Abstract
Interleukin- (IL-)10 and IL-13 play important roles in Th2 cell differentiation and production of autoantibodies in patients with (SLE). However, the mechanisms leading to IL10 and IL13 overexpression in SLE patients are not well understood. In this study, we confirm that the levels of both IL10 and IL13 mRNA in CD4+ T cells and of serum IL10 and IL13 proteins are increased in SLE patients. We show that the DNA methylation levels within IL10 and IL13 gene regulatory domains are reduced in SLE CD4+ T cells relative to healthy controls and negatively correlate with IL10 and IL13 mRNA expression. Moreover, treating healthy CD4+ T cells with the demethylating agent 5-azacytidine (5-azaC) increased IL10 and IL13 mRNA transcription. Together, our results show that promoter methylation is a determinant of IL10 and IL13 expression in CD4+ T cells, and we propose that DNA hypomethylation leads to IL10 and IL13 overexpression in SLE patients.
1. Introduction
Systemic lupus erythematosus (SLE) is a rheumatic autoimmune disease characterized by various immunological abnormalities, including T lymphocyte autoreactivity and the activation of polyclonal B cells, leading to the production of autoantibodies such as antinuclear antibodies (ANA), rheumatoid factor (RF), and anticardiolipin antibodies (aCL) [1]. Defective immune regulation seems to stem from a functional imbalance of T lymphocyte subsets [2]. Within the CD4+ T helper (Th) of patients with SLE, activated Th2 cells overproduce interleukin-6 (IL-6), IL-10, IL-13, and tumor necrosis factor-a whereas Th1 cells underproduce IL-2, interferon-gamma (IFN-γ), and transforming growth factor-β [3–5]. IL-10 is a potent stimulator of B lymphocytes as well as a powerful inhibitor of antigen-presenting cells and T lymphocyte functions [6]. Peripheral blood mononuclear cells (PBMCs) from SLE patients express high levels of IL10 mRNA and protein, and the level of serum IL-10 is correlated with disease activity and severity [7–11]. IL-13 is a cytokine secreted by many cell types but especially Th2 cells. Levels of IL-13 in patients with active SLE are significantly increased and are correlated with disease activity, as defined by the SLE disease activity index of SLE (SLEDAI) [12]. However, little is known about the mechanisms underlying the overexpression of these cytokines in SLE patients.
Previous studies have demonstrated that abnormal hypomethylation of CD4+ T cell DNA can contribute to the pathogenesis of lupus-like diseases [13], and this phenomenon is associated with reduced expression and activity of the DNA methyltransferase enzyme DNMT1 in patient CD4+ T cell samples [14, 15]. The hypomethylation of specific DNA loci in T cells from SLE patients results in the upregulation of methylation-sensitive autoimmune-related genes, such as CD11a (ITGAL), CD70 (TNFSF7), CD40 ligand (TNFSF5), and perforin (PRF1) [16–20]. These data and others show that DNA methylation and chromatin structure can influence the expression of SLE-related genes. We thus hypothesized that the Th2 cytokines IL10 and IL13 are upregulated in SLE due to aberrant DNA methylation of their promoter regions in CD4+ T cells, thereby contributing to the activation of the humoral immune machinery and triggering lupus disease activity.
To test this hypothesis, we examined the DNA methylation status of the IL10 and IL13 genes in CD4+ T cells. We found that the methylation of C/G pairs within the IL10 and IL13 promoter was significantly reduced in T cells from SLE CD4+T cells compared with healthy control samples, and the methylation status was inversely correlated to the levels of IL10 and IL13 transcripts and proteins, as well as the severity of SLE. Furthermore, we found that the treatment of healthy CD4+T cells with the methylation inhibitor 5-azacytidine (5-azaC) induced IL10 and IL13 expression to levels similar to those observed in SLE CD4+T cells. These results indicate an important role of DNA methylation in regulating the expression of the Th2 cytokines in SLE.
2. Materials and Methods
2.1. Subjects
Patient demographics and treatment regimens are shown in Table 1. SLE patients (mean ± SD age 30 ± 6 years) were recruited from outpatient clinics of the Second Xiangya Hospital, Central South University. All patients fulfilled at least 4 of the SLE classification criteria of the American College of Rheumatology [21]. Lupus disease activity was assessed using the SLE Disease Activity Index (SLEDAI) [22]. Healthy controls (mean ± SD age 26 ± 4 years) were recruited from medical staff at the Second Xiangya Hospital. This study was approved by the human ethics committee of the Central South University Xiangya Medical School, and written informed consent was obtained from all subjects. Patients and controls were age and sex matched in all experiments.
Table 1.
Patient demographics, medications.
| Patient | Sex/Age | SLEDAI score | Medication |
|---|---|---|---|
| 1 | F/35 | 6 | Pred 20 mg/d |
| 2 | F/33 | 4 | None |
| 3 | F/22 | 10 | Pred 40 mg/d |
| 4 | F/27 | 6 | Tria 8 mg/d |
| 5 | F/29 | 6 | None |
| 6 | F/26 | 16 | TG 40 mg/d |
| 7 | F/32 | 12 | Pred 35 mg/d |
| 8 | F/18 | 8 | None |
| 9 | F/24 | 4 | Pred 10 mg/d |
| 10 | F/30 | 11 | Pred 20 mg/d |
| 11 | F/31 | 16 | Pred 40 mg/d |
| 12 | F/23 | 6 | Tria 16 mg/d |
| 13 | F/43 | 10 | Pred 20 mg/d |
| 14 | F/41 | 14 | Pred 30 mg/d |
| 15 | F/29 | 10 | Pred 25 mg/d |
F, female; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; Pred: Prednisone; Tria: Triamcinolone; TG: Tripterygium Glycosides.
2.2. Isolation and Culture of CD4+ T Cells
A total of 60 mL of venous peripheral blood was withdrawn from each patient and control subject and preserved with heparin. PBMCs were isolated by density gradient centrifugation, and CD4+ T cells were isolated by positive selection using CD4 beads, according to the protocol provided by the manufacturer (Miltenyi, Germany). The purity of enriched CD4+ T cells was evaluated by flow cytometry and was generally higher than 95%, where indicated PBMCs were stimulated with phytohemagglutinin (PHA), and then cultured with 1 μM 5-azaC (Sigma-Aldrich, USA) in RPMI 1640/10% FCS/IL-2 for an additional 72 hours. After that, CD4+ T cells were isolated from treated PBMCs using CD4 beads. This protocol has been shown to be optimal for inducing expression of other methylation-sensitive T cell genes [16, 18].
2.3. RNA Isolation and Real-Time Quantitative RT-PCR
Total RNA was isolated from CD4+ T cells using the RNeasy minikit (Qiagen, Germany). Real-time quantitative RT-PCR was performed using a Rotor-Gene 3000 (Qiagen, Germany), and mRNA levels were quantified using the One Step PrimeScript RT-PCR Kit (TaKaRa Biotech [Dalian] Co., China). A dilution series of sample RNA was also included to generate a standard curve used to calculate the relative concentrations of transcript present in each sample. Negative controls (in which water was substituted for RNA) were run for each sample. β-actin was also amplified and used as a loading control. The specific primers used for amplification of IL10 were forward, 5′-TGAGAACAGCTGCACCCACTT-3′ and reverse, 5′-TCGGAGATCTC GAAGCATGTTA-3′; IL13: forward, 5′-CAATGGCAGCATGGTATG-3′ and reverse, 5′-ATCCTCTGGGTCTTCTCG-3′; and β-actin: forward, 5′-CGCGAGAAGATGA CCCAGAT-3′ and reverse, 5′-GCACTGTGTTGGCGTACAGG-3′.
2.4. Enzyme-Linked Immunosorbent Assays (ELISAs)
IL-10 and IL-13 protein concentrations in the serum from SLE patients and healthy controls were measured using an ELISA kit (R&D, USA), following the protocol of manufacturer. Optical density (OD) values were read at 450 nm using EL × 800 Absorbance Microplate Reader (BioTek, USA).
2.5. Genomic DNA Extraction and Bisulfite Sequencing
Genomic DNA was isolated from CD4+ T cells using the TIANamp Genomic DNA kit (Tiangen Biotech, Beijing, China). Bisulfite conversion was performed using the EpiTect Bisulfite Kit (Qiagen, Germany). The 192 bp (+3119 to +3310) IL10 enhancer and 290 bp (−2334 to −2045) IL13 promoter fragments contain some important transcription factors binding sites such as STAT5, SP1, and AP-2 predicted using the bioinformatics software Matinspector (http://www.genomatix.de/) and PROSCAN (http://www-bimas.cit.nih.gov/molbio/proscan/). Importantly, these regions encompass enriched CG pairs. These fragments were amplified by nested PCR and cloned into pGEM-T vector (Promega, USA). Five independent clones were sequenced for each of the amplified fragments. Primers used are described in Table 2.
Table 2.
Nest-PCR primers for Bisulfite sequencing.
| Gene | Amplication location | Sequence (5′–3′) |
|---|---|---|
| round I: forward | ||
| (+3063 to +3088 bp) | TTGGGGGTTTAGTTTAGATTTTTTAG | |
| IL10 | round I: reverse | |
| (+3331 to +3307 bp) | TTCACACCCCCTAATATTAACACTC | |
| round II: forward | ||
| (+3119 to +3143 bp) | ATTTAATTTGGAGTTGGTTTAAGTT | |
| round II: reverse | ||
| (+3310 to +3285 bp) | ACTCTATACAATATCTATCCCCAACC | |
| round I: forward | ||
| (−2425 to −2401 bp) | TTGATTGGGTATTATGTTTTTGTTA | |
| round I: reverse | ||
| (−1537 to −1561 bp) | AACTACCTTAATCTATACAACTCCC | |
| IL13 | round II: forward | |
| (−2334 to −2312 bp) | GAGGAAGGGAGGTTTTAATTTTA | |
| round II: reverse | ||
| (−2045 to −2069 bp) | ACCCTAAAAAATAATCTCCAAAAAC | |
2.6. Statistical Analysis
The Student's t-test for equality of means was used to compare values. P-values of less than .05 were considered significant. All analyses were performed with SPSS Version 13.0 software.
3. Results
3.1. Serum IL-10 and IL-13 Levels in SLE Patients and Healthy Controls
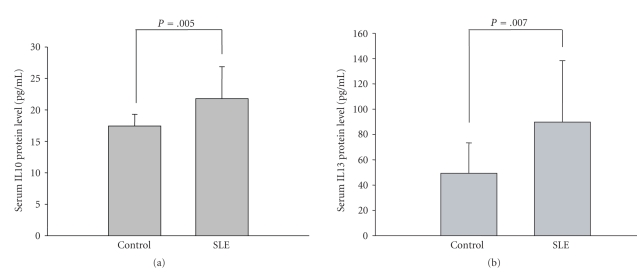
We isolated the serum from peripheral blood samples of 15 SLE patients and 15 healthy controls and then measured the concentration of IL-10 and IL-13 by ELISA. As shown in Figures 1(a) and 1(b), serum protein levels were significantly increased in SLE patients.
Figure 1.
Serum IL-10 and IL-13 protein levels in SLE patients (n = 15) and healthy controls (n = 15). The mean concentration of IL-10 (a) and IL-13 (b) were detected by ELISA (P = .005; P = .007).
3.2. IL10 and IL13 Transcription Levels in SLE CD4+ T Cells Correlate with Disease Activity of SLE
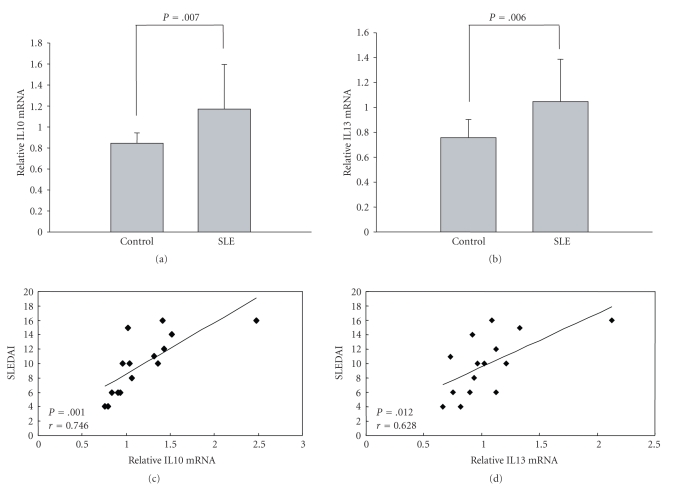
Real-time quantitative RT-PCR was used to detect the mRNA level of IL10 and IL13 in CD4+ T cells from 15 SLE patients and 15 healthy controls. As shown in Figures 2(a) and 2(b), the mean expression levels of IL10 and IL13 relative to β-actin were significantly elevated in patient samples compared to healthy controls. Furthermore, we analyzed the relationship between IL10 and IL13 mRNA levels in CD4+ T cells in individual samples with patient disease activity and found a positive correlation between them (Figures 2(c) and 2(d)).
Figure 2.
IL10 and IL13 mRNA expression in CD4+ T cells from SLE patients (n = 15) and healthy controls (n = 15) and the relationship between expression and disease activity. (a) and (b) IL10 and IL13 mRNA levels in CD4+ T cells were measured by quantitative real-time PCR and normalized to β-actin (SLE versus healthy control, P = .007 and P = .006, respectively). (c) In SLE patients, IL10 mRNA expression correlated (r = 0.746) with SLEDAI score (P = .001). (d) IL13 mRNA expression also correlated with disease activity (r = 0.628, P = .012).
3.3. Regulatory Element Methylation Status in SLE CD4+ T Cells Correlates with IL10 and IL13 Expression
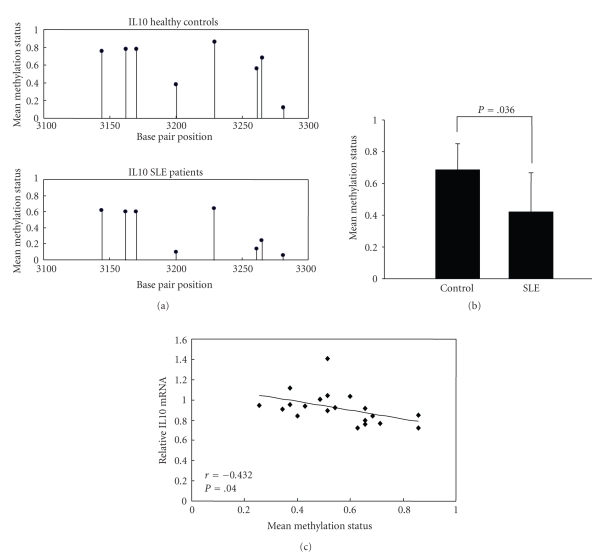
Previous studies have demonstrated that DNA methylation and chromatin structure participate in the regulation of Th2 cytokine genes transcription during Th cell differentiation [23]. To test whether changes in DNA methylation contribute to IL10 overexpression in SLE CD4+ T cells, we detected the methylation status of CG pairs in 192 bp (position +3119 to +3310) CpG inland within the intron 4 enhancer element of the IL10 gene containing the transcription activator STAT5 binding site [24]. Amplified fragments were cloned and five clones were sequenced for each amplification product from each subject (SLE patients, n = 10; healthy controls, n = 10). Figure 3(a) shows the average methylation of each of the 8 CG pairs within the region. Although the mean methylation status of most CG pairs appeared somewhat less methylated in the patient group, there was no statistically significant difference in the overall combined mean methylation status when compared to controls (0.615 ± 0.251 versus 0.375 ± 0.261; P = .083). However, the CG pair at position +3281 was unmethylated in both lupus CD4+ T cells and healthy controls, and when this pair was removed from the overall combined average, the average mean methylation status of the other 7 CG pairs (positions +3144, +3162, +3170, +3200, +3229, +3261, and +3265) was found to be significantly lower in lupus CD4+ T cells than in controls (Figures 3(a) and 3(b)). Moreover, we found that the average methylation status of the 7 methylated CG pairs within the intron 4 CpG island was negatively correlated with IL10 mRNA expression (Figure 3(c)).
Figure 3.
IL10 enhancer methylation patterns in CD4+ T cells from SLE patients and healthy controls. (a) The mean methylation status for each of the 8 CG pairs within intron 4 of the IL10 locus in CD4+T cells from healthy controls (n = 10; upper panel) and SLE patients (n = 10; lower panel). (b) The combined average methylation status of 7 CG pairs (positions +3144, +3162, +3170, +3200, +3229, +3261, and +3265) in IL10 intron 4 in SLE CD4+ T cells was lower than healthy controls (P = .036). (c) Average IL10 promoter DNA methylation status correlated (r = −0.432) with relative mRNA level in SLE patients (P = .040).
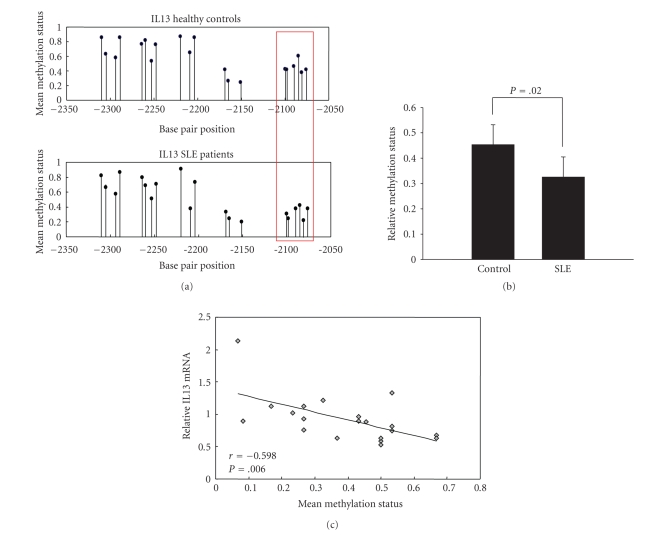
We next investigated the status of a CpG island within a distal promoter region for the IL13 gene in CD4+ T cells from SLE patients (n = 10) and healthy controls (n = 10). Figure 4(a) shows the mean methylation status of each of the 20 CG pairs within this region. We found that the average methylation status of a group of six methylation sites at the proximal end of the detected element (position −2100 to −2076) was significantly lower in the SLE group compared to controls (Figure 4(b)) although there was no significant difference in the overall average methylation level of all 20 CG pairs (0.592 ± 0.206 versus 0.520 ± 0.237; P = .314). Furthermore, we found that the average methylation status of the 6 proximal-end CG pairs (−2100, −2098, −2090, −2085, −2081, and −2076) was inversely correlated with the IL13 mRNA expression (Figure 4(c)).
Figure 4.
IL13 promoter methylation patterns in CD4+ T cells from SLE patients and healthy controls. (a) The mean methylation status for each of the 20 CG pairs within the distal promoter of the IL13 locus in CD4+T cells from healthy controls (n = 10; upper panel) and SLE patients (n = 10; lower). The boxed area indicates the region (positions −2100, −2098, −2090, −2085, −2081, and −2076) with reduced methylation in SLE patients. (b) The combined average methylation status of the 6 hypomethylated CG pairs in the distal promoter of IL13 in SLE CD4+ T cells was reduced compared with the combined average methylation status of these positions in healthy controls (P = .020). (c) Average IL13 promoter (position −2100 to −2076) DNA methylation status correlated (r = −0.598) with relative mRNA level in SLE patients (P = .006).
3.4. Treatment of Healthy CD4+ T Cells with 5-azaC Enhances IL10 and IL13 mRNA Transcription
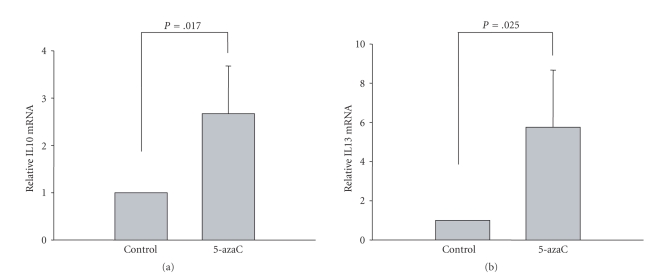
Our previous studies have demonstrated that treating CD4+ T cells from healthy controls with DNA methyltransferase inhibitors such as 5-azaC or PD98059 decreases the DNA methylation level of certain SLE-related autoimmune genes, leading to their upregulation [16–19]. In this study, we asked whether 5-azaC could affect IL10 and IL13 gene expression in T cells from healthy controls. We treated CD4+ T cells with 1 μM 5-azaC for 72 hours and detected the mRNA level of IL10 and IL13 by real-time quantitative RT-PCR. The results showed that IL10 and IL13 increased significantly in the presence of 5-azaC compared with negative controls (Figures 5(a) and 5(b)), suggesting that methylation levels are critical for the normal regulation of IL10 and IL13 in CD4+ T cells.
Figure 5.
Treatment of healthy CD4+ T cells with 5-azaC promotes IL10 and IL13 mRNA transcription. mRNA expression levels of IL10 (a) and IL13 (b) were measured by quantitative real-time PCR and normalized to β-actin. Treatment with 5-azaC results in a 2.6-fold increase of IL10 and a 5.7-fold increase of IL13, relative to untreated controls (P = .017 and P = .025, resp.).
4. Discussion
The imbalance between Th1 and Th2 cells observed in SLE patients is thought to be responsible for provoking a deregulation of cytokine production that contribute to polyclonal B-cell activation observed in SLE patients [1, 3]. In support of this, it has been demonstrated that abnormally high IL-10 secretion by monocytes and lymphocytes is responsible for the heightened production of immunoglobulins of SLE patients [25]. IL-10 production is also enhanced in patients with rheumatoid arthritis (RA) or Sjögren's syndrome, two disorders characterized by prominent B lymphocyte hyperactivity that results in the hyperproduction of immunoglobulins and the increased synthesis of autoantibodies [9]. In this study, we confirmed that IL10 is overexpression in CD4+ T cells isolates from SLE patients compared to healthy controls and IL10 expression positively correlates with disease activity, consistent with previous studies measuring IL10 expression in SLE PBMCs [7–11], suggesting IL-10 plays an important role in the development of SLE.
IL-13 is produced mainly by Th2 cells and is involved in Th2 differentiation [26–28]. It also functions in Th2 cells to stimulate the proliferation and differentiation of B lymphocytes inducing IgM, total IgG [26], IgG4, and IgE [27] synthesis, but not IgA production [26, 27]. Herein we found that serum levels of IL-13 in SLE patients are significantly elevated, in agreement with previous studies [4]. We further demonstrated that IL13 transcription in SLE CD4+ T cells correlated with patient disease activity. These results suggest that both IL-10 and IL-13 play critically important roles in the etiology of SLE and possibly other rheumatic autoimmune disorders; however the mechanism leading to their overproduction is unknown.
Previous studies have confirmed that global genomic methylation is reduced in lupus CD4+ T cells compared with healthy controls and, furthermore, the hypomethylation of specific promoter regions contributes to the overexpression of some SLE-related genes such as CD11a, CD70, CD40L, and Perforin [13, 16–20]. To understand the mechanism(s) underlying the overexpression of IL10 and IL13 in SLE CD4+ T cells, we investigated the methylation status of CpG islands within known regulatory domains for these genes. We found that the methylation status of CG pairs within the enhancer intron 4 of the IL10 genomic locus and within the distal IL13 promoter was significantly reduced in patient samples relative to healthy controls. Furthermore, we found that the average methylation level of hypomethylated CG pairs within these regions negatively correlated with mRNA transcription levels. We further showed that treating CD4+ T cells from healthy controls with the DNMT inhibitor 5-azaC led to a significant increase in IL10 and IL13 transcription, similar to what has been shown for other SLE-related autoimmunity genes, such as CD11a [16], Perforin [17], CD70 [18], and CD40L [19].
A previous study suggested that IL13 expression in Th2 cells is regulated by the methylation status of a region in the proximal promoter encompassing three putative GATA-3 binding sites [23]. This prompted us to investigate the methylation status of the CpG sites around the transcriptional start site of IL13 in T cells. However, we found that all 10 CG sites within the proximal promoter are heavily methylated in both SLE and healthy control CD4+ T cells (data not shown), suggesting that the upregulation of IL13 in SLE patients is not dependent on the demethylation of this region. In contrast, we found that the methylation status of 6 CG pairs (out of a total of 20) within the distal promoter was affected in SLE patients and that demethylation of this sites correlated with IL13 expression in SLE and healthy control CD4+ T cells, suggesting that methylation of these particular sites is an important indicator of SLE disease activity.
DNA methylation modifies gene expression through at least three mechanisms. Methylation of target sequences prevents the binding of some transcriptional activators, such as Sp1 and AP-2, thereby suppressing expression [29]. Methylcytosine binding proteins can also prevent the binding of transcription factors and can suppress gene expression from a distance [30]. Methylcytosine binding proteins also attract chromatin remodeling complexes that modify adjacent histones, resulting in a condensed nucleosome structure, making the locus inaccessible to transcription factors [31]. The methylation-sensitive regions within IL10 intron 4 and the distal promoter of IL13 both contain potential Sp1, AP-2, and STAT5 binding sites predicted using bioinformatics software Matinspector and PROSCAN (Version 1.7). Moreover, it has been demonstrated that the expression of cytokine genes during T-cell differentiation is regulated by acetylation- and methylation-dependent chromatin structural changes [32]. Thus no one mechanism can be ruled out, and further experiments will need to be performed in order to elucidate the underlying cause of methylation-dependent IL10 and IL13 upregulation in CD4+ T cells of patients with SLE.
The reasons why the regulatory element of IL10 and IL13 are hypomethylation remain unclear. Akahoshi reported that there was no difference in the Th1 : Th2 ratio between SLE patients with/without proteinuria and healthy controls, suggesting overexpression and hypomethylation of IL10 and IL13 may be not associated with the number of Th1 and Th2 cells [2]. Our recent studies have found that many factors, such as aberrant expression of specific transcriptional factor RFX1 and nucleus protein Gadd45a, may be involved in regulating the methylation status of methylation-sensitive genes in lupus CD4+ T cells [33, 34].
In summary, disease activity-related increases of IL10 and IL13 expression in CD4+ T cells from SLE patients are associated with demethylation of specific CpG islands within IL10 enhancer and IL13 promoter regions. These findings provide a basis for the design of a new therapy aimed at reversing the epigenetic alternations of gene expression in human SLE patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30730083 and no. 30901300) and the National Basic Research Program of China (973 Plan) (2009CB825605). M. Zhao and J. Tang contributed equally to this work.
References
- 1.Dean GS, Tyrrell-Price J, Crawley E, Isenberg DA. Cytokines and systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2000;59(4):243–251. doi: 10.1136/ard.59.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akahoshi M, Nakashima H, Tanaka Y, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis and Rheumatism. 1999;42(8):1644–1648. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Gómez D, Correa PA, Gómez LM, Cadena J, Molina JF, Anaya J-M. Th1/Th2 cytokines in patients with systemic lupus erythematosus: is tumor necrosis factor α protective? Seminars in Arthritis and Rheumatism. 2004;33(6):404–413. doi: 10.1016/j.semarthrit.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto S, Tokano Y, Kaneko H, Nozawa K, Amano H, Hashimoto H. The increased interleukin-13 patients with systemic lupus erythematosus: relations to other Th1-, Th2-related cytokines and clinical findings. Autoimmunity. 2001;34(1):19–25. doi: 10.3109/08916930108994122. [DOI] [PubMed] [Google Scholar]
- 5.Richaud-Patin Y, Alcocer-Varela J, Llorente L. High levels of TH2 cytokine gene expression in systemic lupus erythematosus. Revista de Investigación Clínica. 1995;47(4):267–272. [PubMed] [Google Scholar]
- 6.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagiwara E, Gourley MF, Lee S, Klinman DM. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-γ-secreting cells in the peripheral blood. Arthritis and Rheumatism. 1996;39(3):379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 8.Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer J-P, Renauld J-C. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4(5):393–395. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 9.Llorente L, Richaud-Patin Y, Fior R, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren’s syndrome, and systemic lupus erythematosus: a potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis and Rheumatism. 1994;37(11):1647–1655. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 10.Llorente L, Richaud-Patin Y, Wijdenes J, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. European Cytokine Network. 1993;4(6):421–427. [PubMed] [Google Scholar]
- 11.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clinical and Experimental Rheumatology. 1998;16(3):283–288. [PubMed] [Google Scholar]
- 12.Xu Z, Chen Y. Determination of serum interleukin-13 and nerve growth factor in patients with systemic lupus erythematosus and clinical significance. Journal of Huazhong University of Science and Technology. 2005;25(3):360–361. doi: 10.1007/BF02828168. [DOI] [PubMed] [Google Scholar]
- 13.Ballestar E, Esteller M, Richardson BC. The epigenetic face of systemic lupus erythematosus. Journal of Immunology. 2006;176(12):7143–7147. doi: 10.4049/jimmunol.176.12.7143. [DOI] [PubMed] [Google Scholar]
- 14.Balada E, Ordi-Ros J, Serrano-Acedo S, Martinez-Lostao L, Rosa-Leyva M, Vilardell-Tarres M. Transcript levels of DNA methyltransferases DNMT1, DNMT3A and DNMT3B in CD4+ T cells from patients with systemic lupus erythematosus. Immunology. 2008;124(3):339–347. doi: 10.1111/j.1365-2567.2007.02771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei W, Luo Y, Yan K, et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scandinavian Journal of Rheumatology. 2009;38(5):369–374. doi: 10.1080/03009740902758875. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Kaplan M, Ray D, et al. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis and Rheumatism. 2002;46(5):1282–1291. doi: 10.1002/art.10234. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, Wu A, Ray D, et al. DNA methylation and chromatin structure regulate T cell perforin gene expression. Journal of Immunology. 2003;170(10):5124–5132. doi: 10.4049/jimmunol.170.10.5124. [DOI] [PubMed] [Google Scholar]
- 18.Lu Q, Wu A, Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. Journal of Immunology. 2005;174(10):6212–6219. doi: 10.4049/jimmunol.174.10.6212. [DOI] [PubMed] [Google Scholar]
- 19.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. Journal of Immunology. 2007;179(9):6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- 20.Oelke K, Lu Q, Richardson D, et al. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis and Rheumatism. 2004;50(6):1850–1860. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythrematosus. Arthritis and Rheumatism. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis and Rheumatism. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 23.Santangelo S, Cousins DJ, Winkelmann NEE, Staynov DZ. DNA methylation changes at human Th2 cytokine genes coincide with DNase I hypersensitive site formation during CD4+ T cell differentiation. Journal of Immunology. 2002;169(4):1893–1903. doi: 10.4049/jimmunol.169.4.1893. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji-Takayama K, Suzuki M, Yamamoto M, et al. The production of IL-10 by human regulatory T cells is enhanced by IL-2 through a STAT5-responsive intronic enhancer in the IL-10 locus. Journal of Immunology. 2008;181(6):3897–3905. doi: 10.4049/jimmunol.181.6.3897. [DOI] [PubMed] [Google Scholar]
- 25.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. Journal of Experimental Medicine. 1995;181(3):839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie ANJ, Culpepper JA, de Waal Malefyt R, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunology Today. 1994;15(1):19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 29.Comb M, Goodman HM. CpG methylation inhibits proenkephalin gene expression and binding of the transcription factor AP-2. Nucleic Acids Research. 1990;18(13):3975–3982. doi: 10.1093/nar/18.13.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bird AP, Wolffe AP. Methylation-induced repression-belts, braces, and chromatin. Cell. 1999;99(5):451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 31.Attwood JT, Yung RL, Richardson BC. DNA methylation and the regulation of gene transcription. Cellular and Molecular Life Sciences. 2002;59(2):241–257. doi: 10.1007/s00018-002-8420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9(6):765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zhao M, Yin H, et al. Gadd45a overexpression contributes to autoimmunity by promoting DNA demethylation in lupus T cells. Arthritis and Rheumatism. 2010;62(5):1438–1447. doi: 10.1002/art.27363. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M, Sun Y, Gao F, et al. Epigenetics and SLE: RFX1 downregulation causes CD11a and CD70 overexpression by altering epigenetic modifications in lupus CD4+ T cells. Journal of Autoimmunity. 2010;35:58–69. doi: 10.1016/j.jaut.2010.02.002. [DOI] [PubMed] [Google Scholar]