Abstract
The indigenous bacteria create natural cohabitation niches together with mucosal Abs in the gastrointestinal (GI) tract. Here we report that opportunistic bacteria, largely Alcaligenes species, specifically inhabit host Peyer's patches (PPs) and isolated lymphoid follicles, with the associated preferential induction of antigen-specific mucosal IgA Abs in the GI tract. Alcaligenes were identified as the dominant bacteria on the interior of PPs from naïve, specific-pathogen-free but not from germ-free mice. Oral transfer of intratissue uncultured Alcaligenes into germ-free mice resulted in the presence of Alcaligenes inside the PPs of recipients. This result was further supported by the induction of antigen-specific Ab-producing cells in the mucosal (e.g., PPs) but not systemic compartment (e.g., spleen). The preferential presence of Alcaligenes inside PPs and the associated induction of intestinal secretory IgA Abs were also observed in both monkeys and humans. Localized mucosal Ab-mediated symbiotic immune responses were supported by Alcaligenes-stimulated CD11c+ dendritic cells (DCs) producing the Ab-enhancing cytokines TGF-β, B-cell-activating factor belonging to the TNF family, and IL-6 in PPs. These CD11c+ DCs did not migrate beyond the draining mesenteric lymph nodes. In the absence of antigen-specific mucosal Abs, the presence of Alcaligenes in PPs was greatly diminished. Thus, indigenous opportunistic bacteria uniquely inhabit PPs, leading to PP-DCs-initiated, local antigen-specific Ab production; this may involve the creation of an optimal symbiotic environment on the interior of the PPs.
Keywords: Alcaligenes, intratissue habitation, Peyer's patch
The intestine is most frequently exposed to a huge number and a wide variety of environmental antigens, including bacteria and food products. As a result, indigenous bacteria create appropriate homeostatic conditions for physiologic processes such as the production of vitamin K and the metabolism of indigestible dietary carbohydrates and polysaccharides (1). In addition to nutritional mutualism, microbial stimulation is required for full maturation of the host immune system, including intestinal secretory IgA (SIgA) production (2). It was demonstrated that germ-free (GF) mice have an immature mucosal immune system, including hypoplastic Peyer's patches (PPs) and diminished numbers of IgA-producing cells and CD4+ T cells (3). Both naturally occurring and acquired Abs in the intestine are of the IgA isotype. SIgA Abs recognize either T cell-independent or -dependent forms of antigens, which may limit the adherence of commensal bacteria to epithelial cells and prevent their penetration into deeper mucosal and systemic lymphoid tissues (4, 5).
Our current understanding is that commensal bacteria in the lumen and intestinal IgA together create natural cohabitation niches in the gastrointestinal (GI) tract (6). However, the nature and location of these cohabitation niches remain to be elucidated because more than 90% of the intestinal microbes have not been cultured. This limits the ability to perform detailed immunologic and bacteriologic analyses of the cohabitation mechanism between the host immune system and commensal bacteria. However, recent advances in the 16S rRNA gene clone library analysis technique have made it possible to study the composition of symbiotic bacteria in the GI tract (7, 8) and thus allow us to understand the molecular and cell biology of bilateral interactions between the mucosal immune system and the intestinal microbiota.
PPs are an example of well-characterized gut-associated lymphoid tissue and contain a wide variety of immunocompetent cells, including dendritic cells (DCs), macrophages, and B and T cells. The tissues continuously take up gut luminal antigens through M cells, including both beneficial and undesired antigens, and initiate antigen-specific immune responses in the host. The numbers of PPs range from 8 to 10 in the murine, and up to 200 in the human, small intestine (4). In a previous study of the interactions between the GI commensal bacteria and mucosal Ab production, luminal bacteria (e.g., Enterobacter cloacae) were shown to be taken up by CD11c+ DCs in the PPs (PP-DCs); this led to the development of the intestinal IgA immune system (9).
Here, we tested the hypothesis that PPs, a major inductive and regulatory site for mucosal immunity (4) and also the entry site for luminal antigens such as indigenous bacteria (9), are one of the intratissue cohabitation niches of the intestinal microbiota necessary for the development of the mucosal immune system. This intratissue colonization may create a state of symbiosis with instructive environmental antigens on the interior of the PPs.
Results
Presence of Indigenous Opportunistic Bacteria on the Interior of PPs.
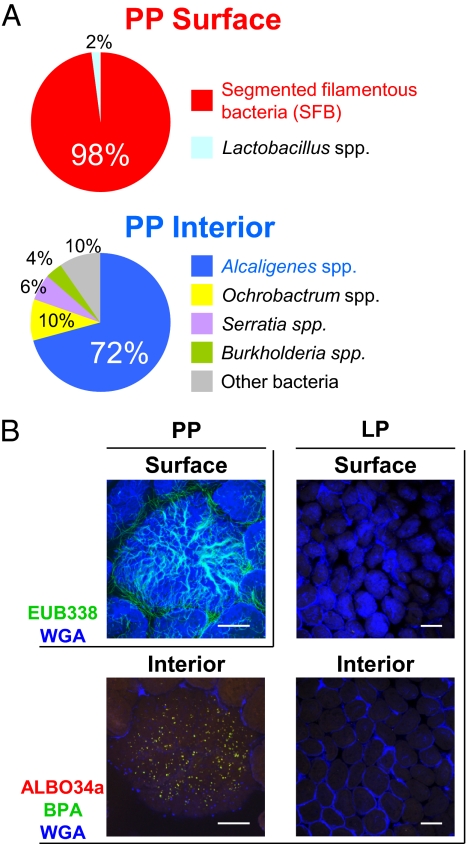
To determine the bacterial composition at the surface and on the interior of PPs in naïve, specific-pathogen-free (SPF) mice, we first used the 16S rRNA gene clone library method. Consistent with a previous report (10), segmented filamentous bacteria were the predominant species detected on the surface of the follicle-associated epithelium covering PPs (Fig. 1A). In contrast, several species of indigenous microbiota, including Alcaligenes spp., Ochrobactrum spp., Serratia spp., and Burkholderia spp., were detected on the interior of PPs. Of these, Alcaligenes, which are opportunistic bacteria (11), were dominant (72%; Fig. 1A).
Fig. 1.
Microbial distribution in the GI immune compartment. (A) Microbial composition at the surface and on the interior of PPs was examined by 16S rRNA gene clone library analysis. (B) The presence of Alcaligenes was visually analyzed by whole-mount FISH at the surface and on the interior of PPs and LP. Data are representative of five independent experiments. [Scale bars, 100 μm (PP), 150 μm (LP).]
To confirm the presence and localization of Alcaligenes on the interior of PPs, we next performed a whole-mount FISH analysis to identify the bacterial distribution in this tissue (12). The microbial cells were visualized by three distinct probes used in several previous studies (12–14) (Table S1). EUB338 is routinely used for detecting bacterial species in an indiscriminate manner (12). ALBO34a is a specific probe for Alcaligenes and Bordetella (13), and BPA is for Alcaligenes, Burkholderia, and Comamonas (14). Thus, Alcaligenes are identified as ALBO34a and BPA double-positive cells.
Consistent with the 16S rRNA analysis (Fig. 1A), EUB338-positive bacteria morphologically similar to segmented filamentous bacteria were observed over the entire surface area of PPs covered by wheat germ agglutinin positive (WGA+) epithelial cells (Fig. 1B). ALBO34a and BPA double-positive Alcaligenes were detected on the interior of PPs, where WGA+ epithelial cells were not observed (Fig. 1B). Sequential analysis through the z axis convincingly showed that Alcaligenes were present on the interior of PPs (Movie S1). We also confirmed the presence of Alcaligenes by the PCR method in a separate study using the 16S rRNA-gene-targeted group-specific PCR primers for Alcaligenes.
In contrast to the preferential localization of Alcaligenes in PPs, this species was essentially absent in the diffuse lamina propria (LP) region of the small intestine (Fig. 1B), whereas EUB338-positive bacteria were scattered throughout the surface layer of the LP (Fig. S1A). Thus, although some antigen-sampling cells [e.g., villous M cells (15) and epithelial DCs (16)] are located in the epithelium covering the more diffuse LP region, it seems that antigen-sampling M cells and DCs in the follicle-associated epithelium of PPs are responsible for the entry of Alcaligenes. Furthermore, the presence of Alcaligenes inside PPs was demonstrated to be a common feature by the characterization of different species of mice housed in various SPF-maintained experimental animal facilities (Fig. S1B). These findings suggest a possibility that commensal bacteria live within the tissues of the organized lymphoid structures associated with the GI tract.
Alcaligenes-Ingested PP-DCs Migrate into Mesenteric Lymph Nodes but not Spleen.
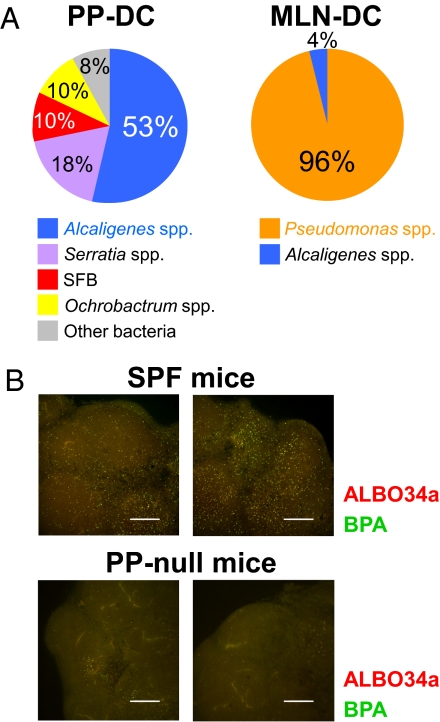
We next investigated the fate of Alcaligenes inhabiting PPs, and particularly their interactions with mucosal immunocompetent cells. When the microbial populations within DCs purified from different tissues were characterized by the 16S rRNA analysis, Alcaligenes were detected within PP-DCs and mesenteric lymph node (MLN) DCs (Fig. 2A) but not splenic DCs (Fig. S2). Our findings support the presence of a restricted PP-MLN axis for migration of DCs that have taken up indigenous microbiota and suggest that MLNs act as reinforcement to help prevent intrusions by indigenous microbiota into the systemic compartment (17). By using FISH analysis, we also found substantial numbers of Alcaligenes in the MLNs of SPF mice (Fig. 2B).
Fig. 2.
PP-MLN migration axis for Alcaligenes-ingested GI tract DCs. (A) CD11c+ DCs were isolated from the PPs and MLNs. Bacterial composition was determined by 16S rRNA gene clone library analysis. (B) Whole-mount FISH was performed to detect Alcaligenes (yellow) in the MLNs of PP-intact and PP-null mice. The confocal images were sequentially captured at 20-μm intervals along the z axis. Data are representative of five independent experiments. (Scale bars, 300 μm.)
To investigate whether PP-DCs are the main source of MLN-DCs harboring Alcaligenes, PP-null mice were generated by in utero treatment with an anti-IL-7 receptor α chain mAb (18). In PP-null mice, negligible numbers of Alcaligenes were detected in their MLNs (Fig. 2B); these bacteria presumably originated from isolated lymphoid follicles (ILFs) (Fig. S1C and Movie S2), which resemble PPs and still develop in PP-null mice (19). This result was identical to previous reports showing that PPs are the major sites for uptake of orally inoculated bacteria and the subsequent induction of host immune responses (e.g., Salmonella typhimurium and Helicobacter pylori) (20, 21).
Preferential Induction of Alcaligenes-Specific Mucosal Ab Responses for the Establishment of Symbiosis.
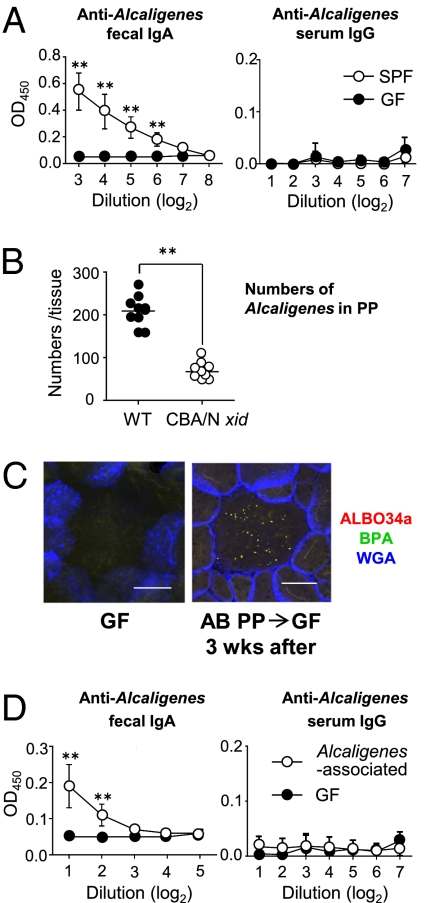
To elucidate whether the intratissue presence of Alcaligenes and their uptake by PP-DCs affect intestinal mucosal Ab responses, we next examined IgA Ab responses to Alcaligenes because IgA is the major isotype of mucosal Abs (4). We used Alcaligenes faecalis subsp. faecalis NBRC (National Institute of Technology and Evaluation Biological Resource Center) 13111T, which was the predominant species in the PPs (Fig. S3A), for the analysis of antigen-specific immune responses. Substantial amounts of Alcaligenes-specific IgA Abs were detected in the feces of SPF mice, whereas GF mice failed to produce this isotype of antigen-specific Abs (Fig. 3A, Left). No serum IgG Abs specific for Alcaligenes were seen in either SPF or GF mice (Fig. 3A, Right). This result reflected the localization of Alcaligenes in PPs, a major mucosal Ab-inductive lymphoid tissue, and not spleen, where systemic IgG Ab responses predominate (Fig. 1 and Fig. S2).
Fig. 3.
Preferential induction of Alcaligenes-specific mucosal Ab responses in the PPs. (A) Alcaligenes-specific fecal IgA and serum IgG Ab responses were determined by ELISA. Data are means ± SD (n = 4). (B) The numbers of Alcaligenes inside PPs were counted in 10 randomly chosen PPs of CBA/N xid and WT mice. Data are representative of three independent experiments. Horizontal bar indicates the mean. (C) Bacterial distribution on the interior of PPs of GF mice. AB, antibiotic-treated mice. Data are representative of three independent experiments. (Scale bars, 100 μm.) (D) Alcaligenes-specific fecal IgA and serum IgG Ab responses in the Alcaligenes-associated ex-GF mice were measured by ELISA. Data are means ± SD (n = 6). **P < 0.01.
In agreement with this finding, an enzyme-linked immunospot (ELISPOT) assay showed that naïve, SPF mice possessed Alcaligenes-specific IgA Ab-forming cells (AFCs) in their intestinal compartments, including PPs and the LP region, but not in the spleen (Table 1). Additionally, no Alcaligenes-specific IgG-AFCs were seen in MLNs or spleen (Table 1). Alcaligenes-specific IgA-AFCs were more commonly observed in the PPs than in the LP region: more than 2% of IgA-AFCs in the PPs were reactive to Alcaligenes, whereas only approximately 0.5% of IgA-AFCs in the LP were specific for Alcaligenes (Table 1). This tissue-specific pattern of Alcaligenes-specific IgA-AFCs was further confirmed by FACS analysis using GFP-Alcaligenes (Fig. S3B): 5.3% of IgA-positive B cells (including 2.3% of IgA plasmablasts) were specific for Alcaligenes in the PPs, whereas only 1.1% of IgA-positive B cells in the LP were specific for this bacterium (Fig. S3B). In addition, when we examined LP-homing properties of local IgA class-switched (or IgA committed) B cells in PPs, Alcaligenes-specific IgA+ B cells expressed fewer gut-homing receptors (α4β7, CCR9, and CCR10) than the rest of the PP-IgA+ B cells (Fig. S3C). Therefore, Alcaligenes-specific IgA-committed B cells most likely remained in PPs, which accounted for the presence of elevated Alcaligenes-specific IgA-AFCs in PPs compared with LP.
Table 1.
Induction of Alcaligenes-specific and total AFCs in Alcaligenes-associated ex-GF mice
| SPF mice | Alcaligenes-associated mice | |||||
| Variable | A (Anti-Alcaligenes) | B (Total) | A/B × 100 (%) | A (Anti-Alcaligenes) | B (Total) | A/B × 100 (%) |
| IgA-AFCs/105 lymphocytes | ||||||
| PP | 28 ± 15 | 1,304 ± 364 | 2.10 ± 0.83 | 10 ± 5 | 625 ± 307 | 1.68 ± 0.46 |
| LP | 52 ± 12 | 9,750 ± 3,350 | 0.57 ± 0.19 | 12 ± 9 | 3,133 ± 1,087 | 0.32 ± 0.20 |
| MLN | 2 ± 1 | 221 ± 64 | 0.63 ± 0.51 | 0 | 20 ± 6 | 0 |
| Spleen | 0 | 36 ± 8 | 0 | 0 | 15 ± 5 | 0 |
| IgG-AFCs/105 lymphocytes | ||||||
| MLN | 0 | 13 ± 7 | 0 | 0 | 10 ± 5 | 0 |
| Spleen | 0 | 15 ± 8 | 0 | 1 ± 1 | 40 ± 18 | 0.77 ± 1.72 |
Alcaligenes-specific and total AFCs in SPF and the Alcaligenes-associated ex-GF mice were enumerated by ELISPOT assay. Data are expressed as means ± SD (n = 6, respectively).
Some intestinal IgA Abs are derived from B1 B cells and recognize T cell-independent antigens commonly expressed by commensal bacteria. Thus, it is possible that Alcaligenes-specific IgA Abs show some cross-reactivity with other commensal bacteria. We tested this possibility by FACS analysis and found that Alcaligenes-specific Abs did not cross-react with other bacteria (e.g., Escherichia coli; Fig. S4A). This view was further supported by the analysis of Alcaligenes-specific IgA mAb (#3E-12A-6D-3G) developed by fusion of B cells from the PPs of SPF mice. This mAb did not cross-react with E. coli. In addition, impaired intestinal IgA Ab responses to Alcaligenes were noted in TCRβ−/− δ−/− mice (Fig. S4B). These data suggest that Alcaligenes-specific IgA Abs are mostly derived from B2 B cells producing T cell-dependent, antigen-specific Abs. This agrees with the evidence that PPs are major sites for the induction of intestinal mucosal Ab responses to T cell-dependent microbial antigens regardless of whether the microbes are commensal or pathogenic (4).
Although PPs are thought to play a major role in the induction of IgA-committed B cells and plasmablasts, but not plasma cells (4), these data suggest that a large part of Alcaligenes-specific fecal IgA Abs are derived from PP IgA-producing cells in a T cell-dependent manner. In fact, markedly decreased levels of anti-Alcaligenes fecal IgA Abs were seen in PP-null mice (Fig. S4C). These findings are in agreement with previous reports demonstrating that PP-DCs are involved not only in the class-switching of IgM+ B cells to IgA+ ones and the determination of gut-tropism via retinoic acid synthesis (22, 23), but also in regulating IgA secretion in the PPs through the stimulation signal provided by the Ab-enhancing cytokine IL-6 (24). We examined IL-6 production by PP cells from GF mice after treatment with Alcaligenes and found that Alcaligenes induced mainly PP-DCs to produce substantial levels of IL-6 (Fig. S5A). When PP-DCs were isolated from WT mice and cocultured with Alcaligenes, the synthesis of the IgA isotype-switching cytokines TGF-β and B-cell-activating factor belonging to the TNF family (BAFF) were also elevated in addition to IgA-enhancing cytokine IL-6 (Fig. S5B).
Taken together, these findings suggest that mucosal Abs, including locally produced, antigen-specific IgA Abs, may play a critical role in the intratissue cohabitation of Alcaligenes in PPs. Supporting this view, Alcaligenes numbers were much lower in the PPs of CBA/N xid mice, which exhibit a B cell defect, than in WT mice (Fig. 3B and Fig. S6A). Further, Alcaligenes levels tended to be lower also in PPs of IgA-deficient mice, although no statistically significant differences were observed (Fig. S6B). Because the IgA-deficient condition did not lead to the complete removal of PP intratissue Alcaligenesis, it is also possible that Alcaligenes-specific IgA Abs may not be fully involved in the presence of Alcaligenes in PPs. Alternatively, this lack of significant differences may offer another explanation due to the compensation of IgA function by IgM Abs in deficient mice because the numbers of anti-Alcaligenes IgM-AFCs was much increased in IgA-deficient mice when compared with WT mice (Fig. S6C).
Ability of Alcaligenes to Colonize the Interior of PPs.
Intratissue cohabitation of Alcaligenes in PPs should be addressed formally and directly by the establishment of a gnotobiotic mouse model monoassociated with Alcaligenes. The current technology, however, does not permit the isolation and culture of Alcaligenes from PPs. Previous studies have shown that Alcaligenes have the distinctive feature of being resistant to multiple antibiotics (25, 26), suggesting to us a unique strategy to directly assess the presence of intratissue Alcaligenes in PPs. By isolating PPs from antibiotic-treated mice under sterile conditions for the preparation of homogenized tissue and its subsequent oral administration to GF mice, we were able to establish PP-derived, Alcaligenes-associated mice. When we examined the antibiotic-treated mice, no bacteria were seen at the intestinal epithelial surface (including the follicle-associated epithelium), whereas Alcaligenes were present inside PPs (Fig. S7A). Three weeks after oral inoculation, Alcaligenes were again noted on the interior of PPs of ex-GF mice (Fig. 3C). The colonization of Alcaligenes in the PPs of ex-GF mice was further supported by the presence of antigen-specific fecal SIgA but not serum IgG Abs (Fig. 3D). A significant increase in antigen-specific IgA- but not IgG-AFCs was also observed in these mice (Table 1). Furthermore, the levels of total IgA were partially increased in the Alcaligenes-associated mice (Fig. S7B). When we examined PPs of GF mice, the numbers of total IgA-AFCs were 143 ± 45 per 105 lymphocytes. On the other hand, the numbers of total IgA-AFCs in PPs isolated from both SPF and the monoassociated mice were 1,304 ± 364 and 625 ± 307, respectively (Table 1). A similar tendency was also seen when total IgA levels were examined in fecal samples taken from monoassociated, GF, and SPF mice (Fig. S7B). These findings further suggest that the intratissue habitation of Alcaligenes in the PPs may contribute to not only the induction of Alcaligenes-specific IgA but also the development of at least a portion of mucosal IgA-associated humoral immunity.
Alcaligenes Were Present on the Interior of Monkey and Human PPs.
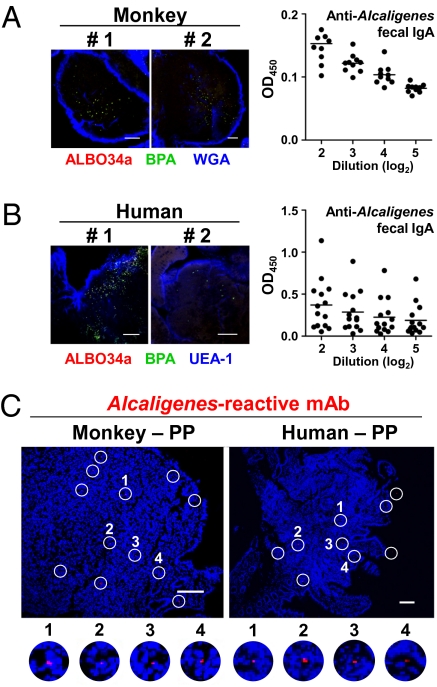
On the basis of the findings demonstrated by a variety of mouse experiments as described above, we next examined the presence of Alcaligenes inside PPs of higher mammals, namely nonhuman primates and humans. This bacterium was observed on the interior of monkey PPs by FISH analysis (Fig. 4A, Left), and anti-Alcaligenes IgA Abs were also detected in the feces of these monkeys (Fig. 4A, Right). To further demonstrate the intratissue habitation of Alcaligenes in monkey PPs, an Alcaligenes-specific mAb (#11E-8C-7A, IgM isotype) was developed. Immunohistochemical analysis with Alcaligenes-specific mAb #11E-8C-7A showed the presence of this bacterium on the interior of primate PPs (Fig. 4C, Left). When human PPs were obtained from noninflamed sites of healthy patients who underwent endoscopic biopsy, the intratissue habitation of Alcaligenes was demonstrated inside human PPs by FISH analysis (Fig. 4B, Left). In addition, anti-Alcaligenes fecal IgA Abs were also detected in human fecal samples (Fig. 4B, Right), consistent with the murine and nonhuman primate studies (Fig. 3A, Left and Fig. 4A, Right), The intratissue habitation of Alcaligenes in human PPs was further confirmed by the use of Alcaligenes-specifc mAb #11E-8C-7A (Fig. 4C, Right).
Fig. 4.
Intratissue habitation of Alcaligenes inside nonhuman primate and human PPs. (A and B) Alcaligenes were detected on the interior of monkey and human PPs by whole-mount FISH (Left). Alcaligenes-specific fecal IgA Ab responses in monkeys and human were examined by ELISA [Right; n = 10 (A), n = 14 (B)]. Horizontal bar indicates the mean. (Scale bars, 100 μm.) (C) Immunohistochemical analysis was conducted in monkey and human PPs with Alcaligenes-reactive #11E-8C-7A mAb and phycoerythrin-labeled anti-mouse IgM Ab. Open circles indicate the presence of Alcaligenes. (Scale bars, 100 μm.)
Discussion
The present study has revealed a unique aspect of intestinal symbiosis between the host immune system and its indigenous microbiota. In this system some opportunistic bacteria, such as Alcaligenes, exploit organized murine mucosal inductive tissues (PPs and ILFs) as their tissue-interior cohabitation niches in vivo. The intratissue habitation of Alcaligenes was further demonstrated by the analysis of PPs from nonhuman primates and humans. Recently, the microbial composition of mucosa-associated lymphoid tissue (MALT) lymphomas was analyzed by the use of a 16S rRNA method and revealed that Alcaligenes were highly detected in those lymphoma tissues (27). This finding also suggests the likelihood that Alcaligenes ordinarily inhabit the human mucosal compartment and that the dysregulation of this mutualism in the organized MALT of the host GI tract may contribute to the development of the MALT lymphoma.
The origin of Alcaligenes involved in this intratissue colonization remains unknown. Alcaligenes are widely present in soil, fresh water, sewage, marine systems, human clinical materials, and the feces of healthy people (11). In this study we attempted to isolate and culture this unique bacterium from PPs of naïve SPF mice, but we unfortunately have not yet developed suitable culture conditions. However, we did confirm that Alcaligenes faecalis NBRC 13111T never entered the PPs after oral inoculation. This may be because Alcaligenes can change their morphology, which includes rod-shaped (0.8–1 × 1–2 μm) and coccoid (0.2–1 μm) forms (11). Similarly, H. pylori exhibits a coccoid form in the specific environment of the small intestine, which is essential for its selective uptake by PPs and the subsequent induction of antigen-specific and pathogenic CD4+ T cells that cause gastritis (21). Thus, it is possible that a specific form, presumably the coccoid form, of Alcaligenes is a prerequisite for its effective transfer into PPs and subsequent establishment of the intratissue cohabitation in the PPs. Supporting this prediction, we detected morphologically small, or presumably coccoid forms of Alcaligenes on the surface of the PP (Fig. S8).
An additional observation in the present study was that the numbers of Alcaligenes decreased in the absence of B cells and mucosal Abs (Fig. 3B and Fig. S6A). These results suggest that Alcaligenes-specific Abs may play a critical role in the PP tissue colonization by these bacteria. An interesting hypothesis would be that the coccoid form of Alcaligenes coated with specific mucosal Abs is selectively taken up by PPs through M cells expressing IgA receptors (28), and formation of the immune complex results in the creation of an appropriate environment for their cohabitation on the interior of PPs.
Another unresolved issue is why Alcaligenes exclusively inhabit the PPs. It has already been demonstrated that Alcaligenes produce antimicrobial substances inhibiting growth of other bacteria, including multidrug-resistant pathogenic bacteria (29–31). Kalimantacins, antibiotics derived from Alcaligenes spp. YL-02632S, were shown to suppress the reproduction of Staphylococcus spp., including Staphylococcus aureus (29). Further, unique antibacterial compounds produced by Alcaligenes spp. FC-88 (30) and M3A (31) were reported to interfere with growth of a wide variety of bacteria, such as E. coli, Streptococcus pyogenes, Pseudomonas aeruginosa, and Staphylococcus aureus. Thus, the presence of Alcaligenes spp. in PPs, the active antigen-sampling site, may be beneficial for the host by eliminating other opportunistic and pathogenic bacteria at their portal of entry.
Physiologically, Alcaligenes are known to bear a nitric oxide (NO) reductase gene and reduce NO (32), which was recently reported to up-regulate IgA class-switch recombination (33). These findings suggest that Alcaligenes possess unique functions to exclusively coexist in the PPs and to create an optimal environment for their cohabitation through the induction and regulation of mucosal Abs. In general, IgM+ B cells, a major source for μ to α class switching, are a dominant B cell fraction in PPs of naïve mice (≈70%) (34). Under the appropriate molecular environment including TGF-β1, CD40L, and IL-4 (4), these B cells undergo class switching to IgA-committed B cells, and thus ≈5% of the total cells in PPs are IgA+ B cells (34). Because NO has been shown to be an additional key regulatory molecule for TNFα/iNOS-producing DC (tip-DC) mediated IgA class switching (33), it is interesting to postulate that NO reductase produced by tissue-inhabiting Alcaligenes may serve as a regulatory molecule for the creation of an optimal and steady rate of IgA+ B cell generation in the PPs.
Unexpectedly, we also detected Pseudomonas spp. (genetically homologous with Pseudomonas fluorescens) and Stenotrophomonas spp. (closely related to Stenotrophomonas maltophilia) within the systemic- (or splenic-) but not PP-DCs of naïve, SPF mice (Fig. S2). These two bacteria are considered to be nosocomial pathogens with low levels of virulence in the natural cohabitation state (35, 36). It has also been reported that they spontaneously emerge in immunocompromised cancer patients in the absence of contamination from their surrounding environment (37, 38). Therefore, our present findings may be of crucial clinical significance for a possible role of the intratissue cohabitation by commensal opportunistic bacteria in systemic lymphoid tissues. This line of investigation is now being intensively studied in our laboratory to further elucidate the significance of commensal microbiota that inhabits both systemic and mucosal lymphoid tissues.
In summary, the present study has indicated a unique aspect of mutualism of indigenous opportunistic bacteria with the host immune system in the GI tract. By cohabiting within the organized lymphoid tissues (e.g., PPs and ILFs), these bacteria affect the development and maturation of the host mucosal immune system. Further, the PP-inhabiting, commensal microbiota are an additional element that contributes to creating and maintaining immunologic homeostasis in the host. The universality for the concept of intratissue habitation of Alcaligenes is shared by mice and primates, and perhaps other mammals, because their presence inside PPs was demonstrated in mice, monkeys, and humans.
Materials and Methods
Animals and Human Samples.
BALB/c and C57BL/6 mice were obtained from CLEA Japan. CBA/N xid and control DBA/2 mice were purchased from Japan SLC. TCRβ−/− δ−/− mice were obtained from the Jackson Laboratory. IgA−/− mice were originally generated by Dr. Gregory Harriman and were kindly provided by the Baylor College of Medicine. Mice were maintained under SPF conditions at the Institute of Medical Science, University of Tokyo and the Immunobiology Vaccine Center, University of Alabama at Birmingham (UAB). GF mouse experiments were performed at the Yakult Central Institute for Microbiological Research. All experiments were conducted in accordance with the guidelines for the Animal Care and Use Committees of the University of Tokyo and UAB.
Nonhuman primate PPs were obtained from cynomolgus macaques housed in the Tsukuba Primate Research Center (TPRC), National Institute of Biomedical Innovation (Tsukuba, Japan). All procedures were conducted in accordance with the guidelines for the Animal Care and Use Committees of the TPRC.
Human PPs were kindly provided by healthy patients without irritable bowel disease who underwent endoscopic biopsy at Osaka University Hospital. All of the subjects provided written informed consent, and the study protocol was approved by the Ethics Committee of Osaka University Graduate School of Medicine (approval no. 08243) and Institute of Medical Science, University of Tokyo (IMSUT) (approval no. 20-67-0331).
16S rRNA Analysis.
The 16S rRNA gene was amplified by PCR with two universal primers (27F: 5′-AGAGTTTGATCCTGGCTCAG-3′; 1492R: 5′-GGTTACCTTGTTACGACTT-3′) ligated into plasmid vector pCR2.1 and transformed into INVαF' competent cells by using a TA Cloning Kit (Invitrogen). Plasmid DNA of randomly selected transformants was prepared by using a TempliPhi DNA Amplification Kit (GE Healthcare) and sequenced by using the primers 27F and 520R (5′-ACCGCGGCTGCTGGC-3′). All sequences were examined by BLAST search to identify the closest relatives. Representative nucleotide sequences obtained in this 16S rRNA gene clone library analysis have been deposited in the International Nucleotide Sequence Database (accession nos. AB453241–AB453250).
Whole-Mount FISH Analysis.
To detect the domain Bacteria or Alcaligenes, oligonucleotide probes were purchased from Invitrogen-Molecular Probes (Table S1). Isolated tissue segments were fixed in 4% paraformaldehyde at 4 °C overnight and washed with PBS. Tissues were hybridized in hybridization buffer [0.9 M NaCl, 20 mM Tris-HCl, 45% (ALBO34a, BPA) or 0% (EUB338) formamide, 0.1% SDS, and 10 μg/mL DNA probe] at 60 °C (ALBO34a, BPA) or 42 °C (EUB338) overnight. After washing twice in washing buffer [0.45 M NaCl, 20 mM Tris-HCl, 45% (ALBO34a, BPA) or 0% (EUB338) formamide, and 0.01% SDS] at 60 °C (ALBO34a, BPA) or 42 °C (EUB338) for 10 min, tissue segments were flushed with PBS. Lectin-labeling experiments were performed Alexa Fluor 633-labeled WGA (Invitrogen-Molecular Probes) and biotinylated UEA1 (Vector Laboratories) followed by Alexa 633-conjugated streptavidin (Molecular Probes) at a concentration of 10 μg/mL for 1 h. After being washed with PBS, the tissue samples were mounted and examined by DM IRE2/TCS SP2 confocal microscopy (Leica Microsystems).
Statistical Analysis.
Data were expressed as the mean ± SD or SEM and evaluated by an unpaired Student's t test. Significance was defined as P < 0.01.
Supplementary Material
Acknowledgments
We thank Mr. Hideyuki Funahashi and Dr. Akemi Imaoka (Yakult Central Institute for Microbiological Research) and Dr. Yuko Takahashi (IMSUT) for the support with GF experimental mice and monkeys, respectively; Dr. Shinichi Nishikawa (RIKEN) for kindly providing us with the anti-mouse IL-7 receptor α chain mAb; and the Tsukuba Primate Research Center, National Institute of Biomedical Innovation for providing us with monkey PPs. This research was supported by Grant-in-Aids for the Scientific Research on Priority Areas, the Global Center of Excellence and the Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a grant from the Ministry of Health and Labor of Japan, and National Institutes of Health Grants DE 12242 and AG 025873.
Footnotes
The authors declare no conflict of interest.
Data deposition: The nucleotide sequences reported in this study have been deposited in the International Nucleotide Sequence Database (accession nos. AB453241–AB453250).
This article contains supporting information online at www.pnas.org/cgi/content/full/1001061107/DCSupplemental.
References
- 1.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 2.Cebra JJ, Jiang HQ, Boiko NV, Tlaskalva-Hogenova H. In: Mucosal Immunology. Mestecky J, et al., editors. San Diego: Academic Press; 2005. pp. 335–368. [Google Scholar]
- 3.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 4.Kiyono H, Kunisawa J, McGhee JR, Mestecky J. In: Fundamental Immunology. Paul WE, editor. Vol 6. Philadelphia: Lippincott-Raven; 2008. pp. 983–1030. [Google Scholar]
- 5.Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–162. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol Immunol. 2002;46:535–548. doi: 10.1111/j.1348-0421.2002.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 8.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 10.Davis CP, Savage DC. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974;10:948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse HJ, Stolz A. Achromobacter, Alcaligenes and Related Genera. In: Dworkin M, et al., editors. Prokaryotes. New York: Springer; 2006. pp. 675–700. [Google Scholar]
- 12.Amann RI, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffels M, Amann R, Ludwig W, Hekmat D, Schleifer KH. Bacterial community dynamics during start-up of a trickle-bed bioreactor degrading aromatic compounds. Appl Environ Microbiol. 1998;64:930–939. doi: 10.1128/aem.64.3.930-939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenzaka T, Yamaguchi N, Tani K, Nasu M. rRNA-targeted fluorescent in situ hybridization analysis of bacterial community structure in river water. Microbiology. 1998;144:2085–2093. doi: 10.1099/00221287-144-8-2085. [DOI] [PubMed] [Google Scholar]
- 15.Jang MH, et al. Intestinal villous M cells: An antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 17.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, et al. IL-7 receptor α+ CD3(-) cells in the embryonic intestine induces the organizing center of Peyer's patches. Int Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz RG, Newberry RD. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- 20.Hashizume T, et al. Isolated lymphoid follicles are not IgA inductive sites for recombinant Salmonella. Biochem Biophys Res Commun. 2007;360:388–393. doi: 10.1016/j.bbrc.2007.06.096. [DOI] [PubMed] [Google Scholar]
- 21.Nagai S, et al. Role of Peyer's patches in the induction of Helicobacter pylori-induced gastritis. Proc Natl Acad Sci USA. 2007;104:8971–8976. doi: 10.1073/pnas.0609014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 24.Beagley KW, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong JL, Shigeno DS, Calomiris JJ, Seidler RJ. Antibiotic-resistant bacteria in drinking water. Appl Environ Microbiol. 1981;42:277–283. doi: 10.1128/aem.42.2.277-283.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ash RJ, Mauck B, Morgan M. Antibiotic resistance of gram-negative bacteria in rivers, United States. Emerg Infect Dis. 2002;8:713–716. doi: 10.3201/eid0807.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam P, et al. [The spectrum of microbiological agents causing pulmonary MALT-type lymphomas. A 16S rRNA-based analysis of microbial diversity] Pathologe. 2008;29:290–296. doi: 10.1007/s00292-008-1068-1. [DOI] [PubMed] [Google Scholar]
- 28.Mantis NJ, et al. Selective adherence of IgA to murine Peyer's patch M cells: Evidence for a novel IgA receptor. J Immunol. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 29.Kamigiri K, et al. Kalimantacins A, B and C, novel antibiotics from Alcaligenes sp. YL-02632S. I. Taxonomy, fermentation, isolation and biological properties. J Antibiot (Tokyo) 1996;49:136–139. doi: 10.7164/antibiotics.49.136. [DOI] [PubMed] [Google Scholar]
- 30.Chen YP. An antibiotic and a haloperoxidase produced by an Alcaligenes microorganism. 2001 World Intellectual Property 009284. [Google Scholar]
- 31.Bacic MK, Yock DC. Antibiotic composition from Alcaligenes species and method for making and using the same. 2001 US Patent 6224863. [Google Scholar]
- 32.Braker G, Tiedje JM. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl Environ Microbiol. 2003;69:3476–3483. doi: 10.1128/AEM.69.6.3476-3483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 34.Gohda M, et al. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer's patches for intestinal IgA responses. J Immunol. 2008;180:5335–5343. doi: 10.4049/jimmunol.180.8.5335. [DOI] [PubMed] [Google Scholar]
- 35.Schroth MN, Hildebrand DC, Panopoulos N. Phytopathogenic Pseudomonads and Related Plant-Associated Pseudomonads. In: Dworkin M, et al., editors. Prokaryotes. New York: Springer; 2006. pp. 714–740. [Google Scholar]
- 36.Senol E. Stenotrophomonas maltophilia: The significance and role as a nosocomial pathogen. J Hosp Infect. 2004;57:1–7. doi: 10.1016/j.jhin.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 37.Hsueh PR, et al. Outbreak of Pseudomonas fluorescens bacteremia among oncology patients. J Clin Microbiol. 1998;36:2914–2917. doi: 10.1128/jcm.36.10.2914-2917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micozzi A, et al. Bacteremia due to Stenotrophomonas maltophilia in patients with hematologic malignancies. Clin Infect Dis. 2000;31:705–711. doi: 10.1086/314043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.