Abstract
The amyloid hypothesis has yielded a series of well-validated candidate drug targets with potential for the treatment of Alzheimer disease (AD). Three proteases that are involved in the processing of amyloid precursor protein—α-secretase, β-secretase and γ-secretase—are of particular interest as they are central to the generation and modulation of amyloid-β peptide and can be targeted by small compounds in vitro and in vivo. Given that these proteases also fulfill other important biological roles, inhibiting their activity will clearly be inherently associated with mechanism-based toxicity. Carefully determining a suitable therapeutic window and optimizing the selectivity of the drug treatments towards amyloid precursor protein processing might be ways of overcoming this potential complication. Secretase inhibitors are likely to be the first small-molecule therapies aimed at AD modification that will be fully tested in the clinic. Success or failure of these first-generation AD therapies will have enormous consequences for further drug development efforts for AD and possibly other neurodegenerative conditions.
Introduction
Effective therapy for Alzheimer disease (AD) is one of the major unmet needs in modern medicine. To date, the options to develop medication for AD have been fairly limited. Novel treatments that address cognitive and behavioral symptoms might complement existing cognition-enhancing therapies, such as the acetyl-cholinesterase inhibitors and the N-methyl-D-aspartate receptor antagonist memantine, but are unlikely to provide sufficient benefit as the disease progresses. Alternatively, a healthy diet and physical or cognitive exercise might prove to be effective interventions that could reduce the incidence of the disease. Both epidemiological studies and experiments in transgenic mice have shown that these ‘lifestyle’ interventions can indeed reduce the incidence of both dementia and dementia-related biochemical changes in the brain.1,2 Clearly, however, lifestyle interventions will not prevent all instances of AD, and treatments that target the pathological processes that underlie this disease are more likely to provide effective treatment strategies.
AD, like many other geriatric disorders, seems to be multifactorial in origin. The prevailing theory, the ‘amyloid hypothesis’, suggests that in the context of failing protection and compensation mechanisms in the aging brain, accumulation of the amyloid-β (Aβ) peptide in protein aggregates triggers numerous pathophysiological changes that ultimately lead to cognitive dysfunction.3 Aβ is produced exclusively by two membrane-bound secretases, and their activity can lead to the accumulation of Aβ in senile plaques, which are one of the defining lesions of AD.4 Furthermore, the consistent emergence of these lesions in the neocortex and hippocampus of patients with AD might indicate that a common biochemical and anatomical pathway exists in this disease, on which the various other risk factors for AD might converge. The amyloid hypothesis also provides the rationale for the current main focus of the pharmaceutical industry, namely to successfully target the Aβ aggregates.
The amyloid hypothesis has been modified and challenged, but is still the only hypothesis with true translational impact. This hypothesis has led to the identification of viable therapeutic targets for treatment or prevention of AD, such as the secretases—proteases that are involved in the generation of Aβ (Figure 1). Successful identification of such targets has enabled the development of drug therapies that inhibit Aβ production. Given that Aβ aggregation is partially a concentration-dependent phenomenon, it is widely believed that such drugs, if shown to lower Aβ in the human brain, might become the first disease-modifying treatments for AD. Whether these drugs will work as therapies should be determined in the next few years. Secretase-based therapies are postulated to be most effective if initiated before disease onset, but whether the regulatory, financial and ethical obstacles to conducting such trials will be overcome in the near future is not clear.
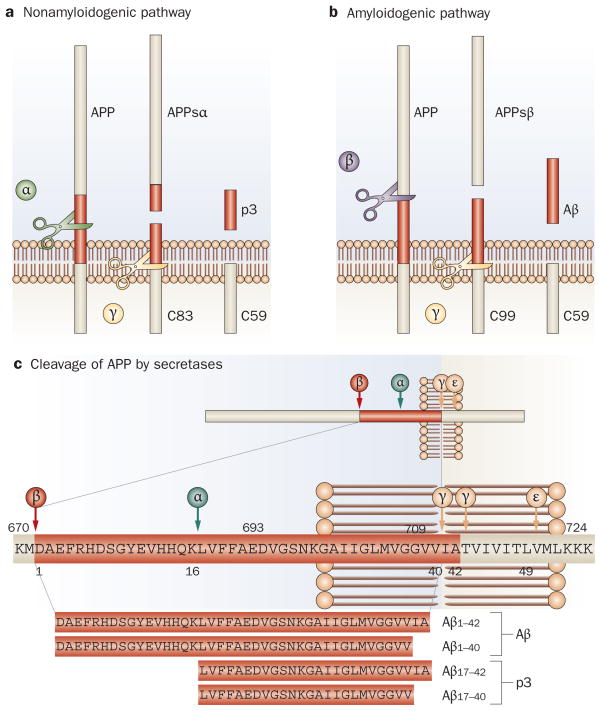
Figure 1.
Processing of APP by the secretases. a | In the nonamyloidogenic pathway, APP is first cleaved by α-secretase within the Aβ sequence, which releases the APPsα ectodomain. Further processing of the resulting carboxyl terminal by γ-secretase results in the release of the p3 fragment. b | The amyloidogenic pathway is initiated when β-secretase cleaves APP at the amino terminus of the Aβ peptide and releases the APPsβ ectodomain. Further processing of the resulting carboxy-terminal fragment by γ-secretase results in the release of Aβ. c | The amino acid residues where the various secretases cleave APP. Aβ and p3 fragments of differing lengths are produced by processing of APP at two different sites by γ-secretase. Abbreviations: Aβ, amyloid-β; APP, amyloid precursor protein; APPsα, soluble amyloid precursor protein-α; APPsβ, soluble amyloid precursor protein-β; C83, carboxy-terminal fragment 83; C59, carboxy-terminal fragment 59; C99, carboxy-terminal fragment 99.
In this Review, we discuss the biology of the three secretases with regard to their viability as potential therapeutic targets for AD drug treatments. We also provide an update on the clinical developments concerning these three drug targets and endeavor to predict how work in this area over the coming years will evolve.
α-Secretases
Biological activity
Outside the CNS, amyloid precursor protein (APP) is preferentially cleaved by α-secretase (Figure 1a).5–7 The α-secretase activity is mediated by a series of membrane-bound proteases (Figure 2a), which are members of the ADAM (a disintegrin and metalloprotease) family. The α-secretases cleave APP within the Aβ sequence itself, between amino acids 16 and 17 (Figure 1c), generating a soluble APPsα ectodomain and a membrane-bound carboxy-terminal fragment, APP-CTFα (Figure 1a). The latter fragment is degraded in lysosomes,8,9 or is further processed by γ-secretase,10 yielding a series of short hydrophobic peptides including Aβ17–40 and Aβ17–42, which are collectively called p3 fragments (Figure 1a,c).11 Processing of APP by α-secretases is postulated to be protective in the context of AD because the enzymes cleave within the Aβ sequence, thereby preventing the production of Aβ. Such a postulate is contingent, however, on multiple unknown aspects of APP and Aβ biology. For example, increasing α-secretase activity would be neuroprotective via the increased shedding of growth-promoting soluble APPα ectodomains only if the p3 fragment produced by α-secretase activity is not pathogenic, and if increasing α-secretase activity actually lowers Aβ production. Several studies have indicated that increased α-secretase-mediated processing of APP reduces the processing of APP by β-secretase and decreases Aβ production;12,13 however, this finding has not been universally replicated.14 Furthermore, the p3 peptide is very hydrophobic and has been reported to be present in diffuse amyloid plaques.15,16 Increasing α-secretase activity, as mentioned above, increases the production of APPsα, and has been reported to be neuroprotective and growth promoting,17 but the consequences of chronically upregulating α-secretase-mediated cleavage of other substrates remains unknown.
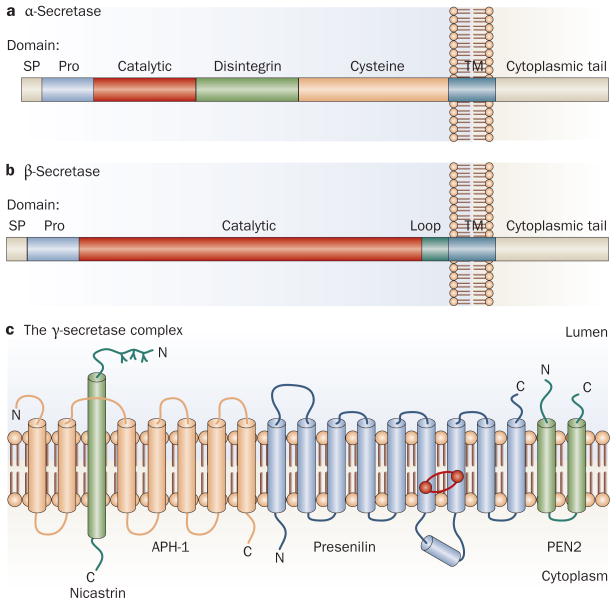
Figure 2.
Structures of the secretases. a | Processing of APP by α-secretase is mediated by a series of proteases, mostly members of the ADAM (a disintegrin and metalloprotease) family, which are all membrane bound and consist of several extracellular domains, including a prodomain, a catalytic domain, a disintegrin domain (which in some instances provides interaction with integrin receptors), and a cysteine-rich domain. b | By contrast, β-secretase activity is specifically mediated by the β-site APP cleaving enzyme 1 (BACE1). BACE1 is also a membrane-bound enzyme that is synthesized with a prodomain. c | The γ-secretase enzyme consists of four proteins, presenilin, nicastrin, anterior pharynx-defective 1 and presenilin enhancer protein 2. Presenilin forms the catalytic core (the catalytic aspartyl residues are indicated by red dots), and the three other proteins are thought to be involved in maturation and stability of the complex. Abbreviations: APH-1, anterior pharynx-defective 1; APP, amyloid precursor protein; PEN2, presenilin enhancer protein 2; Pro, prodomain; SP, signal peptide; TM, transmembrane domain.
From a drug discovery point of view, α-secretase is a peculiar target to choose for the development of AD drug therapies, because it represents an activity that is ascribed to multiple proteases. Furthermore, the exact identity and the relative contribution of each α-secretase to the cleavage of APP in the brain has not been fully elucidated. Transgenic approaches that overexpress the metalloprotease ADAM10 in mice result in increased APPsα generation and are protective against amyloidosis in the human APPV717I transgenic mouse model.18,19 Unfortunately, the extent to which ADAM10 contributes to endogenous α-secretase activity in the human brain is unclear. Furthermore, other proteases of the same family (ADAM9, ADAM17 and ADAM19),18,20–23 as well as β-site APP cleaving enzyme 2 (BACE2),24,25 which shares considerable amino acid sequence homology with β-secretase, have also been shown to cleave APP at or close to the α-cleavage site in APP. Gene deletion studies have also been performed to ascertain the involvement of ADAM9, ADAM10 and ADAM17 in APP processing, but no clear understanding of how these proteins affect APP processing in the brain has been established.26–28 In fact a study has shown that ADAM9 is actually not involved in the non-stimulated secretion of APPsα in neurons.26 Further studies are clearly needed to establish the involvement of these proteases in APP processing. Unfortunately, progress in this area has been hampered by a lack of selective α-secretase inhibitors and the lethality often observed on ablation of the genes that code for these proteases.27,29,30 Deletion of the Adam10 gene, for example, produces a lethal phenotype in mid-gestation. This phenotype is probably caused by vascular defects,27 which in turn are probably a consequence of deficient N-cadherin, E-cadherin and Notch processing. Development of selective inhibitors of the ADAM proteases and conditional transgenic animal models should help to clarify which of the enzymes with α-secretase activity are most important for APP processing.
Drug development
Current evidence suggests that the identified α-secretases demonstrate a high degree of redundancy, and which α-secretases are responsible for APP cleavage in neurons and other brain cells is unclear.27,31 Until this ambiguity is overcome, optimizing the development of compounds that directly activate the α-secretases will be difficult. Stimulating one or more of the signal transduction pathways involved in the regulation of α-secretase activity might be an alternative and indirect method of promoting α-secretase-mediated cleavage of APP. Protein kinase C, mitogen-activated protein kinases, tyrosine kinases and calcium-mediated pathways are all known to be involved in regulating α-secretase activity, and developing compounds that stimulate α-secretase via these pathways is clearly possible.32 Retinoic acid derivatives have been proposed to increase transcription of ADAM10 and could, therefore, also be used to indirectly stimulate α-secretase-mediated cleavage of APP.33
Development of a direct activator of α-secretase as a drug treatment for AD seems unlikely, at least in the short term. Drug treatments that indirectly act as α-secretase stimulators, however, are already being tested in clinical trials. In fact, evidence that indirect activation of α-secretase is successfully achieved by α-7-nicotinic acetylcholine receptor agonists, a 5-hydroxytryptamine receptor 4 agonist, and a γ-aminobutyric acid A receptor modulator has been used to support the clinical development of these agents (Table 1). In many cases, a paucity of data is available to show that these compounds increase α-secretase-mediated cleavage of APP and reduce Aβ levels in vivo. Despite such concerns, the strategy itself is attractive, at least from a theoretical point of view, in that the drug could be approved solely on the basis of symptomatic benefit and later be more extensively studied with respect to its potential for disease modification.34–36 We note, however, that attenuation of AD symptoms by these drugs has not been demonstrated in patients.
Table 1.
Secretase-targeting therapies in human Alzheimer disease trials
| Compound | Company | Clinical trial phase | Comments |
|---|---|---|---|
| α-Secretase activators | |||
| EHT 0202 | Exonhit Therapeutics | II | GABAA receptor modulator that also weakly inhibits phosphodiesterase 4 |
| EVP-6,124 | EnVivo | II | α-7 nicotinic acetylcholine receptor agonist, also in trials for schizophrenia |
| PRX-03,140 | Epix/GlaxoSmithKline | II | Partial 5-HT4receptor agonist |
| β-Secretase inhibitors | |||
| CTS-2166 | CoMentis | I | Selectively binds to the active site of β-secretase; shown to lower plasma Aβ in humans; no cerebrospinal fluid data presented to date |
| γ-Secretase inhibitors | |||
| LY450139 | Eli Lilly | III | Relatively nonselective GSI |
| Begacestat | Wyeth | I | Notch-sparing GSI |
| BMS-708,163 | Bristol-Myers Squibb | II | Notch-sparing GSI |
| MK-0572 | Merck | I | Also being evaluated in combination therapy in breast cancer |
| γ-Secretase modulators | |||
| Tarenflurbil | Myriad | Completed phase III | No effect on cognitive or functional outcomes |
Abbreviations: 5-HT4, 5-hydroxytryptamine 4; Aβ, amyloid-β peptide; GABAA, γ-aminobutyric acid A; GSI, γ-secretase inhibitor.
β-Secretase
Biological activity
The β-secretase enzyme might be a prime therapeutic target for AD, because inhibition of β-secretase should, in theory, decrease production of all forms of Aβ, including the pathogenic 42 amino acid form of Aβ (Aβ42) (Figure 1b,c). In 1999, five different research groups independently reported the molecular cloning of β-secretase and, consequently, this enzyme has been given various names, including β-site APP cleaving enzyme (BACE), aspartyl protease 2 and membrane-associated aspartic protease 2.37–41 The groups used differing isolation methods (for example, expression cloning, protein purification or genomics), yet all identified the same enzyme and concurred that it possessed all the known characteristics of β-secretase.42 For the purposes of this Review, this β-secretase will henceforth be referred to as BACE1.
BACE1 is a type 1 transmembrane aspartic protease (Figure 2b) that is related to the pepsin and retroviral aspartic protease families. BACE1 activity has a low optimum pH and the enzyme is predominantly localized in acidic intracellular compartments (for example, endosomes and the trans-Golgi network). Soon after BACE1 was discovered, a homologous protein, BACE2, was identified. BACE1 and BACE2 share 64% amino acid sequence homology, raising the possibility that BACE2 is also a β-secretase. Unlike BACE1, however, which is highly expressed in neurons of the brain, BACE2 is expressed at low levels in the brain and does not have the same cleavage specificity for APP that BACE1 does, thus indicating that it is a poor β-secretase candidate.
To unequivocally prove that BACE1 was identified correctly as the endogenous β-secretase, knock-out mice, in which the gene encoding BACE1 was deleted, were generated by several groups.43–46 Initial reports indicated that Bace1−/− mice were viable and fertile, suggesting that therapeutic inhibition of BACE1 might produce few mechanism-based adverse effects. Recent studies, however, have shown that Bace1−/− mice exhibit some abnormal phenotypes that are related to the physiological functions of BACE1 (see below). This finding raises concerns that inhibition of BACE1 could produce serious adverse effects in humans. Nevertheless, Aβ generation, amyloid pathology, electrophysiological dysfunction, and cognitive deficits are all reported to be abrogated when Bace1−/− mice are bred to APP transgenics (mice that overexpress the mutant form of APP) to generate Bace1−/−;APP bigenic mice.43,47,48 Bace1−/− mice are devoid of cerebral Aβ, indicating that BACE1 is the main, if not the only, β-secretase enzyme in the brain.49,50 This idea is further supported by reports that lentiviral delivery of Bace1 small interfering RNA attenuates both Aβ amyloidosis and cognitive deficits in APP transgenic mice.48,51 In addition, the rescue of memory deficits in Bace1−/−;APP bigenic mice suggests that therapeutic BACE1 inhibition should improve Aβ-dependent cognitive impairment in patients with AD. Taken together, the BACE1 characterization and validation studies have unequivocally demonstrated that BACE1 is the endogenous β-secretase in the brain and that it is a promising therapeutic target for lowering cerebral Aβ levels in patients with AD.
BACE1 is involved in the processing of numerous other proteins in addition to APP. Identifying these proteins will be necessary for evaluating the possibility of potential mechanism-based toxicity arising from inhibition of BACE1. The inability of soluble BACE1 to efficiently process full-length APP suggests that all BACE1 substrates will probably be membrane-bound proteins.52 In fact, all reported BACE1 substrates, such as Golgi-localized membrane-bound α2,6-sialyltransferase,53 P-selectin glycoprotein ligand 1,54 amyloid-like protein 1 and amyloid-like protein 2,55–57 LDL receptor–related protein (LRP),58 the voltage-gated sodium channel (Nav1) β2 subunit (Navβ2), 59,60 and neuregulin-1 (NRG1) and neuregulin-3 (NRG3),61 are transmembrane proteins. Abrogating the cleavage of NRG1 in BACE1−/− mice reduces myelin sheath thickness of axons of both peripheral sciatic nerves and central optic nerves.62,63 Deficient NRG1 processing in BACE1−/− mice also impairs remyelination of injured sciatic nerves.61 These deleterious effects of attenuated processing of NRG1 by BACE1, coupled with the diverse characteristics of the other BACE1 substrates, suggests that the potential for drug treatments designed to inhibit BACE1 activity to display substantial mechanism-based toxicity is high. Concerns over treatment-linked mechanism-based toxicities has prompted research into whether partial inhibition of BACE1 activity could provide therapeutic benefits for patients with AD while limiting treatment-associated adverse effects. Decreased Aβ deposition was observed in the brains of 12 month-old APPswe;PS1DE9;Bace1+/− mice, in comparison to APPswe;PS1DE9;Bace1+/+ mice (Box 1); however, no substantial differences were observed between these two strains at 20 months.48 Why the older mice in this study did not have reduced amyloidosis is unclear. In a similar study, however, a substantial reduction in Aβ burden was observed in the brains of 13 month-old and 18 month-old PDAPP;Bace1+/− mice (Box 1).49 Although the results of the two transgenic studies differ somewhat, they both suggest that partial inhibition of BACE1 might be sufficient to reduce Aβ burden.
Box 1. Transgenic mouse lines.
APPswe;PS1DE9;Bace1+/− mice are transgenic mice that express mutant forms of amyloid precursor protein (APP) and presenilin, and are also heterozygous for the β-site APP cleaving enzyme 1 (Bace1) gene. These mice have a decreased tendency to generate amyloid-β (Aβ) peptide and amyloid plaques compared with APPswe;PS1DE9;Bace1+/+ mice.
PDAPP;Bace1+/− mice are transgenic mice that express mutant forms of APP under the control of the platelet-derived growth factor promoter, and which are heterozygous for the Bace1 gene. These mice have a decreased tendency to generate amyloid-β (Aβ) peptide and amyloid plaques compared with PDAPP;Bace1+/+ mice.
Drug development
Since the discovery of BACE1, numerous attempts have been made to develop small-molecule BACE1 inhibitors. First-generation BACE1 inhibitors were peptide-based mimetics of the APP β-cleavage site, which had the APP β-site scissile amide bond replaced with a non-hydrolyzable transition state analog, such as statine.40 More recently, nonpeptidergic compounds with high affinities for BACE1 have been generated.64,65
Although initial drug development efforts with peptidomimetic BACE1 inhibitors were encouraging, BACE1 has since proved to be a challenging medicinal chemistry target, for several reasons. The fact that BACE1 has a large substrate binding site is the most obvious one, as this makes inhibiting the enzyme with small nonpeptidergic compounds extremely difficult. Ideally, BACE1 inhibitors should be <500 kDa, orally bioavailable, metabolically stable, intrinsically potent, and highly selective for BACE1 over BACE2 and other aspartic proteases. Potential BACE1 inhibitors must also, in principle, be able to penetrate both the plasma and endosomal membranes to gain access to the intracellular compartments where endogenous BACE1 is localized. An alternative method of delivering BACE1 inhibitors to the intracellular compartments could be achieved by designing BACE1 inhibitors that target and bind to the small fraction of BACE1 expressed at the cell surface. By binding to BACE1 on the cell surface, the inhibitor could be delivered together with BACE1 to the endosomes via endocytosis.66 Efficacious BACE1 drugs would also need to efficiently cross the blood–brain barrier.
Despite these challenges, potent nonpeptidergic small-molecule BACE1 inhibitors have been reported that meet the necessary criteria and show efficacy in lowering cerebral Aβ levels in animal models of AD.65 Moreover, the pharmaceutical company CoMentis recently announced the completion of the first phase I clinical trial of a candidate BACE1 inhibitor drug, and other candidate BACE1 inhibitors might soon be entering clinical trials (Table 1). Reports of successful development of potential nonpeptidergic small-molecule BACE1 inhibitors are encouraging, and suggest that therapeutic approaches involving BACE inhibition for the treatment or prevention of AD might be a reality in the future. Given that BACE1 seems to be involved in other important physiological roles, careful titration of BACE1 drug dosage might be necessary to minimize mechanism-based adverse effects.
γ-Secretase
Biological activity
Despite long-standing knowledge that Aβ was generated through the processing of APP by secretases, the identity of the γ-secretase remained unresolved for many years. Through a convergence of genetic, pharmacological, protein and cell biology studies, it is now clear that γ-secretase is a multi-subunit aspartyl protease that cleaves APP and many other type 1 transmembrane proteins within their transmembrane domains.67,68 Presenilin 1 and presenilin 2 (PS1 and PS2) form the catalytic core of γ-secretase, and three accessory proteins, anterior pharynx-defective 1 (APH-1), nicastrin, and presenilin enhancer protein 2 (PEN2) are also required to complete the γ-secretase complex (Figure 2c).67,69,70 The three accessory proteins seem to be involved in the maturation and stability of the complex. Nicastrin has also been reported to be a substrate receptor;71 however, the data supporting this function is inconclusive and other studies suggest that, like APH-1 and PEN2, nicastrin is required for complex maturation.72
The γ-secretase complex seems to display a high degree of heterogeneity. Humans have two presenilin genes and two APH1 isoforms—APH-1A, which can be alternatively spliced to long and short forms, and APH-1B—and six different functional γ-secretase complexes have been identified.73,74 Rodents have an extra Aph1C gene, which probably arose through duplication of Aph1B.75,76 Rodent γ-secretases might, therefore, display a higher degree of heterogeneity than human γ-secretase. Little is known about the physiological roles of the diverse γ-secretase complexes and how they might process different substrates. One report has shown that in cell free assays, γ-secretase complexes containing APH-1A produce different ratios of intracellular Aβ species from γ-secretase complexes containing APH-1B. In cell culture however, γ-secretase complexes with diverse APH-1 isoforms produce similar ratios of secreted Aβ.77 Deletion of the gene encoding APH-1A is embryonically lethal, almost certainly owing to inhibition of Notch signaling. By contrast, transgenic mice with the genes encoding APH-1B and APH-1C deleted develop normally and have no gross phenotype.
The subunit heterogeneity of the γ-secretase complex suggests that selective targeting of one particular subunit might be an effective alternative treatment strategy to nonselective γ-secretase inhibition. Elective removal of APH-1B and APH-1C in a mouse model of AD has already been shown to decrease Aβ plaque formation and improve behavioral deficits, without adverse effects attributable to impaired Notch signaling being reported.77 This study provides proof of concept that specific inhibition of γ-secretase complexes containing APH-1B in the brain could be a useful strategy for lowering Aβ without inducing Notch-related adverse effects.
γ-Secretase is not only heterogeneous with respect to its protein composition and expression, but it also exhibits a heterogeneous protease activity by cleaving numerous and diverse transmembrane proteins. Two critical asparate residues that are present in opposing trans-membrane domains are believed to catalyze the hydrolysis of the peptide bond in a manner analogous to traditional aspartyl proteases;78 however, this mechanism of catalysis has not been formally proven. Electron microscopy and biochemical studies suggest that γ-secretase forms a pore-like structure in the membrane that allows water to access the active site, although the electron microscopy studies lack the resolution to provide clear insight into the catalytic mechanism.79–82 Furthermore, detailed structural data from nuclear magnetic resonance spectroscopy or X-ray crystallography seem unlikely to emerge in the short term given the complexity of analyzing a molecular complex with 19 transmembrane domains.
Particularly intriguing from a therapeutic perspective is that γ-secretase preferentially cleaves the membrane ‘stub’ of the substrate following ectodomain ‘shedding’, and seems to cleave the substrate with an initial cleavage within the transmembrane domain close to the cytoplasmic border of the membrane, a cleavage often referred to as the ε-cleavage (Figure 1). The ε-cleavage releases the intracellular domain from the membrane, enabling this domain to carry out an intracellular signaling function. For example, following γ-secretase processing, the intracellular domain of Notch translocates to the nucleus, where it binds to various intracellular factors and thereby regulates transcription.83 After the initial cleavage at the ε-cleavage site, γ-secretase cleaves the substrate again (in fact, several times) within the transmembrane domain, a process that ultimately results in the secretion of the amino-terminal portion of the substrate’s membrane stub.84 This sequential cleavage of APP by γ-secretase produces Aβ and seems to be a key determining feature that can increase an individual’s risk of developing AD. In fact, all AD-associated mutations in the presenilin genes and many other mutations in APP increase the proportion of Aβ42 that is produced.85 Aβ42 aggregates more readily than the shorter Aβ peptides and seems to be required for Aβ aggregation and accumulation in vivo.86,87 Moreover, several studies support the concept that shorter Aβ peptides, including the predominant Aβ40 species, inhibit Aβ aggregation and deposition.88,89
Drug development
Unlike β-secretase, γ-secretase has proved to be a highly tractable target for AD drug treatment, at least with respect to the development of orally bioavailable, brain-penetrant γ-secretase inhibitors (GSIs).90 GSIs decrease Aβ production in human and mouse brains, and chronic administration decreases Aβ deposition in APP mouse models.91–94 Multiple orally bioavailable, brain-penetrant GSIs have been identified; however, target-based toxicity has been a major obstacle to the clinical development of these compounds. Target-based toxicity was anticipated on the basis of preclinical studies. The γ-secretase enzyme processes numerous other type 1 transmembrane proteins in addition to APP; for example, γ-secretase processing of Notch1 is crucial for Notch signaling.95 Deletion of PS1 is embryonically lethal in mice, and these mice display a phenotype—prominent skeletal and brain abnormalities—that is also evident in Notch deficient mice.96,97 Moreover, nonselective GSI treatment leads to a variety of toxicities related to Notch inhibition, including, but not limited to, gastrointestinal, skin and immune system abnormalities.98–100 Several dozen γ-secretase substrates have now been identified; however, it is unclear to what extent these other substrates, apart from Notch, contribute to the phenotypes that mice with attenuated γ-secretase processing display. Furthermore, accumulation of APP carboxy-terminal fragments following GSI treatment has been suggested to be toxic, although mice treated with GSIs provided no evidence for this assertion.101
GSIs are now being tested in clinical trials (Table 1). LY450139 is a nonselective GSI, and is currently being tested in a phase III study. Through use of stable isotope labeling combined with cerebrospinal fluid (CSF) sampling to measure the synthesis rate of Aβ, a single oral dose of this compound has been shown to lower CSF Aβ production in healthy men.91,102 Notably, in the same study, enzyme-linked immunosorbent assay analyses of Aβ showed that a substantial decrease in total Aβ was evident only in the treatment group receiving the highest dose of the compound.
As noted previously, the main concern regarding nonselective GSIs is target-mediated toxicity. A narrow therapeutic window exists in which nonselective GSIs substantially reduce Aβ levels in the brain in the absence of Notch-related adverse effects. To overcome these toxicity issues, pharmaceutical companies have been trying to develop ‘Notch-sparing’ GSIs. Such pharmacological selectivity has been shown to be possible;103,104 however, the original compounds were not particularly potent. Two new Notch-sparing inhibitors have recently been described (Table 1), one of which (BMS-708,163) has been shown in phase I clinical trials to lower plasma and CSF Aβ levels.105 No human data have yet been disclosed for the other novel Notch-sparing inhibitor, begacestat.106 The early Notch-sparing GSIs were reported to bind to an adenosine triphosphate site on the γ-secretase complex;103 however, the mechanism by which the second-generation Notch-sparing GSIs work is either not known or has not been disclosed. Some authors have proposed that these GSIs work by binding to the substrate docking sites on γ-secretase that are distinct between Notch and APP. Another possibility is that the compounds selectively target different γ-secretase complexes. At present, the extent to which Notch-sparing GSIs inhibit the processing of the other identified γ-secretase substrates is unclear, and safety concerns around this issue are largely theoretical.
Compounds known as γ-secretase modulators (GSMs) have been shown to alter the profile of the Aβ peptides produced by γ-secretase activity in vitro and in vivo.107,108 Aβ42-reducing GSMs not only decrease levels of Aβ42, but also increase levels of shorter Aβ peptides. Inverse or Aβ42-raising GSMs, on the other hand, increase levels of Aβ42 and decrease the production of shorter Aβ peptides.107,109 Although GSMs have been reported to have varying effects on substrates other than APP, they do not seem to inhibit signaling events mediated by these substrates;107,110 thus, GSMs seem to avoid target-based toxicity. Moreover, given that AD-associated mutations in APP and the presenilin genes modify γ-secretase activity subtly to favor Aβ42 production by as little as 30%, selectively reducing the levels of Aβ42 with GSMs is an attractive alternative treatment strategy to GSI therapy. Tarenflurbil (R-flurbiprofen), the first GSM to be evaluated in humans, was shown to be safe in the long term but recently failed in phase III clinical trials. After 18 months of treatment, patients with AD who received either the active treatment or placebo had almost identical scores on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) score and the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog). This failure was possibly due to low potency ascribed to the compound and poor CNS penetration.111 The recent discovery that certain GSMs bind directly to APP instead of γ-secretase suggests that these compounds might act through a novel mechanism.112 Several GSMs have been reported that are better AD drug candidates than the first-generation GSM compounds, but their mechanisms of action remain unknown.113
Conclusions
The past 10 years have seen substantial progress with regard to the identification and characterization of the secretase enzymes that cleave APP and are involved in the generation of the Aβ peptide in AD. These insights provide a molecular framework for rational drug design. The three secretases have been studied extensively with regard to their physiological importance, and we now have a reasonable understanding of the potential adverse effects associated with the use of drugs that modulate their activities. Compounds targeting each of the three secretases are currently in clinical trials. Whether the currently targeted patients; that is, patients with moderate to severe AD, will show any perceivable benefit from taking such drugs remains an open question, as the brain damage sustained by this stage of disease progression might be irreversible. Moreover, some aspects of the neurodegenerative process, such as intracellular accumulation of tau, might, once induced, become self propagating. Clinicians should, therefore, consider treating patients as soon as possible at a very early stage of the disease, and perhaps even when they are still presymptomatic. The availability of in vivo amyloid imaging might make this approach viable for the prevention of AD in the future.114
Key points
α-Secretase, β-secretase and γ-secretase are proteases that control the production of amyloid-β (Aβ) in the brain
The secretases represent the most promising drug targets for Alzheimer disease therapies
The α-secretase activity is mediated by a series of membrane-bound proteases, further research is needed to identify which of these proteases are most important for processing amyloid precursor protein
β-Secretase and γ-secretase research has progressed enormously and compounds designed to attenuate their activity are in clinic trials
The current trials test only whether elimination or attenuating Aβ in moderate to advanced AD could be beneficial
A real test of the amyloid hypothesis will require drug testing at earlier stages of the disease
Review criteria
The PubMed database was searched for relevant papers using the terms “secretases”, “presenilin”, “ADAM”, “BACE1”, and “Alzheimer’s Disease” and papers were selected based on expert knowledge of the field and of the literature. We have also browsed the website of Alzheimer research forum (http://www.alzforum.org/) for additional updates on drug trials and news from meetings.
Acknowledgments
This work was supported by a NIH/NIA grant (number AG25531) to T. Golde. B. De Strooper was supported by an Artificial SynApse (IWT-ASAP) grant, the Federal Office for Scientific Affairs, Belgium (IUAP P6/43), a Methusalem grant from the Flemish Government and a grant from the European Union (MEMOSAD, F2-2007-200611). R. Vassar received support from the NIH (grant numbers: P01 AG021184, R01 AG022560 and R01 AG030142), the Alzheimer’s Association and the MetLife Foundation.
Footnotes
Competing interests
B. De Strooper declares associations with the following companies: Eisai, Eli Lilly, EnVivo, Johnson & Johnson, Probiodrug, VIB. T. Golde declares an association with the following organization: Mayo Clinic. See the article online for full details of the relationships. R. Vassar declares no competing interests.
Contributor Information
Bart De Strooper, Center for Human Genetics, VIB and KULeuven, Herestraat 49, Box 602, 3000 Leuven, Belgium.
Robert Vassar, Northwestern University, Department of Cell and Molecular Biology, Chicago, IL 60611, USA.
Todd Golde, Mayo Clinic College of Medicine, Department of Neuroscience, Mayo Clinic Florida, 4500 San Pablo Road, Jacksonville, FL 32224, USA.
References
- 1.Knopman DS. Mediterranean diet and late-life cognitive impairment: a taste of benefit. JAMA. 2009;302:686–687. doi: 10.1001/jama.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarov O, et al. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Reinhard C, Hebert SS, De Strooper B. The amyloid-β precursor protein: integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL. Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science. 1990;248:492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- 6.Weidemann A, et al. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases. J Biol Chem. 1999;274:5823–5829. doi: 10.1074/jbc.274.9.5823. [DOI] [PubMed] [Google Scholar]
- 7.Seubert P, et al. Secretion of β-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature. 1993;361:260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- 8.Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992;255:728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- 9.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 10.De Strooper B, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 11.Haass C, Selkoe DJ. Cellular processing of β-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- 12.Hung AY, et al. Activation of protein kinase C inhibits cellular production of the amyloid β-protein. J Biol Chem. 1993;268:22959–22962. [PubMed] [Google Scholar]
- 13.Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM. Protein kinase C-dependent α-secretase competes with β-secretase for cleavage of amyloid-β precursor protein in the trans-golgi network. J Biol Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- 14.Rossner S, et al. Constitutive overactivation of protein kinase C in guinea pig brain increases α-secretory APP processing without decreasing β-amyloid generation. Eur J Neurosci. 2000;12:3191–3200. doi: 10.1046/j.1460-9568.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 15.Gowing E, et al. Chemical characterization of Aβ 17–42 peptide, a component of diffuse amyloid deposits of Alzheimer disease. J Biol Chem. 1994;269:10987–10990. [PubMed] [Google Scholar]
- 16.Higgins LS, Murphy GM, Jr, Forno LS, Catalano R, Cordell B. P3 beta-amyloid peptide has a unique and potentially pathogenic immunohistochemical profile in Alzheimer’s disease brain. Am J Pathol. 1996;149:585–596. [PMC free article] [PubMed] [Google Scholar]
- 17.Ring S, et al. The secreted β-amyloid precursor protein ectodomain APPsα is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammich S, et al. Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postina R, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asai M, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP α-secretase. Biochem Biophys Res Commun. 2003;301:231–235. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- 21.Buxbaum JD, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 22.Koike H, et al. Membrane-anchored metalloprotease MDC9 has an α-secretase activity responsible for processing the amyloid precursor protein. Biochem J. 1999;343:371–375. [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe C, et al. ADAM19 is tightly associated with constitutive Alzheimer’s disease APP α-secretase in A172 cells. Biochem Biophys Res Commun. 2007;352:111–117. doi: 10.1016/j.bbrc.2006.10.181. [DOI] [PubMed] [Google Scholar]
- 24.Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H. BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc Natl Acad Sci USA. 2000;97:9712–9717. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan R, Munzner JB, Shuck ME, Bienkowski MJ. BACE2 functions as an alternative α-secretase in cells. J Biol Chem. 2001;276:34019–34027. doi: 10.1074/jbc.M105583200. [DOI] [PubMed] [Google Scholar]
- 26.Weskamp G, et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol. 2002;22:1537–1544. doi: 10.1128/mcb.22.5.1537-1544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann D, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for α-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 28.Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 29.Maretzky T, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiss K, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and β-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein α-secretases. J Neurosci Res. 2003;74:342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 32.Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Role of the APP non-amyloidogenic signaling pathway and targeting α-secretase as an alternative drug target for treatment of Alzheimer’s disease. Curr Med Chem. 2007;14:2848–2864. doi: 10.2174/092986707782360060. [DOI] [PubMed] [Google Scholar]
- 33.Tippmann F, Hundt J, Schneider A, Endres K, Fahrenholz F. Up-regulation of the α-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 2009;6:1643–1654. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- 34.Caccamo A, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Wolf BA, et al. Muscarinic regulation of Alzheimer’s disease amyloid precursor protein secretion and amyloid β-protein production in human neuronal NT2N cells. J Biol Chem. 1995;270:4916–4922. doi: 10.1074/jbc.270.9.4916. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann M, et al. Acetylcholinesterase inhibitors increase ADAM10 activity by promoting its trafficking in neuroblastoma cell lines. J Neurochem. 2004;90:1489–1499. doi: 10.1111/j.1471-4159.2004.02680.x. [DOI] [PubMed] [Google Scholar]
- 37.Hussain I, et al. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 38.Vassar R, et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 39.Yan R, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 40.Sinha S, et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 41.Lin X, et al. Human aspartic protease memapsin 2 cleaves the β-secretase site of β-amyloid precursor protein. Proc Natl Acad Sci USA. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole SL, Vassar R. The role of amyloid precursor protein processing by BACE1, the β-secretase, in Alzheimer disease pathophysiology. J Biol Chem. 2008;283:29621–29625. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo Y, et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 44.Cai H, et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 45.Roberds SL, et al. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 46.Dominguez D, et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–30806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 47.Ohno M, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 48.Laird FM, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-β amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McConlogue L, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- 50.Nishitomi K, et al. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99:1555–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- 51.Singer O, et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 52.Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer’s β-secretase (BACE1) determines its late Golgi localization and access to β-amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- 53.Kitazume S, et al. Alzheimer’s β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci USA. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichtenthaler SF, et al. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J Biol Chem. 2003;278:48713–48719. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Sudhof TC. Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE 1. J Biol Chem. 2004;279:10542–10550. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- 56.Pastorino L, et al. BACE (β-secretase) modulates the processing of APLP2 in vivo. Mol Cell Neurosci. 2004;25:642–649. doi: 10.1016/j.mcn.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 57.Eggert S, et al. The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves α-, β-, γ-, and ε-like cleavages: modulation of APLP-1 processing by n-glycosylation. J Biol Chem. 2004;279:18146–18156. doi: 10.1074/jbc.M311601200. [DOI] [PubMed] [Google Scholar]
- 58.von Arnim CA, et al. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 59.Wong HK, et al. β Subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J Biol Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 60.Kim DY, et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu X, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willem M, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 63.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 64.Silvestri R. Boom in the development of non-peptidic β-secretase (BACE1) inhibitors for the treatment of Alzheimer’s disease. Med Res Rev. 2009;29:295–338. doi: 10.1002/med.20132. [DOI] [PubMed] [Google Scholar]
- 65.Hills ID, Vacca JP. Progress toward a practical BACE-1 inhibitor. Curr Opin Drug Discov Devel. 2007;10:383–391. [PubMed] [Google Scholar]
- 66.Rajendran L, et al. Efficient inhibition of the Alzheimer’s disease β-secretase by membrane targeting. Science. 2008;320:520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- 67.De Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 68.Wolfe MS, Kopan R. Intramembrane proteolysis: theme and variations. Science. 2004;305:1119–1123. doi: 10.1126/science.1096187. [DOI] [PubMed] [Google Scholar]
- 69.Takasugi N, et al. The role of presenilin cofactors in the γ-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 70.Edbauer D, et al. Reconstitution of γ-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 71.Shah S, et al. Nicastrin functions as a γ-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 72.Chavez-Gutierrez L, et al. Glu(332) in the nicastrin ectodomain is essential for γ-secretase complex maturation but not for its activity. J Biol Chem. 2008;283:20096–20105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- 73.Hébert SS, et al. Coordinated and widespread expression of γ-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol Dis. 2004;17:260–272. doi: 10.1016/j.nbd.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H. Identification of distinct γ-secretase complexes with different APH-1 variants. J Biol Chem. 2004;279:41340–41345. doi: 10.1074/jbc.M405768200. [DOI] [PubMed] [Google Scholar]
- 75.Ma G, Li T, Price DL, Wong PC. APH-1a is the principal mammalian APH-1 isoform present in γ-secretase complexes during embryonic development. J Neurosci. 2005;25:192–198. doi: 10.1523/JNEUROSCI.3814-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serneels L, et al. Differential contribution of the three Aph1 genes to γ-secretase activity in vivo. Proc Natl Acad Sci USA. 2005;102:1719–1724. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serneels L, et al. γ-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer’s disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolfe MS, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 79.Osenkowski P, et al. Cryoelectron microscopy structure of purified γ-secretase at 12 A resolution. J Mol Biol. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazarov VK, et al. Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores. Proc Natl Acad Sci USA. 2006;103:6889–6894. doi: 10.1073/pnas.0602321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tolia A, Chavez-Gutierrez L, De Strooper B. Contribution of presenilin transmembrane domains 6 and 7 to a water-containing cavity in the γ-secretase complex. J Biol Chem. 2006;281:27633–27642. doi: 10.1074/jbc.M604997200. [DOI] [PubMed] [Google Scholar]
- 82.Sato C, Morohashi Y, Tomita T, Iwatsubo T. Structure of the catalytic pore of γ-secretase probed by the accessibility of substituted cysteines. J Neurosci. 2006;26:12081–12088. doi: 10.1523/JNEUROSCI.3614-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kopan R, Ilagan MX. γ-Secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 84.Kakuda N, et al. Equimolar production of amyloid β-protein and amyloid precursor protein intracellular domain from β-carboxyl-terminal fragment by γ-secretase. J Biol Chem. 2006;281:14776–14786. doi: 10.1074/jbc.M513453200. [DOI] [PubMed] [Google Scholar]
- 85.Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim Biophys Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 86.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of β amyloid protein is critical for the seeding of amyloid formation: Implications for pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 87.McGowan E, et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang R, Wang B, He W, Zheng H. Wild-type presenilin 1 protects against Alzheimer’s disease mutation-induced amyloid pathology. J Biol Chem. 2006;281:15330–15336. doi: 10.1074/jbc.M512574200. [DOI] [PubMed] [Google Scholar]
- 89.Kim J, et al. Aβ40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolfe MS. Inhibition and modulation of γ-secretase for Alzheimer’s disease. Neurotherapeutics. 2008;5:391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bateman RJ, et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siemers ER, et al. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-β after inhibition of γ-secretase. Clin Neuropharmacol. 2007;30:317–325. doi: 10.1097/WNF.0b013e31805b7660. [DOI] [PubMed] [Google Scholar]
- 93.Dovey HF, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 94.Abramowski D, et al. Dynamics of Aβ turnover and deposition in different β-amyloid precursor protein transgenic mouse models following γ-secretase inhibition. J Pharmacol Exp Ther. 2008;327:411–424. doi: 10.1124/jpet.108.140327. [DOI] [PubMed] [Google Scholar]
- 95.De Strooper B, et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 96.Wong PC, et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 97.Shen J, et al. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 98.Searfoss GH, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 99.Wong GT, et al. Chronic treatment with the γ-secretase inhibitor LY-411, 575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 100.Li T, et al. Epidermal growth factor receptor and notch pathways participate in the tumor suppressor function of γ-secretase. J Biol Chem. 2007;282:32264–32273. doi: 10.1074/jbc.M703649200. [DOI] [PubMed] [Google Scholar]
- 101.Yankner BA, et al. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science. 1989;245:417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- 102.Siemers ER, et al. Effects of a γ-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 103.Fraering PC, et al. γ-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide-binding site. J Biol Chem. 2005;280:41987–41996. doi: 10.1074/jbc.M501368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Netzer WJ, et al. Gleevec inhibits β-amyloid production but not Notch cleavage. Proc Natl Acad Sci USA. 2003;100:12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fagan T. DC: New γ-secretase inhibitors hit APP, spare Notch. The AlzGene Database. Alzheimer Research Forum [online] 2008 http://www.alzforum.org/new/detail.asp?id=1992.
- 106.Mayer SC, et al. Discovery of begacestat, a Notch-1-sparing γ-secretase inhibitor for the treatment of Alzheimer’s disease. J Med Chem. 2008;51:7348–7351. doi: 10.1021/jm801252w. [DOI] [PubMed] [Google Scholar]
- 107.Weggen S, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 108.Kukar T, Golde TE. Possible mechanisms of action of NSAIDs and related compounds that modulate γ-secretase cleavage. Curr Top Med Chem. 2008;8:47–53. doi: 10.2174/156802608783334042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kukar T, et al. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Aβ42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 110.Weggen S, et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid β42 production by direct modulation of γ-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- 111.Green RC, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kukar TL, et al. Substrate-targeting γ-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pissarnitski D. Advances in gamma-secretase modulation. Curr Opin Drug Discov Devel. 2007;10:392–402. [PubMed] [Google Scholar]
- 114.Klunk WE, Mathis CA. The future of amyloid-beta imaging: a tale of radionuclides and tracer proliferation. Curr Opin Neurol. 2008;21:683–687. doi: 10.1097/WCO.0b013e3283168e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]