Abstract
Background
Parkinson disease is a common neurodegenerative disease. The racial, sex, age, and geographic distributions of Parkinson disease in the US are unknown.
Methods
We performed a serial cross-sectional study of US Medicare beneficiaries aged 65 and older from the years 1995, and 2000–2005. Using over 450,000 Parkinson disease cases per year, we calculated Parkinson disease prevalence and annual incidence by race, age, sex, and county. Spatial analysis investigated the geographic distribution of Parkinson disease.
Results
Age-standardized Parkinson disease prevalence (per 100,000) was 2,168.18 (±95.64) in White men, but 1,036.41 (±86.01) in Blacks, and 1,138.56 (±46.47) in Asians. The incidence ratio in Blacks as compared to Whites (0.74; 95% CI = 0.732–0.748) was higher than the prevalence ratio (0.58; 95% CI = 0.575–0.581), whereas the incidence ratio for Asians (0.69; 95% CI = 0.657–0.723) was similar to the prevalence ratio (0.62; 95% CI = 0.617–0.631). Bayesian mapping of Parkinson disease revealed a concentration in the Midwest and Northeast regions. Mean county incidence by quartile ranged from 279 to 3,111, and prevalence from 1,175 to 13,800 (per 100,000). Prevalence and incidence in urban counties were greater than in rural ones (p < 0.01). Cluster analysis supported a nonrandom distribution of both incident and prevalent Parkinson disease cases (p < 0.001).
Conclusions
Parkinson disease is substantially more common in Whites, and is nonrandomly distributed in the Midwest and Northeastern US.
Key Words: Parkinson disease, Geographical information systems, Environment, Prevalence study, Incidence study
Background
Parkinson disease is a common neurodegenerative disease of the elderly. Previous estimates of Parkinson disease prevalence and incidence have provided conflicting estimates of disease burden among Europeans between 65.6 and 12,500 per 100,000 [1]. Epidemiological data on the distribution of Parkinson disease by race and gender in the US in non-Whites have not been extensively studied. A few studies have suggested a predilection for Whites [2,3,4,5]. Other, similarly powered studies have reported no difference between Whites and non-Whites [6,7]. One study of a diverse urban community, using a disease registry and a subset of Medicare claims data for case identification, found that Black men had the highest incidence [8]. Using a large insurance database for case identification, another study of Parkinson disease and ethnicity found that Hispanics had the highest incidence, and Blacks the lowest. Several studies have suggested that Parkinson disease is more common in men, with a male:female ratio between 1.1 and 2.3 [9].
Although the cause of Parkinson disease is unknown, in most cases environmental factors are suspected. Nevertheless, there is little direct evidence implicating the environment in Parkinson disease. Geographic clustering of Parkinson disease may provide more direct evidence of the environmental role in its pathogenesis. The few studies that have attempted to investigate geographic variation in Parkinson disease have focused on rural living as a risk factor. Many of these case-control studies suggest an increased risk of Parkinson disease in those residing in rural areas [10,11], whereas others report an increased prevalence in industrial areas [12], or no significant difference [4]. However, nonstandard definitions of rurality and small sample size limit these studies.
A Geographic Information System (GIS) combines an event's tenancy in time or space with spatial analysis to examine spatial and temporal relationships. GIS has been used in numerous studies to monitor infectious outbreaks [13], elucidate crime patterns [14], identify health care disparities [15], and investigate environmental exposure-disease relationships [16,17,18]. GIS permits the spatial linkage of demographic, environmental, and clinical databases to create a high-resolution display and facilitate quantitative analysis of the spatial distribution and behavior of a disease. In this study, we report the ethnic, temporal, and geographic trends in Parkinson disease prevalence and incidence using the largest US health care database, Medicare.
Research Design and Methods
This study was approved by the Human Studies Committee at Washington University School of Medicine and by the Centers for Medicare and Medicaid Services.
Study Population
Parkinson disease cases were identified from Medicare research-identifiable files (US Department of Health and Human Services, Centers for Medicare and Medicaid Services, www.cms.hhs.gov/home/rsds.asp/), which contain individual-level data on Medicare benefit recipients including ICD-9 codes, date of birth, race, sex, and zip code of residence. The study population consisted of Medicare beneficiaries living in the United States at any time in the year 1995 or between 2000 and 2005. We identified Parkinson disease cases using ICD-9 codes 332 (Parkinson disease) or 332.0 (paralysis agitans). Beneficiaries who also had diagnoses of secondary parkinsonism (332.1) or other degenerative diseases of the basal ganglia (333.0) were excluded from analysis. Our population data were provided by Medicare denominator files, which contain individual date of birth, race, sex, residence, zip code, and mortality data for all US Medicare-eligible individuals, comprising 98% of all Americans over the age of 65 (Centers for Medicare and Medicaid Services, 2008). Medicare-eligible individuals who were enrolled in a health maintenance organization were removed from the denominator file. These organizations may not submit case claims to Medicare and their inclusion could artificially lower disease rates. Sex and ethnicity were identified using standard Medicare sex and race codes. Race-specific data on beneficiaries with race declarations of ‘Native American’, ‘unknown’ or ‘other’ are not reported due to small numbers of subjects or race ambiguity. Age was determined using the date of birth. Commonly employed age strata 65–69, 70–74, 75–79, 80–84, and 85+ were used to determine age-group-specific disease rates. Beneficiary state and county residence information was used for geographic analyses.
Demographic Analysis
All calculations were performed on beneficiaries who qualified for Medicare by virtue of being 65 years of age or older. To calculate crude Parkinson disease prevalence, we divided the number of Parkinson disease cases by the total number of eligible Medicare beneficiaries in each year for the years 1995 and 2000–2005. We also calculated the annual incidence rates for the years 2002–2005, designating a Parkinson disease case as incident if an individual over the age of 67 had 2 or more years of preceding Medicare data without a Parkinson disease diagnosis claim. Crude Parkinson disease incidence and prevalence were compared by age strata. To compare Parkinson disease incidence and prevalence by race or sex, we first performed age standardization using the direct standardization method with all Medicare beneficiaries as the standard population.
All prevalence and incidence values are reported per 100,000 Medicare beneficiaries. Prevalence and incidence ratios were calculated with Whites as the reference group. Standard deviations are reported for prevalence and incidence, and 95% confidence intervals are reported for prevalence and incidence ratios. Study population characteristics are reported in table 1.
Table 1.
Population demographics (US Medicare beneficiaries, year 2003)
| Population |
||
|---|---|---|
| n | % | |
| Race | ||
| White | 25,581,561 | 86.6 |
| Black | 2,356,271 | 8.0 |
| Hispanic | 593,234 | 2.0 |
| Asian | 470,024 | 1.6 |
| Native American | 96,262 | 0.3 |
| Unknown | 66,448 | 0.2 |
| Other | 367,034 | 1.2 |
| Sex | ||
| Male | 12,316,801 | 41.7 |
| Female | 17,214,033 | 58.3 |
| Age | ||
| 65–69 | 8,060,318 | 27.3 |
| 70–74 | 7,053,376 | 23.9 |
| 75–79 | 6,134,222 | 20.8 |
| 80–84 | 4,417,038 | 15.0 |
| 85+ | 3,865,880 | 13.1 |
| Total | 29,530,834 | |
Rural versus Urban Analysis
The United States Department of Agriculture's rural-urban continuum classification system (United States Department of Agriculture Economic Research Service, 2008) defines rurality by absolute population and classifies each county in the US by degree of rurality in a rank order fashion using a nine-tier scale from a population of less than 2,500 to a population of greater than 1 million. This system separately classifies less populated areas which are adjacent to large urban areas, such as suburbs.
To determine the relationship between rurality and Parkinson disease, we applied the rural-urban continuum classification system to county level age and race standardization for Parkinson disease prevalence and incidence from the year 2002. We compared the mean prevalence and incidence across these categories of rurality using a Kruskal-Wallis test. The prevalence and incidence for the most rural counties (= population less than 2,500) were also compared to those of the most urban counties (= population greater than 1 million) with a two-tailed t test.
Spatial Analysis of Parkinson Disease: Bayesian Modeling and Mapping of Parkinson Disease Incidence and Prevalence
To produce smoothed disease maps, we applied a conditionally autoregressive model to predict county level incidence and prevalence rates of Parkinson disease. This model is derived from a traditional multilevel design, but takes into account spatial adjacency relationships between counties by assuming that neighboring areas had similar spatial variation.
To capture the random effects between counties, we computed the median relative risk [19] and interquartile relative risk [20]. The median relative risk is always greater than or equal to 1 and higher values suggest greater spatial variance. The interquartile relative risk reflects a difference of relative risks between the highest (87.5%) and lowest (12.5%) quartiles of incidence or prevalence. We also performed a high/low Getis-Ord General G cluster analysis to determine the probability of nonrandom grouping of either high or low county prevalence or incidence.
Spatial Analysis of Smoking Behavior
Since studies have consistently demonstrated a protective effect of smoking on incident Parkinson disease, we examined the geographic distribution of smoking behavior and Parkinson disease using data from the National Center for Health Care Statistics (Department of Health and Human Services, National Center for Health Statistics, 2008). ‘Ever smoker’ (= current smoker plus former smoker) rates for those survey respondents aged 65 and above were calculated for each state from the years 2000 to 2006 and compared to mean state Parkinson disease prevalence from 2000 to 2005 using a Spearman correlation analysis.
Statistical Analysis
Statistical analyses were performed using SPSS v.16 and SPSS v.15 with statistical significance at p <0.05. Spatial data management and preparation were performed in SAS 9.1. Bayesian model fitting was conducted in WinBUGS v.1.4.3., ArcGIS v.9.1 was used for mapping.
Results
Prevalence/Incidence of Parkinson Disease in the US
The mean prevalence of Parkinson disease per 100,000 Medicare beneficiaries over age 65 from 1995, and 2000–2005 was 1,588.43 (±97.41) or approximately 1.6% of the elderly population. The mean annual incidence from 2002 to 2005 was 445.79 (±1.85). The mean prevalence of Parkinson disease steadily increased with age, with no apparent plateau, from 553.52 (95% CI = 537.12–569.92) between ages 65–69, to 2,948.93 per 100,000 (95% CI = 2,550.66–3,347.14) at ages 85 and above. Similarly, mean Parkinson disease incidence also appears to increase with age, from 124.22 (95% CI = 121.39–127.05) between ages 65–69 to 970.19 (95% CI = 944.91–995.47) among those greater than 85 years of age. The incidence and prevalence of Parkinson disease have remained stable over the 10-year period from 1995 to 2005 (table 2).
Table 2.
Parkinson disease prevalence and annual incidence (per 100,000) by age among US Medicare beneficiaries
| Age groups | Parkinson disease prevalence |
Parkinson disease annual incidence |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | mean prevalence (95% CI) | 2002 | 2003 | 2004 | 2005 | mean annual incidence (95% CI) | |
| 65–69 | 500.31 | 557.14 | 556.54 | 561.36 | 569.37 | 564.27 | 565.68 | 553.52 (537.12–569.92) | 121.03 | 128.49 | 125.19 | 122.17 | 124.22 (121.39–127.05) |
| 70–74 | 1,017.62 | 1,113.97 | 1,110.98 | 1,106.82 | 1,095.28 | 1,102.62 | 1,108.89 | 1,093.74 (1,070.31–1,117.09) | 307.04 | 344.73 | 331.15 | 332.29 | 328.80 (315.43–342.17) |
| 75–79 | 1,799.95 | 1,934.41 | 1,893.22 | 1,891.85 | 1,881.64 | 1,854.40 | 1,897.84 | 1,881.39 (1,851.99–1,910.61) | 525.66 | 577.90 | 554.06 | 555.21 | 553.21 (535.05–571.37) |
| 80–84 | 2,534.77 | 2,869.13 | 2,862.55 | 2,808.60 | 2,777.13 | 2,763.63 | 2,720.18 | 2,757.02 (2,678.34–2,835.66) | 785.59 | 847.06 | 822.59 | 793.55 | 812.20 (788.31–836.09) |
| 85+ | 2,861.29 | 3,197.98 | 3,217.96 | 1,649.02 | 3,193.97 | 3,131.35 | 3,169.95 | 2,948.93 (2,550.66–3,347.14) | 937.20 | 1,009.60 | 966.83 | 967.11 | 970.19 (944.91–995.47) |
Race/Sex Demographics of Parkinson Disease
The mean age-standardized prevalence of Parkinson disease from 1995, and 2000–2005 was highest among White men (2,168.18 ± 95.64) and lowest among Asian women (963.91 ± 38.74) (table 3). The mean age-standardized prevalence of Parkinson disease in Blacks and Asians was approximately 50% lower than the prevalence in Whites, with crude prevalence ratios of 0.58 (95% CI = 0.575–0.581) and 0.62 (95% CI = 0.617–0.631), respectively, as compared to Whites. Parkinson disease incidence also varied by race, but not as markedly in Blacks, who had a crude incidence ratio of 0.74 (95% CI = 0.732–0.748) (table 3). The incidence ratio for Asians was similar to the prevalence ratio (0.69; 95% CI = 0.657–0.723). The age-standardized prevalence and incidence were greater in men than in women for all races, with a mean prevalence sex ratio of 155 males per 100 females and a mean incidence sex ratio of 146 males per 100 females.
Table 3.
Age-adjusted Parkinson disease prevalence and annual incidence (per 100,000) by ethnic group
| Race | 1995 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | Mean ± SD | Prevalence or incidence ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| White | |||||||||
| Males | |||||||||
| Prevalence | 1,963.67 | 2,253.31 | 2,231.67 | 2,203.38 | 2,187.41 | 2,159.57 | 2,178.26 | 2,168.18 ± 95.64 | − |
| Incidence | − | − | − | 537.36 | 569.71 | 563.26 | 571.35 | 560.42 ± 15.76 | − |
| Females | |||||||||
| Prevalence | 1,247.94 | 1,419.43 | 1,414.31 | 1,407.52 | 1,397.26 | 1,379.77 | 1,379.78 | 1,378.00 ± 59.44 | − |
| Incidence | − | − | − | 367.70 | 387.64 | 383.20 | 381.44 | 379.99 ± 8.60 | − |
| Both sexes | |||||||||
| Prevalence | 1,505.46 | 1,723.43 | 1,715.86 | 1,703.34 | 1,693.74 | 1,675.62 | 1,683.99 | 1,671.63 ± 75.18 | Ref. |
| Incidence | − | − | − | 450.57 | 454.63 | 450.24 | 452.03 | 451.87 ± 2.00 | Ref. |
| Black | |||||||||
| Males | |||||||||
| Prevalence | 1,039.55 | 1,269.05 | 1,281.34 | 1,314.08 | 1,311.14 | 1,295.80 | 1,341.12 | 1,264.58 ± 101.97 | − |
| Incidence | − | − | − | 409.31 | 422.87 | 428.96 | 447.53 | 427.17 ± 15.87 | − |
| Females | |||||||||
| Prevalence | 749.45 | 924.402 | 919.17 | 939.22 | 945.153 | 949.42 | 989.99 | 916.69 ± 77.24 | − |
| Incidence | − | − | − | 301.72 | 319.72 | 328.16 | 341.33 | 322.73 ± 16.59 | − |
| Both sexes | |||||||||
| Prevalence | 848.86 | 1,042.10 | 1,041.59 | 1,068.18 | 1,072.28 | 1,069.63 | 1,112.27 | 1,036.418 ± 6.01 | 0.58 (0.575–0.581) |
| Incidence | − | − | − | 354.10 | 354.64 | 361.93 | 377.02 | 361.92 ± 10.68 | 0.74 (0.732–0.748) |
| Hispanic | |||||||||
| Males | |||||||||
| Prevalence | 1,583.95 | 1,787.17 | 1,790.35 | 1,892.74 | 1,921.25 | 1,911.72 | 1,986.20 | 1,839.05 ± 133.36 | − |
| Incidence | − | − | − | 512.57 | 569.04 | 519.14 | 553.32 | 538.52 ± 27.08 | − |
| Females | |||||||||
| Prevalence | 1,201.13 | 1,337.8 | 1,327.65 | 1,314.08 | 1,361.15 | 1,411.30 | 1,463.02 | 1,345.16 ± 82.25 | − |
| Incidence | − | − | − | 390.78 | 436.30 | 439.23 | 442.90 | 427.30 ± 24.50 | − |
| Both sexes | |||||||||
| Prevalence | 1,356.59 | 1,513.07 | 1,511.76 | 1,562.38 | 1,581.16 | 1,608.43 | 1,671.32 | 1,543.53 ± 99.33 | 0.89 (0.881–0.896) |
| Incidence | − | − | − | 462.9 | 486.92 | 468.14 | 486.29 | 476.06 ± 12.36 | 1.07 (1.047–1.084) |
| Asian | |||||||||
| Males | |||||||||
| Prevalence | 1,313.19 | 1,344.10 | 1,307.56 | 1,401.90 | 1,463.11 | 1,448.29 | 1,468.02 | 1,392.31 ± 70.40 | − |
| Incidence | − | − | − | 382.79 | 424.86 | 392.59 | 407.00 | 401.81 ± 18.30 | − |
| Females | |||||||||
| Prevalence | 971.64 | 904.53 | 922.62 | 968.39 | 975.95 | 984.36 | 1,019.87 | 963.91 ± 38.74 | − |
| Incidence | − | − | 293.63 | 279.01 | 292.27 | 306.32 | 292.81 ± 11.16 | − | |
| Both sexes | |||||||||
| Prevalence | 1,113.23 | 1,085.96 | 1,080.69 | 1,144.22 | 1,173.45 | 1,171.76 | 1,200.61 | 1,138.56 ± 46.47 | 0.62 (0.617–0.631) |
| Incidence | − | − | − | 338.34 | 338.60 | 332.69 | 346.94 | 339.14 ± 5.87 | 0.69 (0.657–0.723) |
Geographic Distribution of Parkinson Disease in the US
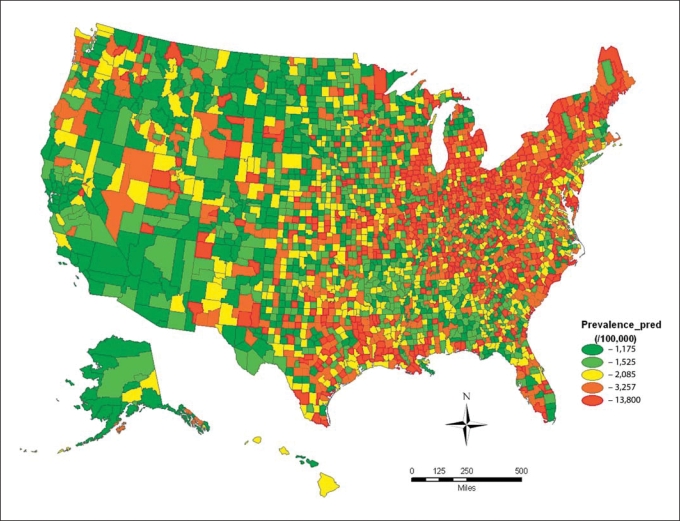
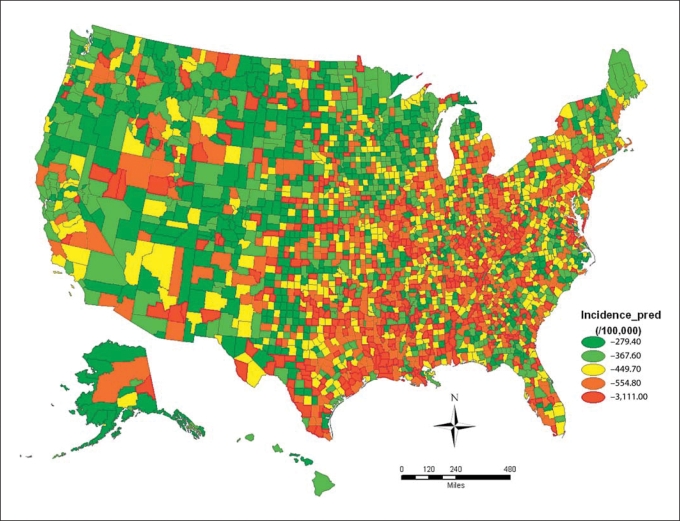
The incidence and prevalence of Parkinson disease varied significantly across the United States. Disease rates were highest in the Midwest and Northeast regions, where the incidence and prevalence of Parkinson disease were 2–10 times greater than the rates of many Western and Southern US counties. The median relative risk and interquartile relative risk indicated that the spatial variations of the incidence and prevalence were considerable (table 4; fig. 1, 2).
Table 4.
Age- and race-standardized Parkinson disease prevalence and annual incidence (per 100,000) by rural-urban continuum classification code, year 2003
| Rural-urban continuum code description (2003) | Mean Parkinson disease prevalence (95% CI) | Mean Parkinson disease annual incidence (95% CI) |
|---|---|---|
| Urban, population >1 million | 1,706.27 (1,671.14–1,741.26) | 476.81 (462.07–491.55) |
| Urban, population 250,000 to 1 million | 1,580.44 (1,508.45–1,583.15) | 436.64 (419.34–453.92) |
| Urban, population <250,000 | 1,545.86 (1,502.44–1,589.16) | 411.06 (449.50–489.82) |
| Rural, adjacent to urban area, population >20,000 | 1,654.44 (1,603.99–1,704.81) | 469.62 (447.94–491.30) |
| Rural, not adjacent to urban area, population >20,000 | 1,526.84 (1,449.39–1,604.21) | 418.58 (386.67–450.47) |
| Rural, adjacent to urban area, population 2,500 to 1 million | 1,503.54 (1,468.31–1,538.69) | 424.28 (409.44–439.70) |
| Rural, not adjacent to urban area, population of 2,500–19,999 | 1,440.31 (1,397.49–1,483.11) | 399.35 (378.22–420.46) |
| Rural, adjacent to urban area, population <2,500 | 1,432.39 (1,362.76–1,501.84) | 391.44 (360.13–422.73) |
| Completely rural, population <2,500 | 1,371.60 (1,303.23–1,439.97) | 413.24 (379.10–447.48) |
Fig. 1.
County level age- and race-standardized prevalence (per 100,000) of Parkinson disease among Medicare beneficiaries in the United States (year = 2003).
Fig. 2.
County level age- and race-standardized incidence (per 100,000) of Parkinson disease among Medicare beneficiaries in the United States (year = 2003).
Cluster analysis of the 2003 data supported a nonrandom grouping of county level prevalence and incidence in these areas (prevalence: z test statistic = 13.93, p < 0.0001; incidence: z test statistic = 12.65, p < 0.0001). We found no significant correlation between the state level ever smoker rates and Parkinson disease prevalence (Spearman's rho = −0.213, p = 0.138), suggesting that the nonrandom geographic clustering of Parkinson disease was not due to differential regional smoking patterns among the elderly in the US.
Parkinson Disease and Rurality
To test the hypothesis that Parkinson disease is associated with rural living, we used the United States Department of Agriculture's rural-urban continuum classification system to classify residence for Medicare beneficiaries from the year 2003 and determined the mean prevalence and incidence per rural category. There was a significant difference in age- and race-standardized Parkinson disease prevalence and incidence between the rural classification categories (Kruskal-Wallis test, prevalence: p < 0.001, incidence: p < 0.001) (table 4). Post hoc analysis revealed that Parkinson disease prevalence for the most urban counties (population greater than 1 million: 1,706.27; 95% CI = 1,671.14–1,741.26) was significantly greater than that for the most rural counties (population <2,500: 1,371.60; 95% CI = 1,303.23–1,439.97) (two-tailed t test; p < 0.01). Similarly, Parkinson disease incidence for the most urban counties (476.81; 95% CI = 462.07–491.55) was significantly greater than that for the most rural counties (413.24; 95% CI = 379.10–477.48) (two-tailed t test; p < 0.01).
Discussion
This study provides large-scale data on Parkinson disease incidence and prevalence using the most inclusive, population-based US health care database. An important strength of this study is the substantial sample size of the Medicare data set, providing an opportunity to quantify the impact of ethnic and geographic risk factors on Parkinson disease prevalence and incidence. In previous US descriptive epidemiological Parkinson disease studies, Parkinson disease cases did not exceed 400 [21]. In comparison, this study utilized Medicare data sets with over 450,000 Parkinson disease cases per year, with data spanning 10 years. We believe that this sample size permits more precise measurements of Parkinson disease prevalence and annual incidence allowing the detection of differences between ethnic groups, age strata, and geographic regions. Moreover, our study is subject to less referral or selection bias than those which have drawn cases from inpatient hospitalization records, neurology clinics, movement disorder clinics, or death certificates. Our study provides more definitive evidence of the impact of age, ethnicity, and geography on disease prevalence and incidence. In the future, the use of this data set may permit the detection of Parkinson disease environmental risk associations with much greater precision and sensitivity.
There are several important findings in our study. Parkinson disease prevalence and annual incidence appear to continue to increase into very old age without any plateau as reported previously in some studies [22,23,24], but in contrast to others [25,26,27]. This has important public health implications for the allocation of health care resources. The aging of the ‘baby boom’ population will further strain Medicare budgets, especially as new and costly Parkinson disease treatments are used in older patients with advanced Parkinson disease. Our findings highlight the urgent need for neuroprotective and neuropreventive interventions.
Another important finding of our study is that Whites have a substantially higher prevalence and incidence of Parkinson disease than Blacks or Asians. This argues for differences in Parkinson disease genetic susceptibility. Interestingly, the incidence ratio between Blacks and Whites is greater than the prevalence ratio, suggesting differential survival of Blacks with Parkinson disease. In addition to the known relative increased mortality from diseases such as diabetes, heart disease, and stroke (Department of Health and Human Services, National Center for Health Care Statistics, www.cdc.gov/nhs/), Blacks may have greater Parkinson disease-related morbidity than Whites. Differences in smoking rates by ethnicity probably do not explain this substantial difference in Parkinson disease prevalence/incidence, since Whites and Blacks over the age of 65 have similar rates of ever tobacco use, i.e. 21.3% for Whites versus 23.3% for Blacks (Department of Health and Human Services, National Center for Health Statistics, 2008).
Rural living has often been cited as a risk factor for Parkinson disease, largely based upon case-control studies, but studies have shown an inconsistent association with rural or urban living. We used a standardized definition for rurality based on population and proximity to a population center, and made the a priori assumption that a true relationship would be manifest by increasing prevalence and incidence with increasing rurality. We failed to find a convincing relationship between either prevalent or incident Parkinson disease and rural living across this spectrum. Conversely, the most urban counties had a significantly higher prevalence and incidence of Parkinson disease than the most rural counties. We believe that rurality may not be as useful a research variable in Parkinson disease epidemiology studies as previously suggested, as the many available definitions of rurality are largely based on economic and political variables and involve few if any quantifiable or specific risk factors that have been shown to be important in Parkinson disease pathogenesis.
Previously, the most convincing data implicating environmental factors in Parkinson disease were derived from a population-based twin study which found similar concordance for monozygotic and dizygotic twins in older-onset Parkinson disease [28]. Our study indicates that there is a ‘Parkinson disease belt’ with high prevalence and incidence of Parkinson disease involving the Midwest and Northeast regions. This nonrandom disease distribution argues strongly for an environmental influence on the pathogenesis of Parkinson disease. Commonly cited Parkinson disease environmental risk factors, such as pesticides and herbicides, are used in these regions (Environmental Protection Agency, 2009; United States Geological Survey Pesticide National Synthesis Project, 2009) [9]. However, given the prominent role of the upper Midwest and Northeast regions in industrialization, our data may also argue for a role of byproducts of industrialization as risk factors in the pathophysiology of Parkinson disease, and supports the hypothesis that a complex interaction of different types of toxin exposures may be associated with higher disease rates. We believe that this concentration of Parkinson disease is not due to regional differences in tobacco use since Parkinson disease rates did not correlate with elderly smoking rates.
There are several caveats to consider with this study. Examining all Medicare claims for Parkinson disease rather than selecting cases that have been referred to specialists may result in an increased number of false positives, due to the miscoding of Parkinson plus syndromes, or secondary parkinsonism as Parkinson disease, but this number is likely small given the relatively low prevalence of these conditions [29,30]. It is also possible that nonrelated, ethnically similar individuals with a genetic susceptibility to Parkinson disease reside in the areas of the country where Parkinson disease rates are the highest for cultural reasons, resulting in case clustering. Decreased access to care and lower quality of care could increase the disease rates determined for Blacks and Asians by increasing the false-negative rate in those groups. However, Hispanics, who suffer from similar health care access disparities, had disease rates nearly equal to Whites (United States Geological Survey Pesticide National Synthesis Project, 2009; Agency for Healthcare Research and Quality, www.ahrq.gov/). Nonetheless, Blacks and Asians may have a difference in diagnosis rates that is, in part, explained by socioeconomic factors or cultural standards. The roles of disproportionate access to advanced care and increased mortality from Parkinson disease for Blacks in prevalent/incident Parkinson disease are unclear but we believe that this is also an issue for rural, White Medicare beneficiaries. Although this study utilized data from 98% of Americans over the age of 65, the demographic and geographic distribution of the unsampled 2% is unknown. However, one would only expect the missing 2% of the population to contain 1,000 Parkinson disease cases, which would not likely alter our findings. Finally, Parkinson disease cases less than 65 years of age are not included in this study. While this may result in lower incidence and prevalence data, the benefit of this selection is that the impact of young-onset Parkinson disease on our incidence and prevalence trend data is minimized, which is important as most inherited forms of Parkinson disease have younger age at onset.
Despite these caveats, our study provides valuable data from which numerous hypotheses can be tested regarding the pathogenesis of Parkinson disease. Future analyses will investigate the potential causes of the geographic variation in Parkinson disease rates including community level exposures to specific environmental toxins such as herbicides, pesticides, and heavy metals. Further studies of racial differences in Parkinson disease rates will investigate the role of differential comorbidities in survival.
Acknowledgements
This study was supported by National Institute for Environmental Health Sciences K24ES017765 and R01ES013743, National Institute of Neurological Disorders and Stroke 5T32NS007205-27, National Center for Research Resources (NCRR0) and National Institutes of Health Roadmap for Medical Research UL1 RR024992, both components of the National Institutes for Health. Additional funding support came from the American Parkinson Disease Association, the St. Louis Chapter of the American Parkinson Disease Association, a grant from Walter and Connie Donius, and the Robert Renschen Fund.
References
- 1.von Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, Oertel W, Siebert U, Berger K, Dodel R. Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol. 2005;15:473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzke JF, Goldberg ID. Parkinsonism death rates by race, sex, and geography. Neurology. 1988;38:1558–1561. doi: 10.1212/wnl.38.10.1558. [DOI] [PubMed] [Google Scholar]
- 3.Lanska DJ. The geographic distribution of Parkinson's disease mortality in the United States. J Neurol Sci. 1997;150:63–70. doi: 10.1016/s0022-510x(97)05371-9. [DOI] [PubMed] [Google Scholar]
- 4.Lilienfeld DE, Sekkor D, Simpson S, Perl DP, Ehland J, Marsh G, Chan E, Godbold JH, Landrigan PJ. Parkinsonism death rates by race, sex and geography: a 1980s update. Neuroepidemiology. 1990;9:243–247. doi: 10.1159/000110780. [DOI] [PubMed] [Google Scholar]
- 5.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 7.Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H, Tang MX, Lantigua R, Wilder D, Gurland B. The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 8.Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H, Tang MX, Lantigua R, Wilder D, Gurland B. The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 9.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 10.Newman EJ, Grosset KA, Grosset DG. Geographical difference in Parkinson's disease prevalence within West Scotland. Mov Disord. 2009;24:401–406. doi: 10.1002/mds.22359. [DOI] [PubMed] [Google Scholar]
- 11.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: a meta-analysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 12.Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson's disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 13.French NP, Berriatua E, Wall R, Smith K, Morgan KL. Sheep scab outbreaks in Great Britain between 1973 and 1992: spatial and temporal patterns. Vet Parasitol. 1999;83:187–200. doi: 10.1016/s0304-4017(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 14.Ernst JS. Mapping child maltreatment: looking at neighborhoods in a suburban county. Child Welfare. 2000;79:555–572. [PubMed] [Google Scholar]
- 15.Nkhoma ET, Hsu CE, Hunt VI, Harris AM. Detecting spatiotemporal clusters of accidental poisoning mortality among Texas counties, US, 1980–2001. Int J Health Geogr. 2004;3:25. doi: 10.1186/1476-072X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.English P, Neutra R, Scalf R, Sullivan M, Waller L, Zhu L. Examining associations between childhood asthma and traffic flow using a geographic information system. Environ Health Perspect. 1999;107:761–767. doi: 10.1289/ehp.99107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reif JS, Burch JB, Nuckols JR, Metzger L, Ellington D, Anger WK. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environ Res. 2003;93:248–258. doi: 10.1016/s0013-9351(03)00131-2. [DOI] [PubMed] [Google Scholar]
- 18.Nerriere E, Zmirou-Navier D, Desqueyroux P, Leclerc N, Momas I, Czernichow P. Lung cancer risk assessment in relation with personal exposure to airborne particles in four French metropolitan areas. J Occup Environ Med. 2005;47:1211–1217. doi: 10.1097/01.jom.0000181757.82556.f7. [DOI] [PubMed] [Google Scholar]
- 19.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161:81–88. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 20.Chaix B, Merlo J, Subramanian SV, Lynch J, Chauvin P. Comparison of a spatial perspective with the multilevel analytical approach in neighborhood studies: the case of mental and behavioral disorders due to psychoactive substance use in Malmo, Sweden, 2001. Am J Epidemiol. 2005;162:171–182. doi: 10.1093/aje/kwi175. [DOI] [PubMed] [Google Scholar]
- 21.Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson's disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 22.de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG, Hofman A. Prevalence of Parkinson's disease in the elderly: the Rotterdam Study. Neurology. 1995;45:2143–2146. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 23.de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, Manubens-Bertran JM, Alperovitch A, Rocca WA. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson's Disease. J Neurol Neurosurg Psychiatry. 1997;62:10–15. doi: 10.1136/jnnp.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tison F, Dartigues JF, Dubes L, Zuber M, Alperovitch A, Henry P. Prevalence of Parkinson's disease in the elderly: a population study in Gironde, France. Acta Neurol Scand. 1994;90:111–115. doi: 10.1111/j.1600-0404.1994.tb02689.x. [DOI] [PubMed] [Google Scholar]
- 25.Marttila RJ, Rinne UK. Epidemiology of Parkinson's disease in Finland. Acta Neurol Scand. 1976;53:81–102. doi: 10.1111/j.1600-0404.1976.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 26.Taba P, Asser T. Incidence of Parkinson's disease in estonia. Neuroepidemiology. 2003;22:41–45. doi: 10.1159/000067105. [DOI] [PubMed] [Google Scholar]
- 27.Benito-Leon J, Bermejo-Pareja F, Rodriguez J, Molina JA, Gabriel R, Morales JM. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord. 2003;18:267–274. doi: 10.1002/mds.10362. [DOI] [PubMed] [Google Scholar]
- 28.Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 29.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 30.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology. 1997;49:1284–1288. doi: 10.1212/wnl.49.5.1284. [DOI] [PubMed] [Google Scholar]