Abstract
The atheroprotective potential of n-3 α-linolenic acid (ALA) has not yet been fully determined, even in murine models of atherosclerosis. We tested whether ALA-derived, n-3 long chain polyunsaturated fatty acids (LCPUFA) could offer atheroprotection in a dose-dependent manner. Apolipoprotein B (ApoB)100/100LDLr−/− mice were fed with diets containing two levels of ALA from flaxseed oil for 16 weeks. Fish oil- and cis-monounsaturated-fat-enriched diets were used as positive and negative controls, respectively. The mice fed cis-monounsaturated fat and ALA-enriched diets exhibited equivalent plasma total cholesterol (TPC) and LDL-cholesterol (LDL-c) levels; only mice fed the fish-oil diet had lower TPC and LDL-c concentrations. Plasma LDL-CE fatty acid composition analysis showed that ALA-enriched diets lowered the percentage of atherogenic cholesteryl oleate compared with cis-monounsaturated-fat diet (44% versus 55.6%) but not as efficiently as the fish-oil diet (32.4%). Although both ALA and fish-oil diets equally enriched hepatic phospholipids with eicosapentaenoic acid (EPA) and ALA-enriched diets lowered hepatic cholesteryl ester (CE) levels compared with cis-monounsaturated-fat diet, only fish oil strongly protected from atherosclerosis. These outcomes indicate that dietary n-3 LCPUFA from fish oil and n-3 LCPUFA (mostly EPA) synthesized endogenously from ALA were not equally atheroprotective in these mice.
Keywords: atherosclerosis, cholesteryl ester, lipoprotein metabolism, liver, long chain polyunsaturated fatty acid
A large body of evidence indicates the importance of dietary n-3 long chain polyunsaturated fatty acids (n-3 LCPUFA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) to cardioprotection (1, 2). As a consequence, increased consumption of food-based sources of EPA and DHA has been recommended in most nutritional guidelines issued by government and health organizations, such as American Heart Association (3). Although oily fish is the richest dietary source of EPA and DHA, its consumption in most Western countries is low and inadequate to provide the recommended daily intake (250–500 mg of EPA and DHA) (3). Vegetable oils, such as flaxseed, soybean, and canola oil, and nuts, such as English walnuts, that are enriched in α-linolenic acid (ALA) can serve as alternative sources of EPA and DHA. ALA is referred to as the essential precursor of n-3 LCPUFA because its further elongation and desaturation leads to the formation of EPA and DHA (4). However, the rate of conversion of ALA to EPA is low (∼5%) and to DHA even lower (<1%) and can be modified by several factors, such as dietary cholesterol, n-6 PUFA intake, and gender-related hormones (5).
The atheroprotective potential of ALA has been questioned for a long time but not yet extensively investigated. To date, studies in humans have reported that (a) ALA supplementation results in its rapid incorporation into lipoproteins and elevated plasma ALA, EPA, and docosapentaenoic acid (DPA) but not DHA concentrations (6, 7); (b) ALA intake is inversely associated with nonfatal acute myocardial infarction (8, 9); and (c) ALA supplementation provides mild protection against ischemic heart disease (10). Overall, benefits associated with ALA consumption appear to be modest compared with those reported after fish-oil supplementation, even though studies addressing a direct comparison between ALA and dietary EPA and DHA from fish oil in humans are scarce.
In hypercholesterolemic animal models, the potential atheroprotection of ALA has been mainly investigated by using whole flaxseeds (11–13). However, as stated in a recent review by Prasad, suppression of atherosclerosis by flaxseed is likely more attributable to the lignan content of flaxseed rather than ALA (14). It still remains unclear whether ALA, per se, may be beneficial in atheroprotection by exhibiting cardioprotective properties similar to those observed with dietary EPA and DHA from fish oil. Previously we found that in a mouse model of LDL-driven atherosclerosis [apolipoprotein B (apoB)100/100LDLr−/− mice], ALA from flaxseed oil limited both plasma and hepatic cholesteryl ester (CE) deposition while enriching hepatic phospholipids (PL) with EPA (unpublished observations). These outcomes suggested a potential for ALA as an atheroprotective fatty acid. However a large dietary study in the same mouse model showed that ALA-derived flaxseed oil was not atheroprotective as was dietary fish oil (15).
In this study we tested the hypothesis that ALA atheroprotection may occur in a dose-dependent manner such that higher ALA amounts would confer as much benefit as dietary EPA and DHA from fish oil. Here we show for the first time in a head-to-head comparison that EPA derived from ALA, although incorporated into tissue lipids to an extent comparable to EPA derived from fish oil, is not nearly as effective as dietary EPA from fish oil in protecting against atherosclerosis.
MATERIALS AND METHODS
Mice, diets, and study outline
ApoB100/100LDLr−/− female mice were created as previously described (15). Mice were of a mixed genetic background (∼75% C57BL/6 and ∼25% 129Sv/Jae). To minimize genetic heterogeneity, sibling controls were used. At 7–8 weeks of age, mice were switched from a diet of regular chow to one of four experimental diets containing 20% calories from fat and 0.02% cholesterol by weight. Two levels of ALA (2% and 4% of energy) from flaxseed oil (Bioriginal Food and Science Corp., Saskatoon, Canada) were used. Isocaloric diets enriched either in cis-monounsaturated fatty acids (mainly oleic acid) (AC HUMKO, Memphis, TN) or fish oil (Omega Protein, Inc., Houston TX) were used as negative and positive controls, respectively. The diet groups consisted of 12 animals, and the diets were administered for 16 weeks. The diets were balanced for all the ingredients; the only variable was the fatty acid composition. See supplementary data for a detailed description of the dietary fatty acid composition (Tables IA and IB) and dietary ingredients (Table II). At baseline and after 2, 4, 8, and 16 weeks of diet, a blood sample was taken by submandibular vein puncture and placed into a tube containing a protease inhibitor cocktail (Sigma) dissolved in 5% EDTA, 5% NaN3. Plasma was separated after centrifugation at 12,500 g for 15 min at 4°C. After being fed the experimental diets for 16 weeks, mice were fasted for 4 h and then euthanized. At the time of sacrifice, a terminal blood sample was drawn via heart puncture, and the carcass was flushed with saline. Liver samples were collected, weighted, snap-frozen in liquid N2,and stored in an ultralow freezer at −80°C. The heart with attached aorta (beginning at the aortic sinus and ending at the iliac bifurcation) was removed from the body and stored in 10% neutral buffered formalin. All animals were housed in our American Association for the Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility. All procedures involving mice had prior approval from the Institutional Animal Care and Use Committee of Wake Forest University School of Medicine.
Plasma lipid and lipoprotein measurements
Plasma total cholesterol (TPC) and triglyceride (TG) concentrations were measured using enzymatic assays as previously described (15). Cholesterol concentrations in VLDL, LDL, and HDL were determined after separation of individual plasma samples (n = 12 mice per diet group) by HPLC with a Pharmacia Superose 6 column run at a flow rate of 0.5 ml/min as previously described (15). LDLs were isolated from fresh aliquots of plasma from individual mice and measured for lipid and fatty acid composition. Briefly, lipoproteins were isolated from plasma samples by adjusting the solution density to 1.225 g/ml with KBr. The plasma samples were then centrifuged for 4 h at 100,000 g in a TLA 120.2 rotor in an Optima MAX-E ultracentrifuge (Beckman Instruments). The floated lipoproteins were harvested using a tube slicer. The lipoprotein mixture was injected onto a Superose 6 chromatography column, which was subsequently run at 0.5 ml/min with 0.9% NaCl containing 0.05% EDTA (pH 7.4) and 0.05% NaN3. Fractions corresponding to LDL were collected, pooled according to elution time, and measured for lipid concentration and fatty acid composition as previously described (15). LDL particle diameter was determined by dynamic light scattering using a zetasizer nano S (Malvern Instruments, United Kingdom).
Liver lipid measurements
Liver lipid concentrations were estimated after performing chloroform-methanol extraction on ∼80 mg of liver tissue as previously described (16). To determine the fatty acid compositions, liver CE, TG, and PL were separated by thin layer chromatography, and the corresponding bands were scraped and methylated. Fatty acids were then quantified by gas-liquid chromatography.
Quantification of atherosclerosis
The extent of atherosclerosis was measured by quantifying the accumulation of CE in the aorta, including the entire vessel from the heart to the iliac bifurcation, according methods previously described (17). Briefly, the aortas were cleaned by removing all adherent adipose and connective tissue, and lipids were extracted in chloroform-methanol using 5α-cholestane as an internal standard. Cholesterol mass was measured by gas-liquid chromatography. Aortic CE (esterified cholesterol × 1.67) was calculated as the difference between free and total cholesterol as measured before and after saponification by extraction of the sterol into hexane. The delipidated tissue protein was digested in 1N NaOH, and total protein content was determined according to the Lowry method (18).
Quantification of mRNA levels
Real-time PCR analyses of liver tissue were performed according to methods previously published (19). Briefly, total mRNA was extracted from ∼80 mg of liver with Trizol (Invitrogen Life Technologies). After resuspension in 300 μl of diethyl pyrocarbonate water, mRNA was reverse transcribed into cDNA using Omniscript reverse transcriptase (Qiagen) under the following conditions: 37°C for 1 h and 93°C for 5 min. RT-PCR was done in triplicate with 5 μl of cDNA, 12.5 μl of SYBR GREEN PCR master mix (Applied Biosystems), 5.5 μl of diethyl pyrocarbonate water, and 1 μl of forward and reverse primer for a final reaction volume of 25 μl. PCR was then run on the Sequence Detection System 7000 under the following conditions: 50°C for 2 min; 94°C for 10 min; and 40 cycles of 94°C for 10s and 60°C for 1 min. The fluorescence measurement used to calculate threshold cycle (Ct) was made at the last time point. A dissociation step was run to ensure a single amplification product. The arbitrary unit value was calculated as follows: 1 × 109 × e(−0.6931 × Ct). All values were normalized to cyclophillin mRNA concentration of the sample to take total mRNA concentration into account. Primer sequences used for qPCR are shown in supplemental Table III.
Statistical analyses
Data are expressed as the mean ± SEM. Results were analyzed using one-way ANOVA followed by Tukey's test for post hoc analyses. Differences were considered significant at P < 0.05. All analyses were performed using GraphPad Prism (version 4) software.
RESULTS
In the current study apoB100/100LDLr−/− mice were chosen because they are prone to develop diet-induced atherosclerosis and exhibit a plasma lipoprotein profile similar to that seen in humans. Cis-monounsaturated fat was selected as the atherogenic comparator fat because in several different animal models and experimental conditions it consistently promoted the highest aortic cholesteryl ester deposition which is the chemical endpoint used to quantify the extent of atherosclerosis in this study (15, 16, 20, 21). At baseline, animals exhibited TPC concentrations about 350 mg/dl with LDL being the predominant plasma lipoprotein class.
ALA-enriched diets are ineffective in lowering plasma cholesterol levels compared with fish-oil diet
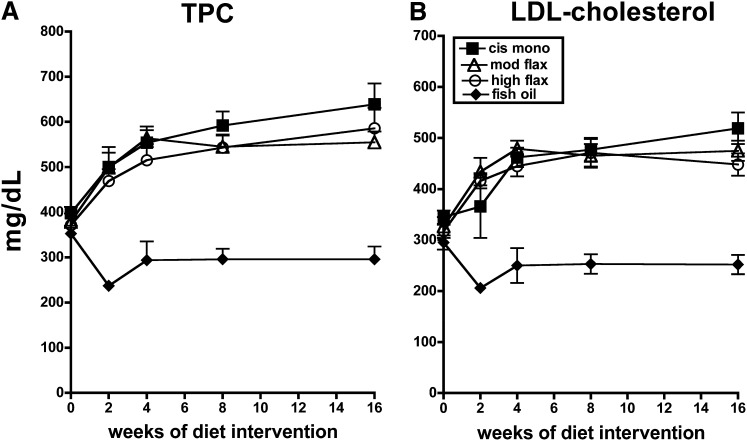
TPC responses to the different fatty acids during the intervention period are illustrated in Fig. 1A. In as early as two weeks of diet intervention, dietary fat-specific differences were found among the diet groups. Both cis-monounsaturated fat and ALA-enriched diet-fed animals showed a significant increase in TPC compared with baseline, while fish oil-fed mice maintained lower TPC concentrations. This trend continued at 4, 8, and 16 weeks, indicating that increased amounts of ALA (high-flax diet) were unable to provide additional benefit compared with cis-mono and moderate flax diets. A similar pattern was observed when the cholesterol concentration in the LDL fraction was determined (Fig. 1B). At two weeks into the study, groups fed cis-mono and ALA-enriched diets exhibited LDL-cholesterol (LDL-c) concentrations of about 400 mg/dl, which were higher than the LDL-c values in the fish-oil group (∼206 mg/dl). At 4, 8, and 16 weeks, cis-mono and ALA-enriched diet-fed animals showed further increases in LDL-c, reaching values near 500 mg/dl at the end of the study, while fish-oil-fed animals did not show increasing LDL-c concentrations and values remained similar throughout the intervention period. When cholesterol concentrations were measured in the VLDL fraction, lower VLDL-c levels (24.3 mg/dl) were found in mice fed the high-flax diet compared with cis-mono and moderate-flax diet-fed animals (61 and 37 mg/dl, respectively), but VLDL-c levels were still higher compared with those measured in fish-oil-fed animals (5.7 mg/dl). Fish-oil diet was effective in reducing plasma triglyceride levels compared with baseline (111.2 mg/dl versus 61.2 mg/dl), and the hypotriglyceridemic effect persisted during diet intervention. No effect on plasma TG was measured in either cis-mono or ALA-enriched diet-fed animals (data not shown).
Fig. 1.
A: Total plasma cholesterol (TPC) and (B) LDL-c concentrations measured at 0, 2, 4, 8, and 16 weeks in mice fed different fatty acids–enriched diets during the intervention period. The data points represent mean ± SEM for 12 mice for each diet group.
ALA incorporation in plasma LDL lowers cholesteryl oleate percentage and enriches LDL-CE with EPA
Studies in nonhuman primates and transgenic mice have reported alterations in LDL particle size and composition of lipid and fatty acid induced by dietary fat type (15, 16, 20, 21). The core lipid of LDL particles was somewhat different in composition in the fish-oil group, with the triacylglycerol percentage being the lowest compared with cis-mono and ALA-enriched diets (1.7% versus 3.67% and 2.5%, respectively) while a slightly lower cholesteryl ester percentage (41% versus 44.6%) was also found. Particle diameters, assessed by light scattering means, were not different among dietary fat groups, reflecting the modest differences in lipid composition (data not shown).
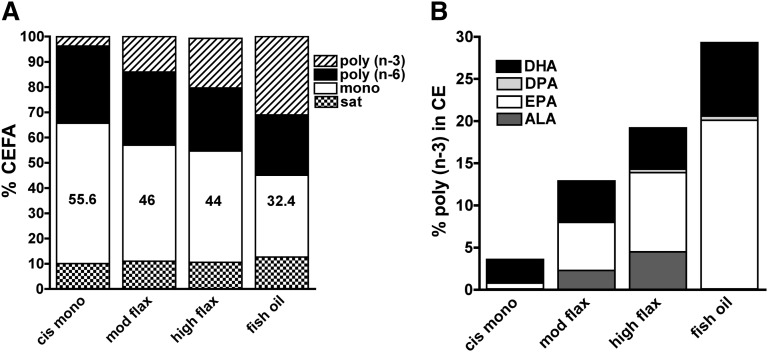
LDL cholesteryl ester fatty acid (CEFA) composition has been reported to be predictive of atherosclerosis (20–22). Fig. 2 illustrates LDL CEFA composition measured after 16 weeks of diet intervention. As shown in Fig. 2A, the predominant fatty acids in CE were monounsaturated fatty acids (mostly oleic acid), which made up close to 50% of the total. Compared with the cis-mono diet, the ALA-enriched diet resulted in a lower percentage of cholesteryl oleate (44% versus 55.6%), while the lowest percentage was found in mice fed the fish-oil diet (32.4%). Similar saturated and n-6 polyunsaturated CE percentages were found among diet groups. In both ALA- and fish-oil-diet groups, a significant enrichment in n-3 polyunsaturated CE was found. To identify which n-3 fatty acid contributed the most, the percentages of ALA, EPA, DPA, and DHA were determined as shown in Fig. 2B. In both ALA and fish-oil groups, EPA was the predominant n-3 fatty acid, even though the extent of n-3 enrichment of LDL-CE was quite different. In the moderate- and high-flaxseed-oil groups, EPA made up to 5.7% and 9.4%, respectively, of LDL-CE fatty acids, while in fish-oil group, 20% of LDL-CE fatty acid was represented by EPA. In mice fed ALA-enriched diets, a dose-dependent increase in ALA and EPA percentages was observed without a change in DHA percentages. Fish-oil-fed animals exhibited the greatest enrichment in both EPA and DHA, which together made up close to 28% of the total fatty acids in CE.
Fig. 2.
LDL cholesteryl ester fatty acid (CEFA) composition measured in mice (n = 9 per diet group) fed different fatty acid-enriched diets for 16 weeks. A: The bars represent the mean percentages of saturated, monounsaturated, polyunsaturated CE in LDL. Inset indicates the pattern that corresponds to each type of CE. B: Enrichment of LDL-CE with n-3 polyunsaturated fatty acids. Bars represent the mean percentages of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). Inset indicates the pattern that corresponds to each type of n-3 fatty acid.
ALA lowers hepatic lipids and is incorporated into lipids as EPA
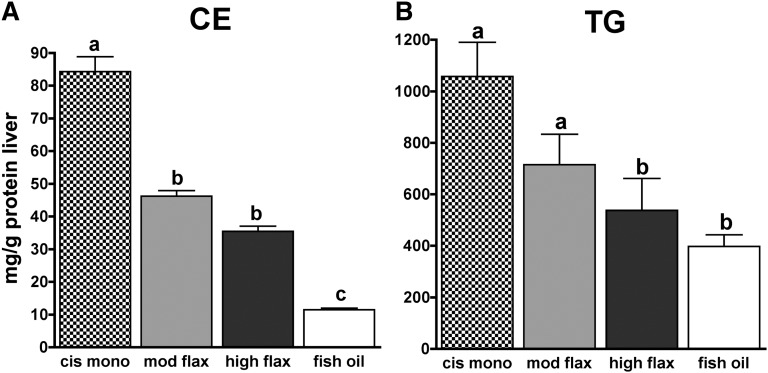
The primary source of cholesteryl oleate in plasma has been identified to be the hepatic enzyme acyl-CoA:cholesterol acyl-transferase 2 (ACAT2) (23–25). ACAT2 esterifies cholesterol using fatty acyl-CoA, mainly oleoyl-CoA. ACAT2 is the isoform of ACAT responsible for the synthesis of CEs incorporated into apoB-containing lipoproteins secreted by the liver and intestine (26, 27). Studies in hypercholesterolemic transgenic mice have elegantly demonstrated that alterations of dietary fatty acids shift the hepatic composition of fatty acyl-CoAs toward the diet composition so that diets enriched in PUFA provide less oleoyl-CoA available for esterification by ACAT2 (16). This shift would presumably result in a lower hepatic CE deposition in ALA- and fish-oil-fed animals. Fig. 3 shows the effect of dietary ALA and n-3 PUFA from fish oil on hepatic neutral lipid (CE and TG) concentrations. The fish-oil group had the lowest CE concentration while the cis-mono group had the highest hepatic CE accumulation (Fig. 3A). Although both ALA groups had lower hepatic CE concentrations compared with the cis-mono group (46 and 35.5 mg/g protein liver, respectively, versus 84 mg/g protein liver), the CE concentrations were higher than that seen in livers from the fish-oil group. A similar pattern was shown for hepatic TG concentrations (Fig. 3B), although in this case, the high-flax-diet group was as effective as fish oil in maintaining lower hepatic TG levels. We have previously observed that moderate amounts of ALA enriched hepatic lipids differently, with ALA being the main n-3 fatty acid in neutral lipids (CE and TG) and EPA the predominant fatty acid in phospholipids (PL) (unpublished observations). We analyzed the hepatic lipid fatty acid composition to determine whether higher amounts of ALA would provide a greater n-3 PUFA enrichment of hepatic lipids (Fig. 4). In agreement with our previous results, the extent of n-3 PUFA enrichment was different among hepatic lipids, with PL being the most enriched and CE the least enriched. In both ALA-diet groups, ALA was the predominant n-3 PUFA in neutral lipids, but it was absent from PL. ALA was incorporated in a dose-dependent manner in both CE and TG, and small percentages of EPA and DHA were found in these fractions as well. The n-3 PUFA enrichment of CE and TG was greater in the fish-oil group with DHA being the main n-3 PUFA. Phospholipid fatty acid composition differed from that of neutral lipids, and PL of both ALA groups had similar EPA and DHA percentages that made up close to 25% of the total fatty acids in PL; this pattern of n-3 fatty acid enrichment was not significantly different from that obtained by the fish-oil diet. In summary, compared to moderate, higher amount of ALA modestly increased the ALA and EPA percentages in CE and TG, but a higher amount of ALA provided an n-3 PUFA enrichment of hepatic PL as EPA that was comparable to that measured in the fish-oil group.
Fig. 3.
Hepatic cholesteryl ester (A) and triglyceride (B) levels in mice fed the experimental diets for 16 weeks (n = 12 per diet group). Letters represent signifi cant differences among groups (P < 0.05 by post hoc Tukey test).
Fig. 4.
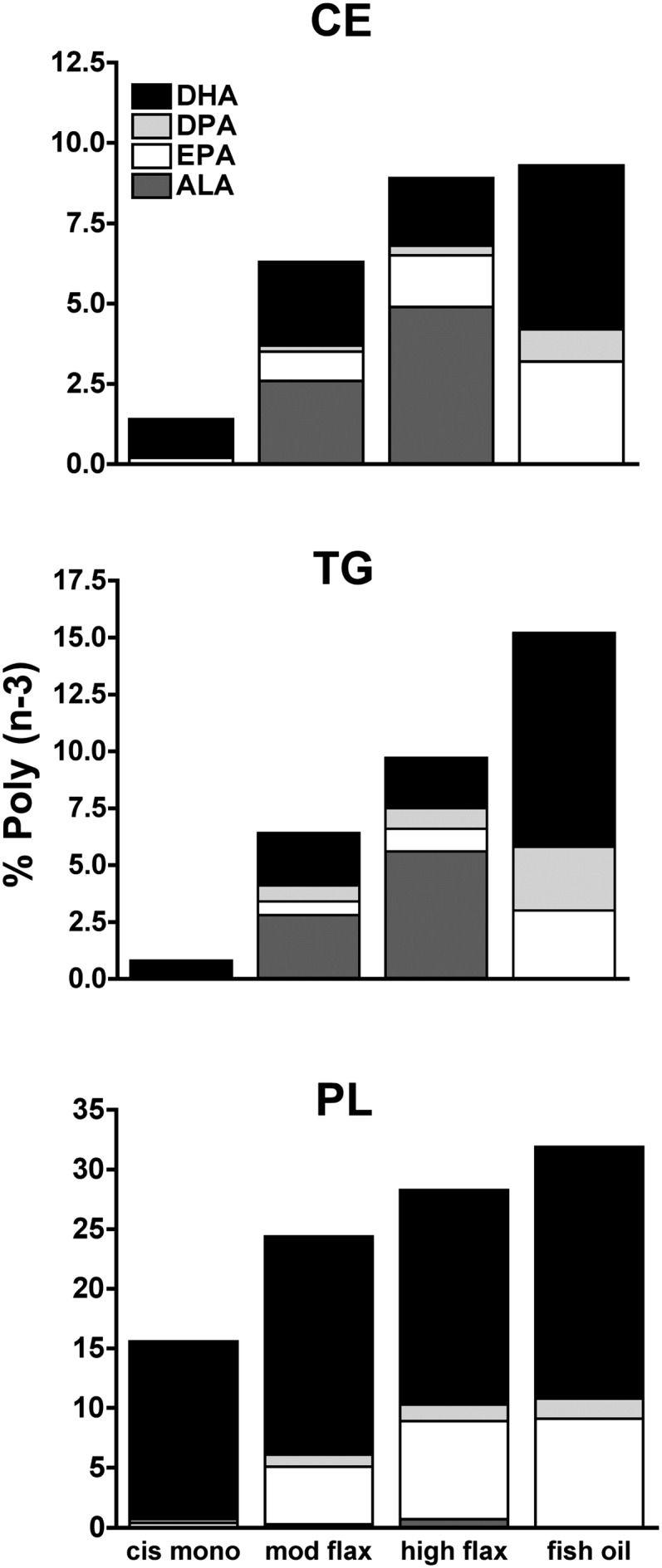
Hepatic lipid enrichment in n-3 polyunsaturated fatty acids measured after 16 weeks of diet intervention (12 mice per diet group). The bars represent the mean percentages of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) in CE, TG, and PL fractions. Inset indicates the color that corresponds to each type of n-3 fatty acid. CE, cholesteryl ester; PL, phospholipids; TG, triglyceride.
ALA does not protect mice from aortic CE deposition
Previous studies have shown that fatty acid compositional changes can translate into effects on atherosclerosis (15, 20). In this study we measured the accumulation of cholesteryl ester in the entire aorta as a chemical endpoint to quantitatively measure the extent of atherosclerosis after 16 weeks of diet intervention. Fig. 5 illustrates the atherogenic response to dietary fatty acids in the aorta. In agreement with previous reports (16, 21), cis-monounsaturated fat-rich diet promoted the highest aortic CE concentration at about 30 mg/g. Plasma and hepatic n-3 PUFA enrichment observed in both ALA groups did not result in a significant benefit to atherosclerosis, as indicated by aortic CE levels that were similar to those measured in the cis-mono group. As anticipated from earlier studies, the fish-oil group had a significantly lower aortic CE concentration.
Fig. 5.
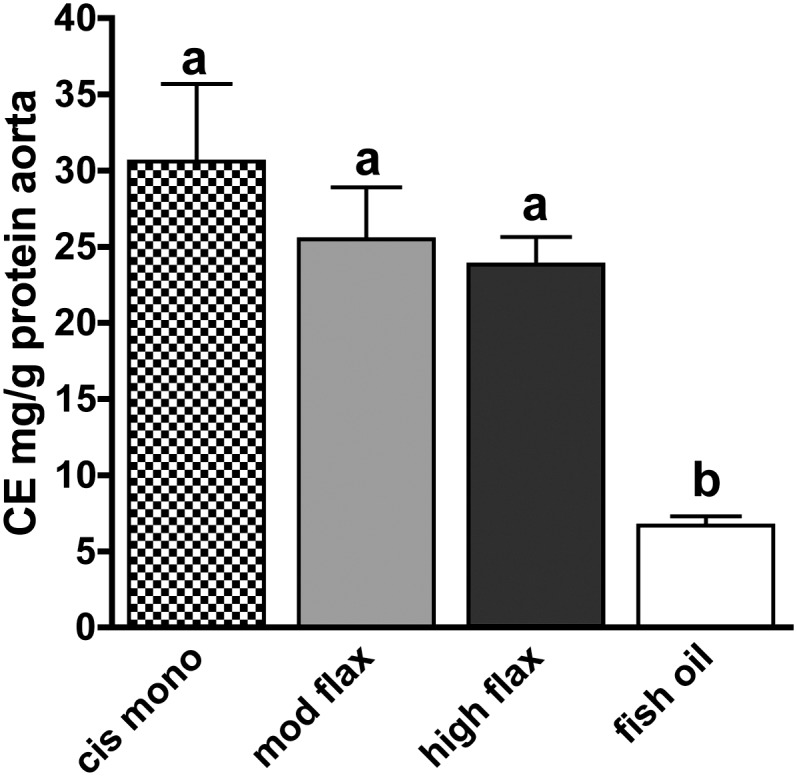
Quantification of atherosclerosis extent in diet-fed animals. Atherosclerosis was measured by gas-liquid chromatography in 12 animals for each diet group. Results are expressed as mg CE/g aortic protein. Letters represent signifi cant differences among groups (P < 0.05 by post hoc Tukey test).
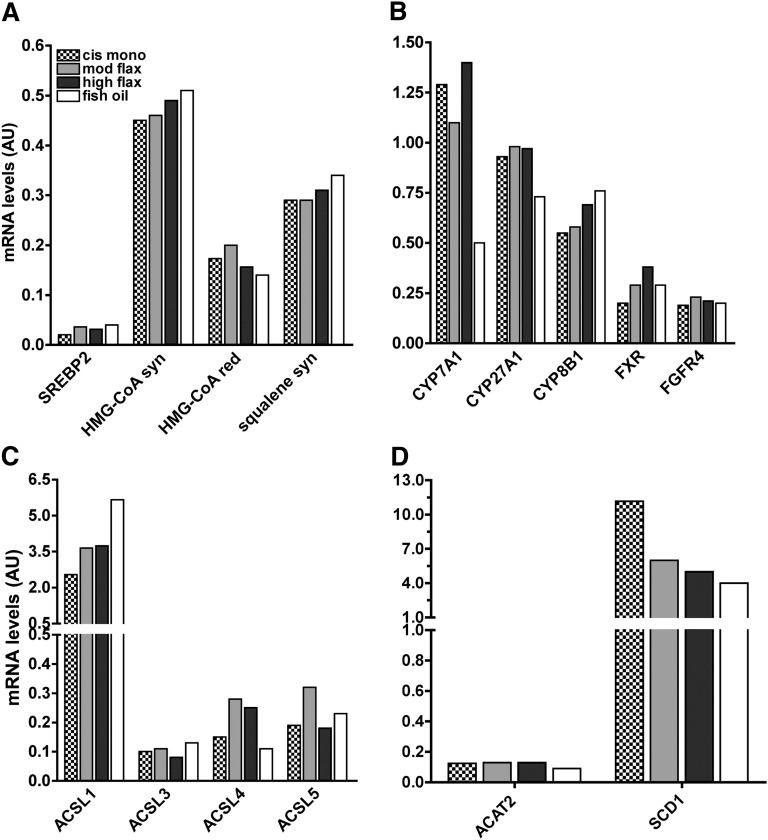
Fish-oil-fed animals had significantly lower hepatic CE levels (Fig. 3) compared with both cis-mono- and ALA-fed animals, and this pronounced hypocholesterolemic effect, along with the lowest LDL-c concentration (Fig. 1B) and LDL cholesteryl oleate percentage (Fig. 2A), apparently contributed to the atheroprotection (Fig. 5). The cholesterol-lowering effect of fish oil can likely be attributed to one or more mechanisms, including reduced cholesterol synthesis and esterification, lowered fatty acyl-CoA synthesis, and increased cholesterol catabolism by conversion into bile acids. Several hepatic genes influence these processes and were analyzed by RT-PCR. No major differences were found among livers of the four diet groups in cholesterol synthesis or bile acid metabolism-related genes, with the exception of cholesterol-7-α-hydroxylase (CYP7A1) (Fig. 6A, B). CYP7A1 transcript was found lower (up to 70%) in fish-oil-fed mice compared with the other diet groups, which is opposite from the directional change anticipated. Both dietary and endogenously synthesized fatty acids are required to be converted into fatty acyl-CoAs by long chain acyl-CoA synthetases (ACSL) before being incorporated into CE, TG, and PL. Four isoforms (ACSL1, ACSL3, ACSL4, and ACSL5) have been found in rodent liver, and few reports have indicated that they might exert independent functions and exhibit differential affinity toward fatty acids (28). As indicated in Fig. 6C, only ACSL1 of the four ACSL isoforms was different among diet groups. As previously reported, no significant differences were found in ACAT2 mRNA levels among dietary fat groups (16, 29) while stearoyl-CoA-desaturase 1 (SCD1) transcript was found lower when polyunsaturated fat-enriched diet was fed compared with the cis-mono diet (Fig. 6D).
Fig. 6.
Hepatic mRNA abundance of cholesterol synthesis (A), bile acid metabolism (B), fatty acyl-CoA synthesis (C), and cholesterol esterification (D) related genes. Measurement of mRNA levels was conducted by RT-PCR on pooled samples (n = 5 per pool) of mice fed the experimental diets for 16 weeks.
To date, a shorter dietary study (4 weeks) carried out under the same experimental conditions produced almost identical outcomes with regard to plasma and hepatic lipid concentrations and n-3 PUFA enrichment, suggesting that diet-induced alterations take place rapidly and contribute to the subsequent aortic response (data not shown).
Collectively these data indicate that in a mouse model of LDL-driven atherogenesis, robust reductions in TPC, LDL-c concentrations, and LDL cholesteryl oleate percentage are required for atheroprotection to be achieved. Fish-oil-fed animals experiencing all of these benefits were the only group protected from atherosclerosis.
DISCUSSION
This report provides original, clear documentation of the distinct effects of n-3 LCPUFA derived from ALA versus dietary EPA and DHA from fish oil on atherosclerotic lesion formation in mice. The major finding of this study was that although ALA gets converted into EPA (mostly) and DHA in amounts that equally enrich hepatic phospholipids with EPA from dietary fish oil, ALA did not confer atheroprotection. This was true even though ALA-enriched diets provided some lowering of atherogenic plasma cholesteryl oleate and hepatic CE levels. Given the large potential supply of ALA achievable from plant sources (compared with the more limited availability of EPA and DHA from marine sources), the data from the current study may have implications when considering increased ALA intake because it does not appear to be an adequate option to fish oil for providing atheroprotection. Further, this outcome was seen when the dietary levels of ALA, particularly in the high-flaxseed-oil group, were much higher than what is likely to be consumed by human beings.
Several potential mechanisms by which ALA could be beneficial to atheroprotection have been proposed. First, increased ALA consumption has been reported to elevate tissue ALA, EPA, DPA, and in some cases, DHA content (5, 6, 30); this effect could result in a plethora of cardioprotective actions, such as lowering plasma lipids, reducing blood pressure, and preventing arrhythmias. Second, ALA competes for the same metabolic enzymes (desaturases and elongases) as does linoleic acid (LA), so increased ALA intake may lead to reduced arachidonic acid (ARA) content and lower biosynthesis of proinflammatory eicosanoids (e.g., PGE2 and TBX2). Third, ALA may directly interact with nuclear receptors and transcription factors, such as peroxisome proliferator-activated receptor α (PPARα) or sterol regulatory element binding protein (SREBP)-1 and 2, and lead to alteration of lipid metabolism. Most of the cited mechanisms have been investigated both in vitro and in vivo (31–34), but results were not consistent and the potential for benefit to atherosclerosis were not conclusive. In our study, the atherosclerosis endpoint was specifically documented but found not to be particularly responsive to even very high levels of dietary ALA.
Controlled dietary studies in nonhuman primates and transgenic mouse models of atherosclerosis have consistently indicated that robust reductions in plasma LDL-c levels, plasma LDL cholesteryl oleate percentage, and hepatic cholesteryl ester concentrations apparently lead to reduced aortic cholesteryl ester deposition. Thus, these plasma and liver measures have been relevant endpoints that illustrate the atherogenic or atheroprotective potential of dietary fatty acids (15, 16, 20, 21).
To define the likelihood that a potential benefit of ALA occurs in atherosclerosis, all of the aforementioned endpoints were carefully measured in mice-fed diets with two different levels of ALA, and the outcomes were compared with those obtained in fish-oil-fed animals. In agreement with previous data (15, 20, 35), the fish-oil diet protected from atherosclerosis (Fig. 5). It apparently accomplished this through a number of effects, such as by significantly lowering plasma TC and LDL-c concentrations (Fig. 1), reducing LDL cholesteryl oleate percentage, enriching LDL-CE with EPA (Fig. 2), and decreasing hepatic CE levels (Fig. 3). In this study, cis-monounsaturated fat had none of these effects; it is a highly atherogenic fatty acid as has been previously documented in this and other animal models of atherosclerosis (15, 20, 21, 36). A dose-dependent n-3 PUFA enrichment of plasma LDL-CE (Fig. 2) and hepatic phospholipids (Fig. 4) was measured in ALA-fed mice along with a modest reduction of hepatic CE levels compared with cis-mono-fed animals. Interestingly, however, no significant reduction in atherosclerosis was seen with the degree of change induced by the ALA-enriched diets (Fig. 5).
The failure of ALA-derived n-3 PUFA to protect against atherosclerosis possibly can be attributed to its inability to alter LDL-c concentration. Previous studies reported that fish-oil–enriched diets significantly lower plasma LDL-c levels by increasing hepatic LDL uptake through an LDLr or LRP-independent mechanism (37) or additionally by decreasing precursor VLDL lipid secretion (38). If, as demonstrated by Vasandani (37) in LDLr−/− mice, reduced VLDL lipid secretion and hepatic cholesteryl ester levels account for ∼67% of the decrease in plasma LDL-c concentration, one would expect LDL-c concentrations in ALA diet groups to be lower than those in cis-mono group. ALA-fed mice had lower VLDL-cholesterol (data not shown) and hepatic CE and TG levels (Fig. 3) compared with cis-mono–fed animals, but the average plasma LDL-c concentration was very similar in cis-mono- and ALA-diet groups (Fig. 1B). One could hypothesize that a less efficient clearance of LDL particles, maybe through an LDLr-independent pathway, is occurring in ALA-diet group compared with the fish-oil-diet group, although a mechanism for this remains unclear. Intravascular remodeling of VLDL to LDL, as well as VLDL catabolism, can contribute to plasma LDL concentrations. Specific characteristics (size, apoE, apoC, and cholesteryl ester content) of VLDL determine whether the particle will be removed from the circulation or undergo conversion to LDL (39). Newly secreted VLDL, as isolated from liver perfusate from fish oil- or ALA-fed animals could be used in the future to identify which particle component may most effectively alter VLDL conversion to LDL. Furthermore, radiolabeled LDL isolated from fish-oil- or ALA-fed mice could be simultaneously injected into control mice to monitor differential clearance rates from the plasma compartment. Such studies possibly could extend our understanding of how dietary fatty acids affect intravascular lipoprotein remodeling and subsequent plasma lipoprotein levels. Note that n-6 PUFA has been shown to protect against atherosclerosis (15, 20) without sharing with n-3 PUFA from fish oil the ability to lower TPC and LDL-c (37), suggesting that the LDL-c-lowering effect may not always be necessary to provide atheroprotection.
Efficiency of ALA conversion to n-3 LCPUFA has been considered responsible for any effect of ALA on atheroprotection (10, 33). However, in our mouse model, ALA conversion to EPA and DPA and to some extent DHA occurred, resulting in a greater enrichment of plasma and hepatic lipids with n-3 PUFA. Similar percentages of EPA and DHA were found in hepatic PL from ALA- and fish-oil-fed animals, suggesting that the intracellular disposal of ALA-derived n-3 PUFA rather than its availability might have a more profound impact on atheroprotection. Phospholipid enrichment in polyunsaturated fatty acids has been proposed to promote a redistribution of cholesterol from the plasma membrane to the endoplasmic reticulum thus resulting in limited SREBP activation of cholesterol and fatty acid synthesis (40). The discovery of four isoforms of ACSL in mouse liver suggests the possibility that intracellular pool(s) of fatty acids may be governed differently according to their metabolic fate, selective incorporation into lipids, and ACSL substrate specificity (28). At present, mechanisms for ACSL regulation in mouse liver are unknown and do not allow more than speculation. It is possible that both dietary and ALA-derived EPA and DHA are incorporated in hepatic PL (Fig. 4) in two (or more) separate pools, with some supplying the lipoprotein secretion machinery (ALA-derived n-3 PUFA enriched plasma LDL-PL) and the others acting at the nuclear level to regulate the abundance of cholesterol and fatty acid-related genes. Although the data in Fig. 6 do not provide evidence for this hypothesis, it is possible that the hepatic response to dietary fat is rapid and gets blunted by physiological adaptations that occur within weeks of dietary intervention. Preliminary data from ongoing, short (number of days on diet) studies support this possibility (unpublished observations).
A comment has to be made about the amount of ALA fed in this study. Daily ALA intake has been estimated to be close to 1.6 g in the Western diet. In previous studies [Bell III et al. (15) and unpublished observations], mice were fed amounts of ALA equal to 1.9% of energy, which might be considered close to the average daily intake. For this study we used higher ALA concentrations, corresponding to 2.3% and 4.4% of energy, respectively, for the moderate- and high-flax diets, to investigate whether we could maximize ALA conversion to EPA and DHA. These concentrations should be considered above those typically achieved with the consumption of a Western-type diet and suggest that even by greatly increasing the amount of dietary ALA, little atheroprotection can be guaranteed.
Our data may provide support to the observation made by Prasad that flaxseed lignan content rather than ALA could be the major contributor to atheroprotection by flaxseeds. It has been shown that flaxseed lignan and secoisolariciresinol diglucoside (SDG) may exert antioxidant and hypotensive activity and may provide beneficial effects on inflammatory mediators [interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α] (14). A large body of evidence suggests that limiting inflammation and cytokine production by dietary n-3 PUFA (EPA and DHA) represents one of the potential mechanisms by which fish oil is atheroprotective (41, 42). Although we did not directly measure cytokine production and proinflammatory mediators, it cannot be excluded that a reduced secretion of proinflammatory cytokines and increased production of anti-inflammatory prostaglandins, such as PGE3, occurred in fish-oil-, but not in ALA-fed animals, thus promoting the prevention of atherosclerosis. We cannot explain why the ALA-derived EPA present in tissues at levels comparable to the EPA present in fish-oil–fed animals would not exert similar anti-inflammatory actions to the extent that is a factor. Indeed, the paradox of the data presented here is that dietary ALA does not provide a similar atheroprotection in spite of significant conversion into EPA and DHA over the course of atherosclerosis development.
How relevant to humans are the endpoints measured in this study? The observation that fish oil, by lowering LDL-c concentration, fosters atheroprotection does not fully reproduce the human situation. In both normal and hypertriglyceridemic patients, fish oil can lower plasma TG and VLDL-c levels, while increasing or leaving LDL-c concentrations unchanged (43). However, compared with ALA, fish oil has consistently provided cardioprotection in both primary and secondary studies. As mentioned previously, studies addressing a direct comparison between ALA and fish oil on cardiovascular risk are scarce, but there might be a significant advance in this field soon. The Alpha-Omega Trial (# NCT00127452) is an interventional, randomized study designed to measure as primary endpoint the coronary mortality in 4800 Dutch heart disease patients (men and women) receiving daily placebo or 400 mg of EPA/DHA, or 2 g of ALA, or both for 40 months; omega-3 fatty acids are provided through daily use of 20 g of margarine (spread) on bread (44). The study, which began in May 2002 and was expected to be completed by December 2009, should provide more definitive information about the potential for benefits of ALA on cardiovascular disease.
Any demonstration of a beneficial effect of increased dietary ALA intake could have a tremendous impact on the risk of coronary heart disease, but the results of our study clearly indicate that dietary EPA and DHA from fish oil, not from ALA-derived n-3 LCPUFA, are able to trigger (or modulate) molecular and cellular responses that ultimately result in an atheroprotective profile. The gift from the land, as Harris (45) named ALA, has not yet proven to be as precious as the gifts from the sea (EPA and DHA).
Supplementary Material
Acknowledgments
The authors greatly appreciate the gift of the flaxseed oil from Bioriginal Food and Science Corp. (Saskatoon, Canada) and the gift of OmegaPure refined menhaden oil (fish oil) from Omega Protein Inc. (Houston, TX ).
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- ALA
- alpha-linolenic acid
- ACAT2
- acyl-CoA-cholesterol:acyltransferase 2
- ACSL
- long chain acyl-CoA-synthetase
- CE
- cholesteryl ester
- CEFA
- cholesteryl ester fatty acid
- CYP7A1
- cholesterol-7-alpha-hydroxylase
- CYP27A1
- sterol-27-alpha-hydroxylase
- CYP8B1
- sterol-12-alpha-hydroxylase
- DPA
- docosapentaenoic acid
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- FGFR4
- fibroblast growth factor receptor 4
- FXR
- farnesoid X receptor
- HMG-CoA-synthase
- hydroxy-methyl glutaryl-CoA-synthase
- HMG-CoA-reductase
- hydroxy-methyl-glutaryl-CoA-reductase
- LCPUFA
- long chain polyunsaturated fatty acids
- LDL-c
- LDL-cholesterol
- PL
- phospholipid
- PPAR
- peroxisome proliferator-activated receptor
- SCD1
- stearoyl-CoA-desaturase 1
- SREBP
- sterol regulatory element binding protein
- TG
- triglyceride
- TPC
- plasma total cholesterol
- VLDL-c
- VLDL-cholesterol
This study was supported by National Institutes of Health Grant HL-49373 and National Center for Complementary and Alternative Medicine (NCCAM) and Office of Dietary Supplements (ODS) Grant P50AT0027820. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three tables.
REFERENCES
- 1.Kris-Etherton P. M., Hecker K. D., Binkoski A. E. 2004. Polyunsaturated fatty acids and cardiovascular health. Nutr. Rev. 62: 414–426. [DOI] [PubMed] [Google Scholar]
- 2.Demaison L., Moreau D. 2002. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell. Mol. Life Sci. 59: 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein A. H., Appel L. J., Brands M., Carnethon M., Daniels S., Franch H. A., Franklin B., Kris-Etherton P., Harris W. S., Howard B., et al. 2006. Diet and lifestyle recommendations revision 2006. A scientific statement from the American Heart Association Nutrition Committee. Circulation. 114: 82–96. [DOI] [PubMed] [Google Scholar]
- 4.Jump D. B. 2002. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 277: 8755–8758. [DOI] [PubMed] [Google Scholar]
- 5.Williams C. M., Burdge G. 2006. Long-chain n-3 PUFA: plant v. marine sources. Proc. Nutr. Soc. 65: 42–50. [DOI] [PubMed] [Google Scholar]
- 6.Burdge G. C., Calder P. C. 2005. α-linolenic acid metabolism in adult humans: the effects of gender and age on conversion to longer chain polyunsaturated fatty acids. Eur. J. Lipid Sci. Technol. 107: 426–439. [Google Scholar]
- 7.Sanders T. A., Younger K. M. 1981. The effect of dietary supplements of omega 3 polyunsaturated fatty acids on the fatty acid composition of platelets and plasma choline phosphoglycerides. Br. J. Nutr. 45: 613–616. [DOI] [PubMed] [Google Scholar]
- 8.Baylin A., Kabagambe E. K., Ascherio A., Spiegelman D., Campos H. 2003. Adipose tissue alpha-linolenic acid and non-fatal acute myocardial infarction in Costa Rica. Circulation. 107: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 9.Campos H., Baylin A., Willett W. C. 2008. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 118: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu F. B., Stampfer M. J., Manson J. E., Rimm E. B., Wolk A., Colditz G. A., Hennekens C. H., Willett W. C. 1999. Am. J. Clin. Nutr. 69: 890–897. [DOI] [PubMed] [Google Scholar]
- 11.Prasad K. 1997. Dietary flaxseed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis. 132: 69–76. [DOI] [PubMed] [Google Scholar]
- 12.Dupasquier C. M., Dibrov E., Kneesh A. L., Cheung P. K. M., Lee K. G. Y., Alexander H. K., Yeganeh B. K., Moghadasian M. H., Pierce G. N. 2007. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am. J. Physiol. Heart Circ. Physiol. 293: H2394–H2402. [DOI] [PubMed] [Google Scholar]
- 13.Pellizzon M. A., Billheimer J. T., Bloedon L. T., Szapary P., Rader D. J. 2007. Flaxseed reduces plasma cholesterol levels in hypercholesterolemic mouse models. J. Am. Coll. Nutr. 26: 66–75. [DOI] [PubMed] [Google Scholar]
- 14.Prasad K. 2009. Flaxseed and cardiovascular health. J. Cardiovasc. Pharmacol. 54: 369–377. [DOI] [PubMed] [Google Scholar]
- 15.Bell T. A., III, Kelley K., Wilson M. D., Sawyer J. K., Rudel L. L. 2007. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiecy in apoB100 only LDLr−/− mice. Arterioscler. Thromb. Vasc. Biol. 27: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 16.Bell T. A., III, Wilson M. D., Kelley K., Sawyer J. K., Rudel L. L. 2007. Monounsaturated fatty acyl-coenzyme A is predictive of atherosclerosis in human apoB-100 transgenic, LDLr−/− mice. J. Lipid Res. 48: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 17.Willner E. L., Tow B., Buhman K. K., Wilson M., Sanan D. A., Rudel L. L., Farese R. V., Jr 2003. Deficiency of acyl-CoA: cholesterol acyltransferase 2 prevents atherosclerosis in apoliprotein E-deficient mice. Proc. Natl. Acad. Sci. USA. 100: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 19.Temel R. E., Lee R. G., Kelley K. L., Davis M. A., Shah R., Sawyer J. K., Wilson M. D., Rudel L. L. 2005. Intestinal cholesterol absorption is substancially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46: 2423–2431. [DOI] [PubMed] [Google Scholar]
- 20.Rudel L. L., Parks J. S., Sawyer J. K. 1995. Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects African green monkeys from coronary artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15: 2101–2110. [DOI] [PubMed] [Google Scholar]
- 21.Rudel L. L., Kelley K. L., Sawyer J. K., Shah R., Wilson M. D. 1998. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human apoB100-overexpressing transgenic mice. Arterioscler. Thromb. Vasc. Biol. 18: 1818–1827. [DOI] [PubMed] [Google Scholar]
- 22.Degirolamo C., Shelness G. S., Rudel L. L. 2009. LDL cholesteryl oleate as predictor for atherosclerosis: evidence from human and animal studies on dietary fat. J. Lipid Res. 50(Suppl): S434–S439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee R. G., Willingham M. C., Davis M. A., Skinner K. A., Rudel L. L. 2000. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J. Lipid Res. 41: 1991–2001. [PubMed] [Google Scholar]
- 24.Carr T. P., Parks J. S., Rudel L. L. 1992. Hepatic ACAT activity in African green monkeys is highly correlated to plasma LDL cholesteryl ester enrichment and coronary artery atherosclerosis. Arterioscler. Thromb. 12: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 25.Rudel L. L., Haines J., Sawyer J. K., Shah R., Wilson M. D., Carr T. P. 1997. Hepatic origin of cholesteryl oleate in coronoary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J. Clin. Invest. 100: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee R. G., Kelley K. L., Sawyer J. K., Farese R. V., Jr., Parks J. S., Rudel L. L. 2004. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95: 998–1004. [DOI] [PubMed] [Google Scholar]
- 27.Parini P., Davis M., Lada A. T., Erickson S. K., Wright T. L., Gustafsson U., Sahlin S., Einarsson C., Angelin B., Tomoda H., et al. 2004. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 110: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 28.Coleman R. A., Lewin T. M., Van Horn C. G., Gonzalez-Baró M. R. 2002. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J. Nutr. 132: 2123–2126. [DOI] [PubMed] [Google Scholar]
- 29.Seo T., Oelkers P. M., Giattina M. R., Worgall T. S., Sturley S. L., Deckelbaum R. J. 2001. Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry. 40: 4756–4762. [DOI] [PubMed] [Google Scholar]
- 30.Burdge G. C., Jones A. E., Wootton S. A. 2002. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 88: 355–363. [DOI] [PubMed] [Google Scholar]
- 31.Kelley D. S., Nelson G. J., Love J. E., Branch L. B., Taylor P. C., Schmidt P. C., Mackey B. E., Iacono J. M. 1993. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids. 28: 533–537. [DOI] [PubMed] [Google Scholar]
- 32.Harper C. R., Edwards M. J., Defilipis A. P., Jacobson T. A. 2006. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J. Nutr. 136: 83–87. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Harris W. S., Chung M., Lichtenstein A. H., Balk E. M., Kupelnick B., Jordan H. S., Lau J. 2006. n-3 fatty acids from fish or fish oil-supplements but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am. J. Clin. Nutr. 84: 5–17. [DOI] [PubMed] [Google Scholar]
- 34.Brenna J. T. 2002. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care. 5: 127–132. [DOI] [PubMed] [Google Scholar]
- 35.Parks J. S., Rudel L. L. 1990. Effect of fish oil on atherosclerosis and lipoprotein metabolism. Atherosclerosis. 84: 83–94. [DOI] [PubMed] [Google Scholar]
- 36.Brown J. M., Shelness G. S., Rudel L. L. 2007. Monounsaturated fatty acids and atherosclerosis: opposing views from epidemiology and experimental animal models. Curr. Atheroscler. Rep. 9: 494–500. [DOI] [PubMed] [Google Scholar]
- 37.Vasandani C., Kafrouni A. I., Caronna A., Bashmakov Y., Gotthardt M., Horton J. D., Spady D. K. 2002. Upregulation of hepatic LDL transport by n-3 fatty acids in LDL receptor knockout mice. J. Lipid Res. 43: 772–784. [PubMed] [Google Scholar]
- 38.Parks J. S., Wilson M. D., Johnson F. L., Rudel L. L. 1989. Fish oil decreases hepatic cholesteryl ester secretion but not apoB secretion in African green monkeys. J. Lipid Res. 30: 1535–1544. [PubMed] [Google Scholar]
- 39.Rudel L. L., Parks J. S., Johnson F. L., Babiak J. 1986. Low density lipoproteins in atherosclerosis. J. Lipid Res. 27: 465–474. [PubMed] [Google Scholar]
- 40.Bordoni A., Di Nunzio M., Danesi F., Biagi P. L. 2006. Polyunsaturated fatty acids: from diet to binding to PPARs and other nuclear receptors. Genes Nutr. 1: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renier G., Skamene E., DeSanctis J., Radzioch D. 1993. Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arterioscler. Thromb. 13: 1515–1524. [DOI] [PubMed] [Google Scholar]
- 42.Blok W. L., Katan M. B., Van der Meer J. W. M. 1996. Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J. Nutr. 126: 1515–1533. [DOI] [PubMed] [Google Scholar]
- 43.Harris W. S. 1997. N-3 fatty acids and serum lipoproteins: human studies. Am. J. Clin. Nutr. 65: 1645S–1654S. [DOI] [PubMed] [Google Scholar]
- 44.Alpha Omega Trial: A Randomized, Placebo Controlled, Double Blind Intervention Study of the Effect of Low Doses of Omega-3 Fatty Acids on Cardiovascular Diseases in Patients with a History of Myocardial Infarction. http:\\www.clinicaltrials.gov\ct2\show\NCT00127452?term=alpha+omega+trial&rank=1. [Google Scholar]
- 45.Harris W.S. 2005. Alpha-linolenic acid: a gift from the land? Circulation. 111: 2872–2874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.