Abstract
Burkitt lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma with a consistent MYC translocation. Epstein-Barr virus (EBV) has been associated with BL at different frequencies, depending on the clinical variant and geographic regions. This is a large-scale study of BL in Brazil, including 234 patients from 5 geographic regions that are widely disparate socioeconomically, including pediatric (61.1%) and adult (37.6%) populations. EBV was present in 52.6% of all BL cases, varying from 29% (12/42) in the South to 76% (13/17) in the North. Most of the cases were EBV type A. The frequency was higher in the pediatric group, and EBV association within this age range predominated in all regions except the South. Expression of p53 protein was observed in 16.2%, and only rare cases showed p63 expression. BL in Brazil is regionally distinct and has a low incidence of p53 overexpression and a higher-than-expected association with EBV in sporadic cases.
Keywords: Burkitt lymphoma, Brazil, Epstein-Barr virus, Tissue microarray, p53, p63, Immunohistochemistry, Polymerase chain reaction
Burkitt lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma (NHL) first described by Burkitt in 1958 in African children from areas holoendemic for malaria.1 BL is composed of a monomorphic population of medium-sized B cells with basophilic cytoplasm and an extremely high proliferative rate.2 Morphologically, in addition to classical BL, the World Health Organization (WHO) recognizes 2 cytologic variants: BL with plasmacytoid differentiation and atypical BL/Burkitt-like. The latter is characterized by greater pleomorphism in nuclear size and shape than the classical type.
Immunophenotypically, all types are similar and express CD20, CD10, bcl-6, and membranous IgM but not IgD, bcl-2, or terminal deoxynucleotidyl transferase (TdT). The Ki-67 proliferation index generally approaches 100%.3 All cases carry a translocation involving the c-MYC gene at 8q24 with the immunoglobulin heavy chain (lGH) gene on 14q32 (80%) or, less commonly, with the κ light chain locus (IGK) at 2p11 (15%) or the λ light chain locus (IGL) at 22q11 (5%).4,5 This brings the proto-oncogene under the influence of powerful immunoglobulin promoters, resulting in deregulated MYC transcription.6 TP53 mutations have also been reported in BL, although these appear to be more frequent in BL cell lines.7
Three main clinical variants of BL have been described: endemic, sporadic, and immunodeficiency-associated. Endemic BL refers to the form that occurs in African children, usually 4 to 7 years old. The tumor frequently involves the mandible and other facial bones (50% of cases), as well as kidneys, gastrointestinal tract, ovaries, breast, and other extranodal sites. The incidence of BL in Africa is estimated to be 50 times higher than in the United States.8 Sporadic BL occurs worldwide and accounts for 1% to 2% of all lymphomas in Western Europe and the United States and approximately 30% to 50% of all childhood lymphomas. In sporadic BL, the abdomen, especially the ileocecal area, is the most common site of involvement.2 The immunodeficiency-associated variant is frequently observed in the setting of HIV infection and has also been reported in allograft recipients and in congenital immunodeficiency. BL accounts for about a third of NHL in HIV+ patients.9 BL in an HIV+ patient generally occurs in patients with relatively high CD4 counts, in contrast with large cell immunoblastic lymphoma, and often represents the initial AIDS-defining illness.8,9
Epstein-Barr virus (EBV), a member of the human herpesvirus family, was initially isolated from a cultured BL cell line by Epstein et al10 in 1964 and was the first description of a virus involved in the pathogenesis of a human tumor.8 EBV has since been linked to many human neoplasms, including hematopoietic (including B-cell and T-cell natural killer and Hodgkin lymphomas), epithelial (nasopharyngeal carcinoma, a subset of gastric carcinoma), and mesenchymal (inflammatory pseudotumor of the liver and HIV-associated smooth muscle neoplasms) tumors.11 The association between BL and EBV occurs at varying frequencies depending on the clinical variant. EBV is present in the majority of endemic cases of BL (up to 100%), in fewer than 30% of cases of sporadic BL,2 and in 30% to 40% of AIDS-related BL.12 Small studies in Brazil and other South American countries have suggested that this association is intermediate between sporadic and endemic variants.13–19
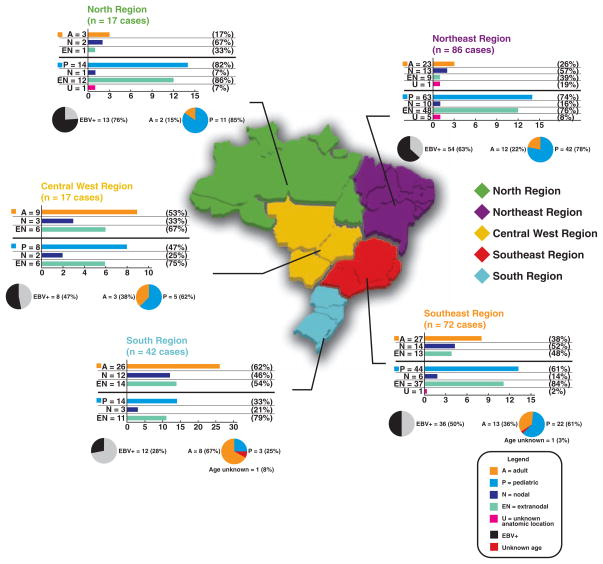
Brazil is the largest country in South America, located at +5°17′ to −33°45′ latitude and −34°48′ to −73°60′ longitude, and has a population of 180 million people. Brazil is characterized by widely disparate socioeconomic conditions among its citizens and regional climatic variation. The country is divided into 5 geographic regions: North, Northeast, Central West, South, and Southeast Figure 1. The South is generally more affluent, while the North and Northeast are less so. Similarly, tropical and infectious diseases are more common in the latter areas.20,21 There are few epidemiologic and no large-scale studies of BL in Brazil, and previously published works on association of EBV with BL have been predominantly focused on pediatric BL from the Northeast,15,16 Southeast,13,14,17,18 and South.19 In the present study, we report the clinical features, immunophenotype, tumor suppressor (p53 and p63) protein expression profile, and association with EBV (by in situ hybridization [ISH] and polymerase chain reaction [PCR]) in 234 well-characterized cases of BL from all 5 geographic regions of Brazil in pediatric and adult populations. To perform this extensive analysis, we used tissue microarrays (TMAs) generated from our extensive tumor bank.
Figure 1.
Brazilian map with the 5 geographic regions showing the distribution of Burkitt lymphoma cases and the frequency of pediatric and adult Burkitt lymphoma related to anatomic location and Epstein-Barr virus (EBV) status.
Materials and Methods
Case Material and Clinical Data
The initial study population was from a total of 595 cases of high-grade B-cell lymphoma with a tentative diagnosis of BL selected between June 1997 and December 2007 from the archives of Consultoria em Patologia, a large national reference consultation service in anatomic pathology located in Botucatu, Brazil. In 99 cases, paraffin blocks were not available and these cases were excluded from the study. In the remaining 496 cases, the original H&E-stained and immunostained slides of each case were reviewed by 3 of us (E.M.Q., G.G., and C.E.B.), and representative areas were selected for the construction of the TMAs. After immunohistochemical analysis, all bcl-2+ and CD10− cases were excluded (135 cases; bcl-2+ cases are being analyzed in another study). Another 127 cases were also excluded from the study after fluorescence in situ hybridization (FISH) analysis as these cases showed inconclusive results or had results inconsistent with BL (exclusion criteria are explained later). In total, 234 cases of BL, confirmed by morphologic, immunohistochemical, and molecular analyses, are described in this report. Nodal and extranodal BL cases were included. Clinical data including sex, age at diagnosis, anatomic location of the tumor, and HIV status were obtained from the referring pathologists or oncologists and/or pathology reports. A morphologic sub-classification of the cases was performed based on variants included in the 2001 WHO classification.2 This study was approved as a whole by the Ethical Committee of University of São Paulo Medical School, São Paulo, Brazil.
TMA Construction
Eight TMA blocks were constructed using a tissue arrayer (Beecher Instruments, Sun Prairie, WI). The number of cases in each TMA block varied from 19 to 84 (not including control cores). Each individual case was represented by 3 tumor cores of 0.6 mm that had been obtained from the original paraffin blocks. Proper positive and negative control cores for each marker were also included in the array block: tonsil (CD20, CD3, CD10, bcl-2, bcl-6, Ki-67, PAX-5, CD5, and CD23), EBV+ Hodgkin lymphoma (latent membrane protein [LMP]-1 and EBV early RNA [EBER1]), tonsillar squamous epithelium (p63), breast ductal carcinoma showing p53 overexpression (p53), and lymphoblastic lymphoma (TdT).
Immunohistochemical Analysis and ISH
Immunohistochemical analysis was performed for each TMA using Novolink polymer (Novocastra, Newcastle upon Tyne, England) as the detection system, and an epitope-retrieval method was applied as needed for each specific antibody; diaminobenzidine was the chromogen. The primary antibodies used reacted against the following antigens: CD20, CD3, CD10, bcl-6, Ki-67, bcl-2, EBV-LMP-1, PAX-5, TdT, CD5, CD23, p53, and p63 Table 1. For p53 and p63 immunostaining, only tumors with more than 10% and more than 5%, respectively, of neoplastic cells showing nuclear expression were considered positive. For p63 immunostaining, cases were divided into 4 groups according to the following scores: negative, 5% or fewer; 1+, more than 5% to 10%; 2+, more than 10% to 50%; and 3+, more than 50%.
Table 1.
Primary Antibodies Used for Immunohistochemical Staining in Paraffin Sections of Burkitt Lymphoma Cases*
| Antigen | Clone | Dilution | Antigen Retrieval Method | Source |
|---|---|---|---|---|
| CD20 | L26 | 1:1,200 | MW; CB | DAKO, Carpinteria, CA |
| CD3 | SP7 | 1:200 | S; CB | NeoMarkers, Lab Vision, Fremont, CA |
| CD10 | 56C6 | 1:100 | S; CB | Novocastra, Newcastle upon Tyne, England |
| BCL-2 | 124 | 1:400 | MW; CB | DAKO |
| BCL-6 | PG-B6P | 1:100 | T + S | DAKO |
| Ki-67 | MIB-1 | 1:4,800 | PC; CB | DAKO |
| Terminal deoxynucleotidyl transferase | Polyclonal | 1:1,600 | PC; EDTA | DAKO |
| PAX-5 | 24 | 1:400 | S | NeoMarkers |
| p53 | DO-7 | 1:2,000 | PC | DAKO |
| CD5 | 4C7 | 1:150 | MW | Novocastra |
| CD23 | SP23 | 1:400 | MW | NeoMarkers |
| p63 | 4A4 | 1:300 | PC | NeoMarkers |
| Latent membrane protein-1 | CS1-4 | 1:500 | S; CB | DAKO |
CB, citrate buffer, pH 6; MW, microwave oven; PC, pressure cooker; S, steamer; T, trypsin.
Heat-induced epitope retrieval was used.
Sections from all TMAs were examined for the expression of EBER1 by ISH using a 30-base oligonucleotide EBER1 probe complementary to a portion of the EBER1 gene, as previously described.14 Appropriate positive (cases of EBV+ BL and EBV+ Hodgkin lymphoma) and negative control (EBV− tissue) cores were also included in the arrays.
PCR Study for EBV Typing
Subtyping for EBV by PCR amplification of the EBV-encoded nuclear antigen (EBNA)2 region was performed in 123 cases. DNA isolation was performed as follows: formalin-fixed, paraffin-embedded histologic sections were submitted to deparaffinization by successive xylene baths and dehydration with 100% ethanol. After digestion and DNA purification,22 PCR was performed to investigate the quality of the extracted DNA, using a set of control primers for human genes that amplify products by 100, 200, 300, 400, and up to 600 base pairs (bp) depending on the integrity of the extracted DNA.23 For EBV typing, 2 primers encompassing a region (E2 up, 5′-AGGCTGCCCACCCTGAGGAT-3′ and E2 low, 5′-GCCACCTGGCAGCCCTAAAG-3′) containing a 16-bp deletion in EBV type A were used. PCR with these primers yields amplification product of 170- and 186-bp fragment length for types A and B, respectively. In some cases, a seminested reamplification was performed using the E2 up and E2R low (5′-GCTGCCACCTGGCGGAAT-3′) primers rendering amplification products of 111 bp and 127 bp for types A and B, respectively, according to Araujo et al.15
PCR-amplified products for both studies were analyzed in a 7% polyacrylamide gel by silver staining.
Fluorescence In Situ Hybridization
FISH was performed using a 3-μm-thick tissue section of each array block, as previously described.3 For the detection of breakpoints in the c-MYC locus, the LSI MYC Dual-Color Break-apart Rearrangement probe (Vysis, Abbott, Abbott Park, IL) was applied. The slides were evaluated using SpectrumOrange and SpectrumGreen filters (Chroma Technology, Fuerstenfeldbruck, Germany) on a Zeiss Axio Imager M1 fluorescence microscope (Carl Zeiss, Göttingen, Germany) and the assistance of Isis FISH Imaging Software (Metasystems, Altlussheim, Germany). Using an extended focus–tile sampling method, tiles with distant unpaired signals (≥10 pixels in distance) were considered positive, and the percentage of tiles containing positive signals was calculated. The threshold for positivity was established from a group of immunophenotypically characterized cases (tonsils) that did not contain the translocation. A positive case was defined as a case in which the mean number of positive tiles detected was 3 SDs above the mean of this negative control group.24 The threshold established was 2.19% (the mean of this negative control group was 0.73%).
Statistical Analysis
Calculations were performed using R (http://www.r-project.org), version 2.5.1, on a Macintosh computer with OS v.10.5.2 (Apple, Cupertino, CA). Significance was calculated using the Fisher exact test for factor comparisons and a general linear model (logistic regression) for comparisons involving age.
Results
Clinical Features
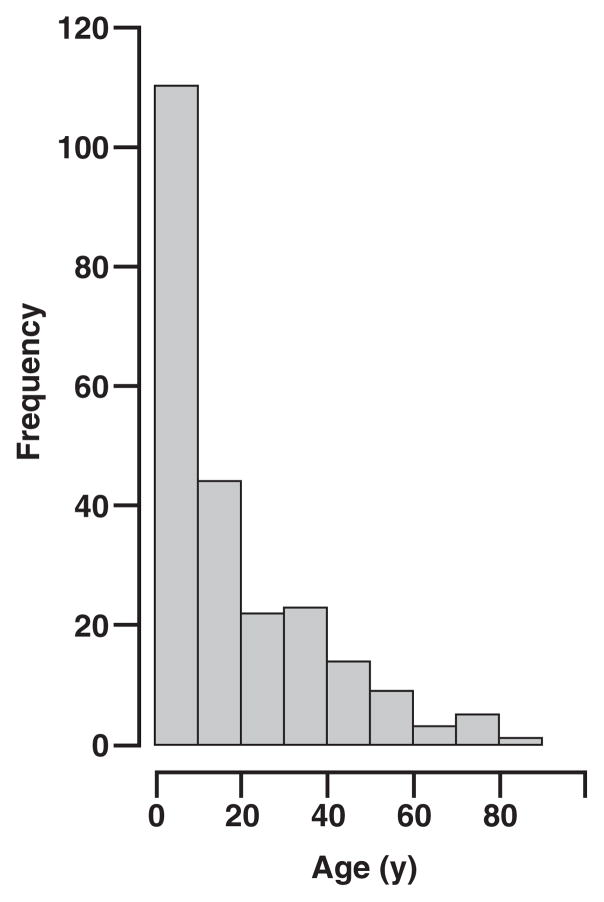
Of the 234 BL cases (CD10+ and bcl-2−), 173 (73.9%) were in males and 61 (26.1%) in females (male/female ratio 2.8:1). The patients ranged in age from 7 months to 81 years (mean, 19.1 years). The distribution of the cases in age subgroups is shown in Figure 2. There was no statistically significant age difference between male and female patient cohorts. In 3 cases (1.3%), the age was unknown. Of the patients, 143 (61.1%) were 16 years or younger (pediatric group) and 88 (37.6%) were older than 16 years (adult group). Extranodal BL comprised 67.9% of the cases (159 cases), while primary lymph node involvement was observed in 28.6% of the cases (67 cases). In 8 cases (3.5%) it was not possible to determine nodal or extranodal involvement. In the extranodal cases, the most common site of involvement was the gastrointestinal tract (59 cases); 49 cases were referred to as extranodal abdominal tumor without further specification; involvement of liver, oropharynx/nasopharynx, jaw/maxilla, ovaries, and central nervous system were observed in 7, 6, 5, 4, and 2 cases, respectively. Among the patients with primary lymph node disease, the most common sites of involvement were the cervical region (32 cases) and the axilla (15 cases). In the pediatric population, primary extranodal disease was observed in 113 cases (79.0%); in contrast, the adult population had extranodal involvement in 43 cases (49%).
Figure 2.
Age distribution of 234 Burkitt lymphoma cases.
The distribution of the cases in each of the 5 geographic regions of the country (including mean age, age range, sex, age group, site of involvement, association with EBV by ISH, and EBV typing by PCR) is summarized in Figure 1 and Table 2. As expected, EBER1 positivity was inversely associated with increased age (P ≤ .006), but was not significantly associated with sex. In contrast with the other regions, in the South and Central West regions, the number of cases of the adult group was greater than the number of pediatric cases. In these 2 geographical regions (South and Central West), extranodal involvement (14 cases and 6 cases, respectively) in the adult group (26 cases and 9 cases, respectively) was more frequent than a nodal presentation (12 cases and 3 cases, respectively).
Table 2.
Distribution of Burkitt Lymphoma Cases in Each Geographic Region*
| Northeast (n = 86 [36.7%]) | Central West (n = 17 [7.3%]) | South (n = 42 [17.9%]) | Southeast (n = 72 [30.8%]) | North (n = 17 [7.3%]) | Total (n = 234) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (y) | 15.4 | 27.5 | 28.6 | 18.3 | 10.3 | 19.1 |
| Range | 7 mo–79 y | 3–75 y | 3–81 y | 2–80 y | 3–51 y | 7 mo–81 y |
| Sex | ||||||
| Male | 64 (74) | 12 (71) | 29 (69) | 54 (75) | 14 (82) | 173 (73.9) |
| Female | 22 (26) | 5 (29) | 13 (31) | 18 (25) | 3 (18) | 61 (26.1) |
| M/F ratio | 2.78 | 2.4 | 2.2 | 3 | 4.6 | 2.8 |
| Age group | ||||||
| Adult | 23 (27) | 9 (53) | 26 (62) | 27 (38) | 3 (18) | 88 (37.6) |
| Pediatric | 63 (73) | 8 (47) | 14 (33) | 44 (61) | 14 (82) | 143 (61.1) |
| Unknown | 0 (0) | 0 (0) | 2 (5) | 1 (1) | 0 (0) | 3 (1.3) |
| Location | ||||||
| Nodal | 23 (27) | 5 (29) | 15 (36) | 21 (29) | 3 (18) | 67 (28.6) |
| Extranodal | 57 (66) | 12 (71) | 27 (64) | 50 (69) | 13 (76) | 159 (67.9) |
| Unknown | 6 (7) | 0 (0) | 0 (0) | 1 (1) | 1 (6) | 8 (3.5) |
| EBV (ISH) | ||||||
| Positive | 54 (63) | 8 (47) | 12 (29) | 36 (50) | 13 (76) | 123 (52.6) |
| Negative | 32 (37) | 9 (53) | 30 (71) | 36 (50) | 3 (18) | 110 (47.0) |
| Inconclusive | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (0.4) |
| EBV type (PCR) | ||||||
| A | 40/54 (74) | 4/8 (50) | 11/12 (92) | 31/36 (86) | 9/13 (69) | 95/123 (77.2) |
| B | 10/54 (19) | 4/8 (50) | 1/12 (8) | 5/36 (14) | 4/13 (31) | 24/123 (19.5) |
| Inconclusive | 4/54 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4/123 (3.3) |
EBV, Epstein-Barr virus; ISH, in situ hybridization; PCR, polymerase chain reaction.
Data are given as number (percentage) or number/total (percentage) unless otherwise indicated.
Among the 234 cases, 14 (6.0%) were known to be in HIV+ patients. The clinical features of this group of patients, including association with EBV and morphologic type, are given in Table 3. The age at the time of diagnosis ranged from 2 to 55 years (mean, 34 years). Overall, and as expected, HIV positivity was associated with increased age (P ≤ .005). There were 11 men (79%) and 3 women (21%), and nodal involvement (8 cases [57%]) was more frequent than an extra-nodal presentation (6 cases [43%]).
Table 3.
Clinical Features, Morphologic Findings, and EBV Association in HIV+ Burkitt Lymphoma Group
| Case No./Sex/Age (y) | Location | EBV ISH | EBV Subtype | Morphologic Type |
|---|---|---|---|---|
| 1/M/2 | Salivary gland | Positive | A | Classic |
| 2/F/7 | Neck LN | Negative | — | Classic |
| 3/F/25 | Axillary LN | Positive | B | Classic |
| 4/M/25 | Duodenum | Positive | A | Classic |
| 5/M/30 | Mesentery | Positive | B | Classic |
| 6/M/32 | Axillary LN | Positive | A | Classic |
| 7/M/34 | Neck LN | Negative | — | Classic |
| 8/M/37 | Axillary LN | Positive | A | Classic |
| 9/M/40 | Retroperitoneum | Positive | A | Classic |
| 10/M/42 | Ileum | Negative | — | Classic |
| 11/M/44 | Skin (head) | Positive | A | Classic |
| 12/M/51 | Axillary LN | Positive | A | Classic |
| 13/F/52 | Abdomen | Positive | A | Classic |
| 14/M/55 | Axillary LN | Negative | — | Classic |
EBV, Epstein-Barr virus; ISH, in situ hybridization; LN, lymph node.
Morphologic Features
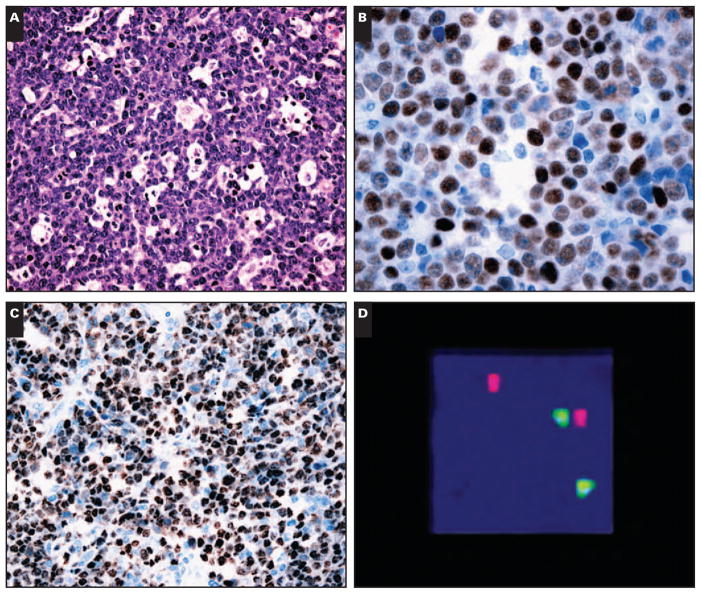
In the original material reviewed to select the areas for TMA, approximately 99% of BLs showed diffuse architecture, and only rare cases exhibited a focal nodular pattern (2 cases). Most of the cases were of classic type (213 [91.0%]) characterized by a monotonous proliferation of medium-sized cells with round nuclei, multiple nucleoli, deeply basophilic cytoplasm, and numerous mitotic figures with a starry-sky pattern Image 1A. Morphologic variants were distributed as follows: plasmacytoid differentiation, 7 cases (3.0%); and atypical BL, 14 cases (6.0%). All HIV+ cases of BL exhibited classic morphologic features.
Image 1.
Morphologic, immunohistochemical, in situ hybridization, and fluorescence in situ hybridization in Burkitt lymphoma. A, Classic Burkitt lymphoma with a prominent starry-sky pattern (H&E, ×200). B, Burkitt lymphoma with high expression of p53 protein by immunohistochemical analysis (×400). C, Expression of Epstein-Barr virus (EBV) in nuclei of neoplastic cells of Burkitt lymphoma (in situ hybridization for EBV early RNA [EBER1], ×200). D, Fluorescence in situ hybridization study using a c-MYC break-apart rearrangement probe showing dissociation of the red and green signals, indicating the presence of a chromosomal breakpoint in the c-MYC locus (×1,000).
Immunohistochemical Analysis and ISH
All cases had an immunophenotype consistent with BL according to the 2001 WHO classification,2 ie, CD20+, CD10+, bcl-6+/−, Ki-67 >95%, CD3−, and bcl-2−. CD20 and CD10 were positive in all cases; bcl-6 was positive in 203 cases (86.8%); and PAX-5 expression was observed in 225 cases (96.2%). TdT and bcl-2 were negative in all cases. All 44 cases studied with antibodies against CD5 and CD23 showed negative results. LMP-1 expression was observed in only 1 (0.4%) of 234 cases. Nuclear expression of p63 protein was seen in 9 cases (3.8%), all classified as 1+. Expression of p53 protein (above the cutoff of 10%) was noted in 38 cases (16.2%) Image 1B. In only 1 case was there coexpression of p63 and p53. All control cores showed the expected immunostaining expression pattern. A higher proportion of EBER1+ BLs were bcl-6+ (P ≤ .06 overall). This effect was even more pronounced in HIV− cases in the pediatric group (P ≤ .04). Of note, p53 positivity, which is customarily interpreted as accumulation of mutant p53, was inversely correlated with EBV status.
ISH for EBV was positive in 123 cases (52.6%). In each of these cases, all or virtually all of the neoplastic cells stained for EBER1 Image 1C. The majority of the EBV+ cases were in the pediatric group (83 cases [67.5%]), and 38 cases (30.9%) occurred in patients older than 16 years. In 2 EBV+ BLs, the age was unknown (1.6%). In the pediatric group (143 cases), 58.0% were EBV+, while in the adult group (88 cases), 38 cases (43%) were EBV+. The mean age of patients in the EBV+ group was 16 years, while the mean age in patients in the EBV− group was 23 years. In 1 case, ISH for EBV was inconclusive owing to lack of sufficient neoplastic tissue in the cores.
The lowest association between BL and EBV was seen in cases from the South region of Brazil, where 12 (29%) of 42 were EBV+. In the other regions, this association varied from 47% (Central West, 8 cases) to 76% (North, 13 cases). In the group of 14 known HIV+ cases, 10 were EBV+ (71%).
PCR Study for EBV Typing
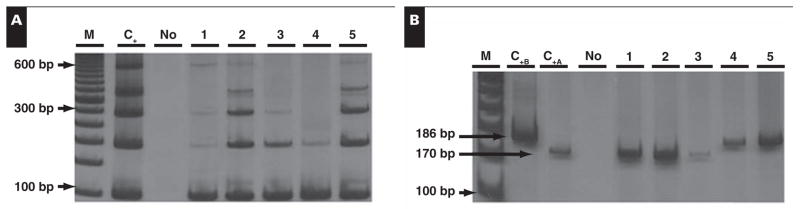
The EBV molecular subtyping analysis in the 123 EBV+ cases by ISH showed that 95 (77.2%) of the cases were EBV type A, and 24 (19.5%) were type B. In 4 cases (3.3%), the DNA obtained from the paraffin blocks was too degraded and yielded inconclusive results for EBV subtyping. No case demonstrated both EBV subtypes in the same sample Image 2.
Image 2.
Epstein-Barr virus (EBV) molecular subtyping. A, Size control polymerase chain reaction (PCR). B, EBV subtyping specific PCR. M, DNA molecular weight marker; No, DNA absence; C+B, positive control for EBV type B, fragment length, 186 base pairs (bp); C+A, positive control sample for EBV type A, fragment length, 170 bp; 1–5, positive samples for EBV.
EBV type A was predominant in 4 of the 5 geographic regions of Brazil. The only exception was observed in the Central West region, which revealed an equal distribution of EBV types A and B in the 8 EBV+ BLs (Table 2). Also, a predominance of EBV type A (8/10 [80%]) was found in HIV+ BL (Table 3).
Fluorescence In Situ Hybridization
All 361 cases of the 8 TMA blocks were studied by FISH using the LSI MYC Dual-Color Break-apart Rearrangement probe. However, 127 were excluded, most of them (89 cases) owing to poorly fixed material. Only 38 of the 127 excluded cases demonstrated results inconsistent with BL, ie, mean number of positive tiles fewer than 2.19%. In the remaining 234 cases, the range for positive signals obtained was 2.2% to 43.2% (mean, 12.5%) Image 1D.
Discussion
BL is an aggressive B-cell lymphoma with a variable incidence in different geographic regions of the world. According to Rabkin et al,25 in endemic areas (Equatorial Africa), the incidence rate varies between 5 and 10 per 100,000 children younger than 15 years, while in the United States this value is closer to 2 per million. In Brazil, the epidemiologic status of BL remains to be elucidated.17 Previous studies on BL in this country have been predominantly from the Northeast,15,16 Southeast,13,14,17,18 and South,19 with an emphasis on the pediatric population. This is the first large-scale study of BL in Brazil, including pediatric and adult populations, and also the first to analyze cases from the Central West and North regions. Brazil is a remarkably diverse country. The South and Southeast regions are more developed socioeconomically and have greater percentages of European descendants. The North includes the Amazon rain forest and is endemic for malaria and other tropical and infectious diseases.20 It should be emphasized, however, that the cases of BL studied herein were received as consultation cases. So, a bias in the number of cases from different regions could influence the low number of cases from the North region. In further studies, we intend to refine the characterization of BL in the North region of Brazil where malaria is a public health problem.
In our study of BL, there was a male predominance (male/female ratio, 2.8:1), and pediatric cases (n = 143) outnumbered adult cases (n = 88). We also observed a higher frequency of extranodal (159 cases) than nodal (67 cases) involvement. In the cases with extranodal involvement, intra-abdominal organs were affected in 129 (81.1%) of 159 cases, while in only 5 cases (3.1%) was jaw/maxilla the initial site. This is clearly distinct from the typical mandibular presentation found in Africa.2,8,12 Lymph node involvement was more common among the adult than the pediatric population, in concordance with the literature.26 Therefore, in this extensive analysis of BL from Brazil, the clinical features were similar to the sporadic form of BL seen in the United States and Europe.2,26,27 Only in the Central West and South regions did the adult group outnumber the pediatric group.
It is well established that there is a significant association of BL with EBV with a variable frequency, depending on the clinicopathologic variant. EBV is present in the majority of endemic cases of BL (up to 100%) and in only 15% to 30% of sporadic cases from the United States.2,12,28 In Brazil, there are few studies that have analyzed the frequency of association of EBV in BL. Araujo et al15 and Sandlund et al16 studied this association in the Northeast region of Brazil in a pediatric population and reported that 87% (47 of 54 cases) and 73% (8 of 11 cases), respectively, were EBV+. In our study, in this geographic region, we observed a lower frequency of association between EBV and BL (63%; 54 EBV+ cases of 86 BL cases evaluated), although this included adult and pediatric cases. In the Southeast region, Gutierrez et al,13 Bacchi et al,14 Klumb et al,17 and Hassan et al18,29 obtained different frequencies of association between BL and EBV, varying from 58% to 72%. In this particular region, we observed that half of the cases of BL were EBV+ (36/72 cases). The South region had the lowest frequency of association (29%), a result that is similar to sporadic BL in developed countries.2,28 However, in a previous study in this same region, Haralambieva et al19 reported that 50% of their BL cases were EBV+. In the Central West region, 47% of the cases were EBV+. The highest frequency of association was observed in the North, in which 76% of the cases of BL were EBV+. This establishes the existence of a substantial group of classical, non-AIDS associated BL cases in Brazil that occurs at a frequency that is much higher than that of EBV−, sporadic BL cases as observed in the United States and Europe.
Our study on BL showed a total of 123 EBV+ cases (by ISH) in the overall population (52.6%) and, as previously demonstrated in Brazil,15,17 the EBV+ group (mean age, 16 years) was younger than the EBV− group (mean age, 23 years). These results, and those of previous studies,2,12 demonstrate that Brazil as a country has an intermediate association of BL with EBV, although this association is much more pronounced in the more tropical regions. Among adult cases of BL in our series, there was a notably higher percentage of EBV+ tumors than has been reported among sporadic variants of the tumor in the United States and Europe.30 Therefore, the pathogenesis of adult cases of BL in Brazil and other equatorial countries may differ from that seen in wealthier nations. This also may have important therapeutic implications, given that antiviral nucleosides may have activity in EBV-associated lymphomas.31,32
EBV strains can be categorized into 2 types (A and B), and a geographic prevalence of these strains has been observed.15 Analysis of the coding region of the EBNA2 gene in endemic BL has revealed a high prevalence of both EBV types. Type B EBV is also identified at high frequency in healthy people in Equatorial Africa,33 while type A EBV is almost exclusively found in the peripheral blood of people from developed Western countries34 and is more frequently found in sporadic BL.35 It is also known that patients with AIDS have an increased prevalence of infection with type B EBV.35,36 By studying the EBNA2 gene, we determined that 95 (77.2%) of the EBV+ BLs contained type A EBV and 24 (19.5%) contained type B EBV, a pattern intermediate between that observed in endemic BL33 and North American cases.35 The South region of Brazil showed the lowest percentage of EBV type B (only 8%), a result similar to sporadic BL in the United States.35 In contrast, the Central West region showed the highest percentage of EBV type B (50%; 4 cases), a pattern similar to that found in endemic BL.33 The distribution of EBV strains in the Northeast and Southeast regions (Table 2) revealed similar results to previous reports in these same geographic regions.15,17,18,29 Among our 14 HIV+ BLs, 10 were EBV+ (by ISH) and only 2 cases (20%) were EBV type B, in contrast with results reported in the literature.35,36
A relationship between immunodeficiency and neoplasia had been recognized for longer than a decade before the emergence of the AIDS epidemic.37 HIV infection is associated with a high incidence of NHL (100–400 times higher than among the general population), Kaposi sarcoma, anal human papillomavirus, and cervical carcinoma. The 2 most common types of this NHL are diffuse large B-cell lymphoma and BL,9 the latter being the first NHL to be described in association with HIV infection and corresponding to about 30% of NHLs in HIV+ patients.8 Our HIV-associated BL showed a male/ female ratio of 3.6:1. Among the 14 HIV+ BL cases, 12 were adults with only 2 pediatric cases, and nodal involvement at diagnosis was more frequent than extranodal involvement. Studies38,39 have demonstrated that the frequency of EBV association in HIV-associated BL varies from 25% to 50%. In 1996, we studied 24 AIDS-related lymphoma cases in Brazil40; 5 were BLs and all were in adults. Only 2 cases (40%) were EBV+. In this study, we observed a greater association between EBV and HIV-associated BL (71%; 10 cases) as compared with the findings of previous studies38–40 but similar to the frequency reported by Lazzi et al41 in Africa, who demonstrated 6 EBV+ cases (75%) among 8 HIV-associated BLs.
Most EBV+ BL cases (whether endemic, sporadic, or AIDS-associated) typically display more restricted forms of latency, usually latency type I, only expressing EBER1 and EBER2 and EBNA1,11,12 and, as we and others showed, at least the EBV BART microRNAs.42 LMP-1 is not typically expressed in BL. In our study, only 1 case of BL was positive for LMP-1 protein by immunohistochemical analysis in a few cells. Similar results have also been described in the literature; Niedobitek et al43 observed expression of LMP-1 protein in a variable proportion of tumor cells in 2 cases of endemic BL. In Brazil, Araujo et al15 and Chen et al34 reported LMP-1 expression in 2 cases and 1 case of BL, respectively. At this point, the significance of this observation with regard to clinical prognosis, outcome, or molecular mechanism remains to be elucidated.
TP53 (also called p53) is a key tumor suppressor gene that is mutated or lost in approximately 50% of all human cancer cases worldwide, including hematologic malignancies. It is activated in response to a variety of cellular and genotoxic stress conditions, leading to the induction of growth arrest, apoptosis, DNA repair, senescence, and differentiation.44 It has been reported that 30% of endemic BL tumors and up to 70% of long-established BL lines carry mutations in p53.12
Bhatia et al45 found p53 mutations in 37% of cases of BL from Argentina and Brazil and concluded that the presence of mutated p53 in BL is independent of the geographic origin of the tumor, the 8;14 chromosomal breakpoint locations, and EBV association. Studies have shown that in some high-grade NHLs, the occurrence of positive immunostaining does not reflect point mutations in the p53 gene and vice versa.46 Villuendas et al47 found overexpression of p53 protein in 5 (63%) of 8 BL cases, and Klumb et al48 reported p53 overexpression in 13 (46%) of 28 pediatric BL cases, although the mutation was demonstrated, by PCR, in only 7 of these 13 cases. In our study, p53 protein expression was observed in 38 cases (16.2%), fewer than in previous studies. This lower percentage can be explained, in part, by the cutoff of 10% used herein, while previous studies used a cutoff of 5%.47,48 More important, we studied a far greater number than any of the prior studies, which we believe yields a more accurate representation of p53 expression in BL (at least in Brazil).
We have sequenced primary pediatric BL cases obtained from the Northeast region of Brazil. The first 13 cases analyzed were wild-type by sequence (data not shown). We also noted a trend toward p53 overexpression in EBER1 ISH-negative BLs. Therefore, the association between mutant p53 and BL may be less than originally thought, and other mechanisms, such as EBV viral genes or epigenetic modifications, may inactivate the p53 pathway. Alternatively, the p53 pathway may be evaded by MYC mutations, as has been demonstrated in a BL animal model.49
p63 is a transcription factor that contains multiple isoforms with various biologic activities.50 The p63 gene locus at chromosome 3q28 bears significant homology to the tumor suppressor gene p53 and to the related gene p73. Both p63 and p73, considered p53-related genes, encode various isoforms with transactivation, DNA binding, and tetramerization domains.51 p63 protein exhibits a consistent expression pattern in normal tissues such as squamous epithelia, urothelium, basal cells of prostatic and breast glands, and reticular epithelium of the normal thymus and also in a subset of lymphocytes in the germinal center of morphologically normal lymph nodes.52,53 Among hematolymphoid neoplasms, p63 expression has been reported in blast crisis in chronic myelogenous leukemia,54 follicular lymphoma, diffuse large B-cell lymphoma,51 isolated cases of chronic lymphocytic leukemia, marginal cell lymphoma,52 and in 44% of anaplastic large cell lymphoma.55 There are few articles reporting the expression of p63 in lymphoid malignancies, and, to the best of our knowledge, this is the first study to evaluate its expression in a large number of BL cases. We observed that only 9 (3.8%) of the 234 BL cases expressed p63 protein, and coexpression of protein p53 and p63 was observed in only 1 case.
In this study of the largest number of cases from Latin America analyzed to date, we have demonstrated that BL in Brazil is diverse and regionally distinct. EBV association is sporadic, and, perhaps, HIV-associated BL is higher than seen in the United States and Europe. Overexpression of p53 was infrequently observed, and dysfunction of this critical tumor suppressor in BL may be linked to other factors rather than inactivating mutations. Prospective studies of BL in patients undergoing standardized chemotherapy regimens are now underway.
Upon completion of this activity you will be able to:
define the 3 major types of Burkitt lymphoma and their patterns of incidence and organ involvement.
describe the range of association with Epstein-Barr virus and tumor suppressor protein expression (p53 and p63) in various Burkitt lymphoma subtypes.
describe the range of clinicopathologic characteristics of Burkitt lymphoma in Brazil.
recognize that there may be geographic and regional differences in the patterns and types of Burkitt lymphoma and define an approach with which diagnostic pathologists may investigate and characterize the features of Burkitt lymphoma in their own practice locales.
comment on whether immunochemical staining for LMP-1 and p63 is indicated in Burkitt lymphoma.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit ™ per article.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 1006. Exam is located at www.ascp.org/ajcpcme.
Acknowledgments
Supported in part by grants 5R01CA082274, 5R01CA121935, 5R01CA112217 (to W.J.H.), and DE018304 (to D.P.D.) from the National Cancer Institute (NCI), Bethesda, MD, the NCI AIDS Malignancy Consortium, and the University of Miami Fogarty AITRP grant D43TW000017.
We thank Lucimara Chioato, PhD, and Luciana Hayashi da Silva, MS, for the molecular biology studies. We also thank Marcio Montes and Luciana Ricardi for FISH technical assistance.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Stein H, et al., editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. World Health Organization Classification of Tumours. [Google Scholar]
- 3.Chuang SS, Ye H, Du MQ, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558–564. doi: 10.1309/EQJR3D3V0CCQGP04. [DOI] [PubMed] [Google Scholar]
- 4.Leder P, Battey J, Lenoir G, et al. Translocations among antibody genes in human cancer. Science. 1983;222:765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- 5.Bench AJ, Erber WN, Follows GA, et al. Molecular genetic analysis of haematological malignancies, II: mature lymphoid neoplasms. Int J Lab Hematol. 2007;29:229–260. doi: 10.1111/j.1751-553X.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- 6.Shiramizu B, Barriga F, Neeguaye J, et al. Patterns of chromosomal breakpoint locations in Burkitt’s lymphoma: relevant to geography and Epstein-Barr virus associations. Blood. 1991;77:1516–1526. [PubMed] [Google Scholar]
- 7.Gaidano G, Ballerini P, Gong JZ, et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991;88:5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferry JA. Burkitt’s lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11:375–383. doi: 10.1634/theoncologist.11-4-375. [DOI] [PubMed] [Google Scholar]
- 9.Navarro WH, Kaplan LD. AIDS-related lymphoproliferative disease. Blood. 2006;107:13–20. doi: 10.1182/blood-2004-11-4278. [DOI] [PubMed] [Google Scholar]
- 10.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 11.Rezk SA, Weiss LM. Epstein-Barr virus–associated lymphoproliferative disorders. Hum Pathol. 2007;38:1293–1304. doi: 10.1016/j.humpath.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Kelly GL, Rickinson AB. Burkitt lymphoma: revisiting the pathogenesis of a virus-associated malignancy. Hematology Am Soc Hematol Educ Program. 2007:277–284. doi: 10.1182/asheducation-2007.1.277. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez MI, Bhatia K, Barriga T, et al. Molecular epidemiology of Burkitt’s lymphoma from South America: differences in breakpoint location and Epstein-Barr virus association from tumors in other world regions. Blood. 1992;79:3261–3266. [PubMed] [Google Scholar]
- 14.Bacchi MM, Bacchi CE, Alvarenga M, et al. Burkitt’s lymphoma in Brazil: strong association with Epstein-Barr virus. Mod Pathol. 1996;9:63–67. [PubMed] [Google Scholar]
- 15.Araujo I, Foss HD, Bittencourt A, et al. Expression of Epstein-Barr virus-gene products in Burkitt’s lymphoma in Northeast Brazil. Blood. 1996;87:5279–5286. [PubMed] [Google Scholar]
- 16.Sandlund JT, Fonseca T, Leimig T, et al. Predominance and characteristics of Burkitt lymphoma among children with non-Hodgkin lymphoma in northeastern Brazil. Leukemia. 1997;11:743–746. doi: 10.1038/sj.leu.2400609. [DOI] [PubMed] [Google Scholar]
- 17.Klumb CE, Hassan R, De Oliveira DE, et al. Geographic variation in Epstein-Barr virus–associated Burkitt’s lymphoma in children from Brazil. Int J Cancer. 2004;108:66–70. doi: 10.1002/ijc.11443. [DOI] [PubMed] [Google Scholar]
- 18.Hassan R, Klumb CE, Felisbino FE, et al. Clinical and demographic characteristics of Epstein-Barr virus–associated childhood Burkitt’s lymphoma in Southeastern Brazil: epidemiological insights from an intermediate risk region. Haematologica. 2008;93:780–783. doi: 10.3324/haematol.12424. [DOI] [PubMed] [Google Scholar]
- 19.Haralambieva E, Schuuring E, Rosati S, et al. Interphase fluorescence in situ hybridization for detection of 8q24/ MYC breakpoints on routine histologic sections: validation in Burkitt lymphoma from three geographic regions. Genes Chromosomes Cancer. 2004;40:10–18. doi: 10.1002/gcc.20009. [DOI] [PubMed] [Google Scholar]
- 20.Braga WS, Souza RA, Silva EB, et al. Coinfection between hepatitis B virus and malaria: clinical, serologic and immunologic aspects. Rev Soc Bras Med Trop. 2006;39:27–31. doi: 10.1590/s0037-86822006000100005. [DOI] [PubMed] [Google Scholar]
- 21.Coura JR, Amaral RS. Epidemiological and control aspects of schistosomiasis in Brazilian endemic areas. Mem Inst Oswaldo Cruz. 2004;99:13–19. doi: 10.1590/s0074-02762004000900003. [DOI] [PubMed] [Google Scholar]
- 22.Howe JR, Klimstra DS, Cordon-Cardo C. DNA extraction from paraffin-embedded tissues using a salting-out procedure: a reliable method for PCR amplification of archival material. Histol Histopathol. 1997;12:595–601. [PubMed] [Google Scholar]
- 23.van Dongen JJM, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 24.Cogliati SB, Novak U, Hens S, et al. Diagnosis of Burkitt lymphoma in due time: a practical approach. Br J Haematol. 2006;134:294–301. doi: 10.1111/j.1365-2141.2006.06194.x. [DOI] [PubMed] [Google Scholar]
- 25.Rabkin CS, Ward MH, Maans A, et al. Epidemiology of non-Hodgkin’s lymphoma. In: Magrath IT, editor. The Non-Hodgkin’s Lymphomas. 2. London, England: Arnold; 1997. pp. 171–186. [Google Scholar]
- 26.Boerma EG, van Imhoff GW, Appel IM, et al. Gender and age-related differences in Burkitt lymphoma: epidemiological and clinical data from the Netherlands. Eur J Cancer. 2004;40:2781–2787. doi: 10.1016/j.ejca.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Magrath IT. Therapy of the small non–cleaved cell lymphoma. In: Magrath IT, editor. The Non-Hodgkin’s Lymphomas. 2. London, England: Arnold; 1997. pp. 779–781. [Google Scholar]
- 28.Barriga F, Kiwanuka J, Alvarez-Mon M, et al. Significance of chromosome 8 breakpoint location in Burkitt’s lymphoma: correlation with geographical origin and association with Epstein-Barr virus. Curr Top Microbiol Immunol. 1988;141:128–137. doi: 10.1007/978-3-642-74006-0_18. [DOI] [PubMed] [Google Scholar]
- 29.Hassan R, White LR, Stefanoff CG, et al. Epstein-Barr virus (EBV) detection and typing by PCR: a contribution to diagnostic screening of EBV-positive Burkitt’s lymphoma. Diagn Pathol. 2006;1:17. doi: 10.1186/1746-1596-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spina M, Tirelli U, Zagonel V, et al. Burkitt’s lymphoma in adults with and without human immunodeficiency virus infection: a single-institution clinicopathologic study of 75 patients. Cancer. 1998;82:766–774. [PubMed] [Google Scholar]
- 31.Kurokawa M, Ghosh SK, Ramos JC, et al. Azidothymidine inhibits NF-kappaB and induces Epstein-Barr virus gene expression in Burkitt lymphoma. Blood. 2005;106:235–240. doi: 10.1182/blood-2004-09-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng WH, Hong G, Delecluse HJ, et al. Lytic induction therapy for Epstein-Barr virus–positive B-cell lymphomas. J Virol. 2004;78:1893–1902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young LS, Yao QY, Rooney CM, et al. New type B isolate of Epstein-Barr virus from Burkitt’s lymphoma and normal individuals in endemic areas. J Gen Virol. 1987;68:2853–2862. doi: 10.1099/0022-1317-68-11-2853. [DOI] [PubMed] [Google Scholar]
- 34.Chen WG, Chen YY, Bacchi MM, et al. Genotyping of Epstein-Barr virus in Brazilian Burkitt’s lymphoma and reactive lymphoid tissue: type A with a high prevalence of deletions within the latent membrane protein gene. Am J Pathol. 1996;148:17–23. [PMC free article] [PubMed] [Google Scholar]
- 35.Goldschmidts WL, Bhatia K, Johnson JF, et al. Epstein-Barr virus genotypes in AIDS-associated lymphomas are similar to those in endemic Burkitt’s lymphomas. Leukemia. 1992;6:875–878. [PubMed] [Google Scholar]
- 36.Boyle MJ, Sewel WA, Sculley TB, et al. Subtypes of Epstein-Barr virus in human immunodeficiency virus–associated non-Hodgkin lymphoma. Blood. 1991;78:3004–3011. [PubMed] [Google Scholar]
- 37.Gatti RA, Good RA. Occurrence of malignancy in immunodeficiency disease: a literature review. Cancer. 1971;28:89–98. doi: 10.1002/1097-0142(197107)28:1<89::aid-cncr2820280117>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton-Dutoit SJ, Raphael M, Audouin J, et al. In situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in acquired immunodeficiency syndrome–related lymphomas: correlation with tumor morphology and primary site. Blood. 1993;82:619–624. [PubMed] [Google Scholar]
- 39.Raphael MM, Audouin J, Lamine M, et al. Immunophenotypic and genotypic analysis of acquired immunodeficiency syndrome–related non-Hodgkin’s lymphomas: correlation with histologic features in 36 cases. French Study Group of Pathology for HIV-Associated Tumors. Am J Clin Pathol. 1994;101:773–782. doi: 10.1093/ajcp/101.6.773. [DOI] [PubMed] [Google Scholar]
- 40.Bacchi CE, Bacchi MM, Rabenhorst SH, et al. AIDS-related lymphoma in Brazil: histopathology, immunophenotype, and association with Epstein-Barr virus. Am J Clin Pathol. 1996;105:230–237. doi: 10.1093/ajcp/105.2.230. [DOI] [PubMed] [Google Scholar]
- 41.Lazzi S, Fwerrari F, Nyongo A, et al. HIV-associated malignant lymphomas in Kenya (Equatorial Africa) Hum Pathol. 1999;29:1285–1289. doi: 10.1016/s0046-8177(98)90258-1. [DOI] [PubMed] [Google Scholar]
- 42.Xia T, O’Hara A, Araujo I, et al. EBV microRNA in primary lymphomas and targeting of CXCL-11 by ebv-mir-BHRF1-3. Cancer Res. 2008;68:1436–1442. doi: 10.1158/0008-5472.CAN-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niedobitek G, Agathanggelou A, Rowe M, et al. Heterogeneous expression of Epstein-Barr virus latent proteins in endemic Burkitt’s lymphoma. Blood. 1995;86:659–665. [PubMed] [Google Scholar]
- 44.Margalit O, Amram H, Amariglio N, et al. BCL6 is regulated by p53 through a response element frequently disrupted in B-cell non-Hodgkin lymphoma. Blood. 2006;107:1599–1607. doi: 10.1182/blood-2005-04-1629. [DOI] [PubMed] [Google Scholar]
- 45.Bhatia KG, Gutiérrez MI, Huppi K, et al. The pattern of p53 mutations in Burkitt’s lymphoma differs from that of solid tumors. Cancer Res. 1992;52:4273–4276. [PubMed] [Google Scholar]
- 46.Kocialkowski S, Pezzella F, Morrison H, et al. Mutations in the p53 gene are not limited to classic “hot spots” and are not predictive of p53 protein expression in high-grade non-Hodgkin’s lymphoma. Br J Haematol. 1995;89:55–60. doi: 10.1111/j.1365-2141.1995.tb08911.x. [DOI] [PubMed] [Google Scholar]
- 47.Villuendas R, Piris MA, Orradre JL, et al. p53 protein expression in lymphomas and reactive lymphoid tissue. J Pathol. 1992;166:235–241. doi: 10.1002/path.1711660305. [DOI] [PubMed] [Google Scholar]
- 48.Klumb CE, Hassan R, Zalcberg IR, et al. p53 protein expression does not correlate with EBV status in childhood B non-Hodgkin lymphomas. Pediatr Blood Cancer. 2004;43:115–119. doi: 10.1002/pbc.20069. [DOI] [PubMed] [Google Scholar]
- 49.Hemann MT, Bric A, Teruya-Feldstein J, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flores ER. The roles of p63 in cancer. Cell Cycle. 2007;6:300–304. doi: 10.4161/cc.6.3.3793. [DOI] [PubMed] [Google Scholar]
- 51.Hedvat CV, Teruya-Feldstein J, Puig P, et al. Expression of p63 in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2005;13:237–242. doi: 10.1097/01.pai.0000142160.52670.ce. [DOI] [PubMed] [Google Scholar]
- 52.Di Como CJ, Urist MJ, Babayan I, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 53.Nylander K, Vojtesek B, Nenutil R, et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J Pathol. 2002;198:417–427. doi: 10.1002/path.1231. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi H, Inokuchi K, Sakuma Y, et al. Mutation of the p51/p63 gene is associated with blastic crisis in chronic myeloid leukemia. Leukemia. 2001;15:1729–1734. doi: 10.1038/sj.leu.2402265. [DOI] [PubMed] [Google Scholar]
- 55.Gualco G, Weiss LM, Bacchi CE. Expression of p63 in anaplastic large cell lymphoma but not in classical Hodgkin lymphoma. Hum Pathol. 2008;39:1505–1510. doi: 10.1016/j.humpath.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]