) and the catalytic DNA binding site is unoccupied (Fig. 2B). The N-domain (aa 1–90) is partially disordered and makes no contacts to the 12-bp DNA ligand. The C-domain (aa 91–346) is fully ordered and the DNA is docked on the basal surface far from the active site (Fig. 2B). The C-domains of the bacterial and viral TopIB enzymes comprise two globular lobes (lobes 1 and 2) (Fig. 2). Alignment of the C-domains of the poxvirus TopIB and DraTopIB DNA complexes reveals that the DraTopIB-DNA structure heralds the existence and position of a bona fide secondary DNA-binding site on TopIB. The secondary DNA binding site is located within lobe 1 (Fig. 2B).
Table I.
Diffraction data and refinement statistics
| Data collection | |
| Space group | C2 |
| Cell | 119.7 Å, 53.4 Å, 77.4 Å, β=96.33 |
| Detector type/source | MAR-CCD/APS |
| Wavelength (Å) | 1.00 |
| Resolution (Å) | 1.65 |
| Detector type/source | MAR345/home |
| Wavelength (Å) | 1.5418 |
| Resolution (Å) | 1.92 |
| Merged data sets | |
| Measured reflections | 265,853 |
| Unique reflections | 56,385 |
| Completeness (%)a | 96.2 (89.5) |
| Rsym (%)a,b | 4.8 (27.6) |
| Rmeas (%)a,c | 5.3 (32.5) |
| Redundancy | 4.7 (3.4) |
| Mean(I/σ(I)) | 24.1 (4.1) |
| Refinement | |
| Resolution (Å) | 76 – 1.65 (1.65 – 1.693) |
| Number of reflections: working set/test set | 53,565/2,820 (3,633/209) |
| Rworkf | 19.3 (22.0) |
| Rfreed | 22.2 (23.2) |
| Protein atoms | 2,495 |
| DNA atoms | 487 |
| Water molecules | 278 |
| Other | 12 |
| r.m.s.d. from target values | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.29 |
| Average B-factor (Å2): | |
| Main chain | 16.0 |
| Side chain | 17.7 |
| DNA | 15.2 |
| Solvent | 24.6 |
| Ramachandran plot | |
| Favored (%)f | 99.7 |
| Outliers (%)f | 0.0 |
| Rotamer outliers (%)f | 0.4 |
Numbers in parenthesis represent values in the highest resolution shell.
Rsym=Σ|I−<I>|/ΣI, where I=observed intensity, and <I>=average intensity obtained from multiple measurements.
Rmeas as defined by (Diederichs and Karplus, 1997).
R-factor=Σ||Fo| − |Fc||/Σ|Fo|, where |Fo|=observed structure factor amplitude and |Fc|=calculated structure factor amplitude.
Rfree: R-factor based on 5% of the data excluded from refinement.
Calculated with MolProbity (Davis et al., 2004).
Figure 2. A secondary DNA binding site on DraTopIB.
The C-terminal catalytic domains of poxvirus TopIB (from the structure of the covalent TopIB-DNA intermediate; PDB 2H7F) and DraTopIB (from the present DraTopIB-DNA cocrystal structure) were superimposed and offset horizontally. The tertiary structures, depicted as beige ribbons traces, are homologous throughout. The C-domains consist of two globular lobes (1 and 2, denoted for poxvirus TopIB in panel A). The active sites are located on the superior surface of the C-domain (denoted by the arrow for DraTopIB in panel B); the catalytic amino acid side chains are shown as stick models. The specificity helix is shown in magenta. The duplex DNA segments of the respective DNA ligands are shown as gray spacing-filling models. The DNA duplex is covalently linked to poxvirus TopIB is the primary (catalytic) DNA binding site. By contrast, DraTopIB binds its DNA ligand on the opposite face of the C-domain ~30 Å away from the primary DNA site. See also Figure S1 for a comparison of the DNA contacts of the specificity helix in poxvirus TopIB versus DraTopIB.
DNA binding triggers folding of the “specificity helix”
Comparison of the C-domains of the DraTopIB apoenzyme and the DNA-bound DraTopIB reveals both subtle and profound changes correlated with DNA binding. The C-domains of the two structures superimpose with a rmsd of 1.07 Å for all main chain atoms. However, when the isolated lobes 1 and 2 are superimposed separately, the rmsd values are only 0.87 Å and 0.76 Å, respectively. Thus, whereas the individual lobes are nearly identical in the apoenzyme and DNA-bound DraTopIB, the DNA elicited a subtle reorientation of the lobes with respect to each other. The five catalytic amino acids (Arg137, Lys174, Arg239, Asn280 and Tyr289) are preassembled into an active site in the DNA complex (Fig. 2B) and are located in the same positions as in the apoenzyme. Yet, none of the five catalytic residues make contact with DNA in the crystal lattice.
An important insight from the poxvirus TopIB-DNA cocrystal (Perry et al., 2006) was the identification of a “specificity helix” (131FGKMKYLKENETVG144) that binds the DNA target site in the major groove (Fig. 2B) and makes atomic contacts to nucleobases and phosphate oxygens that are important for cleavage site recognition and DNA transesterification (Yakovleva et al., 2008). This specificity helix is conserved among poxvirus and mimivirus TopIB enzymes and is a distinctive secondary structure feature of the viral/bacterial TopIB clade that is absent in human TopIB (Redinbo et al., 1998). The specificity helix of poxvirus TopIB is protease-sensitive and disordered in the apoenzyme, but adopts a defined secondary structure and becomes protease-resistant when the poxvirus TopIB is in the DNA-bound state (Cheng et al., 1998; Perry et al., 2006; Sekiguchi and Shuman, 1995). Tight docking of the specificity helix in the major groove 5′ of the scissile phosphate aids in placing the catalytic Arg130 residue in the active site.
The DraTopIB equivalent of the poxvirus specificity helix is 138VGSDIYARQHKTYG151. Structure probing of free DraTopIB by limited proteolysis delineated a single trypsin-sensitive site within this segment between Arg145 and Gln146 (Krogh and Shuman, 2002). Moreover, the 139GSDIYARQHK148 peptide is disordered in the crystal structure of apo-DraTopIB (Patel et al., 2006). By contrast, we find presently that in the DraTopIB-DNA cocrystal, the previously disordered peptide segment forms a well-ordered α-helix that mimics the specificity helix of poxvirus TopIB (Fig. 2B). In the poxvirus TopIB, the specificity helix penetrates deeply into the DNA major groove, where it makes multiple side chain and main-chain contacts to the DNA phosphates and bases (Fig. S1A). The equivalent α-helix in DraTopIB makes a single (nonspecific) contact to a backbone phosphate in a symmetry-related 12-mer DNA and one contact to a nucleobase. We attribute these limited contacts to crystal packing (Fig. S1B). The symmetry related 12-mer DNA is not situated in the active site.
The structures of DraTopIB and poxvirus TopIB suggest that several mechanisms exist to trigger the folding of the specificity helix. In poxvirus TopIB, this occurs as a direct response to binding of the cleavage recognition sequence in the catalytic DNA site. In the case of DraTopIB, the equivalent conformation switch occurs either as: (i) a nonspecific response to duplex DNA that does not trigger catalysis, or (ii) an indirect, perhaps allosteric, response to occupancy of the secondary DNA binding site. Because the catalytic Arg137 is already poised in the active site in the DraTopIB apoenzyme, we presume that the induced folding of the specificity helix is critical for recognition of the target site(s) for DNA transesterification, which (though clearly different from that of the poxvirus TopIB) are presently uncharted.
Architecture of the secondary DNA binding site
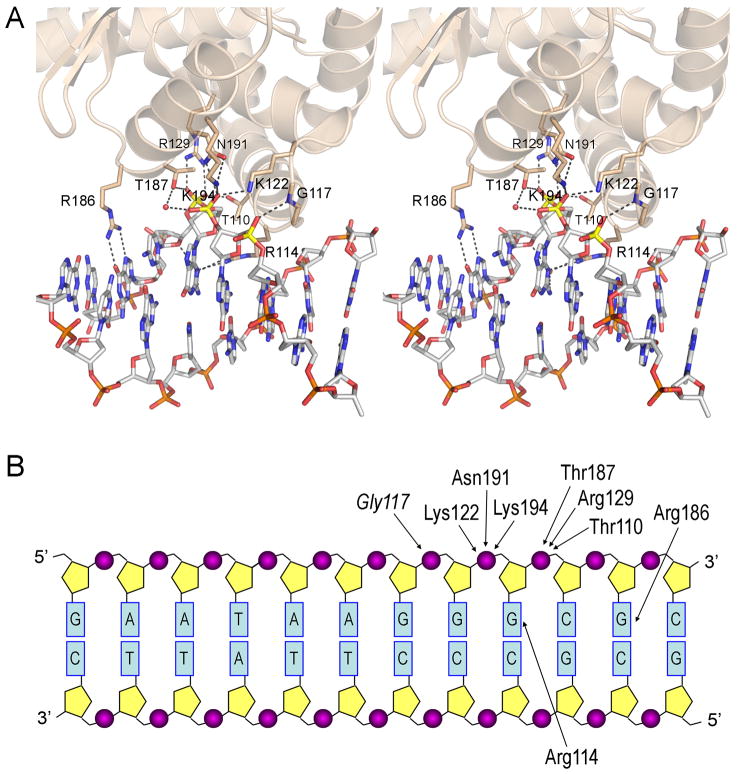
The secondary DNA binding site is located entirely within lobe 1 of the C-domain of DraTopIB. The DNA interface comprises a helix-loop-helix (aa 106–129) that contacts one strand of the 12mer duplex from the minor groove side and another α-helix (aa 186–194) that contacts the same strand from the major groove side (Fig. 3A). The component peptide elements form a groove on the basal surface of the C-domain into which only one strand of the DNA duplex fits. Most of the atomic contacts entail H-bond donation from protein side chain (Thr110, Lys122, Arg129, Thr187, Asn191, Lys194) or main chain (Gly117) atoms to three consecutive DNA phosphates of one DNA strand (Fig. 3B). In addition, Arg116 and Arg186 contact different guanine bases in the same DNA strand (Fig. 3A,B). The “footprint” of the secondary DNA site covers 4 nucleotides (Fig. 3B) and consists of 11 direct hydrogen bonds from protein to DNA (Fig. 3A). In addition, there are at least 8 water-mediated hydrogen bonds from protein to DNA (not shown). The interface area between the protein and DNA is 584 Å2, significantly larger than the 380 Å2 for the interface area between DNA and the specificity helix. The interface area is small in comparison with that of the poxvirus Top IB with DNA (1517 Å2), but this is to be expected for a non-sequence-specific secondary site with a relatively small footprint. Thus, we regard the DNA interactions at the secondary site of DraTopIB as too extensive to ascribe to incidental lattice contacts, unlike the case of the DraTopIB specificity helix discussed above that makes few contacts with the DNA.
Figure 3. Close-up view of the interactions between DNA and DraTopIB.
(A) A stereo image of the secondary DNA binding site is shown. Side chains and a main chain amide that contact the DNA ligand are depicted as sticks. Hydrogen bonds are denoted by dashed lines. (B) The 12mer duplex DNA ligand is depicted as a two-dimensional base-paired ladder. Atomic contacts between the indicated amino acid side chains (or the Gly117 amide) and the DNA phosphates or nucleobases are indicated by arrows.
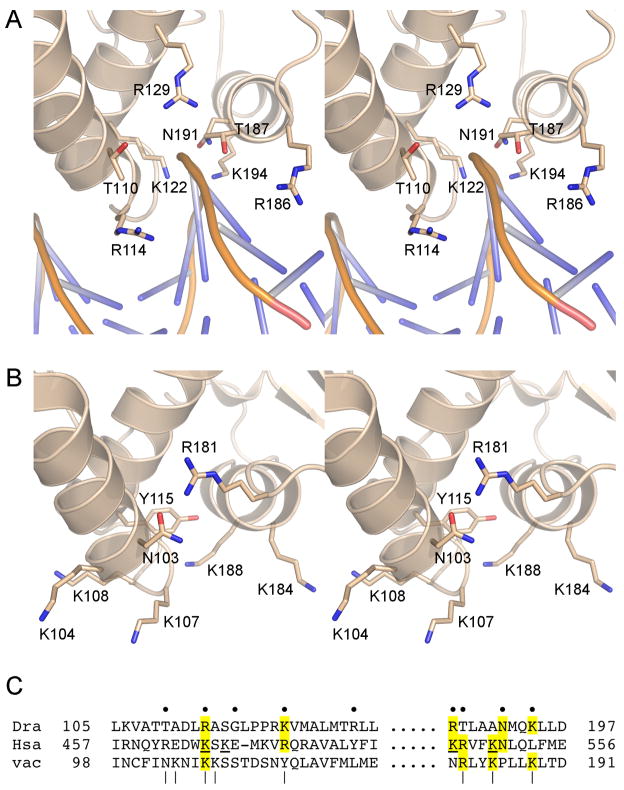
The significance of the secondary DNA binding site defined by the new DraTopIB structure is underscored by its conservation in the poxvirus and eukaryal TopIB. Superposition of the viral and human enzymes on DraTopIB shows that the component α-helices and loops of the secondary DNA site are preserved in all three TopIBs, affording a similar groove to accommodate one strand of duplex DNA (Fig. 4). Also, many of the basic and hydrophilic side chains that contact the DNA in DraTopIB (Fig. 4A) are either conserved in the poxvirus and human enzymes or substituted by a related side chain in a similar spatial position (Fig. 4B and C). The available structural and phylogenetic information suggest that the secondary DNA site is not a unique feature of DraTopIB. Whether other TopIB enzymes can bind DNA using this site remains to be determined (see below). A recent study implicates a cluster of four lysines on the surface of human TopIB as contributory to the preferential binding of TopIB to supercoiled DNA (Yang et al., 2009); these lysines (underlined in Fig. 4C) are located within the putative human TopIB equivalent of the DraTopIB secondary DNA binding site identified presently and three of them are conserved in poxviral and/or bacterial TopIB. These observations lend further credence to a secondary DNA site common to type IB enzymes.
Figure 4. Conservation of the secondary DNA binding site.
(A) A stereo image of the secondary DNA binding site in DraTopIB in shown, viewed from the opposite side of the C-domain as the image in Fig. 3. The DNA is depicted as a cartoon with the phosphate backbone chain shown in yellow and the nucleobases as blue sticks. (B) The corresponding region of the C-domain of poxvirus TopIB is shown in the same orientation as in panel A. The stereo image highlights the basic and polar side chains that are candidates to comprise the secondary DNA site in poxvirus TopIB. (C) This panel shows the aligned amino acids sequences of the two protein segments comprising the actual or imputed secondary DNA binding sites in exemplary bacterial, eukaryal cellular, and poxvirus TopIB enzymes – D. radiodurans (Dra) TopIB, Homo sapiens (Hsa) TopIB, and vaccinia (vac) TopIB, respectively – for which crystal structures are available. The aligment is based on superposition of the tertiary structures. The nine DraTopIB amino acids that contact the DNA in the secondary site are denoted by ●. The amino acids clusters in vaccinia TopIB that, when simultaneously mutated to alanine abolished synaptic plectoneme formation, are denoted by |. The four lysines in HsaTopIB implicated in crossover recognition on the basis of the effects of clustered lysine-to-glutamate changes (Yang et al., 2009) are underlined. Positions of side chain identity/similarity at the actual or imputed DNA binding residues are highlighted in yellow shading.
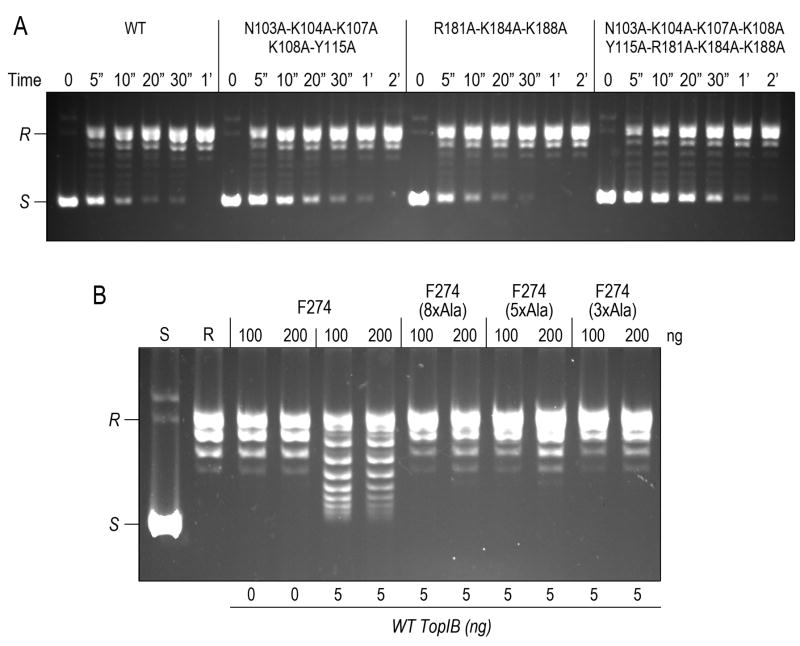
To gain a sense of whether the secondary site might be important for DNA relaxation and/or DNA site recognition by poxvirus TopIB, we engineered three alanine-cluster mutants that eliminated the putative equivalents of the secondary DNA-binding side chains. One cluster (N103A-K104A-K107A-K108A-Y115A; or 5xAla) targeted five amino acids in the helix-loop-helix that engages from the minor groove; the second cluster (R181A-K184A-K188A; or 3xAla) targeted three residues in the α-helix that binds from the major groove. A third cluster (N103A-K104A-K107A-K108A-Y115A-R181A-K184A-K188A; or 8xAla) simultaneously changed all eight amino acids to alanine. The mutated poxvirus TopIBs were produced in E. coli with a C-terminal His6-tag and purified from soluble bacterial lysates by Ni-agarose and phosphocellulose chromatography (Yakovleva et al., 2008) in parallel with wild-type TopIB. The rate of relaxation of supercoiled plasmid DNA by the 3xAla cluster mutant was indistinguishable from the wild-type TopIB, whereas the 5xAla and 8xAla cluster mutants relaxed at about half and one-third of the wild-type rate, respectively (Fig. 5A). The rates of single-turnover cleavage of a short duplex DNA “suicide substrate” containing a single 5′-CCCTT↓ cleavage site for poxvirus TopIB were identical for the wild-type, 3xAla, 5xAla, and 8xAla cluster mutant proteins (not shown). We surmise that individual constituents or grouped subsets of the imputed secondary DNA site of poxvirus are not crucial for topoisomerase activity under the in vitro conditions we employ routinely to assess mutational effects.
Figure 5. Mutations at the secondary binding site of poxvirus TopIB abolish formation of plectonemic synaptic complexes but have little or no effect on supercoil relaxation.
(A) Kinetics of relaxation of 250 ng pUC19 DNA by 2.5 ng of vaccinia TopIB, either wild-type (WT) or the indicated mutants with clustered alanine substitutions at the putative secondary DNA binding site. The DNA products were resolved by native agarose gel electrophoresesis. The supercoiled (S) and relaxed (R) circular DNAs are indicated on the left. (B) Plectonemic supercoiling. Relaxed circular pUC19 DNA was incubated with 100 or 200 ng of vaccinia TopIB-Phe274 or the indicated Ala-cluster mutants thereof, then treated where indicated with 5 ng of wild-type TopIB to relax any supercoils introduced by binding of the F274 protein(s). The products were resolved by native agarose gel electrophoresis.
Implications for TopIB-mediated DNA synapsis
The present discovery of a secondary DNA binding site on DraTopIB lends support for Model B in Figure 1, in which DNA synapsis by poxvirus TopIB reflects capture of a second DNA duplex by DNA-bound proteins. Instructive clues to the process of synapsis were gained by docking the 12-mer DNA duplex bound to DraTopIB into the structure of the poxvirus TopIB bound covalently to its CCCTT target site in duplex DNA as a vanadate transition state mimetic (Perry et al., 2010). This was performed by superimposing the DNA-bound TopIB structures and then subtracting the DraTopIB protein. The resulting model of poxvirus TopIB with the primary and secondary DNA sites occupied in shown in Fig. 6, in three different orientations. The image in Fig. 6C highlights the C-shaped clamp formed by the N- and C-domains around the DNA duplex engaged in the catalytic site. Fig. 6B highlights the groove between the α-helices of the secondary DNA site at the base of the C-domain into which one of the DNA strands fits. The primary and secondary DNA duplexes are oriented similarly, but separated by ~30 Å. The view in Fig. 6A illustrates that the DNAs in the two sites do not have a parallel trajectory; rather their paths cross in this and other planes. Indeed, the angular difference between the paths of the two helices could explain the plectonemic winding of one duplex abound the other that occurs within the synaptic filaments formed by poxvirus TopIB on initially relaxed circular DNAs (Shuman et al., 1997). A similar model built using human TopIB (not shown) also shows the possible formation of a complex with two DNA binding sites, despite the much larger size of the eukaryotic enzyme and the presence of additional protein domains. This binding mode could explain the way eukaryotic TopIB binds to nodes in positively or negatively supercoiled DNA.
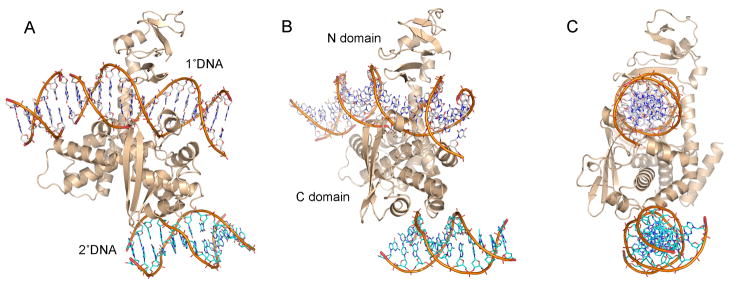
Figure 6. Model of TopIB with primary and secondary DNA sites occupied.
The diagram shows a model of poxvirus TopIB with both DNA binding sites occupied. The model was built by aligning the DraTopIB-DNA complex to the crystal structure of poxvirus TopIB bound covalently to its DNA target site as a vanadate transition state mimetic (Perry et al., 2010; PDB 3IGC) and then deleting the DraTopIB protein to leave just the 12-mer DNA in the secondary site. Three different views of the model are shown in A, B and C. The primary DNA ligand is enveloped within a circumferential protein clamp formed by the N- and C- domains. The secondary DNA ligand is docked at the base of the C-domain with one strand of the duplex fitting into the secondary binding site (B).
The secondary binding site is required for synaptic plectoneme formation by poxvirus TopIB
The structure of DraTopIB in complex with DNA at a secondary binding site provides a blueprint for functional probing of the basis for DNA condensation and synapsis by poxvirus TopIB, especially the plausibility of the model depicted in Fig. 6. Thus, we queried whether the cluster mutations in the secondary site of vaccinia TopIB affect the formation of plectonemic DNA braids, a key biochemical manifestation of TopIB-mediated synapsis (Shuman et al., 1997). To perform this analysis, we modified the wild-type TopIB by changing the Tyr274 nucleophile to phenylalanine, a maneuver that abolishes transesterification without affecting noncovalent DNA binding or intramolecular synapsis (Shuman et al., 1997). As shown in Fig. 5B, the incubation of relaxed plasmid DNA circles with stoichiometric amounts of the Phe274 protein introduced torsional strain, which, after relaxation by catalytic amounts of wild-type TopIB, resulted in the acquisition of up to 8 negative supercoils. As discussed previously (Shuman et al., 1997), this reaction indicates that the TopIB-DNA synaptic complex is a plectonemic supercoil in which the two duplexes encompassed by the protein filament are interwound in a right-handed helix (Shuman et al., 1997). When the same F274 change was introduced into the 5xAla, 3xAla and 8xAla cluster mutants, we found that they were uniformly unable to promote such plectonemic braiding (Fig. 5B). We surmise that the poxvirus equivalent of the secondary DNA site is essential for intramolecular synapsis and its topological sequelae. Our results are consistent with model B in Fig. 1 as the basis for TopIB-mediated synapsis.
In summary, we have demonstrated the existence, structure, and functional relevance of a secondary DNA binding site on bacterial and viral TopIB. This work gives impetus and affords a structural guide to analogous studies of DNA crossover binding by eukaryal cellular TopIB enzymes.
Materials and Methods
DraTopIB was produced and purified as described previously (Patel et al., 2006). Complementary 12-mer DNA oligonucleotides (5′-GAATAAGGGCGC-3′ and 3′-CTTATTCCCGCG-5′) were purified by reverse phase HPLC (Aggarwal, 1990) and then annealed. Initial crystallization trials with mixtures of DraTopIB and 12-bp duplex DNA were performed by the sitting drop vapor diffusion method in 96 well plates set up with a Hydra-II crystallization robot. Small crystals grew in polyethylene glycol at 10°C with a 1:1 molar ratio of protein and DNA. Refined conditions using the hanging drop vapor diffusion method yielded larger crystals in 30% PEG 400 (w/v), 0.1 M sodium acetate (pH 4.5), 0.2 M calcium chloride. Prior to data collection, the crystals were cryoprotected with 25% glycerol by stepwise transfer through cooled (4° C) solutions of the well buffer containing increasing concentrations of glycerol (in 5% glycerol increments per step and soaking for 2 min/step). Crystals were harvested with a rayon crystal-mounting loop, and flash cooled in liquid nitrogen.
A complete diffraction data set was collected initially using a laboratory x-ray source. Later, a higher resolution data set (to 1.65 Å) was collected using synchrotron radiation at DND CAT at the Advanced Photon Source (Argonne National Laboratory). All data were processed using XDS (Kabsch, 1993) and scaled using SCALA (Collaborative Computational Project 4, 1994). The crystals belong to space group C2 with unit cell dimensions a=119.7 Å, b=53.4 Å, and c=77.4 Å, β = 96.3° and had one DraTopIB protomer in the asymmetric unit. Data collection statistics are listed in Table I.
The structure was solved by molecular replacement with the program PHASER (McCoy et al., 2007) using the C-terminal domain of the DraTopIB apoenzyme (PDB 2F4Q) as a search model. Initial electron density maps clearly showed Fo−Fc difference density for DNA, but only weak density for the N-terminal domain. After refinement, the entire DNA and most of the C-terminal domain were built, but some regions of the N-terminal domain were disordered. Refinement was performed with REFMAC5 (Murshudov et al., 1997) and model rebuilding was executed in COOT (Emsley and Cowtan, 2004). The structure has a final Rwork and Rfree of 19.3% and 22.2% respectively with 99.7% of the residues in the favored regions of the Ramachandran plot and no outliers, and good rotamer distributions (Davis et al., 2004). Refinement statistics for the complex are compiled in Table I. The coordinates of the final model and the structure factors have been deposited in the PDB with accession code 3M4A. Figures were made with PyMOL (DeLano 2002).
Wild-type vaccinia virus TopIB and three mutants with clustered alanine substitutions at the secondary DNA binding site (5xAla, 3xAla, and 8xAla) were produced in E. coli with C-terminal His6 tags and purified from soluble bacterial lysates by Ni-agarose and phosphocellulose chromatography (Yakovleva et al., 2008). Topoisomerase reaction mixtures containing (per 20 μl) 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 2.5 mM EDTA, 250 ng supercoiled pUC19 plasmid DNA, and 2.5 ng of TopIB were incubated at 37° C. Aliquots (20 μl) were withdrawn at 5, 10, 20, 30, 60, 120 and 240 s and then quenched immediately with SDS. “Time 0” samples were taken prior to adding TopIB. The mixtures were analyzed by electrophoresis through a 1% horizontal agarose gel in TBE buffer (90 mM Tris-borate, 2.5 mM EDTA). DNA was visualized by staining with ethidium bromide and UV transillumination.
Biochemical analysis of the topological changes in relaxed circular DNA accompanying intramolecular synapsis was performed as described previously (Shuman et al., 1997). To prepare the relaxed plasmid DNA, a reaction mixture (100 μl) containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 25 μg supercoiled pUC19 DNA, and 0.5 μg wild-type vaccinia TopIB was incubated for 30 min at 37° C. The reaction was quenched by adding SDS to 0.2% and EDTA to 20 mM. The mixture was digested with 1 μg proteinase K for 2 h at 37° C, then extracted twice with phenol: chloroform and once with chloroform. The relaxed DNA was recovered by ethanol precipitation and resuspended in 10 mM Tris-HCl (pH 7.5), 1 mM EDTA. Vaccinia TopIB-F274 and F274 variants of the 5xAla, 3xAla, and 8xAla cluster mutants were produced in E. coli with C-terminal His6 tags and purified by Ni-agarose and phosphocellulose chromatography. To assay synaptic plectoneme formation, reaction mixtures (20 μl) containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 2.5 mM EDTA, 250 ng relaxed pUC19 DNA, and 100 or 200 ng of vaccinia TopIB-F274 or its Ala-cluster variants were incubated for 15 min at 37° C. The mixtures were then supplemented where with 5 ng wild-type vaccinia TopIB where specified and incubated for another 15 min at 37° C. The reactions were halted by adding SDS to 0.5%. The mixtures were digested with 10 μg proteinase K for 1 h at 37° C and then analyzed by electrophoresis through a 1% horizontal agarose gel in TBE buffer, in parallel with samples of supercoiled and relaxed pUC19. The DNA was visualized by staining with ethidium bromide.
Supplementary Material
Acknowledgments
Support from the R.H. Lurie Comprehensive Cancer Center of Northwestern University to the Structural Biology Facility is acknowledged. This research was supported by NIH grants GM51350 (to A.M.) and GM46330 (to S.S.). S.S. is an American Cancer Society Research Professor. Portions of this work were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) Synchrotron Research Center at the Advanced Photon Source (APS). DND-CAT is supported by Dupont, DOW and the NSF. Use of the APS is supported by the Department of Energy (DOE). We thank Greg Van Duyne for providing the coordinates of the poxvirus TopIB–DNA vanadate transition state mimetic in advance of publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal AK. Crystallization of DNA binding proteins with oligodeoxynucleotides. METHODS: A Companion to Meth Enzym. 1990;1:83–90. doi: 10.1016/S1046-2023(05)80150-1. [DOI] [PubMed] [Google Scholar]
- Benarroch D, Claverie JM, Raoult D, Shuman S. Characterization of mimivirus DNA topoisomerase IB suggests horizontal gene transfer between eukaryal viruses and bacteria. J Virol. 2006;80:314–321. doi: 10.1128/JVI.80.1.314-321.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Kussie P, Pavletich N, Shuman S. Conservation of structure and mechanism between eukaryotic topoisomerase I and site-specific recombinases. Cell. 1998;92:841–850. doi: 10.1016/s0092-8674(00)81411-7. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Berger JM. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu Rev Biophys Biomol Struct. 2004;33:95–118. doi: 10.1146/annurev.biophys.33.110502.140357. [DOI] [PubMed] [Google Scholar]
- Davies DR, Mushtaq A, Interthal H, Champoux JJ, Hol WG. The structure of the transition state of the heterodimeric topoisomerase I of Leishmania donovani as a vanadate complex with nicked DNA. J Mol Biol. 2006;357:1202–1210. doi: 10.1016/j.jmb.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Davis IW, Murray LW, Richardson JS, Richardson DC. MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 2004;32:W615–619. doi: 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs K, Karplus PA. Improved R-factors for diffraction data analysis in macromolecular crystallography. Nat Struct Biol. 1997;4:269–275. doi: 10.1038/nsb0497-269. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Forterre P, Gribaldo S, Gadelle D, Serre MC. Origin and evolution of DNA topoisomerases. Biochimie. 2007;89:427–446. doi: 10.1016/j.biochi.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr. 1993;26:795–800. [Google Scholar]
- Koster DA, Croquette V, Dekker C, Shuman S, Dekker NH. Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature. 2005;434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- Krogh BO, Shuman S. Catalytic mechanism of DNA topoisomerase IB. Mol Cell. 2000;5:1035–1041. doi: 10.1016/s1097-2765(00)80268-3. [DOI] [PubMed] [Google Scholar]
- Krogh BO, Shuman S. A poxvirus-like type IB topoisomerase family in bacteria. Proc Natl Acad Sci U S A. 2002;99:1853–1858. doi: 10.1073/pnas.032613199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KR, Stewart L, Champoux JJ. Preferential binding of human topoisomerase I to superhelical DNA. Embo J. 1995;14:5399–5409. doi: 10.1002/j.1460-2075.1995.tb00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Herrero F, Holtzer L, Koster DA, Shuman S, Dekker C, Dekker NH. Atomic force microscopy shows that vaccinia topoisomerase IB generates filaments on DNA in a cooperative fashion. Nucleic Acids Res. 2005;33:5945–5953. doi: 10.1093/nar/gki906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Patel A, Shuman S, Mondragon A. Crystal structure of a bacterial type IB DNA topoisomerase reveals a preassembled active site in the absence of DNA. J Biol Chem. 2006;281:6030–6037. doi: 10.1074/jbc.M512332200. [DOI] [PubMed] [Google Scholar]
- Perry K, Hwang Y, Bushman FD, Van Duyne GD. Structural basis for specificity in the poxvirus topoisomerase. Mol Cell. 2006;23:343–354. doi: 10.1016/j.molcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Perry K, Hwang Y, Bushman FD, Van Duyne GD. Insights from the structure of a smallpox virus topoisomerase-DNA transition state mimic. Structure. 2010;18:127–137. doi: 10.1016/j.str.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J, Shuman S. Proteolytic footprinting of vaccinia topoisomerase bound to DNA. J Biol Chem. 1995;270:11636–11645. doi: 10.1074/jbc.270.19.11636. [DOI] [PubMed] [Google Scholar]
- Shuman S, Bear DG, Sekiguchi J. Intramolecular synapsis of duplex DNA by vaccinia topoisomerase. Embo J. 1997;16:6584–6589. doi: 10.1093/emboj/16.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Claeboe CD, Hecht SM, Shuman S. Remote phosphate contacts trigger assembly of the active site of DNA topoisomerase IB. Structure. 2004a;12:31–40. doi: 10.1016/j.str.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Tian L, Claeboe CD, Hecht SM, Shuman S. Mechanistic plasticity of DNA topoisomerase IB: phosphate electrostatics dictate the need for a catalytic arginine. Structure. 2005;13:513–520. doi: 10.1016/j.str.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Tian L, Sayer JM, Jerina DM, Shuman S. Individual nucleotide bases, not base pairs, are critical for triggering site-specific DNA cleavage by vaccinia topoisomerase. J Biol Chem. 2004b;279:39718–39726. doi: 10.1074/jbc.M407376200. [DOI] [PubMed] [Google Scholar]
- Yakovleva L, Chen S, Hecht SM, Shuman S. Chemical and traditional mutagenesis of vaccinia DNA topoisomerase provides insights to cleavage site recognition and transesterification chemistry. J Biol Chem. 2008;283:16093–16103. doi: 10.1074/jbc.M801595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva L, Lai J, Kool ET, Shuman S. Nonpolar nucleobase analogs illuminate requirements for site-specific DNA cleavage by vaccinia topoisomerase. J Biol Chem. 2006;281:35914–35921. doi: 10.1074/jbc.M608349200. [DOI] [PubMed] [Google Scholar]
- Yang Z, Carey JF, Champoux JJ. Mutational analysis of the preferential binding of human topoisomerase I to supercoiled DNA. FEBS J. 2009;276:5906–5919. doi: 10.1111/j.1742-4658.2009.07270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechiedrich EL, Osheroff N. Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J. 1990;9:4555–4562. doi: 10.1002/j.1460-2075.1990.tb07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.