Abstract
Multiple studies have demonstrated that brain-derived neurotrophic factor (BDNF) is a potent modulator of neuronal structure and function in the hippocampus. However, the majority of studies to date have relied on the application of recombinant BDNF. We herein report that endogenous BDNF, released via theta burst stimulation of mossy fibers (MF), elicits a slowly developing cationic current and intracellular Ca2+ elevations in CA3 pyramidal neurons with the same pharmacological profile of the transient receptor potential canonical 3 (TRPC3)-mediated IBDNF activated in CA1 neurons by brief localized applications of recombinant BDNF. Indeed, sensitivity to both the extracellular BDNF scavenger tropomyosin-related kinase B (TrkB)-IgG and small hairpin interference RNA-mediated TRPC3 channel knockdown confirms the identity of this conductance as such, henceforth-denoted MF-IBDNF. Consistent with such activity-dependent release of BDNF, these MF-IBDNF responses were insensitive to manipulations of extracellular Zn2+ concentration. Brief theta burst stimulation of MFs induced a long-lasting depression in the amplitude of excitatory postsynaptic currents (EPSCs) mediated by both AMPA and N-methyl-d-aspartate (NMDA) receptors without changes in the NMDA receptor/AMPA receptor ratio, suggesting a reduction in neurotransmitter release. This depression of NMDAR-mediated EPSCs required activity-dependent release of endogenous BDNF from MFs and activation of Trk receptors, as it was sensitive to the extracellular BDNF scavenger TrkB-IgG and the tyrosine kinase inhibitor k-252b. These results uncovered the most immediate response to endogenously released—native—BDNF in hippocampal neurons and lend further credence to the relevance of BDNF signaling for synaptic function in the hippocampus.
INTRODUCTION
A critical function of the CNS is the ability to assimilate and respond to a range of environmental stimuli; these tasks are reliably accomplished by modulation of synaptic structure and function in response to various neuronal firing patterns. Brain-derived neurotrophic factor (BDNF) is an important modulator of activity-dependent synaptic plasticity in the CNS (Bramham and Messaoudi 2005; Poo 2001), particularly in the hippocampus, wherein resides the foundation for learning and memory. Indeed, it is this region that exhibits some of the highest levels of BDNF protein expression in the brain. Once thought to be important only in developmental processes, BDNF is now a well-known modulator of the synaptic landscape in the postnatal brain, participating in the induction of long-term plasticity of excitatory synaptic transmission in the hippocampus (Figurov et al. 1996; Huber et al. 1998; Kang and Schuman 1995; Korte et al. 1995; Kumura et al. 2000; Minichiello et al. 1999; Patterson et al. 1996) as well as in the density and morphology of dendritic spines, an effect that ultimately translates into a change in the number of excitatory synapses (Tolwani et al. 2002; Tyler and Pozzo-Miller 2001). Moreover, the induction of long-term potentiation (LTP) in area CA1 of the hippocampus is impaired in mice lacking Bdnf (Korte et al. 1995; Patterson et al. 1996; Pozzo-Miller et al. 1999) or its receptor Trkb (Minichiello et al. 1999).
Although numerous BDNF actions have been identified, the underlying mechanisms that bring about these changes are not clearly defined. It is well established that BDNF activation of tropomyosin-related kinase B (TrkB) receptors sets in motion three major intracellular signaling cascades: the Ras-Raf MAPK/ERK cascade, the PI3 kinase cascade, and the PLCγ-IP3 cascade (Segal and Greenberg 1996). The Ras-Raf MAPK/ERK cascade is largely responsible for changes in gene transcription and is necessary for the induction of LTP, hippocampal-dependent associative learning, and BDNF-induced spine formation (Alonso et al. 2004; Atkins et al. 1998; Bading et al. 1993; English and Sweatt 1997; Rosen et al. 1994). At presynaptic terminals in area CA1 of the hippocampus, MAPK/ERK-dependent phosphorylation of synapsin-I results in a higher density of docked neurotransmitter vesicles in glutamatergic terminals, increased frequency of miniature excitatory postsynaptic currents (mEPSC), increased paired-pulse facilitation, facilitated neurotransmitter release during high-frequency activity with consequent increases in LTP, and enhancements in hippocampus-dependent learning (Kushner et al. 2005). The PI3 kinase cascade is responsible for dendritic targeting of BDNF and TrkB mRNA (Righi et al. 2000) and has been implicated in hippocampal-dependent spatial learning (Mizuno et al. 2003). Last, the PLCγ-IP3 cascade also plays a critical role in hippocampal synaptic plasticity as demonstrated by the lack of CA1 LTP in conditional knockout mice carrying a deletion of the PLC-γ adaptor site in Trk receptors (Minichiello et al. 2002).
But what is the most immediate effect of BDNF of these several signaling possibilities? Kafitz et al. (1999) showed that brief (millisecond) recombinant BDNF pulses to neurons in acute brain slices activated a TTX-insensitive, STX-sensitive fast Na+ current later proposed to be mediated by Nav1.9 channels (Blum et al. 2002). On the other hand, Li et al. described the activation of a much slower nonselective cationic conductance in acutely dissociated pontine neurons by bath applied recombinant BDNF—IBDNF—which required TrkB receptors, PLCγ-IP3, intracellular Ca2+ elevations, and canonical transient receptor potential (TRPC) channel activity (Li et al. 1999). TRPC ion channels are activated by stimulation of Gq/G11-type G protein-coupled receptors and by receptor tyrosine kinases such as tropomyosin related kinase (Trk) receptors, leading to PLC-mediated formation of diacylglycerol (DAG) and IP3, followed by activation of IP3 receptors in intracellular Ca2+ stores (Clapham 2003; Montell et al. 2002). We recently described that recombinant BDNF also activates IBDNF in CA1 pyramidal neurons of hippocampal slices and that it requires channels containing TRPC3 subunits (Amaral and Pozzo-Miller 2007a). Both the PLCγ-IP3 and the PI3 kinase cascades play important roles in the activation and persistence of IBDNF, mobilizing Ca2+ from intracellular stores and facilitating transport of TRPC3 containing vesicles to the plasma membrane, respectively (Amaral and Pozzo-Miller 2007a). Moreover, TRPC3 channels are required for BDNF-induced changes in dendritic spine density at CA1 neurons. The aforementioned observations were characterized, however, following application of recombinant BDNF. To confirm that natively expressed endogenous BDNF activates such a membrane conductance, we used theta burst stimulation (TBS), which is known to be an effective stimulus pattern for releasing endogenous BDNF from cultured hippocampal cells (Balkowiec and Katz 2002). Indeed, TBS of Schaffer collaterals in the absence of glutamate and GABA receptor activity evoked a membrane conductance in CA1 neurons that was sensitive to the BDNF scavenger TrkB-IgG, the membrane tyrosine kinase inhibitor k-252a, and the TRPC/SOC (store-operated channels) channel inhibitor SKF-96365 (Amaral and Pozzo-Miller 2007a). Taken together with the dendritic Ca2+ signals associated with IBDNF (Amaral and Pozzo-Miller 2007b), which potentially enable cytoskeletal rearrangements and dendritic spine morphogenesis, we concluded that IBDNF is one of the earliest signaling steps in the BDNF/TrkB cascade (Amaral et al. 2007).
We now focus on the mossy fiber (MF) pathway in area CA3 because these fibers contain the highest levels of BDNF in the brain (Conner et al. 1997; Danzer and McNamara 2004). As we have shown previously in area CA1 (Amaral and Pozzo-Miller 2007a,b), BDNF released from MFs during TBS evokes a membrane current in CA3 neurons that is mediated by TRPC3 channels and associated with Ca2+ influx, which we termed MF-IBDNF. Consistent with such activity-dependent release of BDNF, these MF-IBDNF responses were insensitive to manipulations of extracellular Zn2+ concentration. In addition a single brief TBS of MFs induced a long-lasting depression of the amplitude of EPSCs mediated by AMPA receptors (AMPAR) and NMDA receptors (NMDAR) evoked in CA3 neurons, which is associated with changes in the coefficient of variance (CV2) but not in the NMDAR/AMPAR ratio. TBS-induced depression of NMDAR-mediated EPSCs at MF-CA3 synapses was sensitive to TrkB-IgG and k-252b, indicating a requirement of extracellular endogenous BDNF and TrkB receptor activation. This study yields novel information regarding the role of endogenous BDNF function in the hippocampus, especially its availability for activity-dependent release and subsequent activation of TRPC membrane currents and intracellular Ca2+ elevations, as well as its role in the modulation of excitatory synaptic strength at MF-CA3 synapses.
METHODS
Organotypic slice cultures
Hippocampi were dissected from postnatal day 7–11 Sprague Dawley rats (Harlan, Indianapolis, IN, or Charles River, Wilmington, MA) and cut transversely into ∼500-μm-thick slices using a custom-made wire-slicer fitted with 20-μm-thick Pt wire (Pozzo-Miller et al. 1995). Hippocampal slices were individually plated on Millicell-CM filter inserts (Millipore, Billerica, MA) and cultured in 36°C-5% CO2, 98% relative humidity incubators (Thermo-Forma, Waltham, MA). Slices were maintained in culture media (Neurobasal-A plus B27, InVitrogen, Carlsbad, CA) containing 20% equine serum for the first 4 days in vitro (div). To avoid the confounding effects of hormones and growth factors in the serum, its concentration was gradually reduced over a period of 48 h starting at 4 div (24 h each in 10 and 5% serum). After a period of 24 h in serum free media (Neurobasal-A plus B27), 7–10 div slices were used for electrophysiology (Tyler and Pozzo-Miller 2001). Some slice cultures remained in serum containing culture media (20% equine serum) as described in the original publications (Gahwiler 1981; Pozzo-Miller et al. 1993; Stoppini et al. 1991; Yamamoto et al. 1989). All procedures followed national and international ethics guidelines and were annually reviewed and approved by the Institutional Animal Care and Use Committee at University of Alabama at Birmingham.
Acute slices
Hippocampi were dissected from postnatal day 45–60 C57BL/6 wild-type mice, placed in ice-cold modified artificial cerebrospinal fluid (ACSF, see following text), and cut transversely into 400-μm-thick slices using a vibrating blade microtome (VT1200S, Leica, Nussloch, Germany). For the dissection and storage of acute slices, the modified ACSF contained (in mM) 87 NaCl, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 10 glucose (bubbled with 95% O2-5% CO2; pH 7.4). Slices were first transferred to this modified ACSF warmed to ∼32°C for 30 min and then kept at room temperature in modified ACSF until use for electrophysiology.
Simultaneous electrophysiology and Ca2+ imaging
Individual 7–10 div slice cultures or acute slices were transferred to a recording chamber mounted on a fixed-stage upright microscope (Zeiss Axioskop FS, Oberkochen, Germany) and continuously perfused (2 ml/min) with oxygenated ACSF at room temperature (24°C). For slice cultures, ACSF contained (in mM): 124 NaCl, 2 KCl, 1.24 KH2PO4, 1.3 MgSO4, 17.6 NaHCO3, 2.5 CaCl2, and 10 glucose (adjusted to 310–320 mOsm with sucrose); for acute slices, ACSF contained (in mM) 125 NaCl, 5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, and 25 glucose (adjusted to 300–320 mOsm with sucrose); both ACSF solutions were continuously bubbled with 95% O2-5% CO2 (pH 7.4). Superficial pyramidal neurons in CA3 stratum pyramidale were visualized with a water-immersion ×63 objective (0.9 NA) using IR-DIC microscopy (Dodt and Zieglgänsberger 1990). Simultaneous whole cell recording and microfluorometric Ca2+ imaging was performed as described (Petrozzino et al. 1995; Pozzo-Miller 2006; Pozzo-Miller et al. 1996). Briefly, unpolished patch pipettes contained (in mM) 120 Cs-gluconate, 17.5 CsCl (or KCl), 10 Na-HEPES, 4 Mg-ATP, 0.4 Na-GTP, 10 Na2 creatine phosphate, 2 QX314, and 0.2 Na-EGTA (replaced by 0.2 mM fura-2 for Ca2+ imaging); 290–300 mOsm; pH 7.2 (resistance: 3–4 MΩ). Membrane currents were recorded in the voltage-clamp mode at a holding potential of −65 mV using an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 2 kHz, and digitized at 10 kHz. Recordings were accepted only if access resistance was ≤25 MΩ. Input resistance (Ri) was measured with hyperpolarizing voltage pulses (50 ms, 20 mV), and cells were discarded if any whole cell parameter (i.e., Cm, Ri, Rs) changed by ≥20% during the course of an experiment. A subsample of CA3 pyramidal neurons had Ri of 275.3 ± 77.9 (SD) MΩ and whole cell capacitances of 135.9 ± 30.4 (SD) pF under the preceding recording conditions (n = 31). To reduce the contribution of voltage-gated Ca2+ channels during simultaneous whole cell recording and Ca2+ imaging, neurons were voltage clamped at +40 mV (Malinow et al. 1994; Perkel et al. 1993; Pozzo-Miller et al. 1996). Fura-2 (InVitrogen) was alternatively excited at 360 and 380 nm using a monochromator (Polychrome-IV, TILL Photonics, Munich, Germany), and its emission (>510 nm) was filtered and detected with a frame-transfer cooled CCD camera (Quantix-57, Photometrics/Roper, Duluth, GA); digital image pairs were acquired every 4 s (50 ms exposures for ∼100 × 200 pixel subarrays). Background-subtracted fluorescence intensity measurements were obtained within regions of interest (ROIs) defined over apical primary and secondary dendrites, as well as somata. The average ratio of 360 and 380 nm fluorescence within each ROI was used as an estimate of intracellular Ca2+ concentration as these two parameters are directly proportional to each other (Grynkiewicz et al. 1985). Electrical and optical data were simultaneously acquired on a single G4 Macintosh computer (Apple) running custom-written software (TIWorkBench, written by T. Inoue).
Afferent fiber stimulation was performed with an extracellular patch pipette filled with buffered and oxygenated ACSF (∼2 MΩ) positioned within either the granule cell layer or s. lucidum to stimulate the MFs. Square constant-current pulses of 100 μs duration were produced by an isolated stimulator (ISO-Flex, AMPI, Jerusalem, Israel). High-frequency afferent stimulation was delivered as a theta burst pattern consisting of five bursts at 5 Hz, each burst having four pulses at 100 Hz. A subthreshold concentration of the Na+ channel blocker TTX (10 nM) was included in the ACSF to reduce membrane excitability and prevent polysynaptic responses, common in organotypic slices. The ACSF contained the GABAA receptor antagonist picrotoxin (50 μM), while the K+ channels coupled to GABAB receptors were blocked by intracellular Cs+. The ACSF also contained the noncompetitive antagonists of AMPAR and NMDAR, GYKI-52466 (20 μM) and MK-801 (20 μM), respectively, as well as the antagonist of group-I mGlu receptors LY-367385 (100 μM) and L-type Ca2+ blocker nimodipine (20 μM); additional experiments were performed with the competitive antagonists 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 20 μM) and d,l-2-amino-5-phosphonovaleric acid (d,l-APV, 100 μM). Membrane currents observed in the presence of GYKI-52466 were 111.8 ± 12.5 pA (n = 12), whereas currents in the presence of CNQX were 109.0 ± 11.9 (n = 21; P = 0.8803). The current intensity for afferent stimulation (between 10 and 50 μA) was never larger than 10 times that required to evoke maximal AMPAR-mediated fast EPSCs at −65 mV.
Human recombinant mature BDNF (100 μg/ml; Promega; Madison, WI) dissolved in 0.0001% bovine serum albumin was applied from glass pipettes (∼5 MΩ) using a Pressure System IIe (Toohey, Fairfield, NJ), as described (Amaral and Pozzo-Miller 2007a,b). The application pipette was positioned ∼100 μm above the slice and ∼200 μm away from the soma of the CA3 neuron under recording to avoid pressure and mechanical artifacts and aimed at its apical dendrites within s. lucidum (∼150 μm from the soma) against the direction of ACSF perfusion flow. Every pulse of 25–30 s duration at 20–30 psi delivered a maximum of 2 μl of the BDNF solution on top of the slice.
TRPC3 RNA interference
Custom HuSH small hairpin interference RNA (shRNA) constructs (OriGene, Rockville, MD) were designed to target TRPC3 channel subunits (Gene Bank Accession No. J05181). Short hairpin RNAs encoding the following target sequence 5′-GAGTTCAAGAATGACTACAGGAAGCTCTC 3′ were introduced by transfection with the HuSH construct. The same vector also contained the coding sequence for GFP, and its fluorescence enabled identification of transfected neurons before whole cell recording as described (Amaral and Pozzo-Miller 2007a).
Particle-mediated gene transfer
Hippocampal slices were transfected with a HuSH expression plasmid (OriGene) encoding green fluorescent protein (GFP) and the TRPC3-specific shRNA sequence. A custom-modified Helios gene gun (Bio-Rad; Hercules, CA) was used to perform the biolistic transfection following established protocols (Alonso et al. 2004; Lo et al. 1994). Briefly, the TRPC3-specific shRNA expression vector was precipitated onto 1.6 μm colloidal gold and this mixture was coated onto Tefzel tubing using 0.06 mg/ml polyvinylpyrrolidone. Slices were bombarded using He pressure at 100 psi from a distance of 15 mm. For negative control experiments, gene transfer was performed as in the preceding text using a random shRNA or only enhanced yellow fluorescent protein, except the plasmid encoding eYFP was precipitated onto 1.6 μm colloidal gold at a ratio of 50 μg of DNA to 25 mg of gold.
Drugs
Some compounds were dissolved in DMSO (0.01%) and others directly into the ACSF or intracellular solution; vehicle controls using 0.01% DMSO were routinely performed. When compounds were included in the intracellular solution (e.g., k-252b), a diffusion period of 5–10 min was allowed to elapse before the start of the experiment. All of the chemicals used for these experiments were obtained from Sigma (St. Louis, MO), Calbiochem (San Diego, CA), or Tocris (Ellisville, MO).
Coefficient of variation analysis
The coefficient of variation (CV2) of evoked AMPAR- and NMDAR-mediated EPSCs was calculated after subtraction of the recording noise variance (σ2): [(σ)2 – (σ of noise)2]/mean2, as described (Bender et al. 2009). The fractional change in CV2 (r = CV2 before TBS/CV2 after TBS) was plotted against a synaptic gain factor (f = EPSC amplitude after TBS/EPSC amplitude before TBS), as described (Faber and Korn 1991; Shen and Horn 1996).
Statistical analysis
Averages of multiple measurements are presented as means ±SE; mean ± SD was used to calculate the coefficient of variation (CV). Data were statistically analyzed using unpaired Student's t-test, ANOVA test, and pair-wise correlation and linear regression using the Prism software package (GraphPad Software, San Diego, CA). Probability values <0.05 were considered statistically significant (i.e., P < 0.05, <5% probability that the observations are due to chance). When different than this cutoff value, the actual P values ≤4 decimal points are given in results (rather than just the statement “greater than” or “less than”) to provide readers with more detailed information regarding the outcome of the statistical analyses. Compromise power analyses were performed to determine the statistical power given the number of observations, sample means and SDs, using G*Power (Erdfelder et al. 1996). These power analyses yielded values of statistical Power (1 − β; where β is the type-II error) larger than 0.95 (i.e., 95% confidence of accepting the null hypothesis when is true).
RESULTS
Activity-dependent release of BDNF from MFs evokes MF-IBDNF, a slow membrane current in CA3 pyramidal neurons
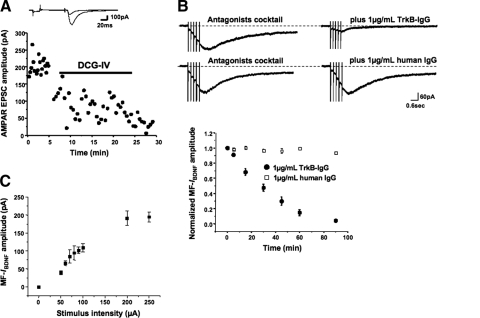
We recently showed that stimulation of afferent Schaffer collaterals activated TRPC-like nonselective cationic currents in CA1 pyramidal neurons by means of the release of endogenous—native—BDNF (Amaral and Pozzo-Miller 2007a). We now focus on the MF pathway in CA3 because these fibers contain the highest levels of BDNF in the brain (Conner et al. 1997; Danzer and McNamara 2004). In the following experiments, the identity of MF-mediated responses was routinely confirmed using the group-II mGluR agonist DCG-IV (Toth et al. 2000). Indeed, EPSC amplitudes decreased significantly after bath application of 1 μM DCG-IV (76.00 ± 5.42 vs. 204.52 ± 5.59 pA, n = 5; P < 0.001; Fig. 1A). TBS has been shown to be an effective stimulation pattern to release GFP-tagged BDNF (Hartmann et al. 2001) or endogenous BDNF from cultured neurons (Balkowiec and Katz 2002). Consistently, stimulation of MFs in CA3 s. lucidum of 7–10 div organotypic slice cultures with a single TBS train evoked slowly activating inward currents in CA3 pyramidal neurons voltage clamped at −65 mV in the presence of ionotropic and metabotropic glutamate and GABA receptor antagonists (20 μM GYKI-52466 or 20 μM CNQX for AMPARs; 20 μM MK-801 or 50 μM d,l-APV for NMDARs; 100 μM LY-367385 for mGluR1/5; 50 μM picrotoxin for GABAARs; Cs+ intracellular solution to block GABAB-coupled K+ channels; 10 nM TTX to reduce polysynaptic activity). The average amplitude of MF-induced IBDNF was 109.02 ± 11.89 pA, and they peaked 1.47 ± 0.23 s following the onset of afferent stimulation (n = 21; Fig. 1B). MF-IBDNF observed in the presence of GYKI-52466 was 111.8 ± 12.5 pA (n = 12), whereas MF-IBDNF in the presence of CNQX was 109.0 ± 11.9 (n = 21; P = 0.8803), demonstrating that kainate receptors did not contribute to the measured responses. The amplitude of MF-IBDNF was very stable, lacking any significant “rundown” during recording times ≤90 min from whole cell access (Fig. 1B). Similar responses were observed in acute slices from 45- to 60-day-old C57BL/6 wild-type mice (117.83 ± 11.85 pA, n = 9; P = 0.6469 compared with MF-IBDNF from organotypic cultures from rat hippocampal slices; Supplemental Fig. S1A).1
Fig. 1.
Release of endogenous brain-derived neurotrophic factor (BDNF) from mossy fibers (MFs) activates MF-IBDNF, a nonselective cationic current in CA3 pyramidal neurons. A: representative examples of AMPA receptor (AMPAR)-mediated excitatory postsynaptic currents (EPSCs) evoked in CA3 pyramidal neurons by MF stimulation, before and after application of DCG-IV. B: representative examples of slow currents evoked by theta burst stimulation (TBS) of MFs in CA3 pyramidal neurons in the presence of antagonists of iono- and metabotropic glutamate receptors as well as GABAA receptors (left). The MF-evoked current (MF-IBDNF) in the same cell was significantly reduced by the extracellular BDNF scavenger tropomyosin-related kinase B (TrkB)-IgG, but not by human control IgG (right). C: input-output curve of slow currents evoked by TBS of MFs.
TrkB-IgG, a chimeric recombinant protein that fuses together the ligand-binding domain of the TrkB receptor with the Fc domain of human IgG (Shelton et al. 1995), was used as an extracellular scavenger to implicate endogenous BDNF binding to membrane receptors in MF-IBDNF activation. Consistent with observations in CA1 (Amaral and Pozzo-Miller 2007a), TrkB-IgG (1 μg/ml, equivalent to 3.8 nM of the receptor dimer) significantly reduced MF-IBDNF amplitude (20.49 ± 6.4 pA, n = 10; P < 0.001 vs. controls; Fig. 1B). As controls, a nonspecific human IgG (1 μg/ml) did not affect MF-IBDNF (147.26 ± 39.25 pA, n = 7; P > 0.05 vs. controls; Fig. 1B). In addition, increasing stimulus intensities evoked larger currents (Fig. 1C), suggesting an activity-dependent process.
Vesicular Zn2+ released during MF stimulation does not contribute to MF-IBDNF
At glutamatergic synapses, neurotransmitter vesicles oftentimes store and release Zn2+ alongside their glutamate content, thereby increasing the extracellular concentration of Zn2+ (Frederickson et al. 2005). Nowhere is this more apparent than in CA3 s. lucidum, the brain region with the highest vesicular Zn2+ content. In addition to its action on NMDA receptors (Mayer and Vyklicky 1989; Mayer et al. 1989), Zn2+ also modulates LTP induction at MF-CA3 synapses: removal of free Zn2+ with Ca-EDTA prevented LTP induction, and exogenous addition of Zn2+ potentiated MF-CA3 extracellular field EPSPs (Li et al. 2001). Because synaptically released Zn2+ was recently proposed to trans-activate TrkB receptors in CA3 (Huang et al. 2008), we experimentally altered its extracellular concentration to determine whether MF-IBDNF is in any way influenced by vesicular Zn2+ released during TBS of MFs.
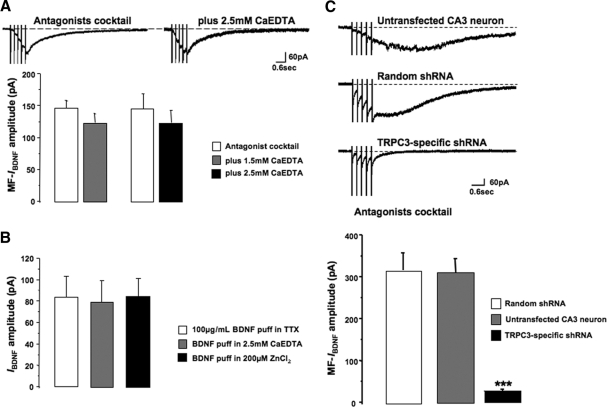
Addition of 1.5 mM of the membrane impermeable Zn2+ chelator Ca-EDTA did not affect MF-IBDNF (123.66 ± 13.81 pA, n = 18; P > 0.05 compared with control responses). Similar results were obtained using 2.5 mM Ca-EDTA (124.51 ± 18.91 pA, n = 34, P > 0.05 compared with controls; Fig. 2A). Considering that the Kd of EDTA for Zn2+ is 1016 (Bers 1982; Vogt et al. 2000), these concentrations of Ca-EDTA are 2 orders of magnitude higher than the apparent affinity constant of Ca-EDTA for Zn2+ (3–4 nM). On a practical note, further attempts to increase the concentration of Ca-EDTA (e.g., to 10 mM) (as in Huang et al. 2008; Li et al. 2001) yielded very unstable whole cell recordings, likely due to excessive buffering of divalent cations that reduced membrane charge screening. Screening and binding of negative charges on the extracellular surface of plasma membranes by divalent cations contributes to the electric field across the membrane, and thus is critical for normal membrane excitability (Hille 2001); in fact, Zn2+ itself exerts very potent charge screening effects (Blaustein and Goldman 1968; Hille et al. 1975).
Fig. 2.
Vesicular Zn2+ released during MF stimulation does not contribute to MF-IBDNF. A: Ca-EDTA did not affect MF-IBDNF. B: neither Ca-EDTA nor ZnCl2 affected IBDNF, the membrane currents evoked by direct application of recombinant BDNF to apical dendrites (in TTX). C: CA3 pyramidal neurons transfected with TRPC3 small hairpin interference RNA (shRNA) show smaller currents than untransfected CA3 neurons in the same slice and cells transfected with a random shRNA (which show currents similar to untransfected cells).
Consistent with the lack of effect of extracellular Zn2+ on MF-evoked responses, membrane currents activated by application of recombinant BDNF to apical dendrites of CA3 pyramidal neurons (in the presence of TTX) were not significantly affected by the addition of 2.5 mM Ca-EDTA (control IBDNF 83.67 ± 19.37 pA, n = 27, vs. IBDNF in Ca-EDTA 79.61 ± 19.67 pA, n = 16; P > 0.05; Fig. 2B). We also tested whether IBDNF was affected by increasing extracellular Zn2+. IBDNF evoked in the presence of 200 μM ZnCl2 was not significantly different from controls (84.19 ± 17.01 pA, n = 11, vs. 83.67 ± 19.37 pA, n = 27; P > 0.05; Fig. 2B). Because recombinant BDNF applied to CA3 s. lucidum did not increase mEPSC frequency in CA3 pyramidal neurons (M. D. Amaral, Y. Li, T. Inoue, G. Calfa, L. Pozzo-Miller, unpublished data), these observations rule out potential artifacts arising from activity-dependent release of vesicular Zn2+ from MFs. Taken together, these results demonstrate that MF-IBDNF is not sensitive to changes in the concentration of extracellular Zn2+.
TRPC3 channels are required for MF-IBDNF
To directly demonstrate the requirement for TRPC3 channels in the activation of MF-IBDNF as we did for recombinant BDNF-induced responses in CA1 (Amaral and Pozzo-Miller 2007a), we used a shRNA that was specifically designed to knockdown TRPC3 channel expression. HuSH constructs encoding TRPC3 shRNA (and GFP used for cell identification) were coated onto gold particles and then biolistically transfected into slice cultures (Alonso et al. 2004; Lo et al. 1994). After 48 h of transfection, GFP or eYFP-expressing CA3 pyramidal neurons were identified by epifluorescence microscopy and the presence of a gold particle (Alonso et al. 2004; Amaral and Pozzo-Miller 2007a).
Consistent with our observations in CA1 neurons, TRPC3 knockdown completely prevented the activation of MF-IBDNF in CA3 pyramidal neurons (27.63 ± 2.92 pA, n = 42, vs. untransfected CA3 neurons within the same slice 316.3 ± 41 pA, n = 12; P < 0.001; Fig. 2C). However, CA3 neurons transfected with a cDNA plasmid encoding eYFP alone or a random shRNA sequence did not affect MF-IBDNF amplitude (311.88 ± 33.34 pA, n = 14; P > 0.05 compared with untransfected CA3 neurons in the same slice; Fig. 2C). These results demonstrate that MF-IBDNF in CA3 neurons is mediated by nonselective cationic channels containing TRPC3 subunits.
Activity-dependent release of BDNF from MFs evokes dendritic Ca2+ signals in voltage clamped CA3 pyramidal neurons
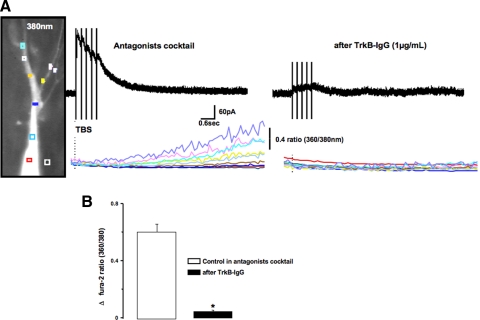
Elevations in the intracellular concentration of Ca2+ ions were the first-described immediate actions of BDNF (Berninger et al. 1993). Activated neurotrophin Trk receptors trigger the formation of IP3 by PLCγ-mediated hydrolysis of phosphatidylinositol bisphosphate (PIP2), which then activates IP3 receptors of intracellular Ca2+ stores (Segal and Greenberg 1996). Consistently, IBDNF evoked by recombinant BDNF in hippocampal CA1 neurons is associated with dendritic transient Ca2+ elevations (Amaral and Pozzo-Miller 2007b). Here we performed simultaneous whole cell recording and microfluorometric imaging in CA3 pyramidal neurons filled with the Ca2+ indicator fura-2 (100 μM) and voltage clamped at +40 mV to reduce the contribution of voltage-gated Ca2+ channels (Perkel et al. 1993; Pozzo-Miller et al. 1996). In the presence of our antagonist cocktail (which also included 20 μM of the L-type Ca2+ channels blocker nimodipine), TBS of MFs within CA3 s. lucidum evoked outward membrane currents followed by slowly developing Ca2+ elevations in apical dendrites (Fig. 3A, left). The 360/380 nm ratio of fura-2 signals had average peak amplitudes of 0.6 ± 0.06 (n = 33, CV = 0.54; Fig. 3B). To determine whether these Ca2+ responses were a result of activity-dependent BDNF release, afferent MFs were stimulated after 30 min incubation with the extracellular BDNF scavenger TrkB-IgG (Fig. 3A, right). Under these conditions, there were no significant changes in the fura-2 ratio (0.05 ± 0.01, n = 14, CV = 0.93) when compared with control responses (P < 0.001; Fig. 3B).
Fig. 3.
MF-IBDNF is associated with elevations in dendritic Ca2+ levels. A, left: representative example of Ca2+ signals evoked during MF stimulation in the presence of TTX, iono- and metabotropic glutamate, and GABA receptor antagonists. Ca2+ elevations occurred throughout the apical dendrites and the soma. (right), The BDNF scavenger TrkB-IgG (1 μg/ml) prevented Ca2+ elevations during MF stimulation in the antagonist cocktail. B: quantification of the changes in peak fura-2 ratios (360 nm/380 nm) during MF stimulation.
TBS of MFs causes a BDNF-dependent depression of excitatory postsynaptic currents in CA3 pyramidal neurons
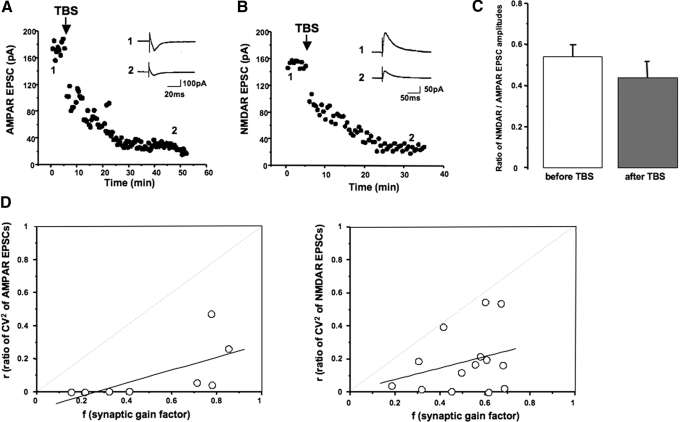
Considering the well-established role of BDNF on hippocampal synaptic plasticity (Bramham and Messaoudi 2005; Poo 2001), we monitored the amplitude of evoked EPSCs recorded from CA3 pyramidal neurons before and after a single brief TBS of afferent MFs. Pharmacologically isolated EPSCs mediated by AMPAR were significantly reduced after TBS of MFs (144.5 ± 26.8 pA vs. 38.52 ± 14.3 pA after 40 min, n = 12; P < 0.001; Fig. 4A); these responses were subsequently blocked by the AMPAR antagonist CNQX (20 μM). Similarly, pharmacologically isolated EPSCs mediated by NMDAR were significantly reduced after TBS of MFs (188.6 ± 13.5 vs. 79.8 ± 10.0 pA after 40 min, n = 30; P < 0.001; Fig. 4B); these responses were subsequently blocked by the NMDAR antagonist d,l-APV (50 μM). This enduring depression of NMDAR-mediated responses unlikely reflects the much short-lived Ca2+-dependent inactivation of NMDARs (Legendre et al. 1993) because it developed even if the monitoring of NMDAR-mediated EPSCs was resumed after a 5 min interval from the theta burst stimulus, long enough for the TBS-induced Ca2+ transient to be dissipated by buffering, sequestration, and extrusion. Note that TBS of MFs also induced a brief posttetanic potentiation (PTP), which is evident when afferent stimulation was resumed immediately after TBS (see Supplemental Fig. S1B).
Fig. 4.
A single brief TBS of MFs causes a BDNF-dependent depression of excitatory postsynaptic currents in CA3 pyramidal neurons. A: representative example illustrating the enduring depression of AMPAR-mediated EPSCs after TBS of MFs. Inset: representative EPSCs before (1) and 50 min after (2) TBS. B: representative example illustrating the enduring depression of N-methyl-d-aspartate receptor (NMDAR)-mediated EPSCs after TBS of MFs. Inset: representative EPSCs before (1) and 40 min after (2) TBS. C: TBS of MFs did not affect the NMDAR/AMPAR ratio. D: plots of the fractional changes in coefficient of variation (CV2, r) vs. the synaptic gain factor (f) after TBS of MFs. Note that all points fall to the right of the diagonal (r = f).
To obtain the ratio of NMDAR/AMPAR EPSCs in the same neuron, 40–50 consecutive synaptic responses in control ACSF were first evoked at Vhold = −60 mV for AMPAR-EPSCs and then another EPSC series at +40 mV for NMDAR-EPSCs. Consistent with the pharmacological approach in different cells, the amplitude of AMPAR-EPSCs and of NMDAR-EPSCs were also significantly reduced after MF TBS (94.4 ± 7.6 vs. 55.7 ± 5.1 pA after 20 min, n = 4; P < 0.001 for AMPAR-EPSCs; 176.2 ± 25.2 vs. 122.5 ± 11.4 pA after 20 min, n = 4; P < 0.05 for NMDAR-EPSCs), while the NMDAR/AMPAR ratio was unchanged (0.5 ± 0.06 vs. 0.4 ± 0.08 after 30 min, n = 5; P = 0.3; Fig. 4C). Such parallel changes in the amplitude of both AMPAR-EPSCs and NMDAR-EPSCs with a constant NMDAR/AMPAR ratio strongly suggest a presynaptic expression mechanism.
Changes in presynaptic function are usually accompanied by changes in the coefficient of variation (CV2) of EPSC amplitudes. Consistent with a decrease in presynaptic transmitter release (e.g., Choi and Lovinger 1997; Shen and Horn 1996), the CV2 of pharmacologically isolated AMPAR-EPSCs was significantly increased after TBS of MFs (n = 8, P = 0.0039 Wilcoxon signed-rank test); a similar increase was observed for the CV2 of pharmacologically isolated NMDAR-EPSCs after MF TBS (n = 14, P < 0.0001 Wilcoxon signed-rank test). Also consistent with a presynaptic change (Faber and Korn 1991; Shen and Horn 1996), the plot of the fractional change in CV2 (r) versus the synaptic gain factor (f) showed a significant correlation with all points to the right of the diagonal (r = f; AMPAR-EPSCs linear regression slope = 0.40, significantly different from unity P = 0.0246, n = 8; NMDAR-EPSCs linear regression slope = 0.35, significantly different from unity P = 0.0012, n = 14; Fig. 4D).
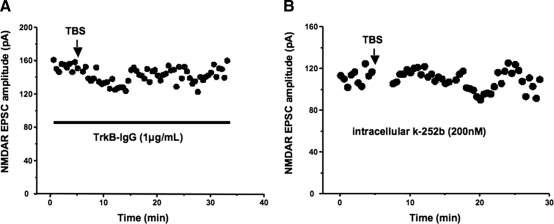
The role of activity-dependent release of endogenous BDNF on this enduring depression of NMDAR-mediated responses was tested using the scavenger TrkB-IgG. Indeed, the amplitude of NMDAR-mediated EPSCs remained unaffected after TBS in the presence of 1 μg/ml of TrkB-IgG in the recording ACSF (165.7 ± 12.8 vs. 142.3 ± 15.3 pA after 20 min, n = 9; P = 0.29; Fig. 5A). Consistent with a requirement of TrkB receptors, intracellular application of the membrane impermeable analog k-252b (200 nM in 0.01% DMSO) (Amaral and Pozzo-Miller 2007a; Knusel and Hefti 1992) also prevented TBS-induced depression of NMDAR-mediated EPSCs (185 ± 9.7 vs. 168.9 ± 7 pA after 20 min, n = 13; P = 0.1763; Fig. 5B). These observations suggest that an enduring depression of glutamatergic synaptic transmission at MF-CA3 synapses is a physiological consequence of activity-dependent release of endogenous BDNF from MFs.
Fig. 5.
The enduring depression of NMDAR-mediated EPSCs was sensitive to the BDNF scavenger TrkB-IgG and the receptor tyrosine kinase inhibitor k-252b. A: representative example illustrating the prevention of MF-TBS-induced depression of NMDAR-mediated EPSCs by bath application of TrkB-IgG. B: representative example illustrating the prevention of MF-TBS-induced depression of NMDAR-mediated EPSCs by intracellular application of k-252b.
DISCUSSION
We describe here several novel insights into the immediate actions of endogenous—native—BDNF on CA3 hippocampal neurons and of its activity-dependent release on excitatory synaptic transmission at MF-CA3 synapses. First, CA3 pyramidal neurons express IBDNF, a nonselective cationic current evoked by TBS of afferent MFs that required TrkB receptors and TRPC3-containing ion channels. Consistent with such activity-dependent release of BDNF, these MF-IBDNF responses were insensitive to manipulations of extracellular Zn2+ concentration. Second, MF-IBDNF was accompanied by dendritic Ca2+ elevations, which were sensitive to the BDNF scavenger TrkB-IgG. Last, TBS of MFs induced a long-lasting depression in the amplitude of EPSCs mediated by both AMPA and NMDA receptors that was associated with changes in the coefficient of variation (CV2) but not in the NMDAR/AMPAR ratio, suggesting a presynaptic expression mechanism. Such enduring depression of NMDAR-mediated EPSCs required activity-dependent release of endogenous BDNF from MFs and activation of Trk receptors, as it was sensitive to TrkB-IgG and the tyrosine kinase inhibitor k-252b.
In the present studies we focused on the MF pathway in CA3 because these fibers contain the highest levels of BDNF in the brain (Conner et al. 1997; Danzer and McNamara 2004). We stimulated these afferent fibers with theta burst patterns because they were shown to be very effective at causing BDNF release (Balkowiec and Katz 2002; Hartmann et al. 2001). The inhibition of these membrane currents by the well-known BDNF scavenger TrkB-IgG (Shelton et al. 1995) but not by a control IgG confirmed that they reflected the activity-dependent release of endogenous BDNF. The fact that TrkB-IgG completely abolished these responses also suggests that the release of vesicular Zn2+ from MFs during TBS did not contribute to the activation of TrkB-initiated, TRPC-mediated membrane currents. This interpretation is also supported by the lack of effect of the membrane-impermeant Zn2+ chelator Ca-EDTA on responses evoked by either endogenous (i.e., MF-IBDNF) or exogenous (i.e., recombinant) BDNF. Furthermore, increasing extracellular Zn2+ also failed to affect membrane currents evoked by direct application of recombinant BDNF. What role vesicular Zn2+ ions play is a matter of intense debate (Frederickson et al. 2005; Kay 2006; Kay and Toth 2008), one that is beyond the main topic of this communication. What is clear from our experiments is that trans-activation of TrkB receptors by synaptically released Zn2+ from MFs (Huang et al. 2008) does not contribute to the activation of TRPC3-mediated currents in CA3 pyramidal neurons.
Activation of a membrane conductance mediated by TRPC channels through the activation of the PLCγ pathway downstream of TrkB receptors seems to be the most immediate action of BDNF in central neurons. Support for this interpretation comes from observations of TRPC currents evoked within tens of seconds by exogenously applied recombinant BDNF in pontine neurons (Li et al. 1999) and CA1 pyramidal neurons (Amaral and Pozzo-Miller 2007a). Albeit with faster activation kinetics, membrane currents evoked by endogenously released BDNF are also sensitive to TrkB and TRPC pharmacological inhibitors (Amaral and Pozzo-Miller 2007a) as well as to shRNA-mediated TRPC3 channel knockdown (Fig. 2A). Similar kinetic differences between TRPC currents evoked by group-I mGluR agonists and endogenously released glutamate have been observed in Purkinje cells (Kim et al. 2003), dopamine neurons of the substantia nigra compacta (Bengtson et al. 2004), and neurons in the lateral amygdala (Faber et al. 2006). The parsimonious interpretation is that the faster activation and smaller amplitude of MF-IBDNF compared with IBDNF reflects spatially and temporally restricted release of a few BDNF granules from MF boutons.
What is the physiological consequence of endogenous BDNF released from MFs onto CA3 pyramidal neurons? The theta burst patterns of MF stimulation that evoked MF-IBDNF in CA3 neurons caused an enduring change in the strength of transmission at MF-CA3 synapses. Indeed, both AMPAR- and NMDAR-mediated EPSCs were significantly depressed after TBS of MFs, an effect prevented by the BDNF scavenger TrkB-IgG and the tyrosine kinase inhibitor k-252b. Because this depression was associated with changes in CV2 but not in the NMDAR/AMPAR ratio, its expression mechanism appears to be a reduction in presynaptic neurotransmitter release (Faber and Korn 1991; Shen and Horn 1996), an unexpected result considering the well-documented enhancement of presynaptic transmitter release by BDNF (Carvalho et al. 2008; Tyler et al. 2002). Regarding the LTP induced in CA3 pyramidal neurons by TBS of MFs reported by Nicholls et al. (2006), we should note that differences in the duration of TBS conditioning stimuli likely account for the different outcomes in that study and in the present communication. Despite the varied mechanisms and loci of induction of bidirectional synaptic plasticity at MF-CA3 synapses, most studies agree on a presynaptic site of expression (Nicoll and Schmitz 2005). For example, MF-LTP of AMPAR-mediated EPSCs is due to enhanced neurotransmitter release by cAMP/PKA-mediated modulation of synaptic vesicle docking and fusion, likely via a GTP-dependent interaction between Rab3A on synaptic vesicles and RIM1α at the active zone (Castillo et al. 1997, 2002), although the direct phosphorylation of RIM1α by PKA is not necessary for presynaptic LTP (Kaeser et al. 2008); it should be noted that MF-LTP of NMDAR-mediated responses is induced and expressed by entirely postsynaptic mechanisms (Kwon and Castillo 2008).
Relevant to the enduring depression of synaptic transmission described here, MF-CA3 synapses during the second postnatal week express two forms of NMDAR-independent LTD that differ in their dependency on mGluR activation (Domenici et al. 1998). However, both of these forms of MF-LTD are induced by prolonged low-frequency stimulation, which is not sufficient to cause BDNF release (e.g., Balkowiec and Katz 2002; Hartmann et al. 2001). MF-CA3 synapses also express a presynaptic form of long-term depression (LTD) of AMPAR-mediated EPSCs (Kobayashi et al. 1996) that involves presynaptic cAMP/PKA signaling (Tzounopoulos et al. 1998), indicating that the above-described presynaptic modulation of synaptic vesicle docking and fusion is bidirectional. Intriguingly, BDNF/TrkB signaling may also involve the active zone protein RIM1α, as the presynaptic effect of BDNF is absent in cultured hippocampal neurons lacking its activator Rab3A in synaptic vesicles (Alder et al. 2005; Thakker-Varia et al. 2001). Because most studies of the presynaptic effects of BDNF were performed either in dissociated cultures of hippocampal neurons (Jovanovic et al. 1996, 2000; Yano et al. 2006)—which almost exclusively contain pyramidal neurons and lack dentate granule cells and their MF boutons—or at CA3-CA1 synapses in hippocampal slices (Figurov et al. 1996; Gottschalk et al. 1998; Pozzo-Miller et al. 1999; Tyler et al. 2006), it remains to be determined whether MF boutons have different intracellular signaling cascades or synaptic vesicle and/or active zone molecular targets than their Schaffer collateral counterparts.
In summary, we uncovered the most immediate response to endogenously released—native—BDNF in hippocampal neurons and its role in the modulation of excitatory synaptic strength at MF-CA3 synapses, lending further credence to the relevance of BDNF signaling for synaptic function in the hippocampus.
GRANTS
This work was supported by International Rett Syndrome Foundation Postdoctoral Fellowships to Drs. Y. Li and G. Calfa, and by National Institute of Neurological Disorders and Stroke Grants NS-40593, NS-057780, and NS-065027 to L. Pozzo-Miller. We also acknowledge University of Alabama at Birmingham Intellectual and Developmental Disabilities Research Center Grant P30-HD38985 and the UAB Neuroscience Cores Grants P30-NS47466 and P30-NS57098.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Alder J, Thakker-Varia S, Crozier RA, Shaheen A, Plummer MR, Black IB. Early presynaptic and late postsynaptic components contribute independently to brain-derived neurotrophic factor-induced synaptic plasticity. J Neurosci 25: 3080–3085, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem 11: 172–178, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Chapleau CA, Pozzo-Miller L. Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling: potential implications for Rett syndrome. Pharmacol Ther 113: 394–409, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Pozzo-Miller L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci 27: 5179–5189, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Pozzo-Miller L. BDNF induces calcium elevations associated with IBDNF, a nonselective cationic current mediated by TRPC channels. J Neurophysiol 98: 2476–2482, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1: 602–609, 1998. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260: 181–186, 1993. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci 22: 10399–10407, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Pugh JR, Jahr CE. Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses. J Neurosci 29: 10974–10978, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. J Physiol 555: 323–330, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Garcia DE, Inagaki N, Hahnel C, Lindholm D. BDNF and NT-3 induce intracellular Ca2+ elevation in hippocampal neurones. Neuroreport 4: 1303–1306, 1993. [DOI] [PubMed] [Google Scholar]
- Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol Cell Physiol 242: C404–408, 1982. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Goldman DE. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol 51: 279–291, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature 419: 687–693, 2002. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76: 99–125, 2005. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol 153, Suppl 1: S310–324, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fiber long-term potentiation in the hippocampus. Nature 388: 590–593, 1997. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature 415: 327–330, 2002. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA 94: 2665–2670, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17: 2295–2313, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, McNamara JO. Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feedforward inhibition of CA3 pyramidal cells. J Neurosci 24: 11346–11355, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt H-U, Zieglgänsberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res 537: 333–336, 1990. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Berretta N, Cherubini E. Two distinct forms of long-term depression coexist at the mossy fiber-CA3 synapse in the hippocampus during development. Proc Natl Acad Sci USA 95: 8310–8315, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem 272: 19103–19106, 1997. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods 28: 1–11, 1996. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J 60: 1288–1294, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience 137: 781–794, 2006. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381: 706–709, 1996. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci 6: 449–462, 2005. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods 4: 329–342, 1981. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain- derived neurotrophic factor in the developing hippocampus. J Neurosci 18: 6830–6839, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. Embo J 20: 5887–5897, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes Sunderland, MA: Sinauer, 2001. [Google Scholar]
- Hille B, Woodhull AM, Shapiro BI. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, pH. Philos Trans R Soc Lond B Biol Sci 270: 301–318, 1975. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57: 546–558, 2008. [DOI] [PubMed] [Google Scholar]
- Huber KM, Sawtell NB, Bear MF. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology 37: 571–579, 1998. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA 93: 3679–3683, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 3: 323–329, 2000. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Sudhof TC. RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci USA 105: 14680–14685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 401: 918–921, 1999. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267: 1658–1662, 1995. [DOI] [PubMed] [Google Scholar]
- Kay AR. Imaging synaptic zinc: promises and perils. Trends Neurosci 29: 200–206, 2006. [DOI] [PubMed] [Google Scholar]
- Kay AR, Toth K. Is zinc a neuromodulator? Sci Signal 1: , 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426: 285–291, 2003. [DOI] [PubMed] [Google Scholar]
- Knusel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem 59: 1987–1996, 1992. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science 273: 648–650, 1996. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92: 8856–8860, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumura E, Kimura F, Taniguchi N, Tsumoto T. Brain-derived neurotrophic factor blocks long-term depression in solitary neurones cultured from rat visual cortex. J Physiol 524: 195–204, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, Cui Y, LeBoutillier JC, Marrone DF, Choi ES, De Zeeuw CI, Petit TL, Pozzo-Miller L, Silva AJ. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci 25: 9721–9734, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 57: 108–120, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Rosenmund C, Westbrook GL. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci 13: 674–684, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24: 261–273, 1999. [DOI] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber –> Ca3 long-term potentiation requires translocation of synaptically released Zn2+. J Neurosci 21: 8015–8025, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo DC, McAllister AK, Katz LC. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 13: 1263–1268, 1994. [DOI] [PubMed] [Google Scholar]
- Malinow R, Otmakhov N, Blum KI, Lisman J. Visualizing hippocampal synaptic function by optical detection of Ca2+ entry through the N-methyl-d-aspartate channel. Proc Natl Acad Sci USA 91: 8170–8174, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L., Jr The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol 415: 351–365, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Jr, Westbrook GL. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol 415: 329–350, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36: 121–137, 2002. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24: 401–414, 1999. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem 10: 108–115, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 108: 595–598, 2002. [DOI] [PubMed] [Google Scholar]
- Nicholls RE, Zhang X-L, Bailey CP, Conklin BR, Kandel ER, Stanton PK. mGluR2 acts through inhibitory Gα subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc Natl Acad Sci USA 103: 6380–6385, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fiber synapses. Nat Rev Neurosci 6: 863–876, 2005. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16: 1137–1145, 1996. [DOI] [PubMed] [Google Scholar]
- Perkel DJ, Petrozzino JJ, Nicoll RA, Connor JA. The role of Ca2+ entry via synaptically activated NMDA receptors in the induction of long-term potentiation. Neuron 11: 817–823, 1993. [DOI] [PubMed] [Google Scholar]
- Petrozzino JJ, Pozzo-Miller LD, Connor JA. Micromolar Ca2+ transients in dendritic spines of hippocampal pyramidal neurons in brain slice. Neuron 14: 1223–1231, 1995. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32, 2001. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller L. BDNF enhances dendritic Ca2+ signals evoked by coincident EPSPs and back-propagating action potentials in CA1 pyramidal neurons. Brain Res 1104: 45–54, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Petrozzino JJ, Connor JA. G protein-coupled receptors mediate a fast excitatory postsynaptic current in CA3 pyramidal neurons in hippocampal slices. J Neurosci 15: 8320–8330, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Petrozzino JJ, Golarai G, Connor JA. Ca2+ release from intracellular stores induced by afferent stimulation of CA3 pyramidal neurons in hippocampal slices. J Neurophysiol 76: 554–562, 1996. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Petrozzino JJ, Mahanty NK, Connor JA. Optical imaging of cytosolic calcium, electrophysiology, ultrastructure in pyramidal neurons of organotypic slice cultures from rat hippocampus. Neuroimage 1: 109–120, 1993. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci 19: 4972–4983, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M, Tongiorgi E, Cattaneo A. Brain-derived neurotrophic factor (BDNF) induces dendritic targeting of BDNF and tyrosine kinase B mRNAs in hippocampal neurons through a phosphatidylinositol-3 kinase-dependent pathway. J Neurosci 20: 3165–3174, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12: 1207–1221, 1994. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19: 463–489, 1996. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, expression of extracellular domain immunoadhesins. J Neurosci 15: 477–491, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WX, Horn JP. Presynaptic muscarinic inhibition in bullfrog sympathetic ganglia. J Physiol 491: 413–421, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37: 173–182, 1991. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci 21: 6782–6790, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 114: 795–805, 2002. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20: 8279–8289, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist 8: 524–531, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249–4258, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Zhang XL, Hartman K, Winterer J, Muller W, Stanton PK, Pozzo-Miller L. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol 574: 787–803, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron 21: 837–845, 1998. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26: 187–196, 2000. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science 245: 192–194, 1989. [DOI] [PubMed] [Google Scholar]
- Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat Neurosci 9: 1009–1018, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.