Abstract
Natural killer T (NKT) cells modulate immune responses against pathogens and tumours, as well as immunological tolerance. We show here that CYLD, a tumour suppressor with deubiquitinase function, has a pivotal and cell-intrinsic function in NKT cell development. Unlike other known NKT regulators, CYLD is dispensable for intrathymic NKT cell maturation but is obligatory for the survival of immature NKT cells. Interestingly, CYLD deficiency impairs the expression of ICOS, a costimulatory molecule required for the survival and homeostasis of NKT cells, and this molecular defect is associated with attenuated response to an NKT-survival cytokine, IL-7, due to reduced expression of IL-7 receptor. We show, for the first time, that IL-7 induces the expression of ICOS in NKT cells, which is largely dependent on CYLD. Interestingly, loss of CYLD causes constitutive NF-κB activation in developing NKT cells, which contributes to their defective IL-7 response and attenuated ICOS expression. These findings establish CYLD as a critical regulator of NKT cell development and provide molecular insights into this novel function of CYLD.
Keywords: CYLD, ICOS, IL-7, NF-κB, NKT
Introduction
Natural killer T (NKT) cells are a subset of innate immune cells that expresses a semi-invariant TCR recognizing glycolipid antigens presented by the non-polymorphic MHC class I-related protein, CD1d (Matsuda et al, 2008). In particular, the type I NKT cells, or invariant NKT cells (hereafter called NKT cells), express TCR repertoire that consists of Vα14-Jα18 (in mice) or Vα24-Jα18 (in human). A well-characterized antigen of NKT cells is α-galactosylceramide (α-GalCer), a synthetic glycolipid originally derived from a marine sponge (Kawano et al, 1997). However, more recent studies have identified NKT-specific glycolipid antigens derived from microbes and self-tissues (Matsuda et al, 2008). In response to antigen stimulation, NKT cells rapidly produce cytokines, most predominantly IL-4 and IFN-γ, thereby modulating the nature and magnitude of immune responses (Florence et al, 2008; Matsuda et al, 2008). Strong evidence suggests that NKT cells also have a function in anti-tumour immunity as well as immunological tolerance (Smyth et al, 2000; Terabe and Berzofsky, 2007; Wu and Van Kaer, 2009).
The development of NKT cells occurs in the thymus and originates from rare CD4+CD8+ double-positive (DP) thymocytes with a rearranged semi-invariant TCR. The positive selection of NKT cells is unique in that it is mediated by the DP thymocytes through recognition of self-glycolipids presented by the CD1d molecule (Bendelac, 1995). After positive selection, immature NKT cells proceed through a multi-stage maturation process, which can be defined based on their surface expression of CD44 and NK1.1 markers (Benlagha et al, 2002; Pellicci et al, 2002). The stage 1 NKT cells (CD44−NK1.1−) first develop into transitional or stage 2 NKT cells (CD44+NK1.1−), which then gain expression of NK1.1 and become mature (stage 3) NKT cells (CD44+NK1.1+). This last step of NKT cell maturation can occur in the periphery or within the thymus. A large set of signalling molecules are known to regulate the maturation process of NKT cells (Godfrey and Berzins, 2007). One such factor is NF-κB, which is required for the progression of NKT cells from NK1.1− to NK1.1+ stages (Elewaut et al, 2003; Sivakumar et al, 2003; Stanic et al, 2004). The canonical NF-kB members have a cell-intrinsic function in driving the maturation of NKT cells (Stanic et al, 2004). On the other hand, the non-canonical NF-κB member, RelB, regulates NKT cell development through its action in thymic stromal cells (Elewaut et al, 2003).
A hallmark of the NK1.1− immature NKT cells is their active proliferation and turnover, as opposed to the largely non-dividing nature of the mature CD44+NK1.1+ population (Benlagha et al, 2002; Matsuda et al, 2002). Recent studies suggest the involvement of c-Myc in mediating the proliferation of early stage NKT cells (Dose et al, 2009; Mycko et al, 2009). The unique intrathymic expansion and survival of immature NKT cells constitute an important step in NKT cell development, although the underlying signalling mechanism is poorly understood. Nevertheless, the cytokine IL-7 has a pivotal function in mediating the survival and homeostasis of developing NKT cells (Matsuda et al, 2002; Matsuda and Gapin, 2005). IL-7Rα knockout (KO) mice have drastically reduced the number of NKT cells in both the thymus and the periphery, despite their competence in NKT cell maturation (Boesteanu et al, 1997). IL-7 promotes the proliferation and survival of stage 1 and stage 2 immature NKT cells (Leite-de-Moraes et al, 2000; Gadue and Stein, 2002). Another cytokine, IL-15, also has a function in the homeostasis of NKT cells, particularly the stage 3 mature NKT population (Lodolce et al, 1998; Kennedy et al, 2000; Matsuda and Gapin, 2005).
Recent studies show that NKT cells constitutively express high levels of ICOS (Kaneda et al, 2005; Akbari et al, 2008; Chung et al, 2008), a costimulatory molecule that is typically expressed on activated T cells (Greenwald et al, 2005). Like CD28, ICOS provides a costimulatory signal to T cells on engagement by its ligand, ICOS ligand (ICOSL), and regulates the expansion and effector functions of CD4 T cells. The constitutive expression of ICOS in NKT cells is crucial for their survival, homeostasis and function (Kaneda et al, 2005; Akbari et al, 2008; Chung et al, 2008). However, despite the critical function of ICOS in NKT cell regulation, the molecular mechanism regulating the homeostatic expression of ICOS in NKT cells is largely unknown.
An emerging signalling mechanism that regulates the development and activation of immune cells is protein ubiquitination, a reversible process controlled by ubiquitinating enzymes and deubiquitinases (DUBs) (Sun, 2008). In particular, lysine 63 (K63)-linked polyubiquitin chains, which are often conjugated on signalling adaptors, function as a platform mediating the recruitment and activation of protein kinases, such as the IκB kinase (IKK), that mediates activation of the transcription factor, NF-κB (Sun and Ley, 2008; Chen and Sun, 2009). On ubiquitin-dependent activation, IKK phosphorylates a major inhibitor of NF-κB, IκBα and triggers IκBα proteolysis, leading to nuclear translocation of activated NF-κB (Sun and Ley, 2008). Recent studies have identified a DUB, CYLD, which specifically deconjugates K63-linked ubiquitin chains and negatively regulates the activation of NF-κB (Sun, 2010). As NF-κB is required for NKT cell maturation (Elewaut et al, 2003; Sivakumar et al, 2003; Stanic et al, 2004), we carried out studies to examine whether CYLD regulates the activation of NF-κB in developing NKT cells and whether CYLD deficiency causes over production of NKT cells. To our surprise, we found that the CYLD KO mice have a severe defect in NKT cell production. However, in contrast to that seen with NF-κB-deficient mice, this defect of the CYLD KO mice is not due to blockade of thymic NKT cell maturation but rather because of massive apoptosis of immature NKT cells. We showed that CYLD regulates homeostasis of immature NKT cells via a novel mechanism that involves regulation of IL-7R signalling and ICOS expression.
Results
CYLD KO mice have a drastic reduction in NKT cell numbers coupled with impaired in vivo NKT cell responses
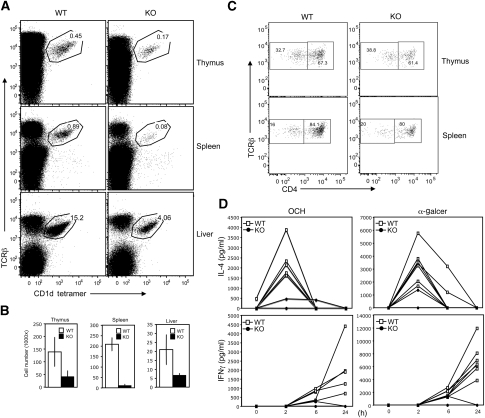
To investigate the function of CYLD in regulating the development and function of NKT cells, we examined the frequency of NKT cells in the wild-type (WT) and CYLD KO mice. The thymus of adult CYLD KO mice had a significant reduction in the frequency of NKT cells (Figure 1A). Similar or more striking NKT defect was detected in the spleen and liver (Figure 1A). The absolute NKT cell number was also drastically reduced in the different organs of CYLD KO mice, with the most profound deficiency seen in the spleen (Figure 1B; Supplementary Figure 1). The severe defect of CYLD KO mice in NKT cell development was not due to genetic background variations, as the experiments were performed using littermate controls generated through breeding the CYLD+/− heterozygous mice. Furthermore, similar results were obtained using CYLD mice that had been backcrossed to C57BL/6 background (Supplementary Figure 2). NKT cells could be divided into CD4+ and CD4−CD8− DN NKT populations. The loss of NKT cells in CYLD KO mice occurred in both of these NKT populations (Figure 1C). Thus, the CYLD deficiency causes severe loss of both CD4+ and DN NKT cells.
Figure 1.
CYLD KO mice have a defect in NKT cell development. (A) Cells were prepared from the indicated organs of CYLD KO and WT mice (6 weeks old) and subjected to flow cytometry to determine the frequency of NKT cells based on their binding to PBS57-loaded CD1d tetramer and expression of TCRβ. Data are representative of four independent experiments. (B) Absolute NKT cell numbers in individual thymuses, spleens and livers of WT and CYLD KO mice (6–8 weeks old). Data are presented as mean±s.d. of three mice. (C) CD1d tetramer positive NKT cells from WT and CYLD KO thymuses were analysed based on the expression of TCRβ and CD4. (D) Age- and sex-matched CYLD KO and wild-type (WT) mice were injected with α-GalCer (100 μg/kg body weight) (seven WT and three KO mice) or OCH (100 μg/kg body weight) (five WT and three KO mice). At the indicated times, blood was drawn and ELISA was performed to determine the concentration of IL-4 and IFN-γ in the sera. The values of individual mice were plotted (note, some of the mutant mouse curves are overlapping). Data are representative of three independent experiments.
On stimulation by antigens, NKT cells rapidly produce IL-4 and IFN-γ (Kawano et al, 1997). Injection of WT mice with α-GalCer induced rapid and transient production of IL-4 and more delayed and persistent production of IFN-γ (Figure 1D). Consistent with their great reduction in NKT cell numbers, the CYLD KO mice had a severe attenuation in α-GalCer-induced production of IL-4 and IFN-γ (Figure 1D). Similar results were obtained with a modified version of α-GalCer, OCH (Miyamoto et al, 2001) (Figure 1D). Parallel intracellular cytokine staining (ICS) assays revealed that the residual CYLD KO NKT cells were able to produce IL-4 and IFN-γ in response to OCH stimulation, albeit with moderately reduced responses (Supplementary Figure 3). Notably, loss of CYLD caused a moderate increase in the basal production of cytokines (Supplementary Figure 3). These results suggest that CYLD has a critical function in regulating the development of NKT cells, although it is largely dispensable for NKT cell activation.
A hallmark of NKT cell TCR repertoire is the involvement of an invariant α chain (Vα14-Jα281 in mice) and a restricted number of V β gene families (Porcelli et al, 1993; Dellabona et al, 1994; Lantz and Bendelac, 1994). To investigate whether the defect of CYLD KO mice in NKT cell development resides in a stage before or after TCR α chain rearrangement, we used transgenic (Tg) mice expressing the rearranged Vα14-Ja281 (Bendelac et al, 1996). The NKT cell development in these Tg mice is more efficient and also bypasses the requirement of TCR α chain rearrangement (Bendelac et al, 1996). We crossed the CYLD KO mice with Vα14Tg mice to generate CYLD KO-Vα14Tg and WT-Vα14Tg littermates. As expected, a substantially increased frequency of NKT cells was detected in the thymus and spleen of the Vα14Tg mice (Supplementary Figure 4). Importantly, the production of Tg NKT cells was also dependent on CYLD, as the CYLD KO-Vα14Tg mice had a greatly reduced frequency of NKT cells in the thymus and spleen (Supplementary Figure 4). This finding suggests that the defect of CYLD KO mice in NKT cell development resides in a stage following the TCR α chain rearrangement.
CYLD KO mice have a cell-intrinsic defect in NKT development
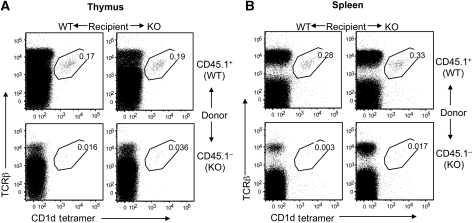
The positive selection of NKT cells is unique in that it requires antigen presentation by cortical DP thymocytes, instead of thymic stromal cells (Kronenberg and Engel, 2007). However, the thymic epithelial cells are nevertheless required for the development of NKT cells (Nakagawa et al, 1997; Elewaut et al, 2003; Sivakumar et al, 2003). To determine whether the NKT defect of CYLD KO mice was intrinsic to the NKT cell lineage or due to abnormal function of antigen-presenting DP thymocytes or thymic environment, we performed mixed radiation bone marrow chimera studies. Bone marrow cells from WT mice (CD45.1+) were mixed with those from CYLD KO mice (CD45.1−) in 1:1 ratio and adoptively transferred into lethally irradiated WT or CYLD KO recipient mice. As the WT bone marrow cells produce WT antigen-presenting cells (DP thymocytes and dendritic cells), impaired NKT cell development within the WT recipients (also possess normal stromal cells) would suggest a cell-intrinsic defect.
The WT bone marrow cells showed a similar potency for NKT cell generation in the WT and KO recipient mice, as comparable frequencies of NKT cells were detected in the thymus (Figure 2A, upper panels) and spleen (Figure 2B, upper panels) of these chimeras. This result suggested that the CYLD deficiency did not affect the overall environment for NKT cell development. Consistent with this finding, the CYLD KO bone marrow cells displayed cell-intrinsic defect in NKT cell development, as a substantially lower frequency of CYLD KO NKT cells (CD45.1−) was generated in both the WT and CYLD KO recipients (Figure 2, lower panels; Supplementary Figure 5). These results establish a cell-intrinsic function of CYLD in the development of NKT cells.
Figure 2.
CYLD has a cell-intrinsic function in regulating NKT cell development. CYLD WT mice were crossed with SJL mice for one generation to obtain the CD45.1 congenic marker. Bone marrow cells derived from the WT/SJL (WT) and CYLD KO (KO) mice were mixed (1:1 ratio) and adoptively transferred into γ-irradiated WT or CYLD KO recipient mice (two per group). After 6 weeks, flow cytometry was performed to determine the frequency of WT (CD45.1+) and KO (CD45.1−) NKT cells in the thymus (A) and spleen (B) of the recipient mice. Data are representative to two WT and two KO recipient mice. Similar results were obtained with multiple recipient mice (Supplementary Figure 5).
CYLD is dispensable for NKT cell maturation
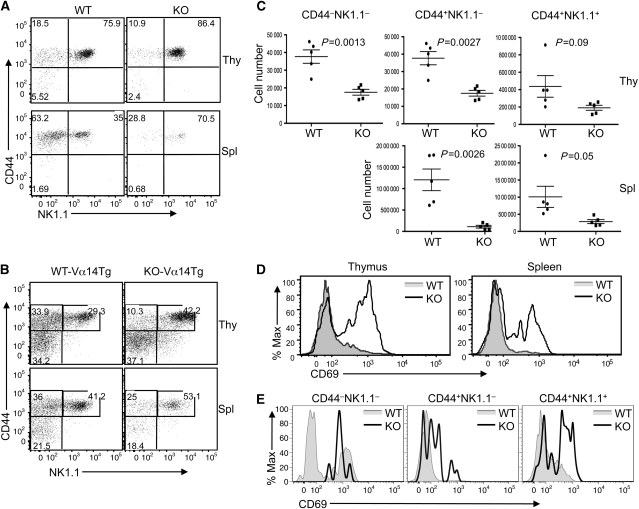
A major post-selection step of NKT cell development is thymic maturation, which is often affected in mutant mice with NKT cell deficiencies (Godfrey and Berzins, 2007). To further understand the mechanism by which CYLD regulates NKT cell development, we examined the maturation of thymic NKT cells in control and CYLD KO mice based on their surface expression of CD44 and NK1.1 markers. In adult WT mice, the majority of thymic NKT cells were mature, stage 3, cells (CD44+NK1.1+) (Figure 3A). In contrast to many other known NKT-regulatory factors, CYLD was dispensable for NKT cell maturation. Despite the reduced numbers of NKT cells in the CYLD KO thymus (Figure 1B), there was no obvious defect in the progression of NKT cells from stage 1 (CD44−NK1.1−) to stage 2 (CD44+NK1.1−) and stage 3 (CD44+NK1.1+). In fact, the frequency of the stage 3 mature NKT cells was increased in the CYLD KO thymus (Figure 3A, upper panels), as well as in the spleen (Figure 3A, lower panels) and the liver (Supplementary Figure 6). Similar results were obtained with the CYLD-Vα14Tg mice (Figure 3B). The increased percentage of mature NKT cells in the CYLD KO mice was apparently due to a dramatic reduction in the number of immature NKT (stage 1 and stage 2) cells (Figure 3C). Consequently, the absolute number of all stages of the NKT cells was reduced in the CYLD KO mice. Thus, the CYLD deficiency does not block NKT cell maturation but seems to cause severe loss of immature NKT cells.
Figure 3.
CYLD deficiency does not block NKT cell maturation and causes a hyper-activated phenotype of NKT cells. (A) Thymocytes (Thy) and splenocytes (Spl) were isolated from adult WT and CYLD KO mice (6 weeks) and subjected to flow cytometry. Gated NKT cells (TCRβ+ and CD1d tetramer+) were analysed for expression of CD44 and NK1.1. (B) Thymic and splenic NKT cells from WT-Vα14Tg and CYLD KO-Vα14Tg mice were analysed as in (A). (C) Absolute numbers of NKT cells of different maturation stages were calculated based on flow cytometry as in (A). Data are presented as mean values of five WT and five CYLD KO mice with error bars representing standard error of mean. Student's t-test was performed to determine the statistical significance. P-value <0.05 is significant. (D, E) CD69 expression was analysed by flow cytometry on gated total thymic and spleen NKT cells (D) or thymic NKT subpopulations (E) from WT and CYLD KO mice.
Loss of CYLD causes a hyper-activation phenotype of developing NKT cells
Thymic NKT cells are characterized by their activated phenotype, displaying constitutive CD69 (Yang et al, 2003). It has been unclear whether the activation of these developing NKT cells is subject to negative regulation. In WT mice, a small proportion of thymic NKT cells exhibited a CD69hi phenotype, which was not detected in the splenic NKT cells (Figure 3D). Interestingly, both the thymic and splenic NKT cells derived from CYLD KO mice expressed considerably higher levels of CD69 than the WT NKT cells (Figure 3D). This hyper-activation phenotype was detected in all three stages of thymic NKT cells derived from both CYLD KO (Figure 3E) and CYLD KO-Vα14Tg (Supplementary Figure 7) mice. These results suggest that the CYLD deficiency causes a hyper-activation phenotype of the developing NKT cells.
CYLD is critical for the survival of developing NKT cells
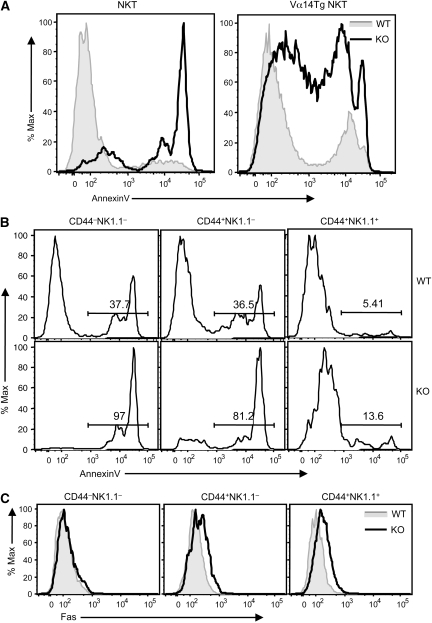
The finding that CYLD KO mice had no defect in NKT cell maturation but nevertheless displayed a severe NKT cell deficiency suggests the possibility that CYLD might regulate the survival and homeostasis of developing NKT cells. We thus examined the effect of CYLD deficiency on the survival of thymic NKT cells. Whereas the WT NKT cells contained a small proportion of apoptotic cells (annexinV positive), it is remarkable that the majority of CYLD KO NKT cells were undergoing apoptosis (Figure 4A, left panel). This result explained the drastic loss of NKT cells in CYLD KO mice. A similar result was obtained with the CYLD KO-Vα14Tg mice, although these Tg mice had a relatively larger population of healthy (annexinVlow) NKT cells than the regular CYLD KO mice (Figure 4A, right panel).
Figure 4.
Enhanced apoptosis in CYLD KO NKT cells. (A) Thymocytes derived from WT and CYLD KO mice (left panel) or WT-Vα14Tg and CYLD KO-Vα14Tg mice (right panel) were subjected to flow cytometry. Gated NKT cells were analysed for apoptosis based on AnnexinV staining. (B) Apoptosis analysis of the indicated stages of thymic NKT cells from WT or CYLD KO mice. (C) Fas expression on the indicated stages of thymic NKT cells of WT-Vα14Tg and CYLD KO-Vα14Tg mice.
We next examined the apoptosis of different stages of the developing NKT cells. Consistent with an earlier study (Benlagha et al, 2002), the stage 1 (CD44−NK1.1−) and stage 2 (CD44+NK1.1−) immature NKT cells were undergoing rapid turnover displaying a high proportion of apoptotic cells, whereas only a small proportion of apoptotic cells was detected in the CD44+NK1.1+ mature NKT population (Figure 4B, upper panels). Importantly, the CYLD deficiency greatly enhanced the level of apoptosis in stage 1 and stage 2 NKT cells (Figure 4B, lower panels). Compared with their WT counterpart, the CYLD KO mature NKT cells also displayed increased levels of apoptosis, but the overall level was considerably lower than that of the immature NKT cells (Figure 4B, lower panels). This result was consistent with the drastic loss of immature NKT cells within the thymus of CYLD KO mice (Figure 3A and C). Thus, CYLD has a critical function in mediating the survival of immature NKT cells.
As the CYLD KO NKT cells displayed a hyper-activated phenotype (Figure 3D and E), we examined the expression of Fas, a death receptor thought to mediate activation-induced apoptosis of peripheral NKT cells (Leite-de-Moraes et al, 2000). The level of Fas was moderately increased in the stage 2 and stage 3 CYLD KO NKT cells; however, the loss of CYLD did not alter the expression of Fas in the stage 1 NKT cells (Figure 4C) despite the massive apoptosis of this subpopulation in CYLD KO mice (Figure 4B). We also analysed the expression of Fas ligand (FasL) expression but did not detect appreciable differences between the WT and CYLD KO NKT cells (Supplementary Figure 8). These results suggest the involvement of additional factors that contribute to the massive apoptosis of the CYLD KO NKT cells.
CYLD regulates IL-7R expression and NKT response to IL-7 stimulation
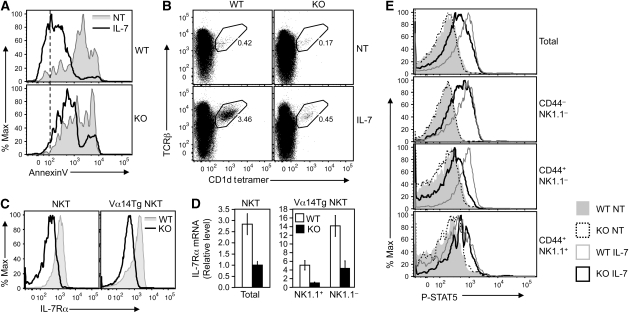
The phenotype of the CYLD KO mice in NKT cell development is reminiscent of that of the IL-7Rα KO mice, which have reduced the numbers of NKT cells without obvious defect in NKT maturation (Boesteanu et al, 1997). As IL-7 is critical for the survival and homeostasis of thymic NKT cells (Matsuda et al, 2002), we examined whether CYLD deficiency affected IL-7-mediated NKT cell survival. As expected, in vitro incubation of thymocytes caused spontaneous apoptosis of both WT and CYLD KO NKT cells (Figure 5A, shaded curve), suggesting the requirement of homeostatic factors for NKT survival. Moreover, the apoptosis of WT NKT cells was almost completely protected by IL-7 (Figure 5A, top panel). Interestingly, however, IL-7 was largely ineffective in protecting the CYLD KO NKT cells from undergoing apoptosis (Figure 5A, lower panel). Parallel thymidine incorporation studies also suggested a function for CYLD in regulating IL-7-stimulated NKT cell proliferation (Supplementary Figure 9). As an alternative way to examine the effect of IL-7 on the homeostasis of NKT cells, we performed flow cytometry to analyse the frequency of NKT cells after being cultured in the absence or presence of IL-7. IL-7 substantially increased the frequency of NKT cells in the WT thymocyte culture but not in CYLD KO thymocyte culture (Figure 5B, left panels). In addition to IL-7, IL-15 also has a function in NKT cell development, particularly the generation of stage 3 CD44+NK1.1+ cells (Lodolce et al, 1998; Kennedy et al, 2000; Matsuda and Gapin, 2005). Loss of CYLD did not appreciably affect IL-15-stimulated STAT5 phosphorylation in NKT cells and only had a minor effect on IL-15-stimulated NKT cell expansion (Supplementary Figure 10). Together, these findings emphasize an important function for CYLD in mediating the survival, and possibly proliferative, responses of NKT cells to IL-7.
Figure 5.
Regulation of IL-7Rα expression and IL-7 signalling by CYLD. (A) Thymocytes derived from WT and CYLD KO mice were enriched for NKT cells by depleting CD8+ cells by magnetic beads. The cells were incubated in vitro either without (NT) or with IL-7 (50 ng/ml) for 48 h, and apoptosis of gated NKT cells was analysed based on AnnexinV staining. (B) NKT-enriched thymocytes were incubated as in (A). After 48 h, the frequency of NKT cells was determined by flow cytometry. (C) IL-7Rα expression on gated thymic NKT cells from WT and CYLD KO mice (left panel) or WT-Vα14Tg and CYLD KO-Vα14Tg mice (right panel). (D) Real-time PCR was performed to determine the relative level of IL-7Rα mRNA in thymic total NKT cells from WT and CYLD KO mice (left panel) and thymic NKT cell subsets from WT-Vα14Tg (WT) and CYLD KO-Vα14Tg (KO) mice (right panel). Data are presented as fold relative to KO sample (left panel) or KO sample of NK1.1+ cell sample (right panel). (E) NKT-enriched thymocytes derived from WT and CYLD KO mice were stimulated with IL-7 (50 ng/ml) for 30 min at 37°C and subjected to intracellular staining of tyrosine-phosphorylated STAT5 (P-STAT5) and NKT surface markers. STAT5 phosphorylation was analysed in the gated populations of NKT cells.
To address the mechanism by which CYLD regulates IL-7-mediated NKT cell survival, we examined the effect of CYLD deficiency on the expression of IL-7R. IL-7R is composed of a specific subunit, IL-7Rα, and the common γc subunit that is shared with several other cytokine receptors (Mazzucchelli and Durum, 2007). The CYLD deficiency did not appreciably affect the expression of γc (data not shown). On the other hand, the CYLD KO NKT cells had substantially lower levels of IL-7Rα, as shown in NKT cells from both CYLD KO (Figure 5C, left panel) and CYLD KO-Vα14Tg mice (Figure 5C, right panel). The reduced IL-7Rα expression was also seen at the mRNA level (Figure 5D), suggesting a positive function for CYLD in regulating IL-7Rα gene expression. It is important to note, though, that the loss of CYLD did not completely block the expression of IL-7Ra (Supplementary Figure 11).
We next examined the function of CYLD in regulating IL-7R signalling by detecting IL-7-stimulated STAT5 tyrosine phosphorylation (Tyr-694), a primary mechanism of STAT5 activation (Shuai, 1999). IL-7 stimulation led to STAT5 phosphorylation in WT thymic NKT cells, as demonstrated by the increased flow cytometric intensity of phospho-STAT5 (p-STAT5) staining (Figure 5E). Although all of the maturation stages of WT NKT cells responded to IL-7, the immature stages (CD44−NK1.1−, CD44+NK1.1−) displayed more robust STAT5 phosphorylation. Consistent with their reduced expression of IL-7Rα and survival response to IL-7, the CYLD KO NKT cells exhibited reduced levels of IL-7-stimulated STAT5 phosphorylation, particularly in the immature stages (Figure 5E). Together, these findings suggest an important function of CYLD in regulating IL-7R expression and, thus, the response of NKT cells to IL-7-stimulated signalling.
Homeostatic expression of ICOS is dependent on CYLD and coupled with IL-7 signalling
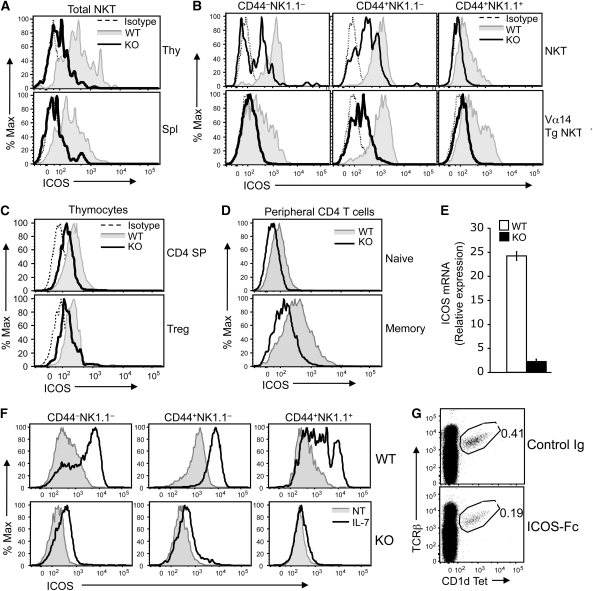
In addition to IL-7, the costimulatory molecule, ICOS, has a critical function in the survival and homeostasis of NKT cells (Akbari et al, 2008; Chung et al, 2008). Notably, a recent Systems Genetics study identified ICOS as a gene whose expression is tightly associated with that of IL-7Rα in thymocytes (Morahan et al, 2008). Because of the critical function of ICOS in NKT regulation, we examined the expression of this costimulatory molecule in the WT and CYLD KO NKT cells. Remarkably, the level of ICOS was drastically reduced in both the thymic and peripheral NKT cells of CYLD KO mice (Figure 6A). This molecular defect was detected in all three maturation stages of the CYLD KO NKT cells, with the immature stages being more prominent (Figure 6B). Moreover, the function of CYLD in regulating ICOS expression was not limited to the NKT populations, as the CYLD KO CD4 SP thymocytes and Treg cells also displayed reduced levels of ICOS expression (Figure 6C). Similarly, the CYLD deficiency also impaired the ICOS expression in peripheral naive and memory CD4 T cells (Figure 6D). CYLD did not seem to regulate the stability of ICOS protein, as incubation of the CYLD KO thymocytes with a proteasome inhibitor, MG132, did not cause an increase in the level of ICOS (data not shown). On the other hand, the CYLD deficiency greatly reduced the expression level of ICOS mRNA in NKT cells (Figure 6E), indicating a function for CYLD in regulating homeostatic expression of ICOS gene expression.
Figure 6.
Impaired ICOS expression in CYLD KO NKT cells and other T-cell subsets. (A) ICOS expression was analysed by flow cytometry on gated NKT cells from the thymus (Thy) and spleen (Spl) of WT or CYLD KO mice. The staining with isotype control Ig was included to determine the background staining level. (B) ICOS expression was analysed on the gated populations of NKT cells derived from WT and CYLD KO mice (upper panels) or WT-Vα14Tg and CYLD KO-Vα14Tg mice (lower panels). (C) ICOS expression was analysed on gated CD4+ single positive (CD4 SP) thymocytes or thymic Treg cells (CD4+CD25+Foxp3+). (D) ICOS expression was analysed on gated naive (CD44loCD62Lhi) and memory (CD44hiCD62lo) CD4+ T cells from spleen of WT and CYLD KO mice. (E) Total NKT cells were purified, by cell sorting, from the thymocytes of WT and CYLD KO mice and subjected to real-time PCR assays. The ICOS mRNA level is presented as fold relative to the KO sample. (F) Thymocytes derived from WT-Vα14Tg or CYLD KO-Vα14Tg were incubated without (NT) or with IL-7 (50 ng/ml) for 48 h. The ICOS expression on the different populations of NKT cells was analysed by flow cytometry. (G) WT mice were injected i.v. with either control Ig or ICOS-Fc (100 μg). After 48 h, the frequency of thymic NKT cells was analysed by flow cytometry.
TCR/CD28-stimulated ICOS expression in peripheral T cells involves NFATc2 (Tan et al, 2006). However, loss of CYLD seemed to specifically regulate the homeostatic expression of ICOS, as the CYLD deficiency had no effect on TCR/CD28-stimulated ICOS expression (data not shown). To date, how the homeostatic expression of ICOS is regulated is largely unknown. As IL-7 signalling has an important function in NKT homeostasis and is regulated by CYLD, we tested the possible involvement of this cytokine in the TCR-independent expression of ICOS. Interestingly, incubation of WT NKT cells in vitro with IL-7 resulted in marked increase in the expression level of ICOS in all three stages of the developing NKT cells (Figure 6F). Furthermore, the IL-7-stimulated ICOS expression was reduced in CYLD KO NKT cells. These results indicate a function for IL-7 in mediating the homeostatic expression of ICOS and partially explain how CYLD regulates both IL-7 signalling and ICOS expression. However, it is important to note that the CYLD KO NKT cells expressed a substantially lower level of ICOS compared with WT NKT cells even when they were cultured without IL-7 (Figure 6F). It is thus likely that the attenuated IL-7 signalling may only partially contribute to the impaired ICOS expression in CYLD KO NKT cells.
ICOS/ICOSL interaction is critical for thymic NKT homeostasis
Recent gene targeting studies reveal an important function for ICOS/ICOSL interaction in mediating the survival and homeostasis of NKT cells in both the periphery (Akbari et al, 2008; Chung et al, 2008) and thymus (Chung et al, 2008). To examine whether somatic disruption of the ICOS/ICOSL interaction affects the homeostasis of thymic NKT cells, we injected WT mice (i.v.) with a recombinant ICOS-Fc fusion protein (Yoshinaga et al, 1999). Remarkably, a single injection of ICOS-Fc led to marked reduction in the frequency of thymic NKT cells (Figure 6G). This effect was specific, as it was not detected in mice injected with a control Fc protein (Figure 6G). These results support the previous finding that ICOS is crucial for NKT cell homeostasis and further emphasize the significance of CYLD-mediated ICOS expression in NKT development.
Loss of CYLD causes hyper-activation of NF-κB in NKT cells, which is responsible for impaired ICOS expression and attenuated IL-7 response
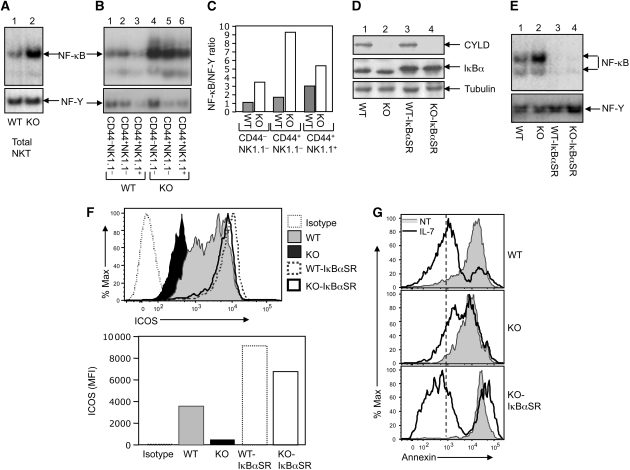
Recent work implicated CYLD as a negative regulator of NF-κB signalling, although this function is cell-type specific (Sun, 2008). We found that CYLD is expressed in all three stages of NKT cells (Supplementary Figure 12). We thus examined whether CYLD controls the activation of NF-κB in NKT cells and whether deregulated NF-κB activation contributes to the impaired ICOS expression and IL-7-mediated NKT cell survival. Whereas WT NKT cells displayed a low-basal level of NF-κB activity, the NF-κB activity was strikingly higher in CYLD KO NKT cells (Figure 7A). The aberrant NF-κB activation was detected in all three stages of the CYLD KO NKT cells (Figure 7B and C) as well as in total thymocytes (Figure 7E). Although CYLD also functions as a negative regulator of bacteria-induced NFAT activation (Koga et al, 2008), loss of CYLD in thymocytes did not cause appreciable activation of NFAT in thymocytes (Supplementary Figure 13). Thus, CYLD has a crucial function in maintaining the normal level of NF-κB in developing NKT cells and possibly other thymic T-cell populations.
Figure 7.
Hyper-activation of NF-κB is responsible for impaired ICOS expression and IL-7 response in CYLD KO NKT cells. (A) Total thymic NKT cells of WT-Vα14Tg and CYLD KO-Vα14Tg mice were purified by flow cytometric cell sorting and immediately subjected to nuclear extract preparation. EMSAs were performed using 32P-radiolabelled probes for NF-κB (upper) and a constitutive transcription factor, NF-Y (lower). (B) The NKT cells from (A) were further separated into the indicated populations by flow cytometric cell sorting and subjected to EMSA as in (A). (C) The bands in (B) were quantified and presented as ratio between NF-κB and NF-Y, using ImageJ software. (D) CYLD KO mice were crossed with IκBαSR transgenic mice to produce the indicated genotypes of littermates. Total cell lysates were prepared with thymocytes derived from the indicated mice and subjected to IB to detect the expression of CYLD and IκBα as well as the loading control tubulin. The larger size of the transgenic IκBα in the IκBαSR mice was probably due to its fusion with a FLAG tag (Boothby et al, 1997). (E) EMSA was performed using nuclear extracts of thymocytes derived from the indicated mice (age-matched). (F) Thymocytes derived from the indicated mice (age-matched) were depleted of CD8+ cells and subjected to flow cytometry to determine the expression level of ICOS on gated NKT (TCRβ+CD1d tetramer+) cells. Data are presented as a flow cytometry profile (upper) and a mean fluorescence index (MFI) bar graph (lower). (G) NKT cell enriched thymocytes from indicated mice were cultured for 48 h either in the absence (NT) or presence of IL-7 (50 ng/ml). NKT cell apoptosis was determined based on annexinV staining on the gated NKT cells.
The deregulated activation of NF-κB in CYLD KO NKT cells was consistent with the hyper-expression of the activation marker CD69 (Figure 3D and E). Furthermore, as NF-κB is required for NKT cell maturation, the deregulated NF-κB activation may explain the accelerated NKT maturation in CYLD KO mice (Figure 3A). To examine whether the deregulated NF-κB activation also contributed to the attenuated expression of ICOS and impaired IL-7R signalling, we used a genetic system involving NF-κB inhibition by an IκBα super-repressor (IκBαSR) transgene. We crossed the CYLD KO mice with a T lineage-specific IκBαSR Tg mouse (Boothby et al, 1997). The aberrant activation of NF-κB in thymocytes was completely blocked in the CYLD KO-IκBαSR mice (Figure 7E), suggesting the involvement of canonical NF-κB signalling. IκBαSR also blocked the basal NF-κB activity in WT-IκBαSR thymocytes (Figure 7E). Importantly, although inhibition of NF-κB by IκBαSR attenuated NKT cell maturation (data not shown), it completely restored the expression of ICOS in the CYLD KO NKT cells (Figure 7F). Expression of IκBαSR in the WT mice also led to a weak increase in the ICOS expression. These results, for the first time, show that NF-κB activation negatively regulates the homeostatic expression of ICOS in NKT cells. Moreover, we found that IκBαSR also rescued the defect of CYLD KO NKT cells in IL-7-stimulated survival (Figure 7G) as well as IL-7Ra expression (Supplementary Figure 14). Thus, although NF-κB is essential for the progression of immature NKT cells to mature NKT cells (Elewaut et al, 2003; Sivakumar et al, 2003; Stanic et al, 2004), the magnitude of NF-κB activation must be tightly regulated, as deregulated NF-κB activation impairs critical molecular events (ICOS expression and IL-7R response) involved in NKT cell survival and homeostasis.
Discussion
Data presented in this paper provide the first example of how negative regulation of NKT cell signalling contributes to NKT cell development. In contrast to the known NKT regulators, CYLD is dispensable for NKT cell maturation. In fact, the CYLD KO mice contain a substantially higher frequency of NK1.1+ mature NKT cells. This phenotype is associated with a hyper-activation phenotype, particularly in the immature NKT populations. However, although loss of CYLD seems to accelerate the process of NKT cell maturation, the CYLD KO mice display a severe reduction in the number of NKT cells in both the thymus and the periphery. This deficiency is due to the massive apoptosis of immature NKT cells. Thus, in contrast to its pro-apoptotic function implicated in other cell types, particularly tumour cells (Sun, 2010), CYLD has a potent anti-apoptotic function in immature NKT cells, which is crucial for NKT cell development.
The hyper-activated phenotype of CYLD KO NKT cells indicated the involvement of an activation-induced mechanism in their apoptosis induction. However, although CYLD KO NKT cells express higher levels of Fas, this is detected mainly in the stage 2 (CD44+NK1.1−) and mature (CD44+NK1.1+) NKT populations. On the other hand, the stage 1 (CD44−NK1.1−) CYLD KO NKT cells do not display a significant increase in Fas expression, despite their strikingly high level of apoptosis. Moreover, the CYLD deficiency does not alter the expression level of FasL. Thus, although Fas/FasL interaction may contribute to thymic NKT apoptosis, the massive NKT cell death in CYLD KO mice clearly involves additional mechanisms. Indeed, the survival defect of CYLD KO NKT cells was associated with attenuated response to IL-7, a cytokine that is crucial for the survival and homeostasis of immature NKT cells (Matsuda et al, 2002; Matsuda and Gapin, 2005). The CYLD KO NKT cells have reduced expression of the IL-7Rα and are hyporesponsive to IL-7-stimulated STAT5 phosphorylation and survival. These findings suggest that modulation of IL-7Rα expression and, thus, IL-7-stimulated signalling may be part of the mechanism by which CYLD regulates the survival and development of NKT cells. Future studies will examine whether overexpression of Bcl2 family of survival factors could rescue the NKT defect in CYLD KO mice.
Another potential mechanism by which CYLD regulates NKT cell development is modulating the expression of ICOS, a costimulatory molecule that is constitutively expressed in NKT cells (Kaneda et al, 2005; Akbari et al, 2008). Gene targeting studies reveal an important function of ICOS in mediating the survival and homeostasis of NKT cells in both the periphery and the thymus (Akbari et al, 2008; Chung et al, 2008). Consistently, we showed that disruption of the ICOS-ICOSL interaction by injecting mice with ICOS-Fc drastically reduces the number of thymic NKT cells. The TCR/CD28-stimulated ICOS gene expression is known to involve NFATc2 (Tan et al, 2006). However, little is known about how the homeostatic expression of ICOS is regulated. Our current study showed a pivotal function for CYLD in regulating the homeostatic, although not TCR/CD28-stimulated, ICOS expression. The expression of both ICOS protein and ICOS mRNA is reduced to near background level in the CYLD KO NKT cells. This novel function of CYLD is not limited to NKT cells, as other CYLD KO thymocyte subsets and peripheral T cells also display reduced ICOS expression. We found that IL-7 is a cytokine that stimulates the expression of ICOS. This latter finding indicates that the reduced IL-7 signalling in CYLD KO NKT cells may contribute to the impaired ICOS expression. However, it is important to note that the defect of CYLD KO NKT cells in ICOS expression seems to be more prominent than that in IL-7Rα expression. Furthermore, the CYLD KO NKT cells also express reduced levels of ICOS in the absence of IL-7. It is thus likely that CYLD-mediated ICOS regulation also involves additional mechanisms.
The activated phenotype of the CYLD-deficient NKT cells, together with the known function of CYLD in NF-κB negative regulation, prompted us to examine the activation status and function of NF-κB in CYLD KO NKT cells. We found that loss of CYLD causes hyper-activation of NF-κB in all three stages of NKT cells. Deregulated NF-κB activation is often associated with enhanced cell survival and activation. However, although the CYLD KO NKT cells display a hyper-activated phenotype, they do not have enhanced survival ability but rather undergo active apoptosis. Our data suggest that this unusual function of NF-κB involves suppression of IL-7 signalling and ICOS expression, as inhibition of NF-κB by IκBαSR rescues the defect of CYLD KO NKT cells in IL-7 response and ICOS expression. It is currently unclear how deregulated NF-κB affects the expression of ICOS and IL-7Rα. Of note, the promoter of both ICOS and IL-7Rα genes contains an atypical NF-κB-binding site (GAGGATCCTC located at −1328 of the ICOS promoter; GGGAATACC located at −200 of the IL-7Rα promoter). As the major NF-κB member deregulated in CYLD KO thymocytes is RelB (Reiley et al, 2007) and data not shown), which is known to function as both a transcriptional activator and repressor (Ruben et al, 1992; Ryseck et al, 1992; Bours et al, 1994; Marienfeld et al, 2003), future studies will examine whether RelB suppresses the expression of ICOS and IL-7Rα gene expression. Our finding that deregulated NF-κB activation contributes to the survival defect of CYLD-deficient NKT cells, together with the previous reports (Elewaut et al, 2003; Sivakumar et al, 2003; Stanic et al, 2004), suggests a complex function of NF-κB in NKT regulation. Consistent with the previous findings that NF-κB is required for NKT cell maturation, rescue of IL-7 signalling and ICOS expression by IκBαSR is insufficient to restore the NKT cell numbers (data not shown). Thus, whereas NF-κB is required for NKT cell maturation (Elewaut et al, 2003; Sivakumar et al, 2003; Stanic et al, 2004), deregulated NF-κB activation interferes with ICOS expression and IL-7 signalling and, thereby, causes detrimental effect on the survival and development of NKT cells.
We have shown earlier that CYLD regulates Lck-mediated TCR-proximal signalling and ERK activation in DP thymocytes and is important for the transition of DP thymocytes to SP thymocytes (Reiley et al, 2006). However, this signalling function of CYLD seems to be specific for DP thymocytes, as TCR-proximal signalling and ERK activation (a downstream target of Lck signalling pathway) are normal in CYLD KO peripheral T cells (Reiley et al, 2007). Similarly, loss of CYLD only slightly diminished the phosphorylation of ERK in thymic NKT cells (data not shown). Consistent with these findings, loss of CYLD does not block the maturation of NKT cells but rather causes a hyper-activation phenotype of the developing NKT cells, further suggesting an Lck-independent function of CYLD in NKT cell development. In summary, our current study establishes CYLD as a critical regulator of NKT cell development and uncovers a novel mechanism that regulates the survival and homeostasis of immature NKT cells.
Materials and methods
Mice
CYLD KO mice were generated as described (Reiley et al, 2006). Heterozygous (CYLD+/−) mice (in C57BL6/DBA mixed genetic background) were intercrossed to generate WT and CYLD KO littermates (Reiley et al, 2007). The experiments were later on also repeated using CYLD WT and KO mice backcrossed to the C57BL6 background for nine generations. Vα14Tg mice expressing the rearranged Vα14-Jα281 TCR α chain (Bendelac et al, 1996) were crossed with CYLD KO mice to generate WT-Vα14Tg and KO-Vα14Tg littermates. IκBαSR Tg mice (in C57BL6 background) express a degradation-resistant form of IκBα (IκBα super-repressor or IκBαSR) under the control of a T-cell specific proximal lck promoter (Boothby et al, 1997) and were obtained from Jackson Laboratory. WT-IκBαSR and KO-IκBαSR mice were generated by crossing CYLD KO with IκBαSR mice. B6.SJL (CD45.1+) mice were obtained from Jackson Laboratory and then crossed to CYLD+/+ (CD45.2) mice to produce CYLD+/+ (CD45.1+CD45.2+) mice. All animal experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center and Pennsylvania State University College of Medicine.
Antibodies and reagents
Functional grade anti-mCD3ɛ (145-2C11) and anti-mCD28 (37.51) antibodies were purchased from eBioscience. Fluorescence-labelled anti-mCD4 (L3T4), anti-mCD25 (PC61.5), anti-mCD62L (MEL-14), anti-CD44 (IM7), anti-mCD69 (H1.2F3), anti-mNK1.1 (PK136), anti-mTCRβ (H57-597), anti-mIL-2Rβ (TM-b1), anti-mICOS (7E.17G9), anti-mIL-7Rα (A7R34), anti-mCD45.1 (A20), anti-T-bet (eBio4B10), anti-mIL-4 (11B11), anti-mIFN-γ (XMG1.2), anti-mFoxP3 (FJK-16s), streptavidin, along with biotinylated anti-mICOS (7E.17G9) and anti-Rat IgG2b isotype control antibodies were purchased from eBioscience. Biotinylated anti-mCD132 (TUGm2), fluorescence-labelled anti-mFas (Jo2), anti-mFasL (MFL3), anti-GATA3 (L50-823) and anti-pSTAT5 (pY694) (clone 47) were from BD Biosciences. ICOS-Fc and hu-Fc control antibodies were provided by Amgen. Anti-CYLD antibody has been described (Reiley et al, 2004). Anti-tubulin (TU-02) was from Santa Cruz. α-GalCer was purchased from Alexis Biochemicals. PBS-57-loaded CD1d tetramers, conjugated to APC or PE, and OCH, an α-GalCer analog, were provided by the NIH Tetramer Core Facility. IL-7 and IL-15 were purchased from Peprotech. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were from Sigma, and monensin was from eBioscience.
Mixed bone marrow chimera
B6.SJL and CYLD+/+mice were crossed for one generation to generate CD45.1+ CYLD+/+ mice (CD45.1+CD45.2+). CYLD+/+ (CD45.1+CD45.2+) and CYLD−/− (CD45.1−CD45.2+) recipient mice were lethally irradiated with 1000 rads in cesium-137 source. At 12 h post-irradiation, CYLD+/+ and CYLD−/− recipient mice were injected intravenously with mixture containing equal proportions of CYLD+/+ (CD45.1+CD45.2+) and CYLD−/− (CD45.1−CD45.2+) T-cell–depleted bone marrow (5 × 106 cells/recipient). Anti-CD90 magnetic beads (Miltenyi) were used for T-cell depletion. Thymic and splenic reconstitution was analysed 6 weeks after injection based on CD45.1 expression (positive in CYLD+/+ cells and negative in CYLD−/− cells).
Lymphocyte preparation and NKT subpopulation purification
Thymocytes and splenocytes were prepared as described (Reiley et al, 2006). To isolate intrahepatic lymphocytes, harvested livers were processed through a metal screen (Bellco) and treated with 20 U/ml collagenase IV (Sigma) for 30 min at 37°C. After digestion, liver slurry was subjected to centrifugation (20 min, 2800 r.p.m.) over 35%/75% Percoll (GE Healthcare). Intrahepatic lymphocytes were isolated from the Percoll interphase. For isolation of total NKT or NKT subset populations, thymocytes were first depleted of CD8+ cells using either CD8 MicroBeads (Miltenyi) or Easysep CD8 positive selection kit (Stemcell). The cells were then stained with CD1d-Tetramer, anti-TCRβ, anti-CD44 and anti-NK1.1 and subjected to flow cytometric sorting. Every effort was made to keep the samples cold during the staining and sorting to prevent TCR signalling. Typical purity of isolation was >98%. For experiments requiring purification of NKT cell subsets, we used CYLD-Vα14Tg thymocytes, due to extremely low frequency of NKT cells in CYLD KO mice (typically pooled from three WT mice and six KO mice).
Real-time quantitative RT–PCR and electrophoresis mobility shift assay
Real-time RT–PCR and electrophoresis mobility shift assay (EMSA) were as described (Chang et al, 2009). The gene-specific primer sets used for real-time RT–PCR assays were: mIL-7Rα, 5′-GCAGACGCGGACCATCACTC-3′ (forward) and 5′-ATTTTTGCAAGTTAAATTCT-3′ (reverse); mICOS, 5′-TACTTCTGCAGCCTGTCCAT-3′ (forward) and 5′-CAGCAGAGCTGGGATTCATA-3′ (reverse); mT-bet, 5′-GCCAGGGAACCGCTTATATG-3′ (forward) and 5′-GACGATCATCTGGGTCACATTGT-3′ (reverse); mGAPDH, 5′-CCGGAATTCAACAGCAACTCCCACTC-3′ (forward) and 5′-CGCGGATCCAGGGTTTCTTACTCCTTG-3′ (reverse).
In vitro NKT proliferation assay
Purified NKT cells were incubated in 96-well plates with medium, IL-7 (50 ng/ml) or IL-15 (50 ng/ml) for 5 days. The cells were then pulsed with 3H-thymidine for 12 h followed by measuring the incorporated 3H activity.
In vivo NKT cell stimulation, ELISA and ICS
CYLD+/+and CYLD−/− mice were injected with 100 μg/kg body weight of α-GalCer or OCH in 200 μl of PBS via the tail vein. Serum cytokine was measured by ELISA according to the manufacturer's recommendations (eBioscience). Splenocytes were isolated 1 h post-α-GalCer/OCH injection and subjected to ICS. Briefly, cells were incubated for 2 h with 2 μM monensin, stained with anti-TCRβ and CD1d-tetramer, and then permeabilized and stained with anti-IL-4 or anti-IFN-γ (BD Biosciences).
Flow cytometry and cell sorting
Flow cytometry was performed as described (Reiley et al, 2006). For phospho-Flow experiments, the cells were either not treated or stimulated as indicated and then subjected to intracellular staining using phospho-specific antibodies. Data were acquired on FACSCanto or LSRII (BD Biosciences) and analysed using FlowJo (Treestar).
ICOS-Fc administration
CYLD+/+ mice were injected intravenously with 100 μg of ICOS-Fc or hu-Fc control in 200 μl of PBS. At 48 h post-injection, thymocytes were isolated for analysis.
Statistical analysis
Two-tailed unpaired t-test statistical analysis was performed using the Prism software. P-values <0.05 represent statistically significant difference.
Supplementary Material
Acknowledgments
We thank Amgen for providing ICOS-Fc and control human Ig proteins and NIH Tetramer Core Facility for OCH and CD1d tetramers. We also thank the personnel from the flow cytometry core facilities of MD Anderson Cancer Center (Karen Martinez David He and Amy Cortez) and the Pennsylvania State University College of Medicine (Nate Schaffer and David Stanford) for technical assistance. This study was supported by a grant from the National Institutes of Health (AI064639, AI057555 and GM084459 to SCS). AJL performed research, analysed data, prepared figures and wrote part of the manuscript; XZ, MC and JH contributed to experimental work; DZ provided the Vα14Tg mice and expertise on NKT cells. RHB supervised research of JH; and SCS supervised the overall experiments and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, DeKruyff RH (2008) ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol 180: 5448–5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A (1995) Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med 182: 2091–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Hunziker RD, Lantz O (1996) Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med 184: 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A (2002) A thymic precursor to the NK T cell lineage. Science 296: 553–555 [DOI] [PubMed] [Google Scholar]
- Boesteanu A, Silva AD, Nakajima H, Leonard WJ, Peschon JJ, Joyce S (1997) Distinct roles for signals relayed through the common cytokine receptor gamma chain and interleukin 7 receptor alpha chain in natural T cell development. J Exp Med 186: 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW (1997) Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med 185: 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V, Azarenko V, Dejardin E, Siebenlist U (1994) Human RelB (I-Rel) functions as a kappa B site-dependent transactivating member of the family of Rel-related proteins. Oncogene 1994: 1699–1702 [PubMed] [Google Scholar]
- Chang M, Lee AJ, Fitzpatrick L, Zhang M, Sun SC (2009) NF-kappa B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J Immunol 182: 3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ (2009) Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell 33: 275–286 [DOI] [PubMed] [Google Scholar]
- Chung Y, Nurieva R, Esashi E, Wang YH, Zhou D, Gapin L, Dong C (2008) A critical role of costimulation during intrathymic development of invariant NK T cells. J Immunol 180: 2276–2283 [DOI] [PubMed] [Google Scholar]
- Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A (1994) An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med 180: 1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, Gounari F (2009) Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci USA 106: 8841–8846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, Turovskaya O, Prigozy TI, Ma L, Banks TA, Lo D, Ware CF, Cheroutre H, Kronenberg M (2003) NIK-dependent RelB activation defines a unique signaling pathway for the development of V{alpha}14i NKT cells. J Exp Med 16: 1623–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence WC, Bhat RK, Joyce S (2008) CD1d-restricted glycolipid antigens: presentation principles, recognition logic and functional consequences. Expert Rev Mol Med 10: e20. [DOI] [PubMed] [Google Scholar]
- Gadue P, Stein PL (2002) NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol 169: 2397–2406 [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Berzins SP (2007) Control points in NKT-cell development. Nat Rev Immunol 7: 505–518 [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23: 515–548 [DOI] [PubMed] [Google Scholar]
- Kaneda H, Takeda K, Ota T, Kaduka Y, Akiba H, Ikarashi Y, Wakasugi H, Kronenberg M, Kinoshita K, Yagita H, Okumura K (2005) ICOS costimulates invariant NKT cell activation. Biochem Biophys Res Commun 327: 201–207 [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M (1997) CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278: 1626–1629 [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 191: 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Lim JH, Jono H, Ha UH, Xu H, Ishinaga H, Morino S, Xu X, Yan C, Kai H, Li JD (2008) Tumor suppressor cylindromatosis acts as a negative regulator for Streptococcus pneumoniae-induced NFAT signaling. J Biol Chem 283: 12546–12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Engel I (2007) On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol 19: 186–193 [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A (1994) An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med 180: 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-de-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M (2000) Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol 165: 4367–4371 [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A (1998) IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9: 669–676 [DOI] [PubMed] [Google Scholar]
- Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M (2003) RelB forms transcriptionally inactive complexes with RelA/p65. J Biol Chem 278: 19852–19860 [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L (2005) Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol 17: 122–130 [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M (2002) Homeostasis of Va14i NKT cells. Nat Immunol 3: 966–974 [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L (2008) CD1d-restricted iNKT cells, the ‘Swiss-Army knife' of the immune system. Curr Opin Immunol 20: 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli R, Durum SK (2007) Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol 7: 144–154 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T (2001) A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413: 531–534 [DOI] [PubMed] [Google Scholar]
- Morahan G, Peeva V, Mehta M, Williams R (2008) Systems genetics can provide new insights in to immune regulation and autoimmunity. J Autoimmun 31: 233–236 [DOI] [PubMed] [Google Scholar]
- Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, MacDonald HR (2009) Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol 182: 4641–4648 [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Iwabuchi K, Ogasawara K, Ato M, Kajiwara M, Nishihori H, Iwabuchi C, Ishikura H, Good RA, Onoé K (1997) Generation of NK1.1+ T cell antigen receptor alpha/beta+ thymocytes associated with intact thymic structure. Proc Natl Acad Sci USA 94: 2472–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI (2002) A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med 195: 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S, Yockey CE, Brenner MB, Balk SP (1993) Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 178: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley W, Zhang M, Sun S-C (2004) Tumor suppressor negatively regulates JNK signaling pathway downstream of TNFR members. J Biol Chem 279: 55161–55167 [DOI] [PubMed] [Google Scholar]
- Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, Leonard TO, Norbury CC, Fitzpatrick L, Zhang M, Sun SC (2007) Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med 204: 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, Sun SC (2006) Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol 7: 411–417 [DOI] [PubMed] [Google Scholar]
- Ruben SM, Klement JF, Coleman TA, Maher M, Chen C-H, Rosen CA (1992) I-Rel: a novel rel-related protein that inhibits NF-κB transcriptional activity. Genes Dev 6: 745–760 [DOI] [PubMed] [Google Scholar]
- Ryseck R-P, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, Bravo R (1992) RelB, a new rel family transcription activator that can interact with p50-NF-κB. Mol Cell Biol 12: 674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K (1999) The STAT family of proteins in cytokine signaling. Prog Biophys Mol Biol 71: 405–422 [DOI] [PubMed] [Google Scholar]
- Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F (2003) Differential requirement for Rel/nuclear factor {kappa}B family members in natural killer T cell development. J Exp Med 197: 1613–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI (2000) Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 191: 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic AK, Bezbradica JS, Park JJ, Matsuki N, Mora AL, Van Kaer L, Boothby MR, Joyce S (2004) NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol 172: 2265–2273 [DOI] [PubMed] [Google Scholar]
- Sun SC (2008) Deubiquitylation and regulation of the immune response. Nat Rev Immunol 8: 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC (2010) CYLD: a tumor suppressor deubiquitinase regulating NF-κB activation. Cell Death Differ 17: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ley SC (2008) New insights into NF-kappaB regulation and function. Trends Immunol 29: 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AH, Wong SC, Lam KP (2006) Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. J Biol Chem 281: 28666–28678 [DOI] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA (2007) NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol 28: 491–496 [DOI] [PubMed] [Google Scholar]
- Wu L, Van Kaer L (2009) Natural killer T cells and autoimmune disease. Curr Mol Med 9: 4–14 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, Yoon JW (2003) Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol 171: 5913–5920 [DOI] [PubMed] [Google Scholar]
- Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM et al. (1999) T-cell co-stimulation through B7RP-1 and ICOS. Nature 402: 827–832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.