Abstract
Rationale
Cardiac muscle adapts to increased workload by altering cardiomyocyte size and function resulting in cardiac hypertrophy. G-protein coupled receptor (GPCR) signaling is known to govern the hypertrophic response through the regulation of ion channel activity and downstream signaling in failing cardiomyocytes.
Objective
Transient receptor potential canonical (TRPC) channels are GPCR operated channels previously implicated in cardiac hypertrophy. Our objective of this study is to better understand how TRPC channels influence cardiomyocyte calcium signaling.
Methods and Results
Here, we used whole cell patch clamp of adult cardiomyocytes to show upregulation of a non-selective cation current reminiscent of TRPC channels subjected to pressure overload. This TRPC current corresponds to the increased TRPC channel expression noted in hearts of mice subjected to pressure overload. Importantly, we show that mice lacking TRPC1 channels are missing this putative TRPC current. Moreover, Trpc1−/− mice fail to manifest evidence of maladaptive cardiac hypertrophy and maintain preserved cardiac function when subjected to hemodynamic stress and neurohormonal excess. In addition, we provide a mechanistic basis for the protection conferred to Trpc1−/− mice as mechanosensitive signaling through calcineurin/NFAT, mTOR and Akt is altered in Trpc1−/− mice.
Conclusions
From these studies, we suggest that TRPC1 channels are critical for the adaptation to biomechanical stress and TRPC dysregulation leads to maladaptive cardiac hypertrophy and failure.
Keywords: Transient receptor potential (TRPC) channels, G-proteins receptor signaling, cardiac hypertrophy
Introduction
Cardiac myocytes respond to changing mechanical workloads by altering the frequency and amplitude of their calcium transients (1, 2). Encoded in these calcium transients are signals that alter not only the immediate contractile response, but also initiate and maintain a remodeling response that adjusts cellular mass, ionic currents, kinetic properties of contractile proteins, and metabolic capacity (3). It is likely that persistence of these signals modulate the calcium signaling events resulting in a hypertrophic response and adverse remodeling. To identify the proximal signals that regulate cardiac hypertrophy, attention has begun to focus on ion channels because they may link mechanical activity to cell signaling (4). Recent work has raised the possibility that hypertrophic agonists linked to G-protein coupled receptors activate calcium entry through transient receptor potential canonical (TRPC) channels (7–12).
TRPC channels encompass a large family of non-selective cation channels found in many different cell types (5). TRPC channels are activated downstream of G-protein receptor through the phospholipase C (PLC) signaling by inositol trisphosphate (IP3) (TRPC1/4/5) or by diacylglycerol (TRPC3/6/7)(6, 7). More recently TRPC1/6 channels were found to be mechanosensitve channels that mediate non-selective cation entry in response to increased membrane stretch (8, 9). These findings raise interesting possibilities about TRPC channels as mechanosensitive channels that may be operative during cardiomyocyte stretch associated with pressure overload. In fact, several groups have linked increased TRPC channel activity to cardiac hypertrophy and failure (10–13). TRPC1/C3/C6 have been found to be upregulated in response to pressure overload and a model of calcineurin-mediated cardiomyopathy. Moreover, transgenic mice overexpressing either TRPC3 or TRPC6 channels in the heart manifest an exaggerated hypertrophic response to pressure overload or die prematurely from heart failure (11, 13). In contrast, a TRPC3 specific small molecule inhibitor prevented the development of cardiac hypertrophy in WT mice subjected to pressure overload (14). Despite some evidence suggesting a link between TRPC channels and cardiac hypertrophy, the molecular mechanism by which TRPC channels contribute to cardiac calcium signaling is not known. We therefore tested the hypothesis TRPC1 channels contribute to biomechanical signaling following pressure overload in the cardiomyocytes and we provide direct evidence that TRPC1 is a key mediator of the cardiac hypertrophic response. Our findings support a concept that therapeutic strategies designed to block TRPC1 channels may have clinical benefit in the hypertrophic failing heart.
Material and Methods
Please see detailed methods section in the online supplement. In brief, Trpc1−/− and WT mice were maintained in a 129 background for more greater than seven generations. Paired mice were used in each study. Cardiac stress was induced using TAC surgery or chronic angiotensin infusion. Adult cardiomyocytes were prepared using Langendorf perfusion to disaggregate single cells. Patch clamp using whole cell technique and calcium imaging with Fura-2 AM were used to measure TRPC1 currents and calcium entry. Cardiac lysates were prepared for biochemical assays of relevant signaling pathways. Cryosectioning of hearts was performed for histologic studies.
Results
Trpc1−/− and cardiac hypertrophy
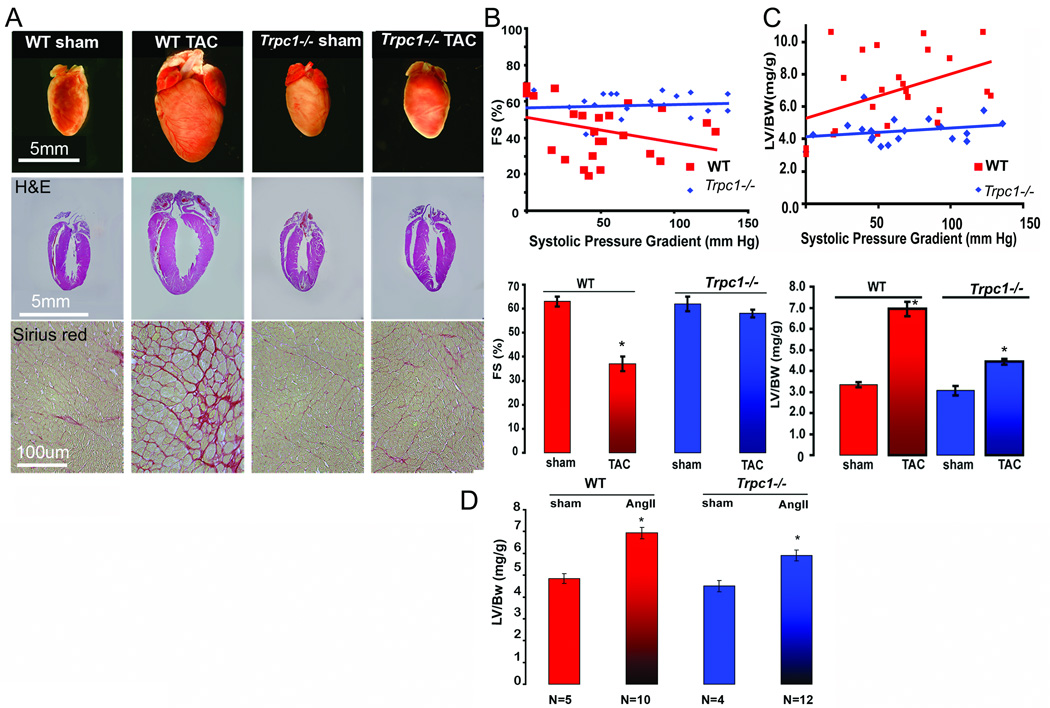
We first designed studies to test the hypothesis that TRPC1 mediated calcium entry in cardiomyocytes activates hypertrophic signaling. Trpc1−/− mice and WT mice were subjected to transverse aortic constriction (TAC) to induce cardiac hypertrophy. Histological sections of hearts from WT mice demonstrated a marked increase in LV (left ventricle) size after eight weeks of TAC compared to sham operated mice (Figure 1A, top row). In contrast, the hearts of Trpc1−/− mice did not demonstrate significant cardiac hypertrophy (Figure 1A, top row) nor was there an increase in collagen deposition as determined by Sirius red staining compared to the heart sections of WT mice (Figure. 1A, bottom row).
Figure 1.
Trpc1 −/− mice are protected from pressure overload and lack a significant hypertrophic response. (A) Photomicrographs of hearts from WT (sham and TAC), Trpc1 −/− (sham and TAC) mice at 16 weeks of age. Histologic sections are stained with hematoxylin/eosin (middle) and Sirius red (bottom) (B) (top) Echocardiographic measurements of fractional shortening (%) as a function of pressure gradient in TAC operated WT and Trpc1−/− mice. *p < 0.05 ( see online table 2). (bottom) Average percent fractional shortening from WT sham (n=6) and TAC (n=30), Trpc1 −/− sham (n=5) and TAC (n=29). (C) (top) LV/BW ratio in WT sham and TAC and Trpc1−/− sham and TAC mice plotted against systolic pressure gradients measured at study termination. (bottom) LV/BW ratio in WT and Trpc1 −/− in sham versus TAC mice. *P < 0.05 in all pairs as analyzed by Tukey-Kramer’s test. (D) WT and Trpc1−/− mice were pumped with 1000ng/kg/min. Ang-II for 4 weeks and then sacrificed to measure their heart weight to body weight ratio. The increase in heart weight to body weight ratio in Trpc1−/− was significantly lower than that of WT; *P<0.01 WT vs Trpc1−/− Ang –II pumped mice.
Serial echocardiograms (performed at 0, 4 and 8 weeks) demonstrated a progressive decline in the fractional shortening (% FS) of WT mice, whereas Trpc1−/− mice maintained preserved % FS over a wide range of systolic pressure gradients (0–150mmHG) (Figure 1B and online table 1). A detailed statistical analysis for fractional shortening and LV/BW ratio has been shown in online table 3 & online figure 2. Invasive LV hemodynamic parameters measured from WT and Trpc1−/− mice showed significant differences in the LV+dP/dtmax and LV-dP/dtmin only after the TAC operation. These data indicate that the loss of TRPC1 is associated with preserved contractility despite a marked increase in pressure load ( online table 1).
Consistent with the observations made by echocardiography and hemodynamics, we found marked difference in cardiac mass between hearts of Trpc1−/− mice subjected to TAC compared to WT mice. The increase in cardiac mass following TAC was significantly reduced in the Trpc1−/− mice as was evident by the change in left ventricular mass/body weight ratio (LV/BW), (3.1±0.23 to 4.5±0.175, before and after TAC respectively) as compared to WT mice (3.3 ±0.14 to 6.8±0.42) (fig. 1C & online table 1). Thus it is apparent from these data that Trpc1−/− mice respond to pressure overload with a modest increase in cardiac mass and preserved cardiac function, whereas the same level of pressure overload in WT mice produced significant cardiac impairment. We also considered whether changes in TRPC2-7 channel expression occurred in the hearts of the Trpc1−/− mice as mechanism for the protection seen in the INSC. In fact, TRPC channels (mRNA or protein) at baseline and after pressure overload did not differ between WT and Trpc1−/− mice.(online figure 1A, B, C&D).
TRPC channels are known to be receptor operated cation channels activated downstream of G-protein coupled receptors, e.g. Angiotensin-II (Ang-II), raising the possibility that Ang-II mediated hypertrophic signaling may influence TRPC signaling (15),(11, 16, 17). WT and Trpc1−/− mice infused with Ang-II (1000 ng/kg/min) for four weeks (28 days) to induce cardiac hypertrophy (Figure 1D and online Table 2).
INSC in adult cardiomyocytes
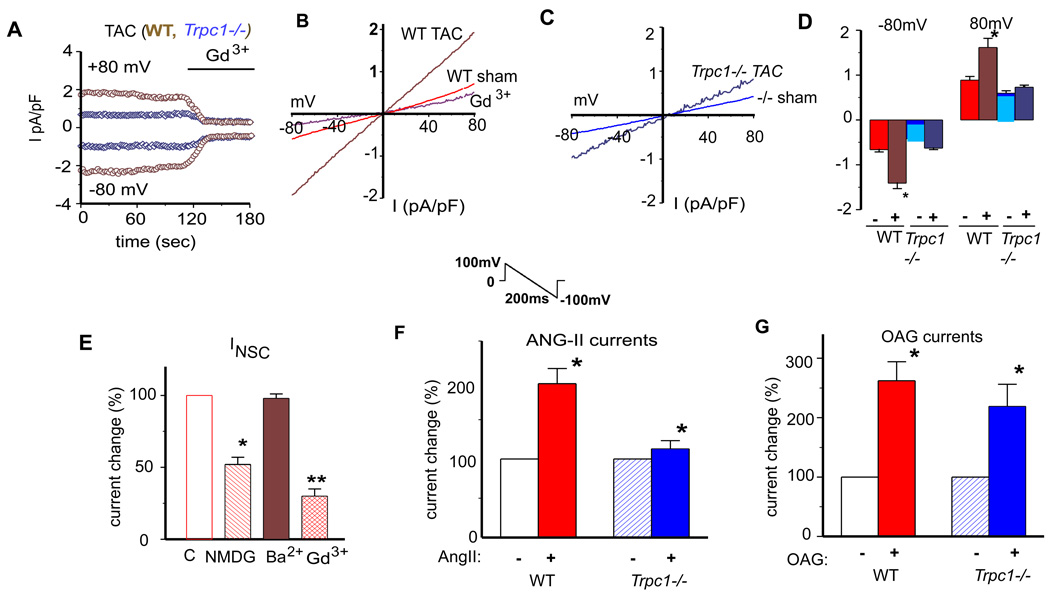
Next, we designed whole cell voltage clamp studies to measure TRPC currents from isolated adult cardiomyocytes taken from WT or Trpc1−/− mice. Solutions were configured so as to limit voltage gated Ca2+ and K+ currents mainly by including inhibitors of L-type calcium channels and Cs+ to block K+ channels. We used an inverse ramp protocol from +100 mV to −100 mV to limit voltage gated Na+ channel currents and holding potential of 0 mV(see online methods)(18). Figure 2A displays current-time plots recorded from WT (brown traces) and Trpc1−/− (blue traces) cardiomyocytes eight weeks after the TAC operation. WT cardiomyocytes displayed a greater current recorded at both +80mV and −80 mV membrane potentials compared to Trpc1−/− cardiomyocytes. The non-selective currents were inhibited by gadolinium (Gd3+), a known TRPC channel blocker (Figure 2A). We noted that the I–V relationship recorded from sham and TAC operated mice was linear in shape with a reversal potential of 0 mV, features reminiscent of non-selective TRPC channels (Figure 2B). Interestingly non-selective currents recorded from cardiomyocytes taken from Trpc1−/− mice displayed similar I–V relations with zero reversal potential (Figure 2C). However the current density of the non-selective currents recorded from Trpc1−/− cardiomyocytes, both sham and TAC operated mice, was markedly reduced compared to the WT cardiomyocytes (Figure 2D).
Figure 2.
Pressure overload induces non-selective whole cell currents (INSC ) in adult cardiomyocytes that are attributed to TRPC1. (A) The examples of membrane current recorded from isolated cardiomyocytes from WT TAC (brown) and Trpc1 −/− TAC (blue). INSC was normalized by membrane capacitance. The currents were recorded at +80mV and −80mV. (B and C) Representative I/V relationship of membrane currents in cardiomyocytes from WT sham and TAC (B) and Trpc1−/− sham and TAC mice (C). (D) Group mean values of INSC at −80mV and +80mV in WT sham (red filled bar, n=25); and TAC (brown bar, n=33); Trpc1 −/− sham (light blue bar, n=27) and TAC (dark blue filled bar, n=27) cardiomyocytes. *, P<0.05, WT TAC vs. Trpc1 −/− TAC. (E) Relative group means of changes of peak membrane currents at −80mV in the presence of barium (2mM, n=11), NMDG (n=12) and Gd3+ (n=10) in the external solutions. Open bar represents control (100%). *, P<0.05; ** P<0.01 versus control. (F) Relative group means of changes (%) of peak membrane current INSC at −80mV caused by perfusion of angiotensin II (Ang-II, 10µM) in WT (red) and Trpc1−/− (blue) cardiomyocytes. * indicates p<0.05 unstimulated versus Ang-II. # indicates p<0.05, WT vs. Trpc1−/− (after Ang-II perfusiosn). (G) Relative group mean changes (%) of INSC at −80mV after perfusion of 1-Oleoyl-2-acetyl-sn-glycerol (OAG 50 µM) in WT and Trpc1−/− cardiomyocytes. p<0.05 WT vs. Trpc1−/−.
To characterize the INSC recorded from cardiomyocytes, we sought evidence for the contributions of TRPC from cation selectivity, pharmacological profiling and gating mechanism (Figure 2E). Replacing Na+ in the external solution with N-methyl-D-glucamine (NMDG) dramatically reduced the non-selective current by greater than 50% from WT cardiomyocytes indicating the current is in part permeable to Na+. In addition, the INSC was rapidly blocked by the addition of gadolinium (Gd3+, 10µM) to the perfusion bath. Trivalent cation block of the INSC is a characteristic feature of TRPC currents (19). We also found that the putative TRPC1 current was equally permeable to calcium and barium which would further suggest this current is in part contributed by TRPC1 (Figure 2E). We found no difference in the Li3+ sensitive currents in cardiomyocytes isolated from WT and Trpc1−/− mice indicating no change in NCX1 currents (not shown) (20). In addition, we found that immunolabeled TRPC1 only partially overlapped with that of NCX1 (online Figure 1E). When considered in total these results indicate that TRPC1 is likely to contribute to the non-selective cation background current recorded in adult cardiomyocytes.
Given the central role of the neurohormone angiotensin-II (Ang-II) in the stretch activated signaling in the cardiomyocyte (23), we tested whether Ang-II application influenced the non-selective cation current attributed to TRPC1. Ang-II stimulation of WT cardiomyocytes resulted in two-fold increase in the current density of the non-selective current (Figure 2F). In contrast, Ang-II failed to augment the TRPC current in cardiomyocytes isolated from Trpc1−/− mice (Figure 2F). We also tested whether TRPC1 currents were activated by 1-oleoyl-2-acetyl-sn-glycerol (OAG), a stable cell permeable analog of DAG (Figure 2G). Here, perfusion of cardiomyocytes with OAG (10µM) activated a non-selective current similar to that seen with Ang-II. We found that the current density of OAG activated currents from Trpc1−/− and WT cardiomyocytes were not different (Figure 2G). These results indicate that TRPC1 contributes to the Ang-II induced non-selective current, whereas TRPC1 does not influence the DAG-induced current. TRPC1 has also been implicated in store operated calcium entry as a SOC channel in many cell types including cardiomyocytes. We however did not find a difference in the rate of Ca2+ entry following store depletion in Trpc1−/− neonatal cardiomyocytes compared to WT cells (online Figure 3). These findings support our model in which TRPC1 channels respond to G-protein signaling that is involved in the pressure overload response.
TRPC1 and the stretch response
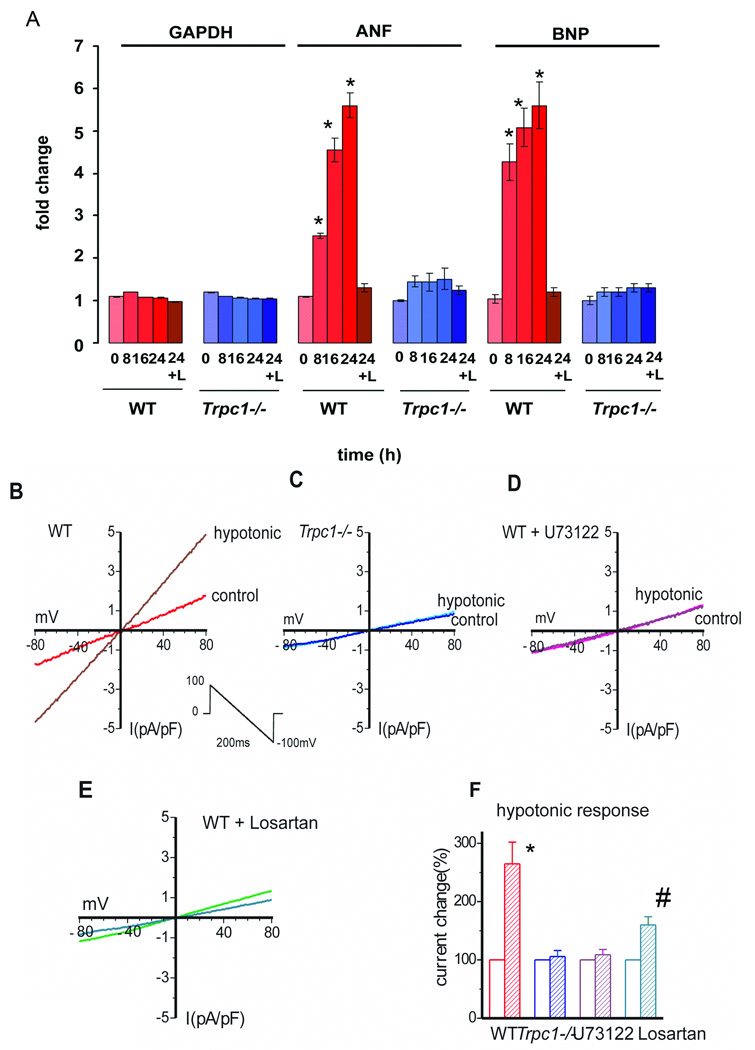
We next determined whether cardiomyocytes from Trpc1−/− mice responded differently to mechanical stretch than WT cardiomyocytes. Isolated neonatal cardiomyocytes subjected to a cyclical stretch at 15% strain at 1Hz frequency for 8–24 hours duration expressed augmented levels of BNP (brain natriuretic factor) and ANF (atrial natriuretic factor) in a time dependent manner (Figure 3A) peaking at 24 hours with a 7 fold increase in the BNP and ANF expression. Interestingly, treating WT cells with losartan to block angiotensin 1 (AT1) receptors prevented the induction of the stretch gene program. Moreover, Trpc1−/− cardiomyocytes showed no change in the expression of BNP and ANF over any time point examined. Thus neonatal cardiomyocytes lacking TRPC1 displayed a blunted response to stretch in comparison to WT cardiomyocytes. The blocking of stretch response by losartan treatment suggests that TRPC1 mediated stretch signaling is downstream of the AT1 receptor.
Figure 3.
(A) TRPC1 influences the stretch activated program. ANF and BNP normalized to GAPDH in response to 8, 16 and 24 h of cyclic stretch (15% strain) in WT and Trpc1−/− neonatal cardiomycytes. *p<0.01 (WT stretched (8,16 and 24 h vs. zero time point for ANF and BNP). Losartan (24+L) (10mM) incubated cardiomyocytes were stretch for 24hours. (B–E) I/V relationships of INSC recorded from WT (B) and Trpc1−/− (C) adult cardiomyocytes in isotonic and hypotonic solutions. Osmotic induced currents were blocked in WT cardiomyocytes by phospholipase C (PLC) inhibition by including 10µM U73122 in the pipette solution or by ATR1 blockade with 10µM losartan. (F) Group mean changes of INSC at −80mV caused by osmotic stress in cardiomyocytes of WT (red bar, n=4) and Trpc1−/− (blue bar, n=6) mice WT CM with PLC blockade U73122 (purple bar, N=5) or ATR1 with Losartan (green bars N=5). * indicates p<0.01, Trpc1−/− vs. WT. # indicates p<0.05 for Losartan stimulated cells versus WT cells.
It has been recently shown that mechanical stretch activates angiotensin receptors (AT1R) in an agonist independent manner that can result in activation of a non-selective cation current in vascular smooth muscle cells (24). These results may explain the recent findings demonstrating TRPC1 and TRPC6 channels as putative stretch activated channels (9). We therefore tested whether stretch influences the TRPC1 current in adult cardiomyocytes. Here, osmotic stress applied to the cardiomyocytes, by changing the osmolarity of the perfusion bath from 305 mOsm to 205 mOsm, increased the non-selective current (Iswell) in WT cardiomyocytes. The IV-relationship of the Iswell from adult cardiomyocytes strongly resembled that observed above for pressure overload and Ang-II. Osmotic stress did not activate the non-selective current in cardiomyocytes lacking TRPC1 (Figure 3B–C). We further characterized the Iswell in WT cardiomyocytes to determine if Ang-II signaling was involved in the osmotic activated currents. Inclusion of the phospholipase C (PLC) blocker, U-73122, in the pipette solution decreased the non-selective current at baseline and following cell swelling induced by osmotic stress (Figures 3D&F). We also demonstrate that the Iswell in WT cardiomyocytes signals through the Ang-II receptor as the current was significantly blocked by losartan (10µM) compared to vehicle treated WT cardiomyocytes (Figures 3E&F). These data indicate Ang-II signaling is fundamental to the current activated by cell swelling. Moreover, WT cardiomyocytes treated with tarantula toxin GsMTx-4, an inhibitor of stretch-activated channels also blocked the non-selective current induced by cell swelling, providing additional evidence that TRPC1 acts as a stretch activated channel (data not shown)(8, 25). Collectively, these studies show that Trpc1−/− cardiomyocytes, in comparison to WT cells, fail to respond to different forms of stretch as indicated by the lack of stretch activated non-selective cation current (swelling and positive pressure) or changes in ANF and BNP mRNA expression (radial stretch). These results provide evidence in support of our model in which TRPC1 channels reside downstream of stretch-activated GPCR signaling to confer stretch dependent signaling associated with cardiac hypertrophy.
Loss of TRPC1 alters hypertrophic signaling
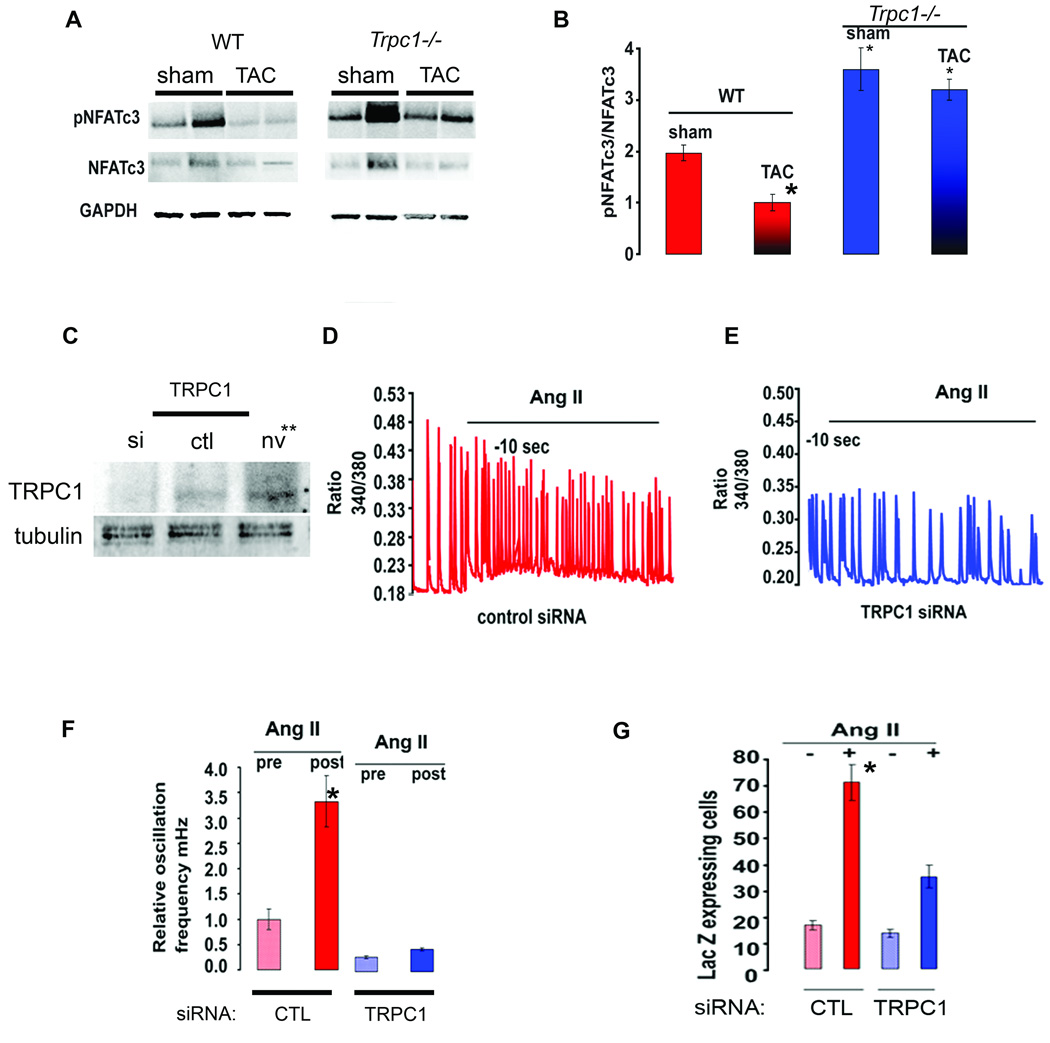
To this point our studies indicated that mice lacking TRPC1 are protected from the deleterious effects of pressure overload. Since it is now well established that calcium entry through TRPC channels influences calcineurin/NFAT signaling in many cell types including cardiomyocytes, we examined the phosphorylation state of NFATC3 in cardiac lysates prepared from WT and Trpc1−/− mice as a marker of calcineurin/NFAT signaling. NFATC3 is dephosphorylated by calcineurin in pressure overloaded hearts as was detected in the WT mice in Figure 4A. We then examined whether the loss of TRPC1 influenced NFAT signaling. First, we found a significant increase in phospho-NFATC3 in the heart lysates prepared from sham operated Trpc1−/− mice compared to WT mice indicating that more NFATC3 exists in the inactive state in the sham operated Trpc1−/− mice (Figure 4A). No differences in total NFAT were seen among WT and Trpc1−/− mice, but a greater fraction of NFATC3 existed in the phosphorylated state in TAC operated Trpc1−/− mice compared to TAC operated WT mice (Figure 4A). These data suggest that calcineurin/NFAT activity in the hearts of mice lacking TRPC1 is less active at baseline and after pressure overload (Figure 4B).
Figure 4.
Calcineurin/NFAT signaling is less sensitized in Trpc1−/− mice following pressure overload. (A) Immunoblotting for p-NFATC3, NFATC3 and GAPDH from cardiac lysates of WT and Trpc1−/− mice. (B) Quantification of p-NFAT/NFATC3 (total) in WT (sham and TAC) Trpc1−/− (sham and TAC) mice * P<0.03 WT TAC versus sham and WT sham versus Trpc1−/− sham. (C) Silencing TRPC1 expression in neonatal cardiomyocytes with adenovirus delivered shTRPC1 compared to non-infected control (NV) and sh-scrambled adenoviral infected cells. (D–E) Ang-II increases the frequency of Ca2+ oscillations in WT cardiomyocytes (D) but not in CM infected with siTRPC1 adenovirus (E). (F) Average relative oscillation frequency (mHZ) from neonatal cardiomyocytes (control and siTRPC1 infected) stimulated with Ang-II. * indicates p<0.03 for control virus infected cells vs. shTRPC1 infected cells stimulated with Ang-II. (G) LacZ was measured from cardiomyocytes isolated from NFAT indicator mice. CM infected with adenovirus for control or shTRPC1 and then stimulated with vehicle or Ang-II for 18 hours. * indicates p<0.01 for control virus infected cells vs. shTRPC1 infected cells stimulated with Ang-II.
We next designed studies using a transgenic NFAT indicator mice (26, 27) to further examine the relationship between TRPC1 and calcineurin/NFAT signaling. Neonatal cardiomyocytes from NFAT indicator mice were infected with adenoviruses carrying either siTRPC1 or siCTL (scrambled) constructs (Figure 4C). Cardiomyocytes were stimulated with Ang-II to alter the frequency of spontaneous calcium oscillations and activate the hypertrophic program (Figure 4D). In cells with TRPC1 silencing, we found a marked attenuation of the calcium oscillations compared to control silenced cells (Figure 4 D, E&F). This change in the oscillation frequency in the siTRPC1 neonatal cardiomyocytes (NCM) corresponded to a reduction in NFAT transactivation as measured by LacZ reporter gene compared to control NCM. These results support our in vivo studies suggesting that TRPC1 channels provide calcium entry needed for hypertrophic signaling through the calcineurin/NFAT signaling pathway in cardiomyocytes (Figure 4G).
TRPC1 and hypertrophic gene expression
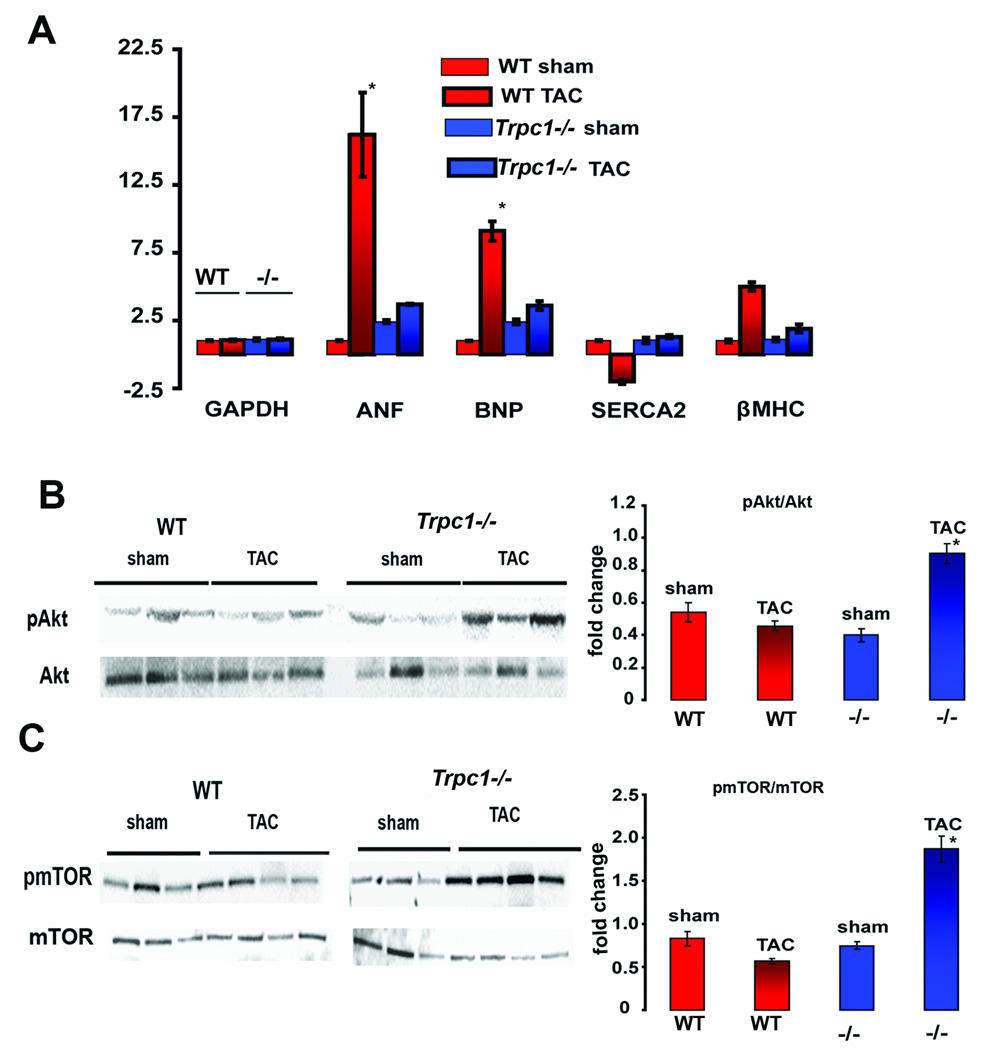
Cardiac hypertrophy with diminished cardiac function has long been associated with changes in profiles of gene expression (28). We therefore measured the mRNA for brain naturetic peptide (BNP) and atrial naturetic (ANF) from the hearts of Trpc1−/− and WT mice (sham and TAC operated). These neurohormones are released by cardiomyocytes subjected to increased wall stress and are important biomarkers for the activation of the fetal gene program in response to cardiac stress (29). WT mice subjected to pressure overload were found to have a nine fold increase in the expression of BNP and 16 fold increase in ANF; whereas Trpc1−/− mice subjected to TAC showed no significant change in BNP and ANF expression (Figure 5A). We also found that the expression of the calcium pump, sarcoplasmic-endoplasmic calcium ATPase (SERCA2a), was decreased in the TAC-WT mice while there was no corresponding reduction in SERCA2a expression in Trpc1−/− mice subjected to TAC. SERCA2a is known to be diminished in heart failure and may contribute to abnormal calcium signaling (30). It is clear from these studies that the attenuated cardiac hypertrophy and preserved cardiac function observed in mice lacking TRPC1 accompanied no changes in the gene expression profile associated with maladaptive cardiac hypertrophy.
Figure 5.
Hypertrophic signaling in WT and Trpc1 −/− mice (A) Relative change in the expression of ANF, BNP, SERCA2a and β-Myosin heavy chain (normalized to GAPDH) in WT and Trpc1−/− mice subjected to sham or TAC operation. * indicates p < 0.03 for WT TAC versus sham mice and Trpc1 −/− TAC versus sham mice (B) Immunoblotting for Akt and p-Akt in cardiac lysates prepared from WT and Trpc1−/− mice. * indicates p<0.03 for WT TAC versus WT sham, Trpc1−/− sham and TAC. (C) Immunoblotting for mTOR and p-mTOR in cardiac lysates prepared from Trpc1−/− (TAC vs. sham mice) and WT (TAC vs. sham mice) *indicates p<0.03 for WT TAC versus WT sham, Trpc1−/− sham and TAC.
To better understand the survival advantage offered to Trpc1−/− mice in response to pressure overload, we next tested the hypothesis that Akt signaling pathways associated with cardiomyocyte survival would be enhanced in the Trpc1−/− mice. In fact, we were unable to discern differences in the level of phospho-Akt between the sham and TAC operation in WT mice. In contrast, Akt phosphorylation was notably increased in heart lysates prepared from Trpc1−/− mice following the TAC operation. Likewise phospho-mTOR was markedly increased in the heart lysates of Trpc1−/− TAC operated mice compared to WT mice. No differences were noted in the total Akt or mTOR expression of WT or Trpc1−/− hearts (Figure 5B & C). These results suggest that the preserved cardiac function and minimal changes to cardiac mass following pressure overload seen in mice lacking TRPC1 may be due in part to the preserved Akt and mTOR signaling which are associated with cell survival.
Discussion
In the present work, we reveal a highly specific role for TRPC1 as a non-selective cation channel in cardiomyocytes that participates in cardiac hypertrophic signaling. In particular, we show that mice lacking TRPC1 are protected from the deleterious effects of increased intra-cardiac pressures imposed by various forms of cardiac stress (pressure overload or chronic Ang-II stimulation). These findings provide the strongest support to date that TRPC channels play a significant role in the pathophysiology of cardiac hypertrophy.
Recent reports have suggested that TRPC1 and TRPC6 are stretch activated cation channels (9, 24). How the TRPC channels sense changes in stretch and what molecular mechanism leads to TRPC1 channel gating is presently unknown. It has been proposed that TRPC channels either respond directly to deformation of the lipids in plasma membranes or as a downstream consequence of AT1R activation (24, 35). We have recently suggested that the scaffolding protein homer-1 links TRPC1 with the cytoskeleton in skeletal muscle (36). In the present study, cardiomyocytes lacking TRPC1 are protected from pressure overload which further supports our model. Under normal hemodynamic conditions TRPC1 plays a role in adjusting cytoskeletal stiffness associated with loading conditions. However, under pathologic workloads TRPC1 currents are augmented in response to more TRPC1 channel expression resulting in the activation of adverse remodeling associated with cardiac hypertrophy. It will be important to know in future studies whether TRPC channels connected to the homer-cytoskeleton are influenced by GPCR activation as has been suggested for TRPC1, homer and mGluR receptors in neurons (38, 39).
TRPC channels are non-selective cation channels and cation flux through TRPC channels have been implicated in other mammalian physiologic processes including learning and memory in the brain and glomerular slit diaphragm function in kidney epithelial cells (40–42). The present work, along with other recent reports, establishes that TRPC channels are located at the sarcolemma where TRPC1 mediated cation flux influences the transition from adaptive to maladaptive cardiac hypertrophy. What is the common link between TRPC channel activity in neurons, podocytes and cardiomyocytes? In neurons TRPC channels are important modulators of the cytoskeleton where growth cones turn and extend processes in response to local growth factor concentrations (41, 43–46). In the podocyte, activating mutations in TRPC6 disrupt the normal function of the foot process which is highly dependent on the integrity of the actin cytoskeleton and GPCR signaling (47, 48). According to our model, TRPC channels act downstream of GPCR signaling that influences membrane-cytoskeletal interactions in order to accommodate specific cellular signaling events. Under conditions of pressure overload, TRPC1 calcium entry is likely to influence hypertrophic signaling resulting in the remodeling response that leads to heart failure. Future efforts to block these channels may offer novel strategies to treat cardiac hypertrophy and failure.
Supplementary Material
Acknowledgements
Special thanks to Dr. Sanjeev Ahuja and Dr. Cary Ward.
Sources of funding: NIH (R01-HL093470) (PBR), Mandel Center at Duke (PBR), and MDA research award (PBR) and in part by the Intramural Program of the NIH, National Institute of Environmental Health Science (JA and LB).
List of Abbreviations
- TAC
Transverse aortic constriction
- Ang-II
Angiotensin II
- LV
left ventricle
- Trpc1
Transient receptor potential canonical 1
- PLC
Phospholipase C
- IP3
inositol phosphate-3
- OAG
1-oleoyl-2-acetyl-sn-glycerol
- FS
fractional shortening
- NCX
Sodium calcium exchanger
- AT1R
Angiotensin1 receptor
- ANF
atrial natriuretic factor
- BNP
brain natriuretic peptide
- INSC
Non-selective current
- Iswell
Non- selective current in response to swelling
Footnotes
Disclosures: None
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Williams RS, Rosenberg P. Calcium-dependent Gene Regulation in Myocyte Hypertrophy and Remodeling. Cold Spring Harb Symp Quant Biol. 2002;LXVII:1–5. doi: 10.1101/sqb.2002.67.339. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Microdomains and elemental events in calcium signalling. Cell Calcium. 1996;20:95–96. doi: 10.1016/s0143-4160(96)90098-6. [DOI] [PubMed] [Google Scholar]
- 4.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat Rev Drug Discov. 2007;6:617–635. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 5.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 8.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 10.Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, Soboloff J, Gill DL, Inesi G. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci U S A. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. Embo J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. Faseb J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 18.Fauconnier J, Lanner JT, Sultan A, Zhang SJ, Katz A, Bruton JD, Westerblad H. Insulin potentiates TRPC3-mediated cation currents in normal but not in insulin-resistant mouse cardiomyocytes. Cardiovasc Res. 2007;73:376–385. doi: 10.1016/j.cardiores.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Clapham DE. Sorting out MIC, TRP, and CRAC ion channels. J Gen Physiol. 2002;120:217–220. doi: 10.1085/jgp.20028618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eder P, Probst D, Rosker C, Poteser M, Wolinski H, Kohlwein SD, Romanin C, Groschner K. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc Res. 2007;73:111–119. doi: 10.1016/j.cardiores.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 23.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 24.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. Embo J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, et al. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol. 2008;28:2637–2647. doi: 10.1128/MCB.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98:540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc Natl Acad Sci U S A. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner JS, Dolmetsch RE. TrpC3 regulates hypertrophy-associated gene expression without affecting myocyte beating or cell size. PLoS ONE. 2007;2:e802. doi: 10.1371/journal.pone.0000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, Bluhm WF, Meyer M, Sayen MR, Swanson E, et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich A, Kalwa H, Fuchs B, Grimminger F, Weissmann N, Gudermann T. In vivo TRPC functions in the cardiopulmonary vasculature. Cell Calcium. 2007;42:233–244. doi: 10.1016/j.ceca.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium. 2008 doi: 10.1016/j.ceca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Dyachenko V, Christ A, Gubanov R, Isenberg G. Bending of z-lines by mechanical stimuli: an input signal for integrin dependent modulation of ion channels? Prog Biophys Mol Biol. 2008;97:196–216. doi: 10.1016/j.pbiomolbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 36.Usui S, Konno D, Hori K, Maruoka H, Okabe S, Fujikado T, Tano Y, Sobue K. Synaptic targeting of PSD-Zip45 (homer 1c) and its involvement in the synaptic accumulation of F-actin. J Biol Chem. 2003 doi: 10.1074/jbc.M210802200. [DOI] [PubMed] [Google Scholar]
- 37.Sonnemann KJ, Fitzsimons DP, Patel JR, Liu Y, Schneider MF, Moss RL, Ervasti JM. Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev Cell. 2006;11:387–397. doi: 10.1016/j.devcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 39.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 40.Winn MP, C.P., Creazzo T, Lynn KL, Farrington MK, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg P. A mutation in the TRPC6 channel causes familial focal segmental glomerulosclerosis. Science. 2005 doi: 10.1126/science.1106215. in press 2005. [DOI] [PubMed] [Google Scholar]
- 41.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 42.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 43.Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shim S, Goh EL, Ge S, Sailor K, Yuan JP, Roderick HL, Bootman MD, Worley PF, Song H, Ming GL. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 46.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 47.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 49.Yang TT, Yu RY, Agadir A, Gao GJ, Campos-Gonzalez R, Tournier C, Chow CW. Integration of protein kinases mTOR and extracellular signal regulated kinase 5 in regulating nucleocytoplasmic localization of NFATc4. Mol Cell Biol. 2008;28:3489–3501. doi: 10.1128/MCB.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.