Abstract
Osteogenesis is synergistically enhanced by the combined effect of complimentary factors. This study showed that Nell-1 and BMP-2 synergistically enhanced osteogenic differentiation of myoblasts and phosphorylated the JNK MAPK pathway. The findings are important because of the osteochondral specificity of Nell-1 signaling and the potential therapeutic effects of coordinated BMP-2 and Nell-1 delivery.
Introduction
BMPs play an important role in the migration and proliferation of mesenchymal cells and have a unique ability to alter the differentiation of mesenchymal cells toward chondrogenic and osteogenic lineages. Signaling upstream of Cbfa1/Runx2, BMPs effects are not limited to cells of the osteoblast lineage. Thus, additional osteoblast-specific factors that could synergize with BMP-2 would be advantageous for bone regeneration procedures. NELL-1 (NEL-like molecule-1; NEL [a protein strongly expressed in neural tissue encoding epidermal growth factor like domain]) is a novel growth factor believed to preferentially target cells committed to the osteochondral lineage.
Materials and Methods
C2C12 myoblasts were transduced with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1+AdBMP-2 overexpression viruses. Effects were studied by cell morphology, alkaline phosphatase activity, osteopontin production, and MAPK signaling. Additionally, in a nude mouse model, viruses were injected into leg muscles, and new bone formation was examined after 2 and 8 wk.
Results
C2C12 myoblasts co-transduced with AdNell-1+AdBMP-2 showed a synergistic effect on osteogenic differentiation as detected by alkaline phosphatase activity and osteopontin production. Nell-1 stimulation on AdNell-1 + AdBMP-2 preconditioned C2C12 cells revealed significant activation of the non-BMP-2 associated c-Jun N-terminal kinase (JNK) MAPK signaling pathway, but not the p38 or extracellular signal-regulated kinase (ERK1/2) MAPK pathways. Importantly Nell-1 alone did not induce osteogenic differentiation of myoblasts. In a nude mouse model, injection of AdNell-1 alone stimulated no bone formation within muscle; however, injection of AdNell-1+AdBMP-2 stimulated a synergistic increase in bone formation compared with AdBMP-2 alone.
Conclusions
These findings are important because of the confirmed osteochondral specificity of Nell-1 signaling and the potential therapeutic effects of enhanced BMP-2 action with coordinated Nell-1 delivery.
Keywords: Nell-1, BMP-2, synergy, osteogenesis, muscle, C2C12
INTRODUCTION
Soluble factors that participate in bone formation include the BMPs, TGFs, fibroblast growth factors (FGFs), and other various cytokines. In particular, the BMPs play an important role in the migration and proliferation of mesenchymal cells and have a unique ability to alter the differentiation of osseous and nonosseous mesenchymal cells toward the osteogenic lineage.(1,2) Osteogenic induction by BMPs has been extensively studied in pluripotent and myogenic cells and can induce bone formation through recombinant protein delivery or viral mediated delivery. BMP-2 is reported to target the core binding factor α 1/runt-related transcription factor 2 (Cbfa1/Runx2), a transcription factor essential to osteoblast and hypertrophic chondrocyte differentiation, and induce osteoblastic gene expression.(3,4) BMPs signal upstream of Cbfa1/Runx2 and their effects are not limited to cells of the osteoblast lineage. This quality makes them both powerful and potentially harmful for therapeutic uses where large doses are needed, and excessive bone formation is undesired.
The process of bone formation is complex and can be enhanced synergistically by the combined effect of complimentary factors. These targeted therapies aim to increase efficacy and reduce the toxicity profile as a rapidly emerging therapeutic strategy. The synergistic actions of growth factors are apparent in many model systems. Synergy in osteogenic differentiation has been reported previously between Runx2/Cbfa1 and BMP-2,(5) BMP-2, -4, and -7,(6) BMP-2 and sonic hedgehog,(7) oxysterols and BMP-2,(8) and C-type natriuretic peptide and BMP-7.(9) Whereas the use of factors that enhance each other’s actions is beneficial, the use of osteoblast-specific factors that would act synergistically with BMPs would be a great advantage for bone regeneration therapies.
NELL-1 (NEL-like molecule-1; NEL [a protein strongly expressed in neural tissue encoding epidermal growth factor like domain]) is a novel growth factor believed to specifically target cells committed to the osteochondral lineage.(10) NELL-1 was isolated and characterized in craniosynostosis patients as specifically upregulated within prematurely fusing sutures.(10-13) The phenotype of the Nell-1 transgenic overexpression mouse revealed cranial suture overgrowth similar to human craniosynostosis,(10) suggesting a distinct role for Nell-1 in bone formation. Conversely, a mouse model with mutated ENU-induced alleles, including Nell-1, resulted in cranial and other vertebral skeletal defects.(14) In committed osteoblasts, Nell-1 up-regulation accelerates osteogenic differentiation and bone formation.(10,11,13,15,16) Interestingly, we have recently shown that human NELL-1 is directly regulated by Cbfa1/Runx2,(17) confirming its osteochondral specificity.(18)
This study was pursued to assess whether Nell-1 and BMP-2 enhance each other’s ability to stimulate osteoblastic cell differentiation in vitro and bone formation in vivo. We hypothesized that Nell-1 would act synergistically with BMP-2 to stimulate osteoblastic cell differentiation, because they signal through different pathways. To test this hypothesis, C2C12 myoblasts co-transduced with AdNell-1+AdBMP-2 overexpression viruses showed synergistic effects on osteogenic differentiation. These results were further confirmed in a nude mouse model. These findings are important because of the osteoblast specificity of Nell-1 signaling and the potential therapeutic effects of enhanced osteogenesis with coordinate BMP-2 and Nell-1 delivery.
MATERIALS AND METHODS
Alkaline phosphatase activity and osteopontin production
C2C12 myoblasts were purchased from the American Type Culture Collection (ATCC). Cells were cultured in DMEM containing 10% FBS, 100 IU/ml penicillin, and 100 IU/ml streptomycin at 37°C in an atmosphere of 5% CO2. On subconfluence, cells were split for expansion and experimentation. The induction of cell morphology changes and alkaline phosphatase (ALP) activity was assessed at days 0, 3, 6, 9, 12, and 15 after transduction of subconfluent C2C12 cells with an adenovirus overexpressing rat Nell-1 (AdNell-1) and/or mouse BMP-2 (AdBMP-2) driven by a CMV promoter(5,10) at a MOI (pfu/cell) of 0, 20, and 40 pfu/cell, with additional AdLacZ to set the overall MOI of each group to 80 pfu/cell. After gene transduction, cells were cultured in culture medium supplemented with 50 μg/ ml ascorbic acid, 10 mM β-glycerophosphate, and 10-8 M dexamethasone. Additional samples were cultured in the presence of 25 μM SP600125 (InSolution JNK Inhibitor II solution; Calbiochem) as previously described.(19) Proteins were harvested in lysis buffer with 0.2% NP-40, and ALP activity was determined with the use of p-nitrophenol phosphate (Sigma-Aldrich, St Louis, MO, USA) as a substrate at an absorbance at 405 nm. Experimental conditions were done in triplicate and normalized with the concentrations of total cellular proteins as determined by BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Enzyme activity was expressed as the fold increase over the day 0 control. Twelve days after gene transduction, the amount of OPN secreted into culture media was measured by ELISA as described by supplier (Assay Designs, Ann Arbor, MI, USA). Absorbance was recorded at 450 nm. Both experiments are presented as the mean result of two biological experiments in triplicate; error bars indicate fluctuations. Student’s t-test was used to assess significant differences, with p ≤ 0.05 considered significant.
Real-time PCR
Total RNA was extracted by Trizol reagent, and DNase-treated RNA was tested for its integrity by agarose gel electrophoresis. One microgram of DNase I treated total RNA was used for reverse transcription as previously described.(10) The product of reverse transcription was used for real-time PCR. Real-time PCR analysis of Nell-1, BMP-2, BMP-receptor1b (R1b), BMP-R2, α-smooth muscle actin (SMA), and GAPDH gene expression was performed with the ABI Prism 7300 real time PCR system, and the primers and probes were purchased as TaqMan primer-probe sets (Applied Biosystems). Analysis was based on calculating the relative expression level of the gene of interest compared with GAPDH(20) and normalized to the expression induced by AdLacZ control at corresponding time-points.
Alizarin red staining and quantification
C2C12 cells were either infected with AdLacZ, AdNell-1, AdBMP-2, or a combination. Uninfected C2C12 cells were treated with rhNell-1 and/or rhBMP-2 or treated at each media change with conditioned media from C2C12 cells that had been viral infected. Samples were cultured for 4 wk before alizarin red staining occurred. Samples were fixed for 15 min in 10% formalin, stained for 2 min in 1% Alizarin red stain, and rinsed repeatedly in water. Photomicrographs were taken with the BX51 Olympus microscope and Picture Frame software. For quantification, alizarin red stain was recovered in 10% acetic acid, heated to 85°C for 10 min and iced, and read in duplicate on a plate reader at 450 nm. All experiments were performed twice in triplicate. Student’s t-test was used to assess significant differences from AdLacZ infected cells or uninfected cells with p < 0.05 considered statistically significant.
Western blot analysis
Cell lysate was harvested 3 or 6 days after C2C12 transduction with AdLacZ, AdNell-1, or AdBMP-2. Forty micrograms of protein was separated on a 4-20% gradient SDS gel (BioRad Laboratories, Hercules, CA, USA). After transferring overnight to a nitrocellulose membrane, proteins were incubated with anti-Nell-1 and anti-BMP-2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by anti-rabbit and anti-goat HRP conjugated antibodies (Pierce Biotechnology, Rockford, IL, USA). Membranes were exposed to Super Signal ECL Western blot detecting agent (Pierce Biotechnology) and visualized on film. Protein loading and transfer efficiency was assessed using the MemCode reversible protein stain kit (Pierce Biotechnology) according to manufacturer’s instructions.
Phosphorylation studies
For examination of receptor phosphorylation, subconfluent cells were transduced with AdLacZ, AdNell-1, AdBMP-2, and/or AdNell-1 + AdBMP-2 at a MOI (pfu/cell) of 40. After gene transduction, cells were cultured in culture medium supplemented with 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and 10-8 μM dexamethasone. On day 5, cells were trypsinized and seeded at 1.5 × 104 cells per well in a 96-well plate and cultured overnight in serum-free medium. The following morning, cells were stimulated with 4 μg/ml rhNell-1 for 10 min to saturate receptors. After fixing, receptor phosphorylation was examined with the Cellular Activation of Signaling ELISA (CASE) kit (Super Array Bioscience, Frederick, MD, USA) for monitoring the activation of the MAPK pathways (p38, c-Jun n-terminal kinase [JNK], extracellular signal-regulated kinase [ERK1/ 2]) according to the manufacturer’s instructions. Immunoreactivity readings (OD450) were normalized by cell number (OD595) and reported as a ratio of phosphorylated to total protein. Experimental conditions were repeated twice in duplicate, and error bars indicate fluctuations.
Muscle injection and μCT imaging
Mature male nude mice were purchased from Charles River (Wilmington, MA, USA). Mice were injected in the thigh muscle with a fixed dose of 1 × 109 pfu each of AdLacZ (n = 7), 1 × 109 pfu AdNell-1 (n = 6), 1 × 109 pfu AdBMP-2 (n = 7), or 5 × 108 pfu AdNell-1 plus 5 × 108 pfu AdBMP-2 (n = 12) diluted in saline. Animals were live imaged at 2, 4, and 8 wk after injection using a μCT scanner (Imtek, Knoxville, TN, USA) to acquire 3D morphometric data as previously described.(21) The large degree of mineralization in AdBMP-2 and AdNell-1 + AdBMP-2 groups rendered some mice immobilized because of fusion between various bones of the legs and torso. Mice were anesthetized with isoflurane, and images were acquired with the X-ray source biased at 35 kVp and 400 μA. Data sets for mice were acquired and reconstructed with resolutions of 200 μm. Visual analyses of the CT data were performed using AVS/Express (vs. 5.1; Advanced Visual Systems, Waltham, MA, USA). Additional samples were harvested after 2 or 8 wk for high-resolution μCT analysis and histology. A high-resolution μCT (μCT40; Scanco, Southeastern, PA, USA) was used as previously published.(10) μCT data were collected at 50 kVp and 160μA. Visualization, reconstruction, and volume analysis of the data were performed using the MetaMorph Imaging System (Universal Imaging, Downingtown, PA, USA) using a threshold of 100.
Histological and histochemical analysis and quantification
Ten-micron-thick paraffin sections of decalcified samples were stained with H&E(22) and Masson’s trichrome stain according to standard protocols. Additional sections were incubated with anti-Cbfa1, anti-Sox9, anti-OSX, anti-OCN (Santa Cruz Biotechnology), and anti-Nell-1(15) primary antibodies followed by biotinylated anti-rabbit and anti-goat IgG secondary antibodies (Vector Laboratories). Positive immunoreactivity was detected using Vectastain ABC kit (Vector Laboratories) and DAB reagent (Sigma-Aldrich). Controls for each antibody consisted of incubation with secondary antibody in the absence of primary antibody. Sections were counterstained with hematoxylin for identification of nuclei. Photomicrographs were taken with the BX51 Olympus microscope and Picture Frame software. Protein production was quantified by ELISA analysis. Total protein from 20 histological sections of 20 μm thickness of paraffin-embedded tissues were extracted as previously reported.(23) Briefly, sections were dissolved in Octane (Fisher), methanol was added, and the dissolved paraffin in Octane was removed. Remaining tissues were incubated at 100°C in standard RIPA buffer for 20 min, followed by 60°C for 2 h and then iced. Proteins were quantified using the BCA Protein Assay Kit (Bio-Rad). For ELISA analysis, flat bottom multiwell plates were coated with 100 ng/well of sample protein in 200-μl coating buffer (0.5 mM sodium carbonate buffer, pH 9.3) overnight at 4°C. Wells were washed with 200 μl of binding buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 2% BSA, and 0.05% Tween20) to block nonspecific binding sites and incubated for 2 h at 37°C. Wells were washed three times, and 0.2 μg/ml of primary antibodies (anti-Cbfa1, anti-Sox9, or anti-OSX; Santa Cruz) were incubated for 1 h at 37°C. Wells were subsequently incubated with biotinylated secondary antibodies and streptavidin-horseradish peroxidase (HRP; Dako). One hundred microliters per well of developing buffer (100 μg/ml tetramethylbenzidine and 0.003% H2O2 in sodium acetate buffer, pH 6.0) was used, and the reaction was stopped with 1 N hydrochloric acid. Binding activity of each antibody to the samples was determined by reading the absorbance at 450 nm. All the experiments were performed on three samples of each group in triplicate. Student’s t-test was used to assess significant differences.
RESULTS
Synergistic effects of Nell-1 and BMP-2 on osteogenic differentiation of C2C12 myoblasts
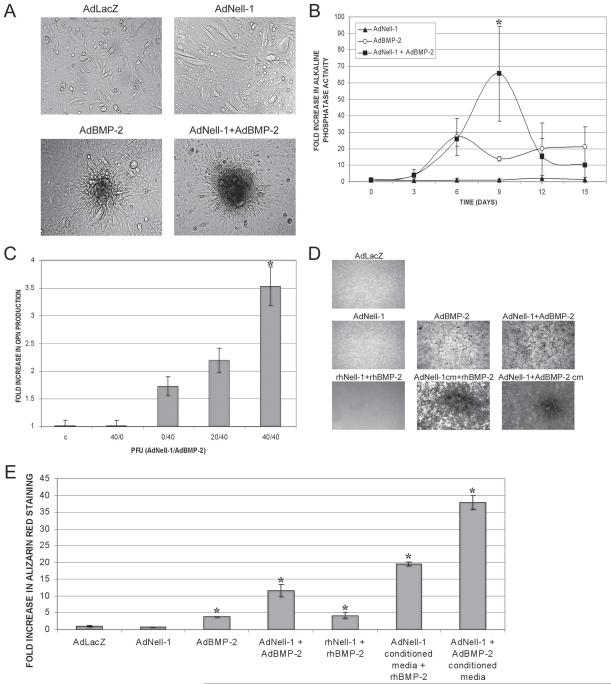
To examine the effects of Nell-1 on myoblasts, we began by studying cell morphology. C2C12 myoblast morphology was examined 6 days after transduction with 40 pfu each of AdLacZ, AdNell-1, AdBMP-2, or AdNell-1+AdBMP-2 (Fig. 1A). C2C12 myoblasts transduced with AdLacZ or AdNell-1 maintained characteristic myoblast morphology with elongated tubular cell bodies. Cells transduced with AdBMP-2 showed a more cuboidal shape and occasional nodule formation, whereas cells transduced with AdNell-1+AdBMP-2 showed cuboidal cells with large and dense nodule formation. Cellular effects were further examined through proliferation rates and myoblast gene expression (data not shown). Proliferation rates remained relatively unchanged in all groups on days 3-15. Furthermore, the expression of α-SMA increased 2.1-fold over day 0 in AdLacZ and AdNell-1 samples on days 3 and 6 after transduction, whereas AdBMP-2-infected samples decrease α-SMA expression to 0.75-fold on day 6 (data not shown). The osteogenic effects of Nell-1 and BMP-2 on C2C12 myoblasts were further examined by ALP activity (Fig. 1B). Previous studies showed that AdBMP-2 alone induced a dose-dependent elevation in ALP activity in C2C12 cells around day 6, which decreased by day 9.(24-26) These results confirmed the previous finding: 40 pfu AdBMP-2 alone stimulated a 27-fold increase in ALP activity on day 6 compared with control (40 pfu AdLacZ). In contrast, 40 pfu AdNell-1 transduction alone did not increase the basal level of ALP activity at any time-point. Importantly, although the AdBMP-2 (40 pfu) and the AdNell-1+AdBMP-2 (40 pfu each) groups had a similar increase in ALP activity on day 6 of 27- and 25.8-fold, respectively, only the AdNell-1+AdBMP-2 group continued to increase significantly on day 9—65.5-fold over control. Osteogenic differentiation was also studied through osteopontin (OPN) protein production on day 12 (Fig. 1C). Similar to the above data, 40 pfu AdNell-1 alone did not increase OPN production, whereas 40 pfu AdBMP-2 alone stimulated an increase (1.7-fold) in OPN production. Again, AdNell-1 acted synergistically with AdBMP-2 to increase OPN production in a dose-dependent manner. OPN production was increased 2.2- and 3.5-fold with AdNell-1/AdBMP-2 at 20/40pfu and 40/40pfu, respectively. Finally, end stage differentiation, through extracellular matrix mineralization, was further examined (Figs. 1D and 1E). Like control uninfected or AdLacZ infected C2C12 cells, AdNell-1 infection did not stimulate mineralization. Significantly upregulated mineralization was detected in samples infected with AdBMP-2 (3.8-fold), with a synergistic upregulation in samples infected with AdNell-1+AdBMP-2 (11.6-fold). Synergistic actions were further examined on uninfected C2C12 cells through the use of recombinant Nell-1 and/or BMP-2 protein or the use of conditioned media (cm) from virus-infected C2C12 cells. Stimulation with 100 ng of both Nell-1 and BMP-2 resulted in a 4.1-fold increase in mineralization, which was lower than that detected in double virus-infected cells. These results suggested that the virus infected cells produced >100 ng of protein each. Because of these findings, we further examined the effects of AdNell-1 and/or AdBMP-2 cm. The use of AdNell-1 cm in addition to rh-BMP-2 revealed a synergistic 19.5-fold increase in mineralization, indicating that very little BMP-2 was needed to promote the effects of Nell-1. Accordingly, the use of cm from AdNell-1+AdBMP-2-infected cells resulted in the most dramatic increase in mineralization, with a 37.9-fold increase, suggesting that viral infection itself somehow reduced the cells ability to mineralize. Thus, the results showed that Nell-1 and BMP-2 acted synergistically through extracellular stimulation to increase mineralization in C2C12 cells.
FIG. 1.
Synergistic effects of Nell-1 and BMP-2 on osteogenic differentiation of C2C12 myoblasts. (A) Cell culture images displaying C2C12 cell morphology 6 days after transduction with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1 + AdBMP-2. Original magnification: ×100. (B) Graph displaying the fold increase of ALP activity in C2C12 myoblasts after transduction with 0, 20, or 40 pfu of AdNell-1 and/or AdBMP-2. (C) Graph displaying the fold increase of OPN protein found in C2C12 culture media 12 days after transduction with 0, 20, or 40 pfu of AdNell-1 and/or AdBMP-2. (D) Images displaying alizarin red staining and (E) a graph quantifying alizarin red staining in C2C12 cells infected with AdLacZ, AdNell-1, AdBMP-2, AdNell-1±AdBMP-2, or uninfected C2C12 cells stimulated with rhNell-1 and/or rhBMP-2 protein, with or without conditioned media from viral-infected C2C12 cells. Data are expressed as the mean ± SE. *Significant differences from control samples of corresponding time-points; p ≤ 0.03.
Mechanism of Nell-1 signaling in C2C12 myoblasts
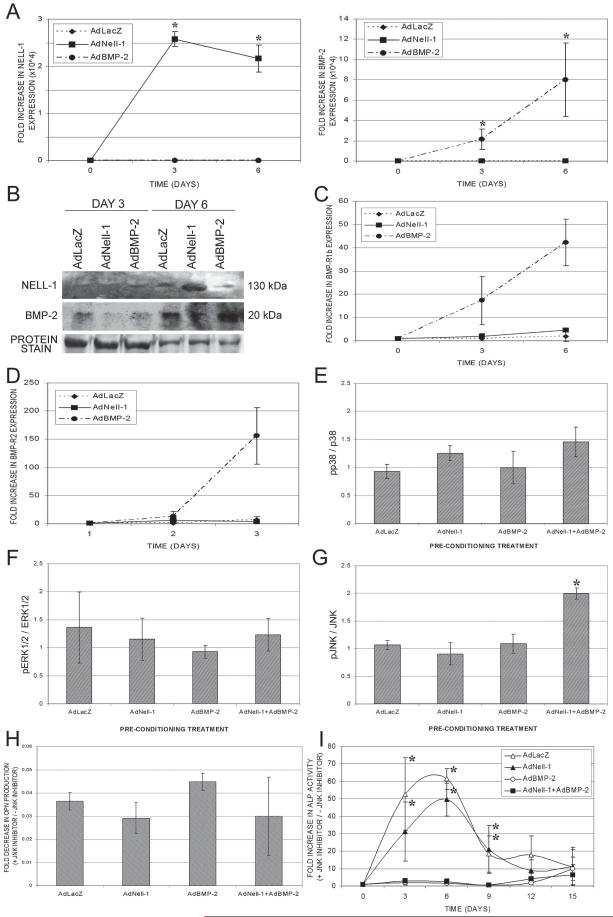
To confirm viral expression in C2C12 myoblast cultures, real-time PCR showed significantly elevated Nell-1 expression only in cultures transduced with AdNell-1 and significantly elevated BMP-2 expression only in cultures transduced with AdBMP-2 on days 3 and 6 (Fig. 2A). Nell-1 and BMP-2 protein production was further confirmed by Western blot analysis (Fig. 2B), with elevated protein production on day 6 in corresponding cultures. Basal production of these proteins was noted and can be attributed to culture conditions including the differentiation media. Based on the synergistic actions of Nell-1 and BMP-2 to induce osteogenic differentiation of C2C12 myoblasts, we further studied the role of Nell-1 and BMP-2 signaling pathways. We first studied the ability of Nell-1 to enhance BMP-2 signaling through an upregulation of BMP receptors (BMP-R). As shown by real-time PCR, 3 and 6 days after transduction, AdNell-1 did not significantly alter the expression of either BMP-R1b and BMP-R2 compared with control, whereas AdBMP-2 transduction increased the expression of BMP-R1b 17- and 42-fold and BMP-R2 13- and 156-fold, on days 3 and 6, respectively (Figs. 2C and 2D). Finally, we studied the possibility that BMP-2 could enhance Nell-1 signaling. Because the receptor for Nell-1 has not been identified, we studied the classical MAPK pathway: p38, ERK1/2, and JNK. The data showed that Nell-1 stimulation resulted in no significant change in the activation of the p38 (Fig. 2E) and ERK1/2 (Fig. 2F) pathways after 6 days of preconditioning with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1+AdBMP-2. In contrast, the results showed a significant Nell-1-induced 2-fold increase in JNK activation after 6 days of preconditioning with AdNell-1+AdBMP-2 (Fig. 2G). Interestingly, JNK activation was not observed in cells preconditioned with AdLacZ, AdNell-1, or AdBMP-2 alone. These data are significant, because they suggest that the preconditioning period involving AdNell-1+AdBMP-2 transduction affected the myoblasts’ ability to respond to Nell-1 stimulation and that Nell-1 and BMP-2 worked synergistically to enhance Nell-1-induced JNK signaling, a signaling pathway not linked to BMP-2 stimulation of myoblasts. JNK signaling was further studied through it’s disruption using SP600125, a specific and direct inhibitor of JNK activity. We first examined OPN production. OPN is a well-recognized osteoblast differentiation marker. During osteogenesis, OPN is found in bone-forming cells almost concomitantly with the appearance of ALP and before osteoid deposition, whereas osteocalcin is still absent during the early stages of mineralization. Blocked JNK signaling almost entirely eliminated OPN production on day 12, with no significant differences between groups in C2C12 muscle stem cells (Fig. 2H). These data suggested that blocked JNK signaling eliminated the cells’ innate ability to produce OPN, even in the presence of AdNell-1 and/or AdBMP-2. Combined data from Figs. 1C, 2G, and 2H suggest that JNK phosphorylation played a key role in the ability of C2C12 cells to produce OPN and to convert myoblasts into osteoblast-like cells. AdNell-1+AdBMP-2 preconditioning allowed Nell-1 to phosphorylate the JNK pathway, which in turn induced OPN expression and an osteoblastic phenotype in C2C12 muscle stem cells. To further verify that Nell-1 + BMP-2 induced C2C12 cells to convert into an osteoblast-like cell through JNK signaling, we examined ALP activity, an early marker for matrix maturation. AdNell-1+AdBMP-2 induced upregulated ALP activity as indicated in Fig. 1B. However, blocking JNK phosphorylation did not inhibit ALP expression in AdNell-1+AdBMP-2-transduced cells; in fact, ALP activity increased 2.9- and 2.6-fold on days 3 and 6, respectively, for this group (Fig. 2I). A perhaps overly simplified explanation is that Nell-1 + BMP-2 may have induced ALP expression through pathways other than JNK. The results also suggest that gene expression and regulatory pathways are distinctive between normal osteoblast differentiation and muscle stem cells “conversion” into an osteoblastic phenotype.
FIG. 2.
Mechanism of Nell-1 signaling in C2C12 myoblasts. (A) Real-time PCR and (B) Western blot analysis for the expression and production of Nell-1 and BMP-2 in C2C12 myoblasts after transduction with 40 pfu AdLacZ, AdNell-1, or AdBMP-2. Protein staining was performed to assess loading and transfer efficiency. Graph displaying fold increase in real-time PCR expression levels of (C) BMP-R1b and (D) BMP-R2 on days 3 and 6 after transduction with AdLacZ, AdNell-1, or AdBMP-2. Graph displaying mean ratios of (E) phosphorylated p38 to total p38, (F) phosphorylated ERK1/2 to total ERK1/2, and (G) phosphorylated JNK to total JNK in C2C12 myoblasts after 10 min of stimulation with Nell-1. Cells had been pretreated by transduction with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1+AdBMP-2 for 6 days. Data are expressed as the mean ± SE of the fold increase of stimulated over unstimulated samples. Graphs displaying the fold increase in (H) OPN protein production or (I) ALP activity in C2C12 myoblasts infected with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1±AdBMP-2 on culture with 25 μM JNK inhibitor divided by results obtained in the absence of JNK inhibitor. *Significant differences; p ≤ 0.05.
Nell-1 and BMP-2 synergistically induce bone in thigh muscle
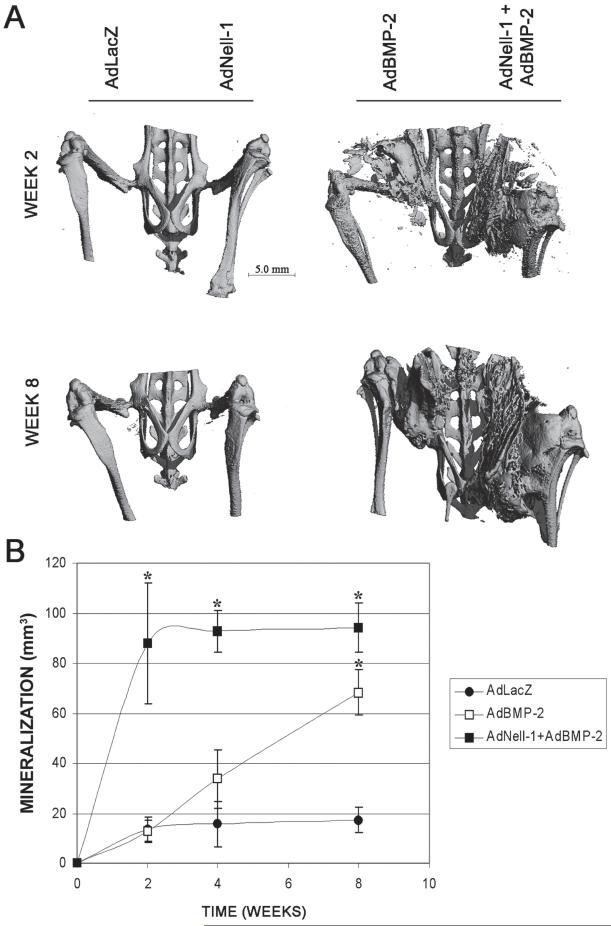
The ability of AdBMP-2 to promote in vivo endochondral bone formation in muscle tissue of immunocompromised mice and rats has been shown previously.(27,28) To analyze the effects of Nell-1 on intramuscular bone formation in vivo, nude mice were injected with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1+AdBMP-2. High-resolution μCT analysis showed no intramuscular mineralization in legs injected with 1 × 109 pfu AdLacZ or AdNell-1 (Fig. 3A), up through a dose of 4 × 109 pfu (data not shown). Mineralization was present in legs injected with 1 × 109 pfu AdBMP-2 or 5 × 108 pfu each AdNell-1+AdBMP-2 on weeks 2 and 8 after injection. Quantification of intramuscular mineralization, through low-resolution live μCT imaging of individual animals over time (Fig. 3B), revealed a slow but steady increase in mineralization volume in AdBMP-2-injected muscle with significantly more intramuscular mineralization at week 8 compared with AdLacZ-injected muscles. Legs injected with AdNell-1+AdBMP-2 showed a significant and rapid increase in mineralization volume with significantly more mineralization at weeks 2, 4, and 8 compared with AdLacZ.
FIG. 3.
High-resolution and live μCT imaging over time. (A) High-resolution μCT images of mouse legs transduced with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1+AdBMP-2 intramuscularly at both 2 and 8 wk after injection. Note the absence of intramuscular mineralization in legs injected with AdLacZ or AdNell-1. (B) Graph displaying mineralization volume found within mouse legs through 8 wk of live μCT imaging. Note the increasing intramuscular bone formation over time in defects transduced with AdBMP-2 or AdNell-1 + AdBMP-2. *Significant differences from AdLacZ samples; p ≤ 0.05.
Histological analysis of bone regeneration
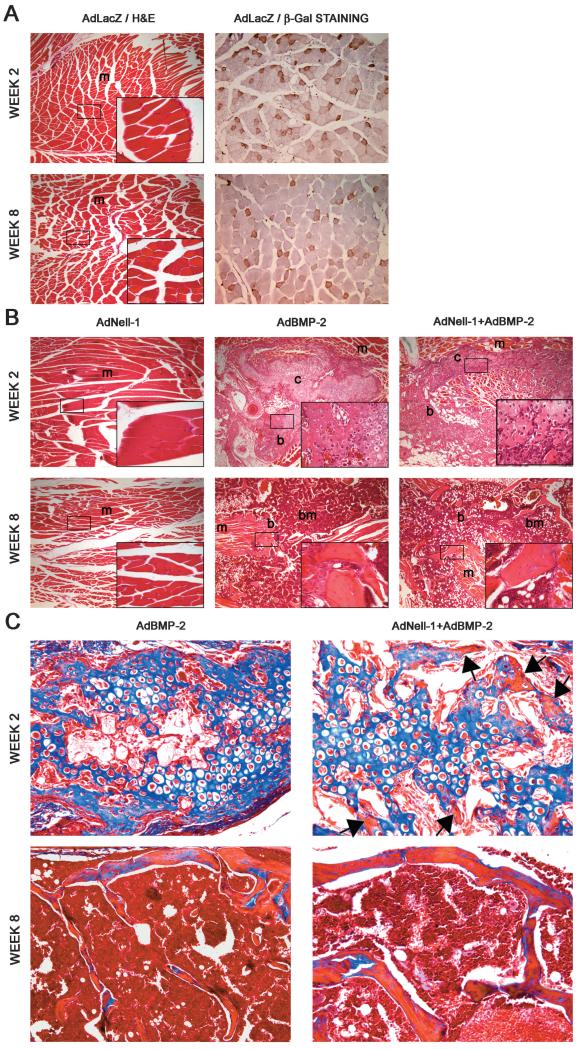
μCT findings were confirmed through histological analysis of thigh muscle. Samples transduced with AdLacZ were found to contain only muscle tissues in the area of injection through H&E staining, although immunohistochemistry for β-Gal detected viral expression through out the muscle at weeks 2 and 8 (Fig. 4A). Similarly, samples transduced with AdNell-1, AdBMP-2, and AdNell-1+AdBMP-2 were studied (Fig. 4B). H&E staining revealed the presence of large zones of chondrogenesis adjacent to zones of osteogenesis and muscle within the thigh muscle of legs injected with AdBMP-2 or AdNell-1+AdBMP-2, but not AdNell-1, at week 2. By week 8, bony struts, bone marrow, and only minimal amounts of cartilage were present within the muscle tissue of AdBMP-2- and AdNell-1+AdBMP-2-injected legs. Trichrome staining confirmed the presence of primarily osteoid (blue) at week 2 and primarily mature bone (red) at week 8 (Fig. 4C). Bone formation was similar in appearance between the AdBMP-2 and AdNell-1+AdBMP-2 samples, although the latter contained areas of immature mineralized bone as early as week 2. In accordance with μCT data, muscle injected with AdLacZ or AdNell-1 did not show bone or cartilage formation at week 2 or 8.
FIG. 4.
Histological analysis of intramuscular bone formation after virus transduction. (A) H&E (left) and β-Gal immunohistochemistry (right) on AdLacZ-transduced muscle at weeks 2 and 8. (B) H&E staining on AdNell-1-, AdBMP-2-, and AdNell-1 + AdBMP-2-transduced muscle at weeks 2 and 8. Note the presence of cartilage in AdBMP-2- and AdNell-1 + AdBMP-2- transduced samples at week 2 and bone at week 8. (C) Masson’s trichrome staining of transduced muscle after injection with AdBMP-2 or AdNell-1 + AdBMP-2. Note the presence of muscle (red), osteoid (blue), and mature bone (red) within samples. Black arrows point to areas of mineralized bone (red) in week 2 trichrome-stained samples. Original magnification: ×40 (A left and B), ×400 (bottom right panel of A left and B), and ×200 (A right and C). M, muscle; c, cartilage; b, bone; bm, bone marrow.
Immunohistological analysis of bone and cartilage markers
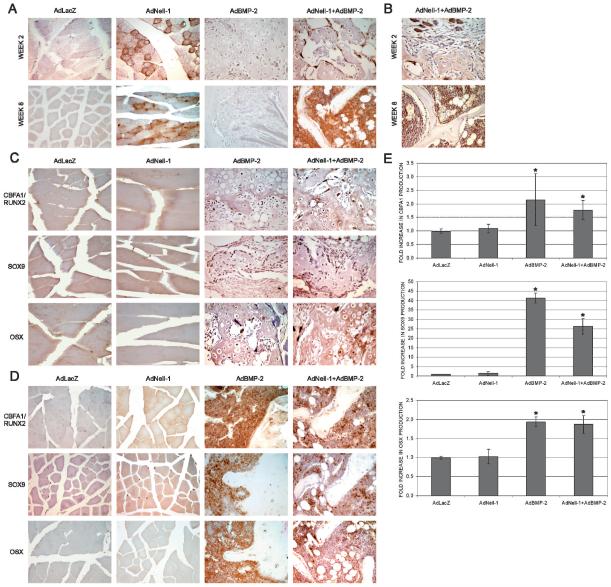
Markers for bone and cartilage formation were further studied by immunohistochemistry. The presence of Nell-1 was only detected in muscle tissues that had been transduced with the AdNell-1 virus (Fig. 5A). BMP-2 production was confirmed in samples transduced with AdNell-1+AdBMP-2 (Fig. 5B). Markers for chondrogenic and osteogenic differentiation were further evaluated. Two molecules, Cbfa1/Runx2 and OSX, are known to be specifically required for osteoblast differentiation, whereas Sox9 is known to keep mesenchymal cells in a proliferative prechondrogenic state. Samples transduced with AdNell-1 showed a similar protein production pattern to AdLacZ control samples, with positive staining only for Sox9 at weeks 2 (Fig. 5C) and 8 (Fig. 5D). Legs injected with AdBMP-2 or AdNell-1+AdBMP-2 showed increased staining for all mesenchymal markers (Cbfa1/Runx2, Sox9, and OSX) at week 2. Nell-1 and BMP-2 production was localized to osteoblasts lining hypertrophic cartilage and osteoid, corresponding with the localization pattern of Cbfa1, Sox9, and OSX. At week 8, Nell-1 and BMP-2 production was primarily detected within the bone marrow space, making it difficult to decipher whether protein production was restricted to pre-osteoblast, mesenchymal stem, or hematopoietic cells. Accordingly, the production of all three proteins was localized to the bone marrow space with a patchy arrangement, indicating that not all cells of the bone marrow were producing these proteins. BMP-2 is know to affect many cell types including pre-osteoblasts and mesenchymal stem cells, so it is not surprising that these cell were affected by the presence of BMP-2. Few mature osteoblasts within new bone produced Nell-1, BMP-2, or the other studied proteins. It is possible that these cells had entered a quiescent state, and thus transgene expression also halted.
FIG. 5.
Immunohistochemistry for markers of cartilage and bone differentiation. (A) Staining for Nell-1 protein in samples transduced with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1 + AdBMP-2. Note that positive Nell-1 staining is only present in samples injected with AdNell-1. (B) Staining for BMP-2 protein in samples transduced with AdNell-1 + AdBMP-2 at weeks 2 and 8. Staining for Cbfa1/Runx2, Sox9, and OSX in samples injected with AdLacZ, AdNell-1, AdBMP-2, or AdNell-1 + AdBMP-2 at (C) 2 and (D) 8 wk after transduction. Original magnifications at ×400 for all images. (E) Graphs displaying the fold increase in Cbfa1, Sox9, or OSX production in histological samples. *Significant differences from AdLacZ samples; p < 0.05.
Immunohistochemistry findings were further quantified by ELISA (Fig. 5E). Whereas AdBMP-2 samples displayed low-intensity generalized Cbfa1 production and AdNell-1+AdBMP-2 samples displayed high-intensity focused production, Cbfa1 quantification revealed no significant differences between these groups (2.15- and 1.77-fold, respectively), although both were significantly higher than AdLacZ samples. The same was true for OSX production (1.93- and 1.87-fold, respectively). The AdBMP-2 group produced significantly more Sox9 than the AdNell-1+AdBMP-2 group (41- and 26-fold, respectively), indicating its strong effects on antimuscle differentiation. Thus, because no significant differences were noted between the AdBMP-2 and AdNell-+AdBMP-2 groups in terms of Cbfa1 or OSX production, the effects of Nell-1 regarding bone formation must be related to later osteoblast activities.
DISCUSSION
Osteogenic differentiation is a complex process involving numerous signaling cascades, which synergize to direct the higher-order functions of matrix mineralization and bone formation. Of particular importance is the hierarchy in signaling, with Cbfa1/Runx2 controlling the progression of osteogenic differentiation and often referred to as the master regulator of mesenchymal cell fate, through temporal activation and/or repression of cell growth and gene expression regulation.(29) BMP-2 is a member of the TGF-β superfamily and has been shown to induce osteogenic differentiation through Cbfa1/Runx2-dependent and -independent pathways.(3,4,30) This signaling hierarchy, related to the regulation of Cbfa1/Runx2 within the osteogenic tree accounts for the ability of BMPs to induce cellular chemotaxis, proliferation, and osteogenic differentiation of both osseous and nonosseous mesenchymal cells.(1,2) Nell-1, on the other hand, has three Cbfa1/Runx2 binding sites (osteoblast-specific cis-acting element 2 [OSE2]) within its promoter and signals downstream of Cbfa1/Runx2.(17,18)
Nell-1’s effects thus far have been osteochondral-specific—inducing premature suture closure in human craniosynostosis, osteoblast differentiation, and hypertrophy of cartilage.(10,11,16,18,31) Additionally, a mouse model with the mutation of ENU-induced alleles, including Nell-1, resulted in cranial and other skeletal defects.(14) Nell-1 is suggested to play a key role as a regulator of craniofacial skeletal morphogenesis, especially in committed chondrogenic or osteoblastic differentiation. Interestingly, unlike Nell-1, BMP-2 has not been reported to induce cranial suture closure in vivo or ex vivo.(32) The objective of this research was to study the potential synergistic actions between Nell-1 and BMP-2 in the induction of osteogenic differentiation of non-osteoblastic cells, specifically myoblasts.
We have replicated several osteoblastic markers and functions in C2C12 myoblasts using the forced expression of Nell-1 and BMP-2. Of particular significance, the combined expression of Nell-1 and BMP-2 resulted in a greater osteogenic response than the addition of either factor alone. Early findings noted differences in cellular morphology as C2C12 myoblasts became more cuboidal on transduction of AdBMP-2 or AdNell-1+AdBMP-2, corresponding to an increase in ALP activity. Synergistic activity was also detected in OPN production and matrix mineralization. Importantly, supplying the conditioned media from virus-infected C2C12 cells to uninfected C2C12 cells resulted in even greater increases in matrix mineralization, revealing that Nell-1 required minimal amounts of BMP-2 for full function on myoblasts and that these two growth factors initiated cellular stimulation extracellularly. In all four experiments of Fig. 1, AdNell-1 transduction alone resulted in no biological response within C2C12 myoblasts, indicating that, in their current state, these cells were unable to respond to Nell-1 stimulation. Additionally, the combined transduction of AdNell-1+AdBMP-2 resulted in ALP, OPN, and mineralization increases above and beyond the result of either AdNell-1 or AdBMP-2 transduction alone.
The simplest interpretation of these results was to assume that Nell-1 and BMP-2 were contributing separate, but complimentary, signals to stimulate osteoblast differentiation. Other possible explanations for our results include Nell-1 stimulation of BMP-2 responsiveness through regulation of BMP receptors or Smad signaling, BMP-2 stimulation of Nell-1 responsiveness though the increased production of the Nell-1 receptor or pathway signaling, or modulation of transcriptional activity by interactions of these two factors on the promoters of target genes. Because of Nell-1’s inability to initiate osteogenic differentiation on myoblasts in the absence of BMP-2, as shown in these data, and that Nell-1 does not significantly change the expression level of BMP receptors or Smads,(10) the data suggest that either BMP-2 somehow prepared the cells for Nell-1’s actions or that Nell-1 and BMP-2 interact at a molecular level to enhance osteogenic differentiation of cells. It is not clear at this time whether Nell-1 signals through a cell surface receptor-mediated pathway, an internalized receptor, or interacts molecularly with other factors. Efforts are still underway to identify the Nell-1 receptor, which is believed to be a membrane-bound protein as evident by preliminary unpublished data showing that Nell-1 binds to the outer surface of osteoblasts but not fibroblasts. Furthermore, the effect of AdNell-1 and AdBMP-2 co-administration is not additive, because Nell-1 alone had no effect on cell morphology, ALP activity, OPN production, or matrix mineralization to contribute to the effects of BMP-2, but rather, synergistic as the addition of Nell-1 more than doubled the effects of BMP-2 alone in a significant manner.
To examine the potential mechanism of synergy, we studied the MAPK signaling pathway. BMP-2 has been reported to activate the p38 and ERK1/2 MAPK pathways in myoblasts,(33,34) but not the JNK MAPK pathway.(35) Furthermore, a Nell-1-related molecule, Nell-2, has shown the activation of the JNK MAPK pathway in neurons.(36) These results clearly show that Nell-1 stimulation only significantly activated the non-BMP-2-associated JNK MAPK pathway in C2C12 myoblasts.(37) Furthermore, this stimulation on preconditioned C2C12 cells only occurred in AdNell-1+AdBMP-2 co-transduced samples. The rational for choosing 6 days of preconditioning for C2C12 cells for this pathway study was based on our results showing drastically altered cellular morphology, ALP activity, and expression patterns of Nell-1, BMP-2, and BMP-Rs at this time-point. Thus, it is logical that Nell-1 + BMP-2 stimulation of C2C12 myoblasts effectively promoted their differentiation and cell surface marker expression levels, rendering these cells sensitive to Nell-1 and other signals. Furthermore, disrupted JNK signaling blocked some, but not all, of Nell-1’s effects. For example, JNK inhibition almost entirely eliminated OPN production in all groups, indicating that the cells’ innate ability to produce OPN was heavily dependent on JNK signaling and that further JNK signaling by Nell-1 was in part responsible for increased OPN production. In addition, ALP activity was increased in all groups on JNK inhibition. Increased ALP activity on JNK inhibition was twice as great in samples stimulated by Nell-1 + BMP-2 compared with BMP-2 alone. These important results highlight that Nell-1 signaling is not entirely caused by JNK signaling and that further studies to identify the Nell-1 specific receptor are crucial. Finally, these results point to the specificity of cellular and molecular signaling by Nell-1. Additionally, the data suggest that Nell-1 and BMP-2 together enhance osteogenic differentiation synergistically, because of their distinct signaling pathways and the observation that their combined activity was greater than would be predicted from the activities of each factor alone.
The situation encountered when animals are injected with AdNell-1 and AdBMP-2 in vivo is considerably more complex, but can still be understood by extrapolating from in vitro results. Similar to the in vitro results, AdNell-1 injection alone resulted in no bone formation, whereas the new bone volume formed in vivo on co-injection of AdNell-1+AdBMP-2 was greater than that formed by a single injection of AdBMP-2 alone. Bone formation was confirmed histologically to reveal the endochondral bone formation process in all samples injected with AdBMP-2 alone or in combination with AdNell-1. Massive islands of cartilage adjacent to new osteoid and bone were present within the muscle as early as 2 weeks after injection. By 8 weeks, hypertrophic cartilage had been replaced by a network of bony struts, confirming μCT findings.
Synergism between treatments can be the result of an interaction between the signaling effects of each monotherapy or direct protein interaction and modulation of each monotherapy. To gain an understanding of the potential downstream cellular effects contributing toward the synergy between Nell-1 and BMP-2, samples were analyzed histologically for regulation of molecules important for osteochondral differentiation. Because bone formation is controlled through a balance between mesenchymal cell proliferation and differentiation, the cellular effects elevated in the combined AdNell-1+AdBMP-2 group compared with the AdBMP-2 group are thought to play a role in establishing synergy between Nell-1 and BMP-2 with respect to osteogenesis in muscle cells. The initial finding confirmed our hypothesis that Nell-1 does not target myoblasts, as the protein production landscape in AdNell-1 transduced muscle resembled that of AdLacZ transduced muscle. These findings are hypothesized to be related to the absence of a Nell-1 receptor on myoblasts.
Whereas the protein profile, as detected by immunohistochemistry, between AdBMP-2 and AdNell-1 + AdBMP-2 groups was similar 2 wk after transduction, differences were noted between the groups in the distribution of Cbfa1/Runx2 and OSX production 8 wk after transduction. Cbfa1/Runx2, known to be targeted by BMP-2 and central to osteochondral differentiation,(30) was produced more intensely by fewer cells in the combined AdNell-1+AdBMP-2 groups compared with AdBMP-2 alone samples that showed less intense generalized production. Nell-1 signals downstream from Cbfa1/Runx2(17) and does not stimulate Cbfa1/Runx2 expression,(10) thus the results suggest that Nell-1 may interact directly with BMP-2 or a component within the BMP-2 signaling cascade to enhance BMP-2’s ability to upregulate Cbfa1/Runx2 production intensely. Additionally, BMP-2 has been reported to stimulate osteogenic differentiation through PKC dependent and independent pathways.(38,39) Nell-1 also interacts with specific PKC isoforms including PKCδ, βI, and ζ, but not PKCα, γ, and ε,(15) to exerts its signaling function in a still unidentified manner. This level of regulation may potentially exhibit cross-talk between the two signaling pathways.
Another molecule required for osteoblast differentiation that signals downstream of Cbfa1/Runx2 is OSX.(40,41) OSX was upregulated more intensely and in a fewer number of cells in AdNell-1+AdBMP-2-transduced muscle at week 8 and corresponded to a greater volume of bone production in these samples. Previous work has revealed that BMP-2 treatment can induce the expression of OSX through Cbfa1/Runx2-dependent and -independent pathways mediated by Dlx5.(41,42) Furthermore, our previous work revealed that Nell-1 monotherapy resulted in a decrease of OSX and an increase in OCN expression in MC3T3-E1 osteoblasts and in critical sized rat calvarial defects.(43) Thus, the increases in OSX production in these samples may have resulted from accentuated BMP-2 signaling resulting from Nell-1 + BMP-2 synergy. Additional studies into other signaling pathways may further provide evidence that Nell-1 and BMP-2 act together synergistically during osteoblast differentiation, and that useful gene or protein therapy based strategies for bone regeneration may be achieved using the approach of overexpressing combinations of factors.
To summarize, we showed that specific and targeted Nell-1 and BMP-2 signaling are highly synergistic in myoblasts in vitro and in vivo. A potential interaction between Nell-1 and BMP-2 at the cellular level in the muscle compartment exists through the regulation of proliferation versus differentiation. In the cellular compartment, BMP-2 may interact at the level of mesenchymal cell chemotaxis, proliferation, and initial differentiation (including an upregulation of Nell-1 responsive elements). Results suggest that the therapeutic benefit of dual stimulation of these pathways in patients may exceed the benefits and minimize the undesired trade-offs expected from the reported effects of Nell-1 or BMP-2 monotherapy and support the significant therapeutic potential of this combination strategy in osteogenic differentiation both within and now outside the craniofacial complex.
ACKNOWLEDGMENTS
The authors thank Dr Renny Franceschi for the kind gift of the AdBMP-2 virus. This work was supported by a grant from the Wunderman Family Foundation, March of Dimes Birth Defect Foundation (6-FY02-163), NIH/NIDCR RO3 DE 014649-01, NIH/NIDCR K23DE00422, NIH DE016107-01, the Thomas R. Bales Endowed Chair, and the NIH T32 UCLA Research Training Grant. Additional funding was provided by the National Natural Science Foundation of China 30400502; Science and Technology Commission of Shanghai Municipality 04Z05601, 05DJ14006, 055407034, 05QMX1426; Shanghai Education Committee 03BC39, 04YQHB081, Y0203; and the UCLA SAIRP NIH-NCI 2U24 CA092865 cooperative agreement.
Footnotes
Drs Ting, Soo, Zhang, and Wu own stock in and serve as cofounders of Bone Biologics. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- 2.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura R, Hata K, Harris SE, Ikeda F, Yoneda T. Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone. 2002;31:303–312. doi: 10.1016/s8756-3282(02)00826-8. [DOI] [PubMed] [Google Scholar]
- 4.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003;18:705–715. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao M, Zhao Z, Koh JT, Jin T, Franceschi RT. Combinatorial gene therapy for bone regeneration: Cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J Cell Biochem. 2005;95:1–16. doi: 10.1002/jcb.20411. [DOI] [PubMed] [Google Scholar]
- 7.Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM, Adelmant G, Baron R, Roman-Roman S. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 8.Kha HT, Basseri B, Shouhed D, Richardson J, Tetradis S, Hahn TJ, Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: Pro-bone and anti-fat. J Bone Miner Res. 2004;19:830–840. doi: 10.1359/JBMR.040115. [DOI] [PubMed] [Google Scholar]
- 9.Yeh LC, Zavala MC, Lee JC. C-type natriuretic peptide enhances osteogenic protein-1-induced osteoblastic cell differentiation via Smad5 phosphorylation. J Cell Biochem. 2006;97:494–500. doi: 10.1002/jcb.20657. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Kuroda S, Carpenter D, Nishimura I, Soo C, Moats R, Iida K, Wisner E, Hu FY, Miao S, Beanes S, Dang C, Vastardis H, Longaker M, Tanizawa K, Kanayama N, Saito N, Ting K. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 2002;110:861–870. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, Kwong E, Bertolami CN, Kawamoto H, Kuroda S, Longaker MT. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, Nakamura Y, Hirai Y, Maekawa H, Takahashi E. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda S, Oyasu M, Kawakami M, Kanayama N, Tanizawa K, Saito N, Abe T, Matsuhashi S, Ting K. Biochemical characterization and expression analysis of neural thrombospon-din-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- 14.Desai J, Shannon ME, Johnson MD, Ruff DW, Hughes LA, Kerley MK, Carpenter DA, Johnson DK, Rinchik EM, Culiat CT. Nell-1 deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. 2006;15:1329–1341. doi: 10.1093/hmg/ddl053. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda S, Tanizawa K. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun. 1999;265:752–757. doi: 10.1006/bbrc.1999.1753. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Carpenter D, Bokui N, Soo C, Miao S, Truong T, Wu B, Chen I, Vastardis H, Tanizawa K, Kuroda S, Ting K. Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development. J Bone Miner Res. 2003;18:2126–2134. doi: 10.1359/jbmr.2003.18.12.2126. [DOI] [PubMed] [Google Scholar]
- 17.Truong S, Zhang X, Pathmanathan D, Soo C, Ting K. Craniosynostosis-associated gene Nell-1 is regulated by Runx2. J Bone Miner Res. 2007;I22:7–18. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- 18.Cowan C, Cheng S, Ting K, Soo C, Walder B, Wu B, Kuroda S, Zhang X. Nell-1 induced bone formation within the distracted intermaxillary suture. Bone. 2005;38:48–58. doi: 10.1016/j.bone.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 21.Berger F, Lee YP, Loening AM, Chatziioannou A, Freedland SJ, Leahy R, Lieberman JR, Belldegrun AS, Sawyers CL, Gambhir SS. Whole-body skeletal imaging in mice utilizing microPET: Optimization of reproducibility and applications in animal models of bone disease. Eur J Nucl Med Mol Imaging. 2002;29:1225–1236. doi: 10.1007/s00259-002-0850-1. [DOI] [PubMed] [Google Scholar]
- 22.Cowan C, Shi Y, Aalami O, Chou Y, Mari C, Thomas R, Quarto N, Contag C, Wu B, Longaker M. Adipose-derived adult stromal cells heal critical-sized mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 23.Shi SR, Liu C, Balgley BM, Lee C, Taylor CR. Protein extraction from formalin-fixed, paraffin-embedded tissue sections: Quality evaluation by mass spectrometry. J Histochem Cytochem. 2006;54:739–743. doi: 10.1369/jhc.5B6851.2006. [DOI] [PubMed] [Google Scholar]
- 24.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Okubo Y, Bessho K, Fujimura K, Iizuka T, Miyatake SI. In vitro and in vivo studies of a bone morphogenetic protein-2 expressing adenoviral vector. J Bone Joint Surg Am. 2001;83(Suppl 1):S99–S104. [PubMed] [Google Scholar]
- 26.Okubo Y, Bessho K, Fujimura K, Iizuka T, Miyatake S. Expression of bone morphogenetic protein-2 via adenoviral vector in C2C12 myoblasts induces differentiation into the osteoblast lineage. Biochem Biophys Res Commun. 1999;262:739–743. doi: 10.1006/bbrc.1999.1281. [DOI] [PubMed] [Google Scholar]
- 27.Alden TD, Pittman DD, Hankins GR, Beres EJ, Engh JA, Das S, Hudson SB, Kerns KM, Kallmes DF, Helm GA. In vivo endochondral bone formation using a bone morphogenetic protein 2 adenoviral vector. Hum Gene Ther. 1999;10:2245–2253. doi: 10.1089/10430349950017220. [DOI] [PubMed] [Google Scholar]
- 28.Sonobe J, Okubo Y, Kaihara S, Miyatake S, Bessho K. Osteoinduction by bone morphogenetic protein 2-expressing adenoviral vector: Application of biomaterial to mask the host immune response. Hum Gene Ther. 2004;15:659–668. doi: 10.1089/1043034041361208. [DOI] [PubMed] [Google Scholar]
- 29.Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/ AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 30.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Cowan CM, Jiang X, Soo C, Miao S, Carpenter D, Wu B, Kuroda S, Ting K. Nell-1 induces acrania-like cranioskeletal deformities during mouse embryonic development. Lab Invest. 2006;86:633–644. doi: 10.1038/labinvest.3700430. [DOI] [PubMed] [Google Scholar]
- 32.Lenton KA, Nacamuli RP, Wan DC, Helms JA, Longaker MT. Cranial suture biology. Curr Top Dev Biol. 2005;66:287–328. doi: 10.1016/S0070-2153(05)66009-7. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L450–L458. doi: 10.1152/ajplung.00206.2005. [DOI] [PubMed] [Google Scholar]
- 34.Takeda M, Otsuka F, Nakamura K, Inagaki K, Suzuki J, Miura D, Fujio H, Matsubara H, Date H, Ohe T, Makino H. Characterization of the bone morphogenetic protein (BMP) system in human pulmonary arterial smooth muscle cells isolated from a sporadic case of primary pulmonary hypertension: Roles of BMP type IB receptor (activin receptor-like kinase-6) in the mitotic action. Endocrinology. 2004;145:4344–4354. doi: 10.1210/en.2004-0234. [DOI] [PubMed] [Google Scholar]
- 35.Izumi M, Fujio Y, Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Oshima Y, Nakaoka Y, Kishimoto T, Yamauchi-Takihara K, Hirota H. Bone morphogenetic protein-2 inhibits serum deprivation-induced apoptosis of neonatal cardiac myocytes through activation of the Smad1 pathway. J Biol Chem. 2001;276:31133–31141. doi: 10.1074/jbc.M101463200. [DOI] [PubMed] [Google Scholar]
- 36.Aihara K, Kuroda S, Kanayama N, Matsuyama S, Tanizawa K, Horie M. A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res Mol Brain Res. 2003;116:86–93. doi: 10.1016/s0169-328x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 37.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–498. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 38.Hay E, Lemonnier J, Fromigue O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028–29036. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- 39.Celil AB, Campbell PG. BMP-2 and IGF-I mediate Osx expression in human mesenchymal stem cells via the MAPK and PKD signaling pathways. J Biol Chem. 2005;280:31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 41.Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309:689–694. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 42.Celil AB, Hollinger JO, Campbell PG. Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem. 2005;95:518–528. doi: 10.1002/jcb.20429. [DOI] [PubMed] [Google Scholar]
- 43.Aghaloo T, Cowan C, Chou Y, Zhang X, Lee H, Miao S, Hong N, Kuroda S, Soo C, Wu B, Ting K. Nell-1 induced bone regeneration in calvarial defects. Am J Pathol. 2006;169:903–915. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]