Abstract
We have developed a nanocarrier drug-delivery system based on micelles formed by a new class of well-defined linear PEGylated two-arm oligomer of cholic acids in aqueous solution. By varying the length of the linear PEG chains and the configuration of cholic acid oligomer, one can easily fine-tune the physicochemical properties of the amphiphilic polymers and the resulting micelles. These include particle size, critical micelle concentration, and drug loading capacity. High level of hydrophobic anticancer drugs such as PTX, etoposide and SN-38 can be readily loaded into such nanocarriers. The loading capacity of the nanocarrier for PTX (PTX) is extremely high (12.0 mg/mL), which is equivalent to 37.5% (w/w) of the total mass of the micelle. PTX-loaded nanocarriers are much more stable than Abraxane® (PTX/human serum albumin nanoaggregate) when stored in bovine serum albumin solution or dog plasma. PTX release profile from the micelles is burst-free and sustained over a period of seven days. The anti-tumor activity of PTX-loaded nanocarriers against ovarian cancer cell line in vitro, with continuous drug exposure, is similar to Taxol® (formulation of PTX dissolved in Cremophor EL and ethanol) or Abraxane®. Targeted drug delivery to tumor site with these novel micelles was demonstrated by near infrared fluorescence (NIRF) imaging in nude mice bearing ovarian cancer xenograft. Furthermore, PTX-loaded nanocarriers demonstrated superior anti-tumor efficacy compared to Taxol® at equivalent PTX dose in ovarian cancer xenograft model.
Keywords: Nanocarrier, cholic acid, micelle, size tunable, high loading, tumor targeting
Introduction
Polymeric micelles self-assembled from amphiphilic block copolymers have been developed as carriers to improve the pharmacokinetic and efficacy of typical anticancer drugs [1-6]. Size control is of great importance for these drug carriers as it plays a crucial role in the interaction with cells and its in vivo fate in the drug delivery system [4, 7]. Drug carriers in the size range of 10–100 nm can passively accumulate in tumor area through the leaky vasculature via the enhanced permeability and retention (EPR) effect [8, 9]. However, it is difficult to control the size and distribution of micelles without a well defined polymer structure, and variability in such size distribution may also cause stability problems during circulation under physiological conditions [10]. In addition, conventional micelle drug delivery systems based on amphiphilic linear block copolymers have many limitations which include: low encapsulation efficiency, fast initial drug release rate, and difficulty of site-specific functionalization [1, 4, 11, 12].
In recent years, amphiphilic linear-dendritic block copolymers have emerged as a novel class of polymers with potential applications in drug delivery [13-16]. The stepwise and orchestrated synthetic approach used to prepare these polymers permits mono or low polydispersity and an excellent control over molecular architecture [14, 15]. Linear-dendritic block copolymers are usually comprised of a hydrophilic linear block (i.e. polyethylene glycol (PEG)) and a relatively hydrophobic dendritic block [15-17]. The micelle formed by these polymers tends to have uniform size [15]. Several hydrophobic drugs and fluorescent probes have been physically encapsulated into these micelles [14-17]. However, the crowded molecular structure of the dendritic block may restrict encapsulation of some drugs into these micelles.
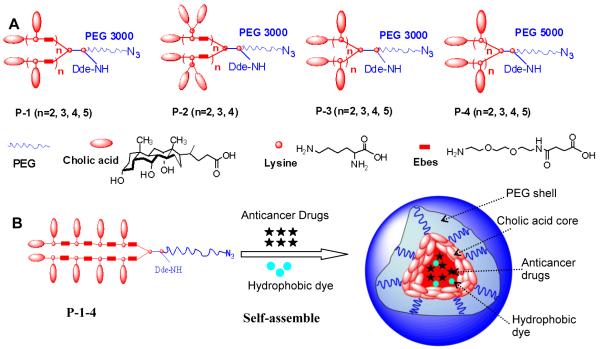
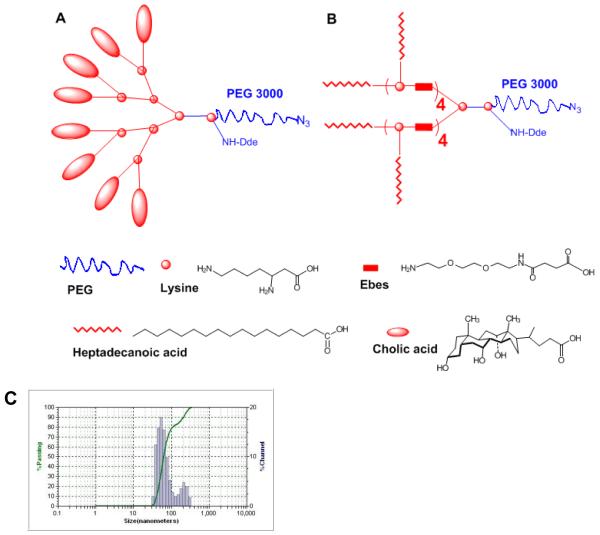
In this study, we designed and synthesized a unique class of well-defined amphiphilic linear-branched block copolymers by stepwise condensation approach of peptide chemistry (Figure 1A, Figure S-1) [14]. Unlike the linear-dendritic structures, these polymers are composed of a hydrophilic linear PEG and a flexible two-arm linear oligomer of cholic acids as a hydrophobic core-forming block (schemes shown in Figure 1A). PEG, L-lysine, Ebes (N-(8-amino-3,6-dioxa-octyl)succinamic acid) [18] and cholic acid (one of the main bile acids) were used as biocompatible building blocks in the construction of the polymers. Cholic acid is a unique facial amphiphilic molecule displaying two methyl groups and three hydroxyl groups on two opposite faces of a rigid polycyclic steroidal scaffold with one carboxyl group at one end for covalent ligation. These features make cholic acid an excellent building block for constructing functionalized molecular architectures with well-defined geometrical properties as well as molecular container for host-guest chemistry [19, 20]. Ebes linkers were introduced as an inert hydrophilic spacer molecule [18] within the oligo-lysine backbone to provide flexibility and to reduce the steric hindrance caused by the bulky cholic acids. The resulting amphiphilic linear-branched block copolymers can self-assemble under aqueous condition to form micelles. Such micelle-based nanocarrier system is size tunable, highly stable, and can encapsulate various hydrophobic anticancer drugs (i.e. PTX, etoposide and SN-38) with superior loading capacity to conventional micelles. Targeted drug delivery to tumor site with these novel micelles was demonstrated in xenograft mouse models utilizing the size-mediated EPR effects.
Figure 1.
(A) Schematic representation of the four series of linear-branched block copolymers; (B) Illustration of a multifunctional micelle formed by self-assembly of the P-1-4 polymer.
Experimental Section
Materials
FmocNH-PEG-COOH (molecular weight: 3000 and 5000 Da, determined by gel permeation chromatography) was purchased from Rapp Polymere (Germany). PTX was purchased from AK Scientific Inc. (Mountain View, CA). Etoposide was purchased from Sigma-Aldrich (St. Louis). SN-38 was purchased from Tokyo Chemical Industry Co., Ltd (Janpan). Taxol® (Mayne Pharma, Paramus, NJ) and Abraxane® (Abraxis Bioscience, LA, CA) were obtained from the Cancer Center of University of California, Davis. (Fmoc)lys(Boc)-OH, (Fmoc)Lys(Dde)-OH, (Fmoc)Lys(Fmoc)-OH, and (Fmoc)Ebes-OH were obtained from AnaSpec Inc. (San Jose, CA). 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD), a hydrophobic near infrared fluorescence dye was purchased from Invitrogen. Cholic acid, MTT [3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromid] and all other chemicals were purchased from Sigma-Aldrich (St. Louis).
Synthesis of linear-branched copolymers
The linear PEGylated two-arm oligomer of cholic acids were synthesized via stepwise peptide chemistry. For example, we started to synthesize P-1 series polymers from linear polyethylene glycol via solution phase reactions (Supplementary material, Figure S-1). 3-azidopropylamine (3 eq.) was coupled onto the carboxylic group of the FmocNH-PEG-COOH (3000 Da) using N-Hydroxybenzotriazole (HOBt 3 eq.)/diisopropyl carbodimide (DIC 3 eq.) as coupling agents in DMF overnight. The polymer was subsequently precipitated and washed with cold ether. After removal of the Fmoc via a 20% piperidine solution in DMF, (Fmoc)Lys(Dde)-OH (3 eq.) was coupled onto the N terminus of PEG using DIC and HOBt as coupling reagents until a negative Kaizer test result was obtained, thereby indicating completion of the coupling reaction. The PEGylated compounds were then precipitated and washed with cold ether. Then (Fmoc)Lys(Fmoc)-OH (3 eq.), (Fmoc)Ebes-OH (6 eq.), (Fmoc)Lys(Boc)-OH (6 eq.) were coupled to the above PEGylated products step by step via the same Fmoc peptide synthesis procedure. The scaffolds of P-1-2, P-1-3, P-1-4 and P-1-5 were built by repeating the coupling of (Fmoc)Ebes-OH and (Fmoc)lys(Boc)-OH 2, 3, 4 and 5 times, respectively. After removal of Boc and Fmoc group, cholic acid NHS ester reacted with the free amino groups of the scaffolds to generate the four polymers in P-1 series. The polymers were precipitated and washed by cold ether and dissolved in water. The polymer solution was filtered and then dialyzed against 4L water in a dialysis tube with MWCO of 3.5 KDa; reservoir water was refreshed completely four times in 24 h. Finally, the polymers were lyophilized. P-2, P-3 and P-4 series were synthesized with the similar strategy by using the different combinations of Fmoc-lys(Fmoc)-OH and Fmoc-lys(Boc)-OH as well as different rounds of coupling and Fmoc deprotection. 1H NMR spectra of the polymers were recorded on an Avance 500 Nuclear Magnetic Resonance Spectrometer using CDCl3 and D2O as the solvents. The concentration of the polymers was kept at 5 ×10−4 M for NMR measurements.
Characterizations of micelles
The morphology of micelles was observed on a Philips CM-120 Transmission electron microscope (TEM) operating at an acceleration voltage of 80 kV. A drop of aqueous micelle solution was deposited onto a copper grid with carbon film for approximately 1 min and then was blotted with a piece of filter paper. The grid was dried at room temperature and stained with 0.1% (w/v) phosphotungstic acid. Finally, the sample was maintained and measured at room temperature. The size and size distribution of the micelles were measured by dynamic light scattering (DLS) instruments (Nanotrac). The micelle concentrations were kept at 20 mg/mL for DLS measurements.
Critical micelle concentration measurements
The critical micelle concentration (CMC) of the micelles was measured through fluorescence spectra as described previously [6, 21]. Pyrene was used as a hydrophobic fluorescent probe. Aliquots of pyrene solutions (6×10−6 M in methanol) were added to flasks, and then the methanol was evaporated. Polymer solutions ranging from 5×10−7 to 5×10−4 M were added to the flasks subsequently. The solutions were equilibrated for 24 h at room temperature. The final pyrene concentration was kept at 6 ×10−7 M. Excitation spectra were recorded ranging from 300 to 360 nm with a fixed emission at 390 nm. Plots of I339/ I334 from the excitation spectra of pyrene against log [C] are flat at the low concentration extreme and sigmoidal in the crossover region. The CMC is taken as the intersection of the tangent to the curve at the inflection with the horizontal tangent through the points at low polymer concentration.
Preparation of Paclitaxel, etoposide or SN-38 loaded micelles
Hydrophobic antitumor drugs, such as PTX, etoposide or SN-38, and PEGylated two-arm oligomer of cholic acids (20 mg) were first dissolved in chloroform. The organic solvent was evaporated under vacuum to form a thin film. PBS buffer (1 mL) was added to hydrate the thin film, followed by 2 hours of sonication. The unloaded drug precipitations were removed by passing the micelles solution through a 0.22 μm filter. The final concentrations of the polymers were maintained at 20 mg/mL for all the drug loading tests. The amount of drug loaded in the micelles was analyzed on a HPLC system (Waters) after releasing the drugs from the micelles by adding 9 times of DMSO and 10 min sonication. The drug loading was calculated according to the calibration curve between the HPLC area values and concentrations of the drug in DMSO. The loading capacity is defined as the highest drug concentration that can be achieved by the micelles in aqueous solution. The loading efficiency is defined as the ratio of drug loaded into micelles to the initial drug content.
Stability of micelles in BSA solution and dog plasma
The stability of Abraxane® and PTX-loaded micelles (PTX concentration: 7.5 mg/mL) was studied in two different medias, PBS containing 5% (w/v) BSA, 50% (v/v) dog plasma. The particle size and size distribution were monitored by dynamic light scattering. The mixture was incubated at physiological body temperature (37 °C) and determined at time interval for up to 96 h. All the measurements were repeated three times. Values reported are the mean diameter ± SD for duplicate samples.
Drug release study
PTX-loaded P-1-4 micelle solution was prepared to determine the in vitro drug release profile. The initial PTX concentration was 7.2 mg/mL and the mass ratio of PTX in micelles was 26.5%. Aliquots of PTX-loaded micelle solution were injected into dialysis cartridges (Pierce Chemical Inc.) with a 3 KDa MWCO. The cartridges were dialyzed against 100 mL sodium salicylate solution (1 M) at 37 °C and rotator rate was set to 100 rpm. The concentration of PTX remained in the dialysis cartridge at various time points was measured by HPLC. The drug release profile of Taxol® (PTX concentration: 6.0 mg/mL) was determined under identical condition for comparison. Values were reported as the means for each triplicate samples.
MTT Assay
SKOV-3 ovarian cancer cells were seeded in 96-well plates a day prior to the treatment at a density of 3000 cells/well. After 72 h incubation with different concentrations of blank micelles or various formulations of PTX in a humidified 37 °C, 5% CO2 incubator, MTT was added to each well and further incubated for 4 h at 37 °C. The absorbance at 570 nm and 660 nm were detected using a microplate ELISA reader (SpectraMax M2, Molecular Devices, USA). Results were shown as the average cell viability [(ODtreat–ODblank) / (ODcontrol–ODblank)×100%] of triplicate wells. The IC50 value was calculated as the concentration of agents that inhibited the cell growth by 50%.
In vivo and ex vivo optical imaging
SKOV-3 ovarian cancer cells (7×106 cells in a total volume of 100 μL PBS and Matrigel, 1:1 v/v) were injected subcutaneously into nude mice to form subcutaneous nodules. The SKOV-3 tumor bearing mouse was injected via tail vein with 100 μL of PTX and DiD (near infrared hydrophobic fluorescence dye, Invitrogen) co-loaded micelles solution (the concentration of PTX and DiD loaded in micelles were both 1mg/mL). The final size of the micelles was 30 nm. In vivo near infrared (NIRF) optical imaging of the mice was obtained by a Kodak Image Station 2000MM at different time points. The mouse was sacrificed at 24 h post-injection and all the major organs and tumor were excised for ex vivo imaging. All animal experiments were performed in compliance with institutional guidelines and according to protocol No. 06-12262 approved by the Animal Use and Care Administrative Advisory Committee at the University of California, Davis.
In vivo therapeutic study
Subcutaneous SKOV-3 ovarian cancer xenografts were implanted into nude mice as described above. The treatments were started when tumor xenograft reached a tumor volume of 100-200 mm3 and this day was designated as day 0. On day 0, these mice were randomly divided into three groups, with five mice in each group. Taxol®, PTX-loaded nanocarriers or PBS (as control) was administered intravenously into the tail vein on days 0, 4, 8, 12, 16 and 20. Taxol® was given at its maximal tolerated dose (MTD), which is 20 mg/kg [22], and PTX-loaded P-1-4 micelles were administered at the same PTX dose (20 mg/kg) for comparison. Injection volume was 0.1 mL for each 10 g of mouse body weight. Tumor size was measured with a digital caliper twice per week. Tumor volume was calculated by the formula (L×W2)/2, where L is the longest, W is the shortest in tumor diameters (mm). To compare between groups, relative tumor volume (RTV) was calculated at each measurement time point (where RTV equals the tumor volume at given time point divided by the tumor volume prior to initial treatment).
Statistical analysis
Statistical analysis was performed by Student’s t-test for two groups, and one-way ANOVA for multiple groups. All results were expressed as the mean ± standard error (SEM) unless otherwise noted. A value of P<0.05 was considered statistically significant.
Results and discussion
Physico-chemical Characterizations of micelles
Four series of amphiphilic polymers with different number of repeating units in the core-forming segments (n=2, 3, 4, 5) have been synthesized (Figure 1A). Polymers are designated as P-1-2, P-1-3, P-1-4, and P-1-5 in the P-1 series corresponding to the number of core-forming units (2, 3, 4 and 5, respectively). P-1-4 is a representative of the novel amphiphilic linear-branched polymers, comprised of a two-arm oligomer of ten cholic acids attached to one terminus of the linear PEG through a poly(lysine-Ebes) backbone (Figure 1B). Cancer targeting ligands can be conjugated to the azide group at the distal termini of the PEGs in the polymers via “click” chemistry [23]. Radionuclides or fluorescent dyes can also be attached to the ε-amino group of the lysine at the junction between the PEG and the oligocholic acid chains after removal of 1-(4,4-dimethyl-2,6-dioxocyclohex-1-yldine)ethyl (Dde) protecting group, (Figure 1B).
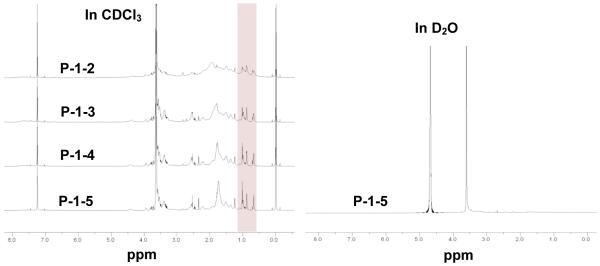
The polymers can self-assemble in aqueous solution to form micelles with a hydrophobic cholic acid core surrounded by a hydrophilic PEG shell (Figure 1B). 1H-NMR was recorded to confirm the polymer chemical structure and micelle formation in aqueous environment. The signals of PEG chains (3.5-3.7 ppm) and cholic acid (0.6-2.4 ppm) could be observed in CDCl3 (Figure 2). The peaks at 0.66, 0.87 and 1.01 ppm in 1H-NMR spectra in CDCl3 correspond to cholic acid methyl protons 18, 19, and 21, respectively. These characteristic peaks increased steadily with the number of core-forming units in the P-1 series polymers when keeping the signals of PEG chains at the same level (Figure 2). The integration of these peaks can be used to calculate their chemical compositions. The number of cholic acids determined by 1H-NMR was consistent with the molecular formula of the target polymer (Table 1). The signals of both PEG chains and cholic acid were detected when the spectrum was obtained in CDCl3. When the NMR experiment was performed in D2O, the cholic acid proton peaks disappeared (Figure 2), indicating that the core-shell structure of the self-assembled block copolymers was well preserved under an aqueous environment, thereby restricting the motion of cholic acid methyl protons facing or within the hydrophobic core.
Figure 2.
1H-NMR spectra of P-1 series polymers at same molar concentrations in CDCl3 and D2O.
Table 1.
Number of cholic acids in molecular formula of P-1 series in comparison to that determined by 1H-NMR
| P-1-2 | P-1-3 | P-1-4 | P-1-5 | |
|---|---|---|---|---|
| Number of cholic acids in molecular formula | 6 | 8 | 10 | 12 |
| Number of cholic acids determined by NMR a | 5.3 | 7.2 | 9.3 | 11.5 |
The number of cholic acids was calculated based on the average integration ratio of the peaks of methyl proton 18, 19, and 21 in cholic acid at 0.66, 0.87 and 1.01 ppm and methylene proton of PEG at 3.5-3.7 ppm in 1H-NMR spectra in CDCl3. The molecule weight of the PEG for P-1 series polymers was 3000 Da.
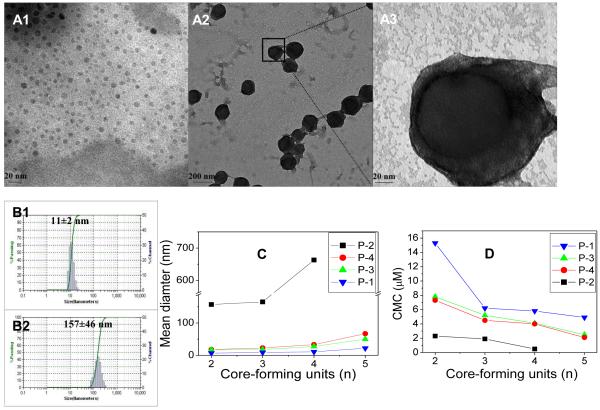
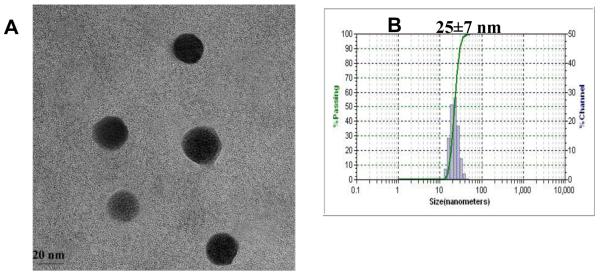
Micelle morphology was observed under a transmission electron microscopy (TEM) after staining the air-dried micelles with phosphotungstic acid (0.1 wt %). As shown in Figure 3 A, both the smaller P-1-4 (~10 nm) and the larger P-2-1 (~150 nm) micelles retained spherical shapes and size uniformity. Under higher magnification, the clear core-shell structure of P-2-1 was evident (Figure 3 A3). The dynamic light scattering (DLS) measurements showed that the mean diameters of P-1-4 and P-2-1 micelles were 11 and 157 nm, respectively (Figure 3 B1, B2). The micelle diameters measured by DLS were consistent with those observed under TEM.
Figure 3.
(A1)TEM images of blank P-1-4 micelles, (A2) blank P-2-1 micelles (low magnification), (A3) blank P-2-1 micelles (high magnification); Size and distribution of (B1) P-1-4 micelles and (B2) P-2-1 micelles, measured by dynamic light scattering (DLS); (C) Mean diameters and (D) critical micelle concentrations (CMC) values versus the number of core-forming units in the polymers. The final polymer concentrations were kept at 20 mg/mL for all DLS measurements.
We found that by varying the length of the linear PEG chains and the number of cholic acids in the hydrophobic core, one can easily tune the properties of micelles formed, such as particle size and critical micelle concentration (CMC) values. Micelle size and polydispersity were determined for P-1, P-2, P-3 and P-4 series by DLS. All micelles were observed to have homogeneous size distributions as shown in Figure 3C. The mean diameters of the micelles of P-1, P-3 and P-4 series were in a range of 7-67 nm while that of P-2 series was considerably larger, from 157 to 660 nm (Figure 3C). The particle size was found to increase slightly when the length of the PEG chain was increased from 3000 (Figure 3C P-3) to 5000 Da (Figure 3C P-4). Increasing the number of core-forming units (n=2-5) or doubling the number of cholic acid molecules attached to the polylysine backbone (Figure 3C; P-1, P-2), however, significantly increased the size of the micelles. As the number of cholic acids and core-forming units increased, the hydrophobicity of the micelle core increased, which led to a higher aggregation number of the polymers and a significant increase in the final micelle size [14]. Introducing hydrophilic spacers between the cholic acids resulted in a decrease in the hydrophobicity of the micelle core and thereby decreased the final size of the micelles (Figure 3C; P-3, P-1).
Pyrene was used as a hydrophobic fluorescent probe to determine the CMC of the PEGylated two-arm oligomer of cholic acids in the P-1, P-2, P-3 and P-4 series [21]. CMC values of these copolymers were in the range of 0.5-15.3 μM. The effect of PEG length on CMC was insignificant (Figure 3D P-3→P-4). However, when the hydrophobicity of the core-forming block was increased (i.e. increasing the number of core-forming units, n=2-5 and doubling cholic acid molecule attachments (Figure 3D P-1→P-2)), the CMC values decreased dramatically. Polymers devoid of hydrophilic spacers resulted in lower CMC values than those with spacers within the oligolysine backbone (Figure 3D P-3→P-1). This was probably due to the large increase in hydrophobicity of the core-forming block that strongly facilitates its self-assembly in water [14]. These properties (size, CMC values, and drug-loading properties) of the assemblies were well-regulated based on the numbers and configuration of how the building blocks (PEG, lysine, linker and cholic acids) are joined together, and could provide important means to optimize the micelles as effective drug carriers.
Paclitaxel loading and release
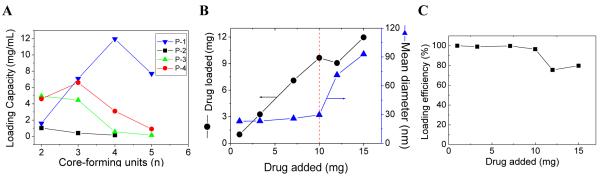
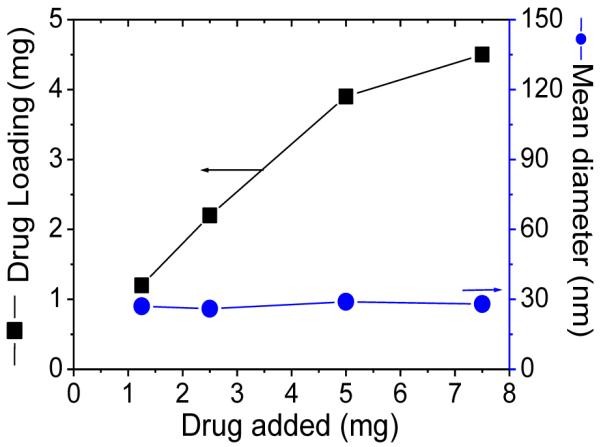
Drug loading capacity and stability are key features in determining whether a micelle system can effectively serve as a drug carrier [4]. We revealed that various anti-tumor drugs can be encapsulated into these micelles efficiently by solvent evaporation method [4]. PTX is a wide-spectrum antineoplastic agent used to treat various malignant tumors [8, 24]. Due to its very low water solubility (1 μg/mL), the clinical preparation of PTX (Taxol®) is formulated in a mixture of dehydrated alcohol and Cremophor EL, which may sometimes cause serious side effects [4, 25]. Abraxane® is a 130 nm albumin-bound particle formulation of PTX [8]. Here we demonstrated that PTX can be easily loaded into our micelles in an aqueous solution (Figure 4A). It was observed that the micelles with CMC values in a range of 4.5-7.8 μM tend to have better PTX loading capacity (≥ 4.5 mg/mL) than micelles with either lower or higher CMC values. P-1-4 micelles (CMC: 5.8 μM) had the highest PTX loading capacity at 12.0 mg/mL (Figure 4B), which was equivalent to 37.5% (w/w) of the total mass of the micelle. This indicated that the P-1-4 micelle formulation of PTX could provide a 12,000 fold increase in the water solubility of this drug. Thus, in terms of drug loading capacity, P-1-4 micelle was far superior to the two clinical PTX formulations [Taxol® (6.0 mg/mL) and Abraxane® (5.0 mg/mL)] and other conventional micelle formulations reported to have PTX loading capacities of less than 25% [26-28]. Moreover, it should be mentioned that at a PTX loading of 10 mg/mL, the loading efficiency was almost 100% and the final particle sizes still remained in the range of 25-30 nm (Figure 4B, 4C). However, beyond 10mg/mL, the particle size increased dramatically (Figure 4B). TEM and DLS measurements showed that the PTX-loaded micelles had a uniform size distribution (Figure 5 A, B).
Figure 4.
(A)The PTX loading capacity of P1, P2, P3 and P4 micelles; (B) PTX loading and diameter change of P-1-4 micelle versus the level of drug added at initial loading; and (C) PTX loading efficiency of P-1-4 micelle versus the level of drug added at initial loading. The volume of the final micelle solution was kept at 1 mL and the final concentration of the polymers at 20 mg/mL.
Figure 5.
TEM image (A) and DLS size distribution (B) of PTX-loaded P-1-4 micelles (PTX loading was 7.3 mg/mL).
Ebes molecules in P-1-4 micelles serve as spacers to facilitate the efficient packing of cholic acid molecules during the drug loading within the self-assembled complex. In comparison to P-1-4, the micelles formed by polymers in the absence of hydrophilic spacers (P-3-4, P-4-4) have relatively low PTX loading capacity (Figure 4 A). We also synthesized a linear-dendritic copolymer, comprised of a dendritic oligomer with eight cholic acids attached to a linear PEG molecule (3000 Dalton) (Figure 6A). PTX loading capacity of this polymer was much lower than those of P-1-4 and P-1-3 micelles, and this is likely attributable to the crowded configuration of cholic acid oligomer (data not shown). When we replaced cholic acid in P-1-4 with heptadecanoic acid, a linear fatty acid (Figure 6B), the PTX loading capacity of the resulting micelle dropped dramatically to 0.06 mg/mL and secondary aggregations were observed (Figure 6C). These results indicate that cholic acid plays a very important role in the stability and drug loading capacity of micelles. In the above micelles, the facial cholic acid molecules, in conjunction with appropriate hydrophilic spacers, may allows the interdigitation of the cholic acids between adjacent polymers and create a large and stable cargo space in the interior, thereby permitting efficient encapsulation of hydrophobic drugs.
Figure 6.
Schematic representation of (A) the linear-dendritic copolymer, comprised of dendritic oligomer of eight cholic acids attached to a linear PEG molecule (3000 Da); and (B) the P-1-4 polymer after replacing cholic acids with heptadecanoic acids. (C) the micelle size of (B).
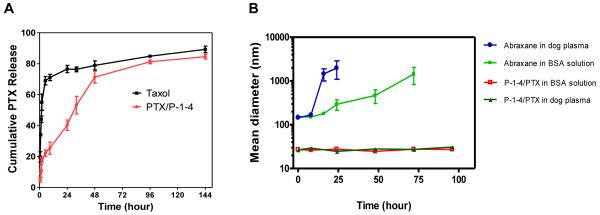
Sodium salicylate solution was used as dialysis medium to maintain an ideal sink condition for in vitro release studies of poorly soluble drugs [29, 30]. PTX release from Taxol® was rapid and about 70% of PTX was released within the first 5 h (Figure 7A). In contrast, PTX release from P-1-4 micelle was much slower and it took 48 h for the same amount of drug to be released from the nanoparticle.
Figure 7.
(A) Cumulative PTX release profile from Taxol® and P-1-4 micelles. 1 M Sodium salicylate solution was used as dialysis medium to maintain an ideal sink condition. The concentration of PTX remained in dialysis cartridge at various time points was measured by HPLC. (B) Dynamic light scattering measurement of the change in the diameters of Abraxane® (PTX loading: 5.0 mg/mL) and P-1-4 micelles loaded with 7.3 mg/mL of PTX in dog plasma 50% (v/v)) and in 45 mg/mL of BSA solution in PBS at 37 °C. Values reported are the mean diameter ± SD for duplicate samples.
Stability study of PTX-loaded micelles
The long-term stability of these micelle formulations at 4°C in PBS, BSA and dog plasma was evaluated. The drug loaded micelles were stored at 4°C in PBS for over 8 months and observed to be very stable in both size and drug content. In contrast, Abraxane® formed larger aggregates and precipitated after two days of storage in PBS (data not shown). P-1-4 micelles with a PTX loading of 7.3 mg/mL was found to retain a uniform size at around 30 nm over the 96 h incubation period in the presence of physiologically relevant concentrations of bovine serum albumin (BSA) (45 mg/mL) and in 50 % (v/v) dog plasma at 37° C (Figure 7B). In contrast, significant size variations were observed for Abraxane® under such conditions. These in vitro stability studies suggest that these PTX-loaded micelles may have a long circulation time under physiological conditions [21]. It has been reported that drug-loaded polymeric micelles generally become more unstable as drug loading increases [31]. However, the micelle formed by P-1-4 was found to be very stable in storage or under physiological conditions even at very high level of drug loading. We believe the combination of hydrophilic uncharged PEG shell and the flexible oligo-cholic acid core of the micelle lessen undesirable interactions of the nanoparticles with plasma proteins [1], and therefore ensure efficient and stable drug encapsulations under physiological condition.
In vitro Cytotoxicity study
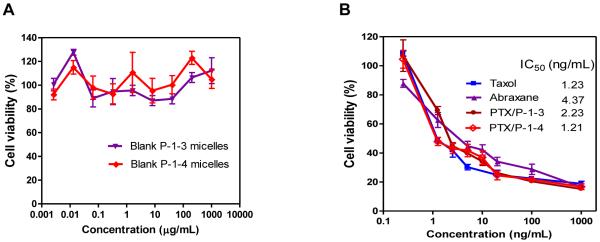
To be clinically useful, the nanocarrier itself should be non-toxic. The four building blocks of the novel polymers are all biocompatible molecules. We confirmed that the nanocarrier itself showed no observable cytotoxicity up to 1 mg/mL against SKOV-3 ovarian cancer cells by MTT assay (Figure 8A). PTX-loaded nanocarriers have comparable in vitro anti-tumor effects against SKOV-3 ovarian cancer cells as the two clinical formulations of PTX (Taxol® and Abraxane®), with IC50 values ranging from 1.21 to 4.37 ng/mL (Figure 8B).
Figure 8.
MTT assays showing the viability of SKOV-3 cells treated with (A) different concentrations of blank P-1-3 and P-1-4 micelles; and (B) Taxol®, Abraxane®, and PTX-loaded P-1-4 micelles, after 72 h incubation. The cell viability was calculated as the ratio of cell number in the treated sample divided by that in the untreated control. Values reported are the mean ± SD for triplicate samples.
Biodistribution of micelles in ovarian cancer xenograft mouse model
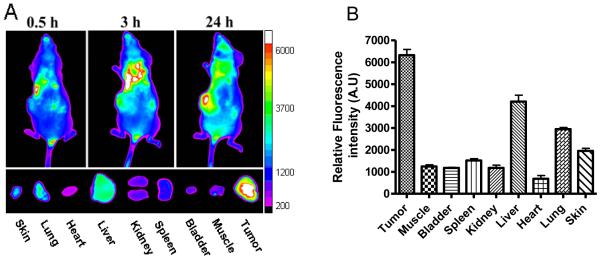
Hydrophobic fluorescent probes can be physically incorporated into micelles for in vivo tracking [32]. Hereby, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-dicarbocyanine perchlorate (DiD), a hydrophobic near infrared (NIR) fluorescent dye, was loaded together with PTX into P-1-4 micelles. The particle size of the final micelle was 30±10 nm as determined by DLS. NIR optical imaging studies were performed to evaluate the biodistribution and tumor targeting ability of the nanocarrier in mice bearing human SKOV-3 ovarian cancer xenograft. 100 μL of DiD and PTX co-loaded micelle solution was injected into mice via tail vein, and then the mice were scanned with a Kodak imaging system (IS2000MM) at different time points. The contrast of fluorescence signal was observed between tumor and background at 3 h post injection, and became more significant at 24 h (Figure 9A). Ex vivo imaging further confirmed that nanocarriers could preferentially accumulate in tumor over normal organs (Figure 9A bottom, 9B). In contrast, no obvious tumor accumulation was observed all the time in mice injected with free DiD dye (data not shown). This is probably due to the prolonged in vivo circulation time of the micelles and the size-mediated enhanced permeability and retention (EPR) effect [1]. Some recent studies showed that micelles might disaggregate upon dilution in the blood stream [33-35]. After injection, the concentration of P-1-4 micelles was estimated to be 100 μM in mouse (the total intravascular volume of a nude mouse was about 2 mL), which was well above the CMC value of P-1-4 micelles (5.8 μM). Under this condition, a large portion of the micelle should remain intact even though the telodendrimers were not cross-linked. This assertion is consistent with our observation that tumor uptake of the DiD was very high when compared to most normal organs. Nanocarrier uptake by the liver and lung was moderate, which is likely attributed to the nonspecific clearance of nanoparticles by the reticuloendothelial system (RES) [1]. The nanocarriers are expected to achieve sustained drug release once they have accumulated in the tumor, which could result in enhanced therapeutic effects.
Figure 9.
In vivo and ex vivo near infra-red (NIR) optical imaging. (A) Top: In vivo NIR optical images of SKOV-3 xenograft bearing mouse were obtained with Kodak imaging system at different time points after i.v. injection of P-1-4 micelles co-loaded with PTX and DiD; Bottom: Ex vivo NIR image of dissected organs and tumor was obtained at 24 h after injection. (B) Quantitative fluorescence intensities of tumor and organs from ex vivo images.
Anti-tumor efficacy in ovarian cancer xenograft mouse model
SKOV-3 tumor bearing mice were randomly divided into three groups (n=5) with similar tumor size in each group. Treatments were started when tumor volume reached about 100~200 mm3. Taxol® and PTX/P-1-4 micelles were administered intravenously at a PTX dose of 20 mg/kg for a total of six doses on days 0, 4, 8 12, 16 and 20. As shown in Figure 10, rapid tumor growth was observed in the control group treated with PBS. Treatment with either PTX formulations resulted in significant anti-tumor effects. However, PTX/P-1-4 micelle was found to be more efficacious than Taxol®. By day 24 after initial treatment, the median RTV was 2.65 for Taxol® while the RTV for PTX/P-1-4 micelles was 1.26. The superior therapeutic efficacy and lower toxicity of PTX/P-1-4 micelles over Taxol® in the SKOV-3 ovarian cancer xenograft mouse model could be attributed to their preferential accumulation at tumor site after i.v. injection (Figure 9) and subsequent sustained drug release profile (Figure 7). In the clinic, Taxol® is often given intravenously over 3 hours at a dose of 175 mg/m2 (approximately 4mg/kg) followed by cisplatin for previously untreated patients with ovarian cancer. The same regimen may be repeated every 3 weeks. Based on our preclinical data, we have good reason to believe that PTX/P-1-4 micelles will be clinically more efficacious but less toxic than Taxol®.
Figure 10.
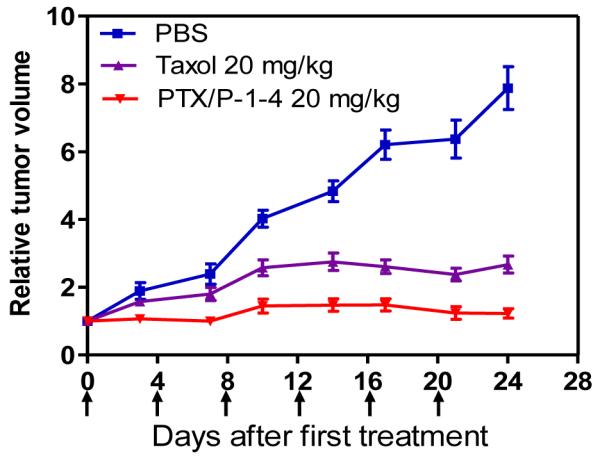
In vivo anti-tumor efficacy after intravenous treatment of different PTX formulations in the subcutaneous mouse model of SKOV-3 ovarian cancer. Tumor bearing mice were administered i.v. with PBS (control), Taxol® and PTX/P-1-4 on days 0, 4, 8,12, 16 and 20 when tumor volume reached about 100~200 mm3. Data represent mean ± SEM of five mice per group.
Etoposide and SN-38 loading
To further explore the potential of the above micelles as drug carriers, we have investigated the loading ability of these micelles for two other important but poorly soluble anticancer agents: etoposide and SN-38. These drugs can also be readily incorporated into our micelles resulting in significant increased in water solubility (Figure 11). Etoposide is an inhibitor of the enzyme topoisomerase II and used for the treatment of many cancers including lung cancer and lymphoma. Its low water solubility does pose a formulation challenge. It has been reported that PCL-PEG-PAMAM and stPCL-PEG32 polymeric micelles could achieve etoposide loading concentrations at 0.95 and 0.50 mg/mL, respectively [36, 37]. We observed P-1-4 micelles could entrap etoposide much more efficiently, reaching a drug concentration of 4.3 mg/mL in water. Interestingly, the size of micelles remained almost the same at around 30 nm with different levels of etoposide loading (Figure 11). We have also demonstrated that SN-38, which is a metabolite of irinotecan (a prodrug) and an extremely potent drug for colon cancer treatment [38], can be loaded into the micelles up to 2.3 mg/mL. The sizes were in the range of 10-100 nm for most of the micelle formulations of etoposide and SN-38 made from the linear-branched block copolymers. This size range enables these drug loaded micelles take full advantage of the EPR effect to accumulate in the tumor [4, 8]. Work is undergoing in our laboratory to evaluate the efficiency of loading many other hydrophobic drugs into our nanocarrier system.
Figure 11.
Etoposide loading and size change of P-1-4 micelle versus the level of drug added at initial loading. The volume of the final micelle solution was kept at 1 mL and the final concentration of the polymers at 20 mg/mL.
In conclusion, we have developed a highly versatile nanocarrier system for hydrophobic anticancer drugs by using novel amphiphilic linear-branched block copolymer micelles. The system has the characteristics of tunable properties (i.e., size and CMC), superior drug loading capacity and stability, targeted drug delivery and ease of site-specific functionalization. This integrated nanomedicine platform presents promising advantages for targeted drug delivery in cancer treatment. Drug-loaded nanocarriers displaying tumor specific targeting ligand are expected to exhibit the double targeting functions: “passively” via the EPR effect and “actively” via cell surface binding and endocytic uptake [39]. Covalently conjugated radionuclides or fluorescent dyes will allow direct monitoring of the biodistribution and final fate of micelle in vivo.
Supplementary Material
Acknowledgments
The authors thank the editorial assistance from Mr. David Olivos and Dr. Wiley Fowler, and financial support from NIH/NCI NCDDG U19 CA113298, R21CA128501, RO1CA115483 and China Scholarship Council (CSC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Sutton D, Nasongkla N, Blanco E, Gao JM. Functionalized micellar systems for cancer targeted drug delivery. Pharmaceutical Research. 2007;24(6):1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- [2].Torchilin VP. Micellar nanocarriers: Pharmaceutical perspectives. Pharmaceutical Research. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- [3].Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable Long-Circulating Polymeric Nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: Drug loading and release. Current Pharmaceutical Design. 2006;12(36):4685–4701. doi: 10.2174/138161206779026263. [DOI] [PubMed] [Google Scholar]
- [5].Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Letters. 2005;5(2):325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- [6].Li Y, Pan S, Zhang W, Du Z. Novel thermo-sensitive core-shell nanoparticles for targeted paclitaxel delivery. Nanotechnology. 2009;20(6):65104. doi: 10.1088/0957-4484/20/6/065104. [DOI] [PubMed] [Google Scholar]
- [7].Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nature Nanotechnology. 2008;3(3):145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- [8].Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- [9].Gao ZG, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Letters. 2002;2(9):979–982. [Google Scholar]
- [10].Florence AT. Preface - Dendrimers: a versatile targeting platform. Advanced Drug Delivery Reviews. 2005;57(15):2104–2105. [Google Scholar]
- [11].Layre AM, Gref R, Richard J, Requier D, Chacun H, Appel M, Domb AJ, Couvreur P. Nanoencapsulation of a crystalline drug. International Journal of Pharmaceutics. 2005;298(2):323–327. doi: 10.1016/j.ijpharm.2005.02.039. [DOI] [PubMed] [Google Scholar]
- [12].Zou T, Li SL, Zhang XZ, Wu XJ, Cheng SX, Zhuo RX. Synthesis and characterization of a biodegradable amphiphilic copolymer based on branched poly(epsilon-caprolactone) and poly(ethylene glycol) Journal of Polymer Science Part a-Polymer Chemistry. 2007;45(22):5256–5265. [Google Scholar]
- [13].Chang Y, Kwon YC, Lee SC, Kim C. Amphiphilic linear PEO-dendritic carbosilane block copolymers. Macromolecules. 2000;33(12):4496–4500. [Google Scholar]
- [14].Gillies ER, Jonsson TB, Frechet JMJ. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. Journal of the American Chemical Society. 2004;126(38):11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- [15].Nguyen PM, Hammond PT. Amphiphilic linear-dendritic triblock copolymers composed of poly(amidoamine) and poly(propylene oxide) and their micellar-phase and encapsulation properties. Langmuir. 2006;22(18):7825–7832. doi: 10.1021/la0607050. [DOI] [PubMed] [Google Scholar]
- [16].Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, Cheng RH, Wang L, Lam KS. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–6016. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stover TC, Kim YS, Lowe TL, Kester M. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials. 2008;29(3):359–369. doi: 10.1016/j.biomaterials.2007.09.037. [DOI] [PubMed] [Google Scholar]
- [18].Song A, Wang X, Zhang J, Marik J, Lebrilla CB, Lam KS. Synthesis of hydrophilic and flexible linkers for peptide derivatization in solid phase. Bioorg Med Chem Lett. 2004;14(1):161–165. doi: 10.1016/j.bmcl.2003.09.067. [DOI] [PubMed] [Google Scholar]
- [19].Zhao Y. Facial amphiphiles in molecular recognition: From unusual aggregates to solvophobically driven foldamers. Current Opinion in Colloid & Interface Science. 2007;12(2):92–97. [Google Scholar]
- [20].Janout V, Regen SL. Bioconjugate-Based Molecular Umbrellas. Bioconjugate Chemistry. 2009;20(2):183–192. doi: 10.1021/bc800296g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jones M, Leroux J. Polymeric micelles - a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- [22].Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, Seo MH. Iin vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001;72(1-3):191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- [23].Lu J, Shi M, Shoichet MS. Click chemistry functionalized polymeric nanoparticles target corneal epithelial cells through RGD-cell surface receptors. Bioconjug Chem. 2009;20(1):87–94. doi: 10.1021/bc8003167. [DOI] [PubMed] [Google Scholar]
- [24].Park YH, Ryoo BY, Choi SJ, Yang SH, Kim HT. A phase II study of paclitaxel plus cisplatin chemotherapy in an unfavourable group of patients with cancer of unknown primary site. Jpn J Clin Oncol. 2004;34(11):681–685. doi: 10.1093/jjco/hyh124. [DOI] [PubMed] [Google Scholar]
- [25].Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, Stoter G, Sparreboom A. Influence of Cremophor El on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res. 2002;8(4):1237–1241. [PubMed] [Google Scholar]
- [26].Le Garrec D, Gori S, Luo L, Lessard D, Smith DC, Yessine MA, Ranger M, Leroux JC. Poly(N-vinylpyrrolidone)-block-poly(D,L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. Journal of Controlled Release. 2004;99(1):83–101. doi: 10.1016/j.jconrel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- [27].Shuai XT, Merdan T, Schaper AK, Xi F, Kissel T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjugate Chemistry. 2004;15(3):441–448. doi: 10.1021/bc034113u. [DOI] [PubMed] [Google Scholar]
- [28].Wang J, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: Preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. Journal of Drug Targeting. 2005;13(1):73–80. doi: 10.1080/10611860400011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cho YW, Lee J, Lee SC, Huh KM, Park K. Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. J Control Release. 2004;97(2):249–257. doi: 10.1016/j.jconrel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [30].Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, Fang X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1-2):176–185. doi: 10.1016/j.ijpharm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- [31].Lee SC, Huh KM, Lee J, Cho YW, Galinsky RE, Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8(1):202–208. doi: 10.1021/bm060307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc Natl Acad Sci U S A. 2008;105(18):6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Savic R, Azzam T, Eisenberg A, Maysinger D. Assessment of the integrity of poly(caprolactone)-b-poly(ethylene oxide) micelles under biological conditions: a fluorogenic-based approach. Langmuir. 2006;22(8):3570–3578. doi: 10.1021/la0531998. [DOI] [PubMed] [Google Scholar]
- [34].Bae YH, Yin H. Stability issues of polymeric micelles. J Control Release. 2008;131(1):2–4. doi: 10.1016/j.jconrel.2008.06.015. [DOI] [PubMed] [Google Scholar]
- [35].Chen H, Kim S, He W, Wang H, Low PS, Park K, Cheng JX. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo forster resonance energy transfer imaging. Langmuir. 2008;24(10):5213–5217. doi: 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- [36].Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J. Synthesis and characterization of star poly(epsilon-caprolactone)-b-poly(ethylene glycol) and poly(L-lactide)-b-poly(ethylene glycol) copolymers: evaluation as drug delivery carriers. Bioconjug Chem. 2008;19(7):1423–1429. doi: 10.1021/bc7004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gaucher G, Poreba M, Ravenelle F, Leroux JC. Poly(N-vinyl-pyrrolidone)-block-poly(D,L-lactide) as polymeric emulsifier for the preparation of biodegradable nanoparticles. J Pharm Sci. 2007;96(7):1763–1775. doi: 10.1002/jps.20833. [DOI] [PubMed] [Google Scholar]
- [38].Sapra P, Zhao H, Mehlig M, Malaby J, Kraft P, Longley C, Greenberger LM, Horak ID. Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory model. Clin Cancer Res. 2008;14(6):1888–1896. doi: 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
- [39].Gao Y, Chen LL, Gu WW, Xi Y, Lin LP, Li YP. Targeted Nanoassembly Loaded with Docetaxel Improves Intracellular Drug Delivery and Efficacy in Murine Breast Cancer Model. Molecular Pharmaceutics. 2008;5(6):1044–1054. doi: 10.1021/mp800072e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.