Abstract
Venous thromboembolism (VTE) is a frequent complication of malignancy, and its incidence has increased markedly in recent years. VTE itself can directly lead to patient mortality, and is the second leading cause of death in patients with cancer. Furthermore, emerging data suggest that activation of coagulation in malignancy is integrally linked with tumor biology, particularly with angiogenesis. The development of the clinical hypercoagulable state is also linked with adverse prognosis in patients with cancer, including patients receiving systemic chemotherapy. This review focuses on the clinical evidence documenting a link between VTE and adverse short-term and long-term prognosis in patients with cancer.
Those clamorous harbingers of blood and death.
-Wm. Shakespeare, Macbeth, Act V, Sc. VI
The association between venous thromboembolism (VTE) and cancer has been known for centuries1. The most important consequence of VTE in the cancer patient is its effect on mortality. Death in cancer patients with VTE can be directly attributable to the thromboembolic event itself; just as importantly, cancer patients who develop VTE also have increased mortality that cannot be directly attributed to thrombosis. The observation of this phenomenon can be traced back to Trousseau, who presciently understood the nature of his own prognosis when self-diagnosing himself with what turned out to be cancer-associated “phlebitis”1.
The most common thrombotic events in cancer patients are deep venous thrombosis (DVT) and pulmonary embolism (PE). Arterial events including stroke, myocardial infarction and peripheral arterial embolism also occur, although the prevalence of such events is lower than for VTE2. Cancer patients on active therapy appear to have the greatest risk for VTE. Of all patients with VTE, cancer patients account for 20%, with patients on chemotherapy accounting for as much as 13% of the total burden of VTE 3,4. Newer anti-cancer drugs, in particular anti-angiogenic agents, are associated with very high rates of thombosis5–7. In a recent analysis of over 1 million hospitalized cancer patients, the rate of VTE increased by 28% from 1995 to 2003 (P < 0.0001)8. The increase was even greater among patients receiving chemotherapy, for whom the rates rose from 3.9% per hospitalization to 5.7%, an increase of 47% (P<0.0001).
Emerging preclinical data suggest that activation of coagulation promotes tumor growth and angiogenesis, lending further credence to the association of the clinical hypercoagulable state and adverse cancer prognosis 9,10. The interplay between the hemostatic system and tumor biology as well as the impact of anti-thrombotic agents on improving survival of cancer patients are outside the scope of this paper, and have recently been reviewed elsewhere11. This narrative review focuses on data from prospective and retrospective cohort studies published in the English language literature identifying the linkage between cancer-associated VTE as a direct cause of death in the cancer patient, and its adverse effect on both short-term (arbitrarily defined here as the 4-month period following diagnosis) and long-term prognosis.
VTE as a Proximate Cause of Death in the Cancer Patient
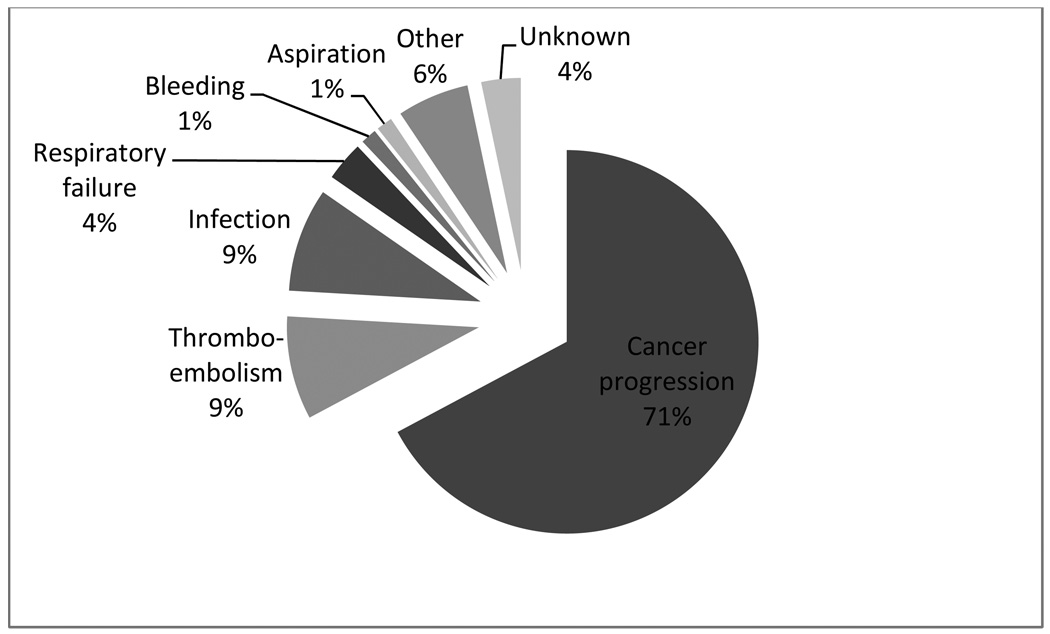
PE can directly lead to death in patients with cancer, as can arterial events. There is a lack of recent data identifying causes of death in patients with cancer. We were able to clarify this issue in a recent analysis of causes of death in cancer patients receiving chemotherapy in a prospective observational study12. Of 4,466 patients enrolled in this study, 141 (3.2%) patients died during the period of observation (median, 75 days). As might be expected, a majority of patients died of progression of underlying cancer (n=100, 70.9%) (Figure 1). Among non-cancer causes of death, thrombosis (including both venous and arterial events) was the leading contributor along with infection (n=13, 9.2% for each). The annualized death rate for VTE in this study was 448 per 100,000 patients which represented a remarkable 47-fold elevation (95% CI, 6–89, p=0.03) over the general population. The annualized death rate for arterial thrombosis in cancer patients enrolled in this study was 716 per 100,000 patients which represented a 2.7-fold elevation (95% CI 0.8–4.5, p=0.08) over the general population.
Figure 1. Thromboembolism and Outpatient Mortality.
Causes of death in 4,466 cancer patients receiving outpatient chemotherapy. Percentages may not add up to 100% due to rounding.
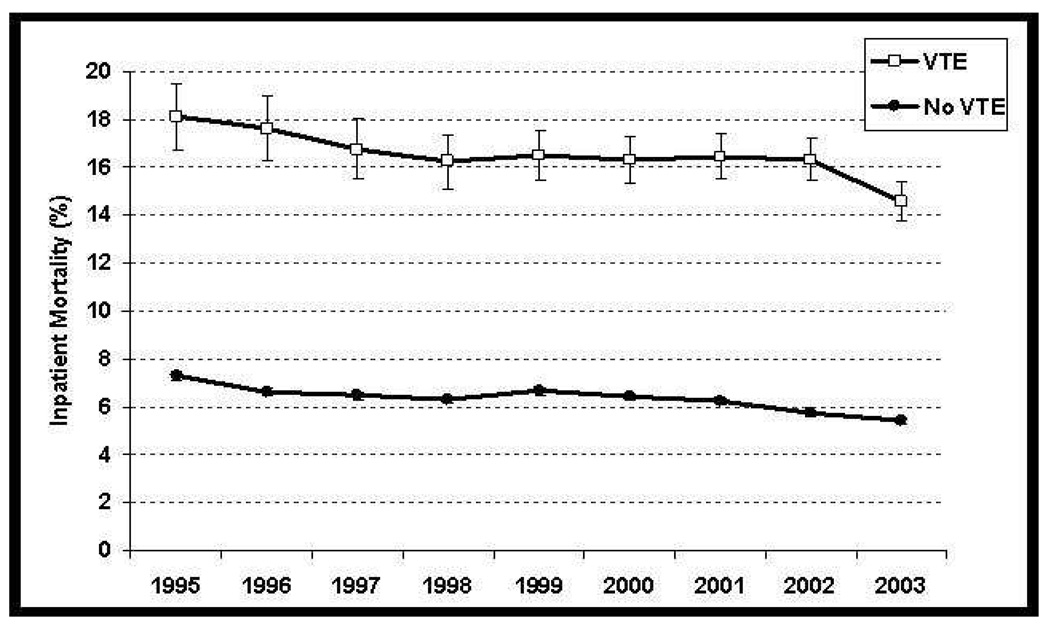
Indirect evidence of VTE leading to mortality in cancer patients is also available from analyses of hospitalized cancer patients. We recently reported an analysis of 1,824,316 hospitalizations in 1,015,598 cancer patients between 1995 and 2003 at 133 United States medical centers8. Mortality was significantly and consistently greater among patients who developed VTE as compared to patients who did not over the duration of study (16.3% versus 6.3%, P<0.0001) (Figure 2). In particular, the mortality rate was higher in patients who developed PE as compared to those who did not (24.8% versus 6.5%, P<0.0001). In a prior study of hospitalized neutropenic cancer patients, those with VTE again had a greater in-hospital mortality rate than patients without such a diagnosis (OR 2.01, 95% CI 1.83– 2.22, p<0.0001). In this study, we had access to data regarding presence or absence of metastatic disease and found a similar risk of mortality even in patients without metastatic disease2. Further indirect evidence is available from a pooled data analysis of 2,743 patients with advanced ovarian cancer enrolled in three prospectively randomized trials of platinum and paclitaxel-based chemotherapy after primary surgery13. Overall survival was significantly reduced in patients with VTE (median, 29.8 v 36.2 months; P = 0.03). In multivariate analysis, symptomatic PE, but not DVT alone, was of prognostic significance suggesting a direct impact on mortality in this postoperative setting. It is important to note that this is still largely circumstantial evidence since causes of death cannot be reliably determined in such large databases. However, these analyses adjusted for all known variables contributing to death and still found an independent association of VTE with mortality.
Figure 2. Thromboembolism And Inpatient Mortality.
Presence of venous thromboembolism was significantly associated with increased inpatient mortality (P <0.0001) over the duration of study. Error bars represent 95% confidence intervals. (From Khorana AA et al8).
VTE and Its Effect on Short-Term Prognosis
A recent prospective observational cohort study of cancer patients initiating a new chemotherapy regimen has shed light on the association of VTE and early mortality during chemotherapy14. The study evaluated 4,458 adult cancer patients with solid tumors or malignant lymphoma between 2002 and 2006 at 115 United States sites. VTE occurred in 93 (2.1%) patients at a median of 38 days following initiation of chemotherapy. One hundred and thirty-seven patients died during the period of observation (median follow-up, 75 days). In multivariate analysis after adjusting for major confounders for mortality, including ECOG performance status, Charlson comorbidity index, age, body mass index, cancer type, stage, delivered chemotherapy dose intensity, study year and practice site, the occurrence of VTE was a significant independent predictor of early all-cause mortality [HR=6.98, 95%CI: 2.83–17.21; P<.0001]. Following additional adjustment for baseline laboratory abnormalities, including elevated serum creatinine, alkaline phosphatase or decreased serum protein, VTE remained significantly associated with early mortality [HR=4.50; 95%CI: 1.61–12.53; P=.004].
We have recently developed and validated a risk assessment score predictive of the development of VTE in cancer patients15. Five predictive variables were identified: site of cancer (2 points for very high-risk site, 1 point for high-risk site), platelet count of 350 × 10(9)/L or more, hemoglobin less than 100 g/L (10 g/dL) and/or use of erythropoiesis-stimulating agents, leukocyte count more than 11 × 10(9)/L, and body mass index of 35 kg/m(2) or more (1 point each). Rates of VTE in the derivation and validation cohorts, respectively, were 0.8% and 0.3% in low-risk (score = 0), 1.8% and 2% in intermediate-risk (score = 1–2), and 7.1% and 6.7% in high-risk (score >/= 3) category over a median of 2.5 months (C-statistic = 0.7 for both cohorts). In a subsequent analysis, we evaluated mortality outcomes based on the risk scores16. VTE was again strongly associated with increased early all-cause mortality during the course of cancer chemotherapy. Death or disease progression was reported in 7%, 18% and 28% of the low, intermediate and high-risk score groups, respectively. The HR for reduced PFS among the intermediate and high-risk score groups compared to the low-risk score group were 2.77 [1.97–3.87] and 4.27 [2.90–6.27], respectively (P<0.0001). Death from all causes within 4 months of treatment initiation was reported in 1.2%, 5.9% and 12.7% patients for the low, intermediate and high-risk score groups. HR estimates for mortality among the intermediate and high-risk score groups were 3.56 [1.91–6.66] and 6.89 [3.50–13.57], respectively (P<0.0001). In multivariate analysis, the risk score and VTE occurrence were both significant independent predictors for early mortality and reduced PFS after adjusting for major prognostic factors. The association of VTE with both disease progression and mortality suggests a strong correlation with underlying tumor behavior, rather than direct mortality from VTE itself. Finally, these data suggest that the VTE can have a strong impact on short-term prognosis in cancer patients actively receiving chemotherapy.
VTE and Its Effect on Long-Term Prognosis
Data regarding the effect of VTE on long-term prognosis in patients with cancer come primarily from large population-based databases. In an analysis of the Danish Cancer Registry, 668 cancer patients with DVT were compared to 5,371 matched control cancer patients 17. In the group with cancer at the time of VTE, the one-year survival rate was 12%, as compared with 36% in the control group (P<0.001), and the mortality ratio for the entire follow-up period was 2.2 (95%CI, 2.05 to 2.40). These findings could not be explained by the type or extent of cancer, age or mortality directly attributable to VTE although patients with cancer-associated VTE did have a greater likelihood of advanced stage. In an analysis of 235,149 cancer cases in the California Cancer Registry, 3775 (1.6%) were diagnosed with VTE within 2 years18. The diagnosis of VTE was associated with an increased risk of death for all stages and cancer types, significant for all but regional and metastatic renal cancer, with a median overall relative risk of 3.7 (range of hazard ratios, 1.3–14.4). Again, the study was unable to analyze whether this worsened prognosis was due to adverse tumor biology, greater underlying comorbidity, or whether VTE was a cause of death. Similarly, in an analysis of older cancer patients in the SEER database, concomitant VTE was associated with a relative increase in the risk of death for 8 of the 10 cancer types studied; the increase in risk tended to range from 20–40% across most cancer types19.
In studies of cancers involving multiple sites, it is possible that some of the effect of VTE on mortality may be attributable to the fact that the types of cancer that are associated with VTE also have a worse prognosis e.g.,, gastrointestinal malignancies or the presence of metastatic disease. However, these criticisms are refuted by multiple studies analyzing the effect of VTE on prognosis in specific malignancies or by specific stage of cancer, including non-metastatic disease, which essentially confirm and extend the findings of the larger studies discussed above. Breast cancer is typically associated with lower rates of VTE among solid tumors. In an analysis of over 100,000 patients with breast cancer from the California Cancer Registry, 1.2% developed VTE in the 2-year period following diagnosis20. In multivariate analysis, VTE was a significant predictor of decreased 2-year survival (HR, 2.3; 95% CI, 2.1 to 2.6) and when stratified by initial cancer stage, the effect was highest in patients with localized (HR, 5.1; 95% CI, 3.6 to 7.1) or regional stage (HR, 3.5; 95% CI, 2.5 to 4.8) cancer compared with patients with metastatic disease (HR, 1.9; 95% CI, 1.5 to 2.4). In a similar analysis of over 90,000 patients with primary lung cancer, the 1-year and 2-year cumulative VTE incidences were 3.0% and 3.4%, respectively21. In multivariate analysis, after adjusting for stage and other variables, VTE was again a significant predictor of death within 2 years for both non-small cell and small cell lung cancers (HR = 2.3, 95% CI = 2.2–2.4, and HR = 1.5, 95% CI = 1.3–1.7, respectively). Amongst patients with colorectal cancers, VTE was a significant predictor of death within 1 year of cancer diagnosis among patients with localized (HR = 1.8; 95% CI, 1.4 to 2.3) or regional disease (HR = 1.5; 95% CI, 1.3 to 1.8) but not among patients with metastatic disease (HR = 1.1; 95% CI, 1.0 to 1.2)22. The association with worsened survival in patients with locoregional disease is interesting. Potential explanations include the possibility that VTE is a surrogate for a biologically more aggressive tumor not captured by routine staging; if so, this distinction would be more apparent in early-stage cohorts. However, limitations of dataset analysis (such as the absence of information regarding acute illnesses that may contribute to both VTE and death) must be kept in mind.
A similar association with survival has been reported for certain hematologic malignancies as well. In our study of high-grade non-Hodgkin’s lymphoma patients, the median survival for stage I and II patients with VTE was 1.04 years (95% CI 0.0–2.64 years), while it was significantly longer and not reached in patients without VTE (log rank test, P = 0.0073)23. In an analysis of patients with acute lymphoblastic leukemia, development of VTE was associated with a 40% increase in the risk of dying within 1 year24. However, in patients with acute myeloid leukemia no such association was observed.
It is important to note, however, that the association of VTE with cancer prognosis is multifactorial. In certain cancers, it is considered likely that tumor cells actively participate in the generation of the hypercoagulable state through procoagulants such as tissue factor (TF). It has been hypothesized, therefore, that the association of the clinical hypercogulable state is a surrogate for adverse tumor biology which in turn is responsible for the adverse association with prognosis, even in patients with early-stage disease. Supporting this hypothesis, TF overexpression and elevated levels of TF have both been associated with adverse prognostic features in patients with pancreatic and ovarian cancers25,26. In contrast, however, under certain clinical circumstancesVTE may be initiated by specific therapeutic agents. One instance of this is multiple myeloma, where rates of VTE rise markedly when thalidomide- or lenalidomide-containing regimens are used but not when these drugs are used as single agents or when other agents are used. It is interesting to note, therefore, that in the setting of myeloma where tumor biology appears less likely to play a role, the development of VTE is not associated with an adverse impact on prognosis27,28.
Conclusions
VTE is a frequent complication of the natural history of cancer and anti-cancer therapies. VTE itself may be directly responsible for death in patients with cancer; in the most recent available data, thrombosis is believed to account for 9% of cancer-related deaths. Even more importantly, the development of VTE serves as a harbinger of poor outcomes for patients with cancer. Cancer patients with VTE have an elevated risk of early mortality during chemotherapy, as well as increased risk of tumor progression and reduced long-term survival. We are just beginning to understand the linkage between the development of the clinical hypercoagulable state and tumor biology. Much work needs to be done to better elucidate the mechanisms responsible and to identify the benefit of anti-thrombotic agents in alleviating the poor prognosis associated with VTE in cancer.
Acknowledgments
Dr. Khorana is supported by grants from the National Cancer Institute K23 CA120587, the National Heart, Lung and Blood Institute 1R01HL095109-01 and the V Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Khorana AA. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost. 2003;1:2463–2465. doi: 10.1111/j.1538-7836.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 3.Caine GJ, Stonelake PS, Lip GY, et al. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 5.Cavo M, Zamagni E, Cellini C, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood. 2002;100:2272–2273. doi: 10.1182/blood-2002-06-1674. [DOI] [PubMed] [Google Scholar]
- 6.Kuenen BC, Levi M, Meijers JC, et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol. 2003;21:2192–2198. doi: 10.1200/JCO.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 8.Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 9.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem. 2000;275:1521–1524. doi: 10.1074/jbc.275.3.1521. [DOI] [PubMed] [Google Scholar]
- 10.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 11.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902–4911. doi: 10.1200/JCO.2009.22.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 13.Fotopoulou C, duBois A, Karavas AN, et al. Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel-containing first-line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. J Clin Oncol. 2008;26:2683–2689. doi: 10.1200/JCO.2008.16.1109. [DOI] [PubMed] [Google Scholar]
- 14.Kuderer NM, Francis CW, Culakova E, et al. Venous thromboembolism and all-cause mortality in cancer patients receiving chemotherapy. Journal of Clinical Oncology. 2008;26 Abstract 9521. [Google Scholar]
- 15.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuderer NM, Khorana AA, Francis CW, et al. Venous Thromboembolism Risk Model Predicts Early Progression and Overall Mortality in Cancer Patients Receiving Chemotherapy. Blood. 2008;2008 Abstract 172. [Google Scholar]
- 17.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 18.Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 19.Gross CP, Galusha DH, Krumholz HM. The impact of venous thromboembolism on risk of death or hemorrhage in older cancer patients. J Gen Intern Med. 2007;22:321–326. doi: 10.1007/s11606-006-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 21.Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6:601–608. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 22.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 23.Komrokji RSUN, Khorana AA, Lyman GH, Kaplan KL, Fisher RI, Francis CW. Venous Thromboembolic Events in Patients with Diffuse Large B Cell Non- Hodgkin's Lymphoma. Blood. 2004;104 Abstract 4542. [Google Scholar]
- 24.Ku GH, White RH, Chew HK, et al. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009;113:3911–3917. doi: 10.1182/blood-2008-08-175745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han LY, Landen CN, Jr, Kamat AA, et al. Preoperative serum tissue factor levels are an independent prognostic factor in patients with ovarian carcinoma. J Clin Oncol. 2006;24:755–761. doi: 10.1200/JCO.2005.02.9181. [DOI] [PubMed] [Google Scholar]
- 26.Nitori N, Ino Y, Nakanishi Y, et al. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2005;11:2531–2539. doi: 10.1158/1078-0432.CCR-04-0866. [DOI] [PubMed] [Google Scholar]
- 27.Zangari M, Barlogie B, Thertulien R, et al. Thalidomide and deep vein thrombosis in multiple myeloma: risk factors and effect on survival. Clin Lymphoma. 2003;4:32–35. doi: 10.3816/clm.2003.n.011. [DOI] [PubMed] [Google Scholar]
- 28.Zangari M, Barlogie B, Cavallo F, et al. Effect on survival of treatment-associated venous thromboembolism in newly diagnosed multiple myeloma patients. Blood Coagul Fibrinolysis. 2007;18:595–598. doi: 10.1097/MBC.0b013e3281067fb2. [DOI] [PubMed] [Google Scholar]