Abstract
Rationale
Recent studies have implicated mitochondrial reactive oxygen species (ROS) in regulating hypoxic pulmonary vasoconstriction (HPV), but controversy exists regarding whether hypoxia increases or decreases ROS generation.
Objective
This study tested the hypothesis that hypoxia induces redox changes that differ among sub-cellular compartments in pulmonary (PASMC) and systemic (SASMC) smooth muscle cells.
Methods and Results
We employed a novel, redox-sensitive, ratiometric fluorescent protein sensor (RoGFP) to assess the effects of hypoxia on redox signaling in cultured PASMC and SASMC. Using genetic targeting sequences, RoGFP was expressed in the cytosol (Cyto-RoGFP), the mitochondrial matrix (Mito-RoGFP), or the mitochondrial inter-membrane space (IMS-RoGFP), allowing assessment of oxidant signaling in distinct intracellular compartments. Superfusion of PASMC or SASMC with hypoxic media increased oxidation of both Cyto-RoGFP and IMS-RoGFP. However, hypoxia decreased oxidation of Mito-RoGFP in both cell types. The hypoxia-induced oxidation of Cyto-RoGFP was attenuated through the over-expression of cytosolic catalase in PASMC.
Conclusions
These results indicate that hypoxia causes a decrease in non-specific ROS generation in the matrix compartment, while it increases regulated ROS production in the IMS, which diffuses to the cytosol of both PASMC and SASMC.
Keywords: Mitochondria, reactive oxygen species, RoGFP, hypoxic pulmonary vasoconstriction
Introduction
Small pulmonary arteries constrict in response to a decrease in alveolar oxygen tension through a physiological response termed hypoxic pulmonary vasoconstriction (HPV). Despite intensive study since the first description of HPV in 1946, the mechanism of O2 sensing remains unclear. Mitochondria have been considered a putative site of cellular oxygen sensing because they consume O2 and therefore represent the intracellular site with the lowest oxygen tension. Mitochondria generate superoxide and hydrogen peroxide during cellular respiration, so changes in the production of reactive oxygen species (ROS) during hypoxia could potentially signal changes in O2 tension and trigger cellular responses. However, whether hypoxia increases or decreases ROS generation during HPV remains unclear.
Using chemiluminescence in whole perfused lungs and endothelium-denuded rings of distal pulmonary arteries (PA), Michelakis, Archer and Weir detected decreases in ROS generation during hypoxia 1-4. Similarly, in cultured pulmonary arterial pulmonary smooth muscle cells (PASMC), Mehta et al. detected a decrease in ROS generation as determined by dihydrodichlorofluorescein diacetate (DCF), dihydroethidium (DHE), and Amplex red 5. By contrast, using cultured pulmonary arterial smooth muscle cells (PASMC), we found that hypoxia triggers an increase in ROS generation 6-8. Our studies employed the ROS-sensitive indicator dichlorofluorescein (DCF), mitochondrial inhibitors, enzymatic and chemical antioxidants and mitochondria-deficient (π0) cells. Other investigators have detected similar increases in ROS generation during hypoxia by employing lucigenin-derived chemiluminescence and electron paramagnetic resonance (EPR) spectrometry in small pulmonary arteries, as well as DCF in PASMC 8,9.
Attempts to resolve the question of whether ROS levels increase or decrease during hypoxia have been hindered by the technical limitations of the tools used to assess oxidant stress. While lucigenin can detect ROS, lucigenin itself is capable of producing superoxide in cells 9. Furthermore, questions regarding how these probes distribute between intracellular and extracellular compartments 5 have led to concerns regarding the interpretation of data arising from their use. Other probes such as DCF and DHE lack specificity and can potentially accumulate within organelles 10. In addition, none of these probes exhibits ratiometric behavior, so changes in fluorescence arising from changes in intracellular dye concentration, excitation intensity, or fluorescence path length caused by changes in cell volume cannot be distinguished from changes in redox state. Finally, obtaining a global measure of tissue ROS production during hypoxia may mask important differences is ROS production occurring among cellular sub-compartments. The goal of the present study was to test the hypothesis that hypoxia differentially alters ROS generation, depending on the subcellular compartment. To address this, we employed a novel redox-sensitive protein sensor, which we separately targeted to the mitochondrial matrix, the mitochondrial inter-membrane space, and the cytosol. This sensor, RoGFP, contains engineered cysteine residues, enabling di-thiol formation in response to oxidant stress 11-15. Oxidation resulting in di-thiol formation causes reciprocal changes in emission intensity when excited at two different wavelengths. Unlike DCF or lucigenin, the fluorescence excitation spectrum of RoGFP is strongly and reversibly dependent upon the redox state of the introduced cysteines 12. Moreover, by exposing cells to strong reducing and oxidizing agents at the conclusion of an experiment, RoGFP oxidation state can be calibrated on a cell-by-cell basis in situ. We used these targeted sensors to assess redox changes in cellular sub-compartments induced by hypoxia. We also compared the hypoxia-induced redox responses in PASMC with those of systemic arterial smooth muscle cells (SASMC).
Methods
Pulmonary microvessel myocyte isolation
The Northwestern University Institutional Animal Care and Use Committee approved all animal studies. PASMC were isolated from rat lungs as described previously 6 using a modification of the method of Marshall et al. 16. Cells isolated by this method were confirmed to be PASMC as previously described 6. Using a similar method, SASMC were isolated from rat renal arteries and confirmed to be SASMC as previously described 6. The isolated cells were cultured for 2 weeks (4 passages) and then used for the next 4 weeks (4-12 passages).
RoGFP Sensors
The RoGFP protein contains two engineered cysteine thiols, as first described by Remington et al. (RoGFP2) 11. The cDNA encoding the protein was created by introducing four mutations in the mammalian GFP expression vector (pEGFP-N1) (C48S, Q80R, S147C, and Q204C) using a QuikChange Multi Site-directed mutagenesis kit (Strategene). The RoGFP construct was ligated into the VQ Ad5CMV K-NpA adenoviral shuttle vector between the KpnI and NotI sites; after sequencing and amplification this plasmid was used to generate a recombinant adenovirus to permit widespread expression in our cells (ViraQuest Inc., North Liberty, IA). The resulting redox-sensitive protein has excitation maxima at 400 and 484 nm, with emission at 525 nm. In response to changes in redox conditions, RoGFP exhibits reciprocal changes in intensity at the two excitation maxima 12, and its ratiometric characteristics render it insensitive to expression levels 13-15. Although RoGFP’s fluorescence behavior is relatively independent of pH and it does not respond to authentic nitric oxide (NO), reduced NADH, or the antioxidant N-acetyl-L-cysteine (NAC), its spectrum is slightly affected by reduced glutathione (GSH) possibly due to thiol-disulfide exchange (Online Figures I and II).
RoGFP was expressed in the mitochondrial matrix (Mito-RoGFP) by appending a 48 bp region encoding the mitochondrial targeting sequence from cytochrome oxidase subunit IV, at the 5′ end of the coding sequence. This construct was then ligated into the VQ Ad5CMV K-NpA plasmid between the KpnI and NotI sites, and used to generate an adenoviral vector. RoGFP was targeted to the mitochondrial inter-membrane space (IMS-RoGFP) by appending it to glycerol phosphate dehydrogenase (GPD). A cDNA construct encoding GPD, an integral protein of the inner mitochondrial membrane whose C-terminus protrudes into the inter-membrane space 17, was ligated in-frame with cDNA encoding RoGFP 17. The corresponding polypeptide includes amino acids 1–626 of GPD, with RoGFP at the carboxy terminus. This method has been used previously to express YFP in the inter-membrane space 18. (See Online Supplemental Material for characterization of the RoGFP sensors and experimental protocols).
Statistics
Changes in RoGFP oxidation and YC2.3 FRET were analyzed by using a two-way ANOVA with repeated measures. A Newman-Keuls multiple-range test was used to evaluate significant differences between groups and times. To control for differences in the hypoxic responses of cultured myocytes, experimental studies and control experiments were always carried out on the same day. Statistical significance was set at p<0.05 23.
Results
Hypoxia induces progressive cytosolic oxidation in pulmonary artery smooth muscle cells
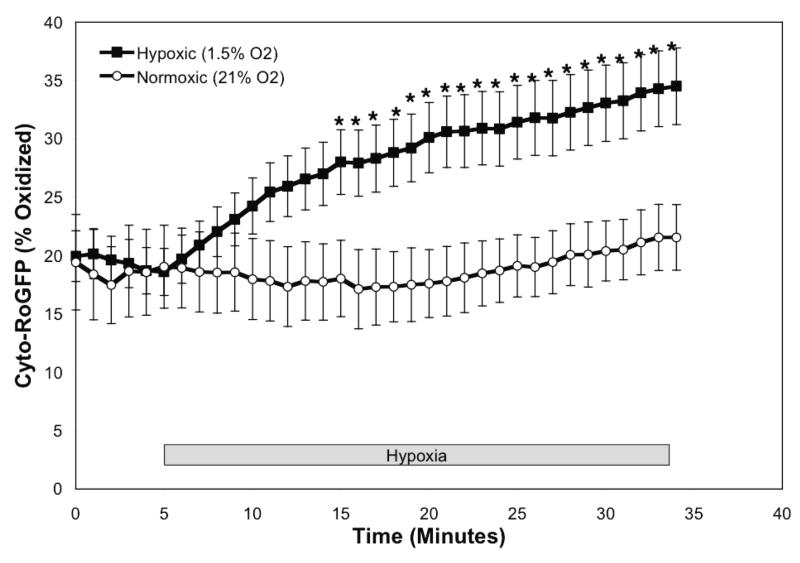
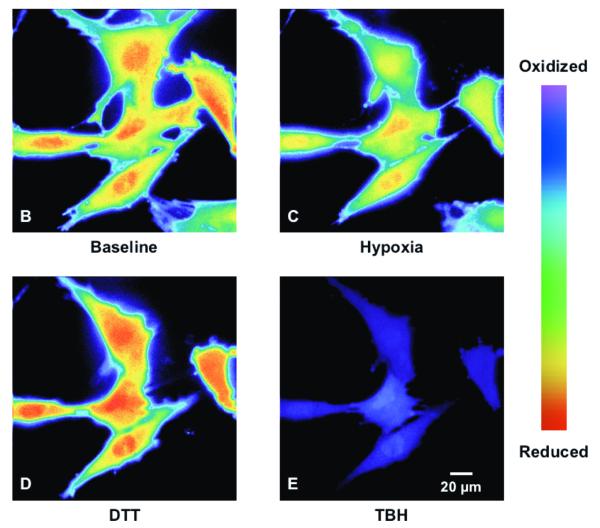
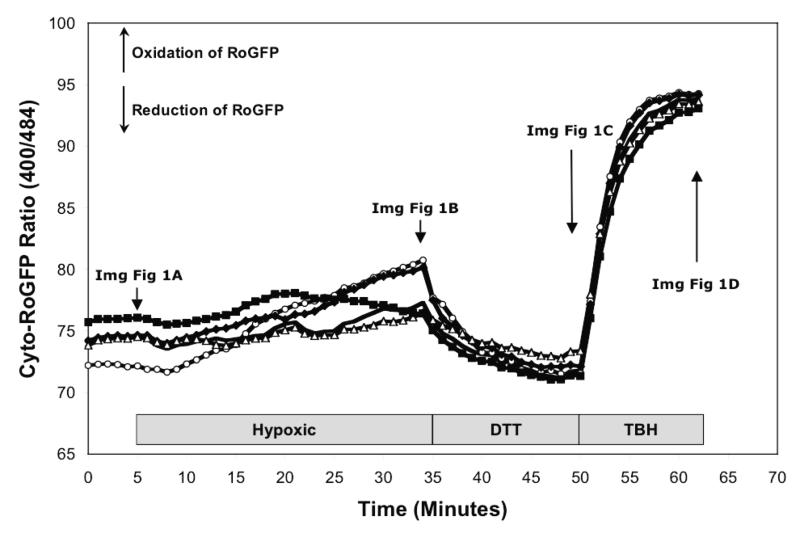
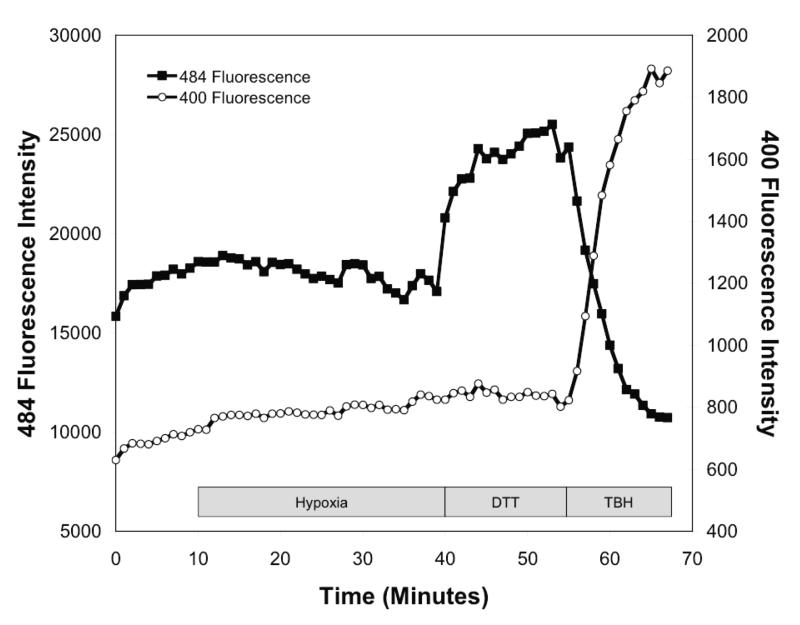
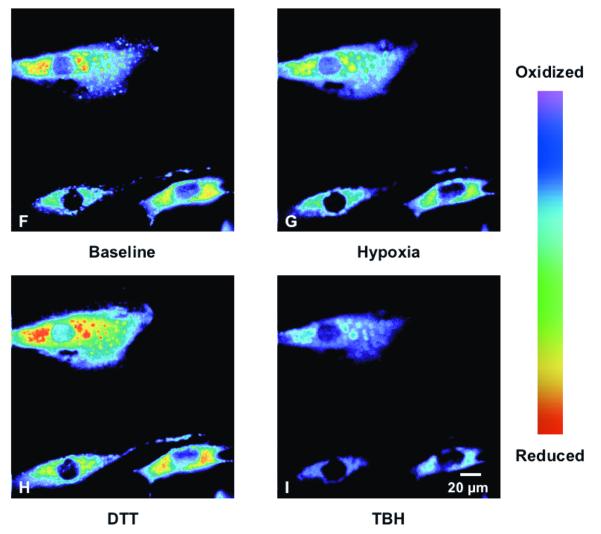
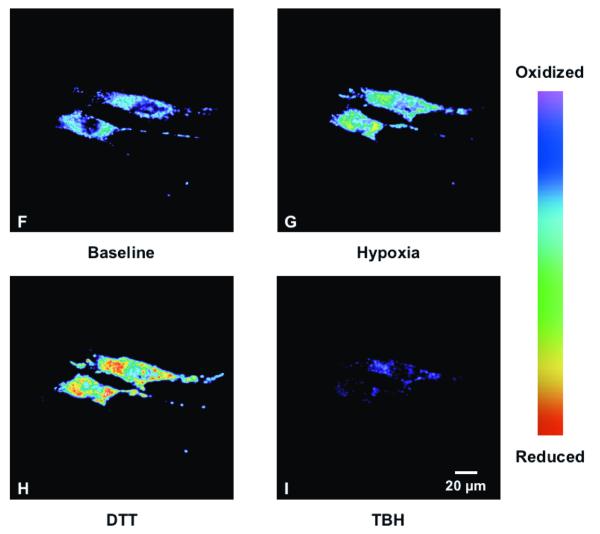
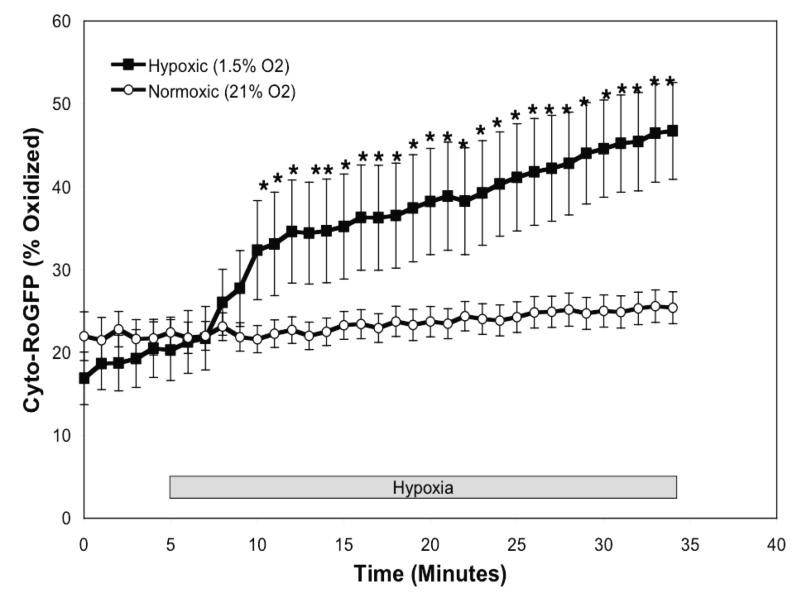
To determine the effect of hypoxia on ROS production in the cytosol, isolated rat PASMC expressing Cyto-RoGFP were subjected to hypoxia. Under baseline normoxic conditions, the Cyto-RoGFP sensor was 18.6±2.0% oxidized (Fig. 1A). During hypoxia, Cyto-RoGFP demonstrated significantly progressive oxidation over a period of 30 minutes compared to normoxic PASMC and reached 34.5±3.2% oxidized by the end of the experiment (Fig 1A). False-color ratiometric representative images (Fig. 1B-E) and quantitative changes of the fluorescence ratio from individual PASMC (Fig. 1F) demonstrate the dynamic responsiveness of the Cyto-RoGFP sensor to hypoxia, DTT (1 mM), and tBH (1 mM). In addition, Figure 1G demonstrates the reciprocal changes in fluorescence intensity for the two excitation maxima (484 and 400 nm) of Cyto-RoGFP in response to hypoxia, DTT, and tBH in a single PASMC. Taken together, these results indicate that hypoxia shifts the cytosol to a more oxidized state.
Figure 1.
Hypoxia shifts the cytosol to a more oxidized state in PASMC. (A) Combined results from multiple experiments in PASMC expressing Cyto-RoGFP and superfused with either normoxic (21% O2) or hypoxic (1.5% O2) media. (B-E) False-color ratiometric images of PASMC demonstrate the dynamic redox range of the Cyto-RoGFP sensor. PASMC expressing Cyto-RoGFP were superfused with normoxic (21% O2) media (B), then with hypoxic (1.5% O2) media for 30 min (C), followed by media containing DTT to fully reduce the sensor (D), and then with media containing tBH to fully oxidize the sensor (E). (F) Representative time course, quantitative analysis of the fluorescence intensity ratio from representative individual PASMC imaged in B-E. ‘Img Fig’ denotes the time at which the images B-E were captured during the experiment. (G) Representative time course, quantitative analysis of the reciprocal changes in Cyto-RoGFP fluorescence intensity at the two excitation maxima (484 and 400 nm) for a single PASMC. Values are means ± S.E., n=6 cover slips, 4-10 cells/cover slip. *p<0.05 compared to normoxia.
The mitochondrial IMS becomes more oxidized during hypoxia in PASMC
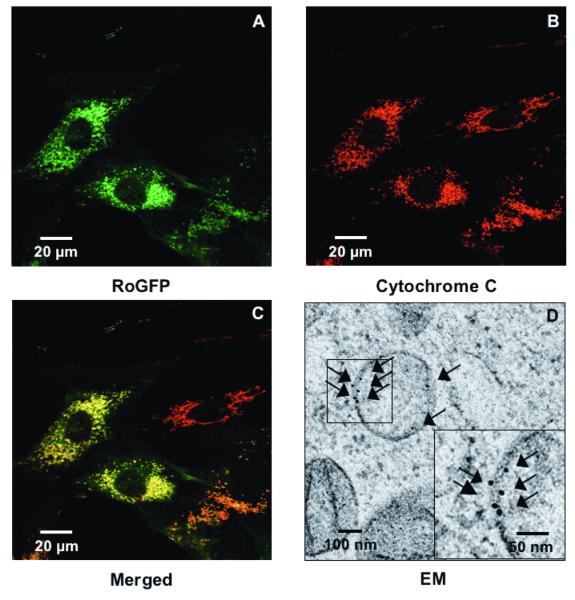
Having confirmed that hypoxia oxidizes thiol targets in the cytosol, we asked whether hypoxia induces ROS production in the IMS of the mitochondria. RoGFP expression was therefore targeted to the inter-membrane space by appending it to GPD, an enzyme localized to the IMS 18. Confocal imaging demonstrated that IMS-RoGFP expression co-localized with mitochondrial cytochrome c, which is known to be localized to the inter-membrane space (Figs. 2A-C). To further verify correct targeting of IMS-RoGFP to the inter-membrane space, we performed immuno-electron microscopy of cells using an antibody against GFP. At the dilutions employed, no background labeling outside mitochondria was observed in multiple images of separate mitochondria. Rather, the immunogold labeling was restricted to the mitochondrial membrane at the mitochondrial periphery and cristae, demonstrating expression restricted to the mitochondrial inter-membrane space (Fig. 2D).
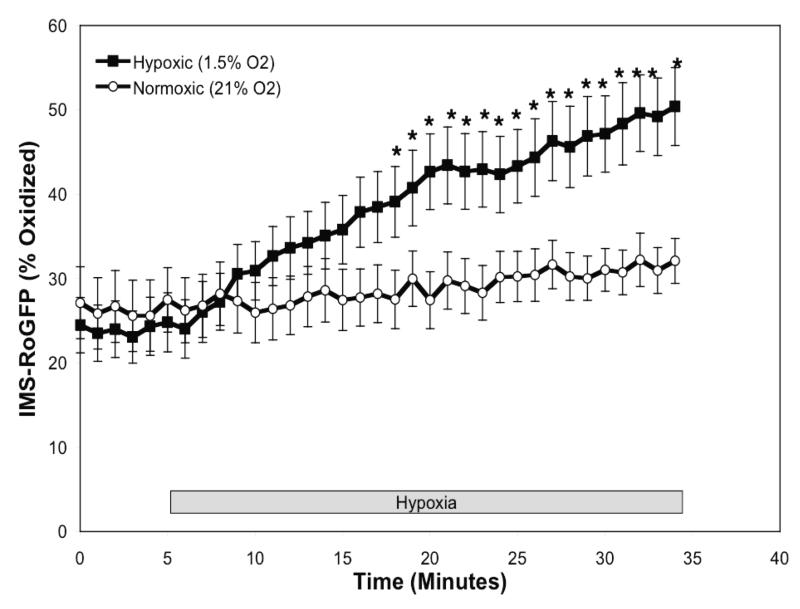
Figure 2.
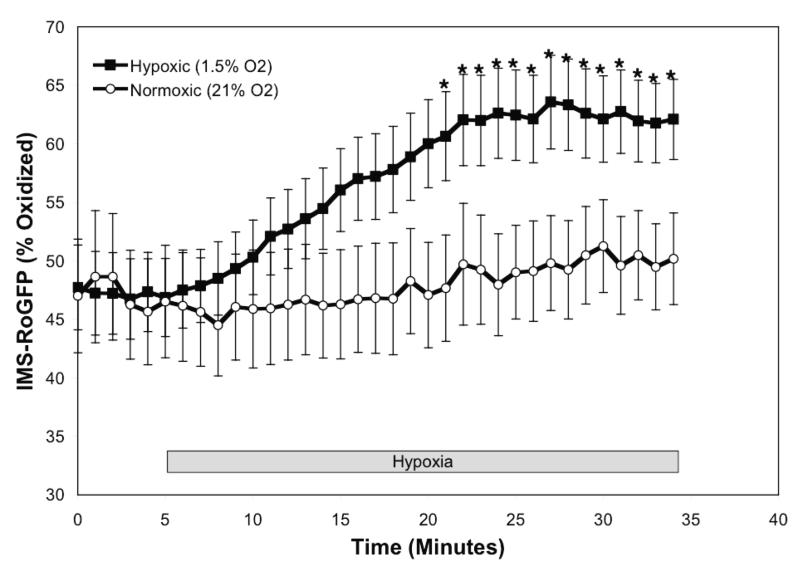
Hypoxia shifts the mitochondrial IMS to a more oxidized state in PASMC. (A-C) Confocal images of PASMC expressing IMS-RoGFP (A), immunostained for cytochrome c (B), and demonstrating co-localization in mitochondria (C). (D) IMS-RoGFP targeted to the mitochondrial inter-membrane space of rat PASMC as determined by electron microscopy. Primary antibody: mouse monoclonal anti-GFP; secondary antibody: goat anti-mouse IgG, conjugated to 10 nm gold particles (arrows). Note the restriction of labeling to the mitochondrial membrane on the mitochondrial periphery. (E) Combined results from multiple experiments in PASMC expressing IMS-RoGFP and superfused with normoxic (21% O2) or hypoxic (1.5% O2) media. (F-I) False-color ratiometric images of PASMC demonstrate the dynamic redox range of the IMS-RoGFP sensor. PASMC expressing IMS-RoGFP were superfused with normoxic (21% O2) media (F), then with hypoxic (1.5% O2) media for 30 min (G), followed by media containing DTT to fully reduce the sensor (H) and then with media containing tBH to fully oxidize the sensor (I). Values are means ± S.E., n=6 cover slips, 4-10 cells/cover slip. *p<0.05 compared to normoxia.
During normoxia, the RoGFP in the IMS was more oxidized (47.7±4.5%; Fig. 2E) compared with the oxidation state demonstrate with Cyto-RoGFP (18.6±2.0%; Fig. 1A). In response to hypoxia, IMS-RoGFP also significantly shifted to a more oxidized state compared to normoxic PASMC and reached a level of 62.1±3.4% oxidized at the end of the experiment (Fig. 2E). These results indicate that hypoxia shifts the mitochondrial IMS to a more oxidized state.
The mitochondrial matrix becomes more reduced during hypoxia in PASMC
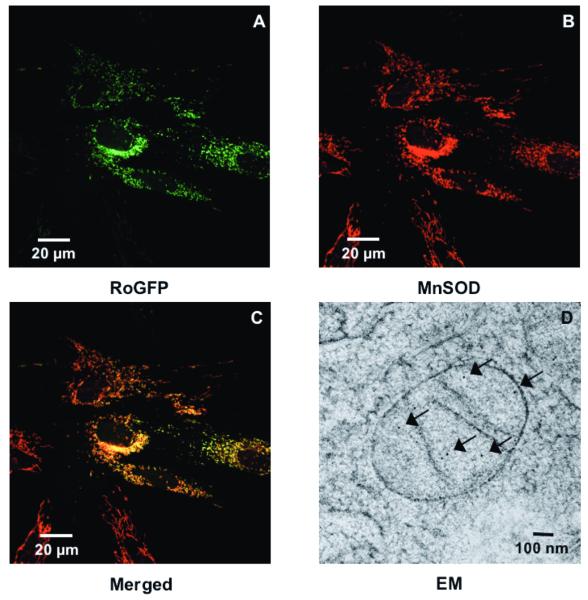
Having found that hypoxia induces RoGFP oxidation in the inter-membrane space, we next assessed the effect of hypoxia on redox status in the mitochondrial matrix. RoGFP was targeted to the mitochondrial matrix using the mitochondrial targeting sequence from cytochrome oxidase subunit IV. Confocal microscopy studies of expression of Mito-RoGFP and endogenous MnSOD, a protein expressed exclusively in the mitochondrial matrix, demonstrated co-localization of these two proteins at the resolution afforded by light microscopy (Fig. 3A-C). Further assessment of its targeting was examined in immunogold-labeled cryosections examined under electron microscopy (Fig. 3D). Unlike the pattern of immunogold labeling seen for IMS-RoGFP (Fig. 2D), the immunogold labeling in Mito-RoGFP-expressing cells was restricted to the matrix and was not associated with either cristae or peripheral mitochondrial membranes.
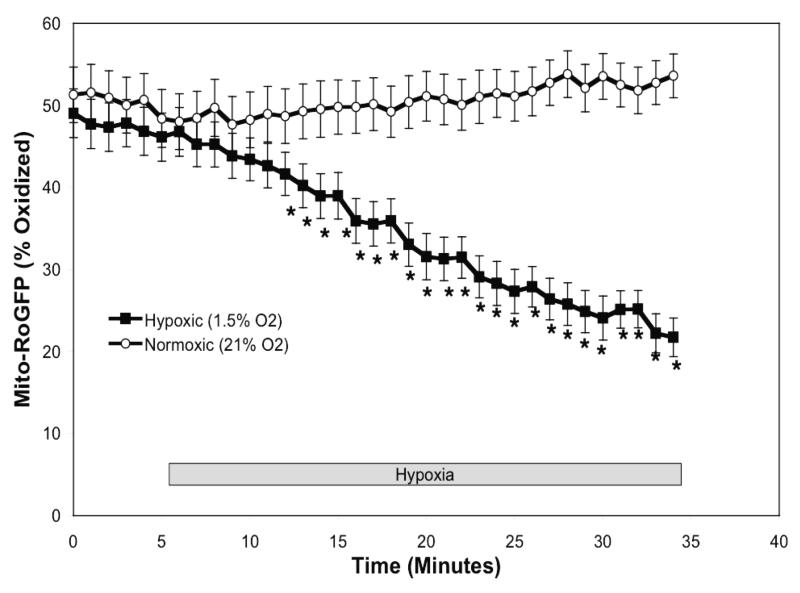
Figure 3.
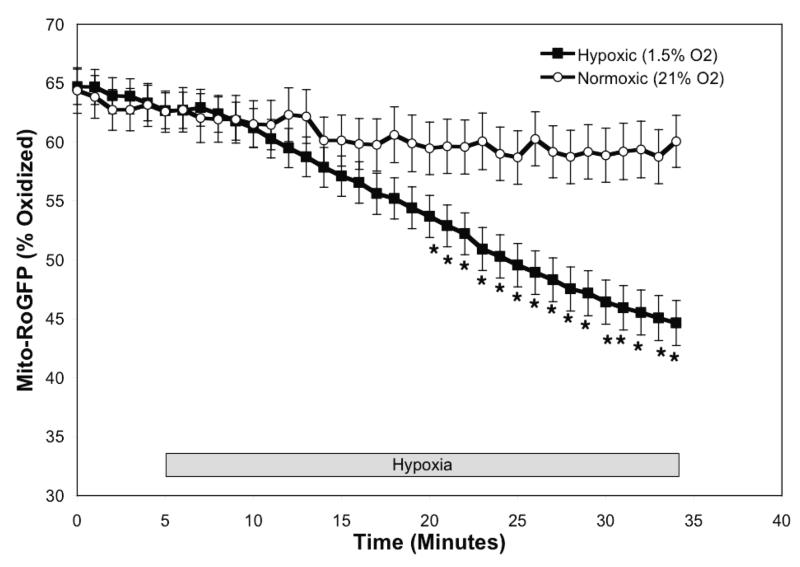
Hypoxia shifts the mitochondrial matrix to a more reduced state in PASMC. Confocal images of PASMC expressing IMS-RoGFP (A), immunostained for MnSOD (B), and demonstrating co-localization in mitochondria (C). (D) Mito-RoGFP targeted to the mitochondrial matrix of rat PASMC as determined by electron microscopy. Primary antibody: mouse monoclonal anti-GFP; secondary antibody: goat anti-mouse IgG, conjugated to 10 nm gold particles (arrows). (E) Combined results from multiple experiments in PASMC expressing Mito-RoGFP and superfused with normoxic (21% O2) or hypoxic (1.5% O2) media. (F-I) False-color ratiometric images of PASMC demonstrate the dynamic redox range of the Mito-RoGFP sensor. PASMC expressing Mito-RoGFP were superfused with normoxic (21% O2) media (F), then with hypoxic (1.5% O2) media for 30 min (G), followed by media containing DTT to fully reduce the sensor (H) and then with media containing tBH to fully oxidize the sensor (I). Values are means ± S.E., n=6 cover slips, 4-10 cells/cover slip. *p<0.05 compared to normoxia.
Under baseline normoxic conditions, the Mito-RoGFP sensor was 62.6±1.5% oxidized (Fig 3E), significantly more oxidized than RoGFP localized to the mitochondrial inter-membrane space (Fig. 2E). However, unlike the Cyto- and IMS-RoGFP, which became more oxidized, during hypoxia Mito-RoGFP became progressively more reduced compared to normoxic PASMC decreasing to 44.4±1.9% oxidized by the end of the experiment (Fig. 3E). These results reveal that the mitochondrial matrix undergoes a reductive stress during hypoxia.
Expression of antioxidant proteins attenuates hypoxia-induced oxidation of RoGFP
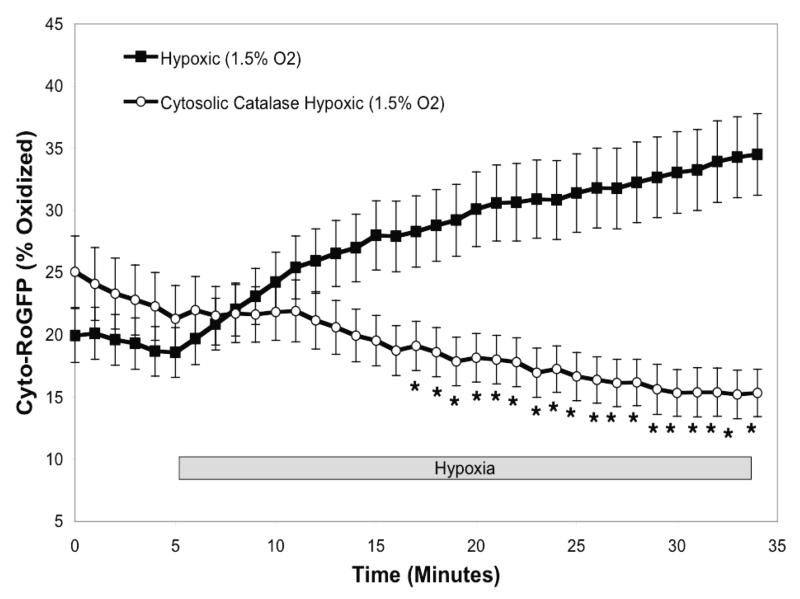
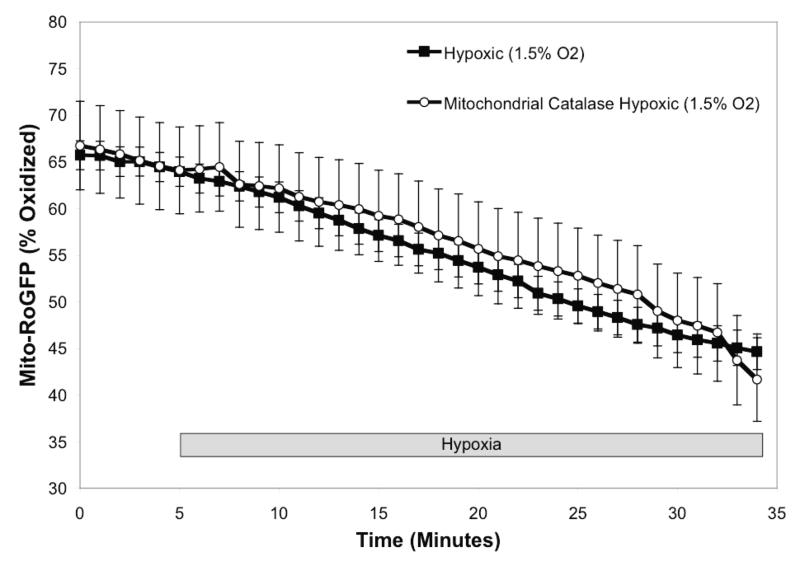
Our observation of hypoxia-induced oxidation of RoGFP in the cytosol and mitochondrial IMS, but not the matrix, suggested that hypoxia-induced ROS generation is localized to the cytosol and and/or inter-membrane space. To determine whether H2O2 signaling contributes to the oxidation response during hypoxia, catalase was first over-expressed in the cytosolic compartment7. Cytosolic catalase over-expression completely blocked the hypoxia-induced increase in cytosolic oxidation (Fig. 4A), indicating that hypoxia-induced oxidative stress in the cytosolic compartment is ultimately H2O2. Over-expression of catalase in the mitochondrial matrix, as previously achieved 7, had no effect on the hypoxia-induced decrease in oxidation of Mito-RoGFP during hypoxia (Fig. 4B). These results indicate that the progressive reduction in the mitochondrial matrix during hypoxia is not mediated by H2O2 signaling.
Figure 4.
Catalase attenuates hypoxia-induced oxidation of Cyto -RoGFP in PASMC. Cytosolic catalase was over-expressed in cells expressing Cyto-RoGFP (A) while mitochondrial catalase was over-expressed in cells expressing Mito-RoGFP (B). Values are means ± S.E., n=6 cover slips, 4-10 cells/cover slip. *p<0.05 compared to hypoxia.
Hypoxia shifts the redox status of subcellular compartments in SASMC
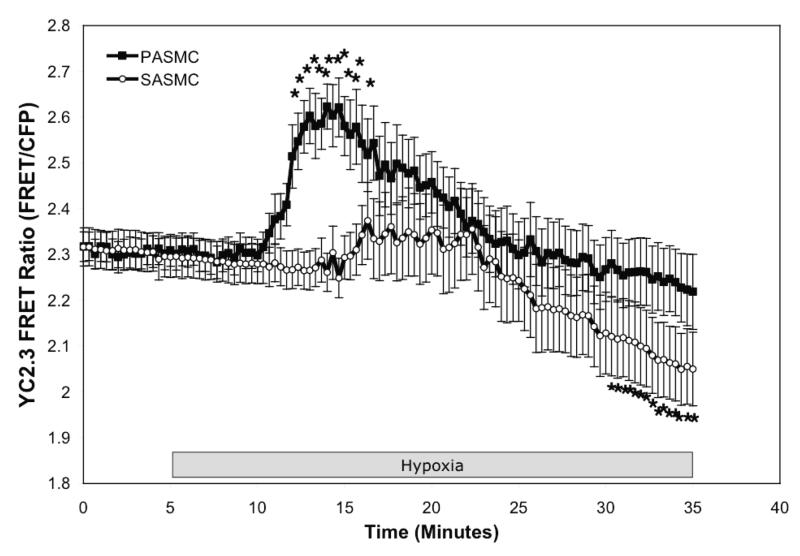
Unlike pulmonary arteries, systemic arteries relax in response to hypoxia 4. We assessed the redox response to hypoxia in smooth muscle cells isolated from renal arteries to determine if they respond differently from PASMC during identical hypoxic stimulation. Just as in the PASMC, hypoxia induced a significant oxidation of Cyto-RoGFP from normoxic baseline (21.6±1.6% to 46.7±5.9% oxidized; Fig. 5A). Similarly, hypoxia also induced oxidation of RoGFP localized to the mitochondrial IMS from normoxic baseline (27.5±3.5% to 50.4±4.6% oxidized, Fig. 5B). Finally, hypoxia significantly reduced RoGFP targeted to the mitochondrial matrix from normoxic baseline (48.7±3.3% to 21.7±2.3% oxidized; Fig. 5C), as we observed in the PASMC. To examine the effect of the hypoxia-induced increase in ROS signaling on cytosolic calcium ([Ca2+]i) signaling during hypoxia, PASMC and SASMC expressing YC2.3 were compared. Hypoxia significantly increased the YC2.3 FRET Ratio in PASMC by 15 min (2.31±0.03 to 2.62±0.05; Fig. 5D) while it significantly decreased the ratio in SASMC within 30 min (2.29±0.04 to 2.05±0.08; Fig. 5D). Taken together, these results indicate that hypoxia induces a similar pattern of redox modulation in SASMC and PASMC, and that there are cell type-specific downstream effectors that respond to this pattern of redox modulation.
Figure 5.
Hypoxia shifts the redox status of subcellular compartments in SASMC. SASMC expressing Cyto-RoGFP (A), IMS-RoGFP (B), or Mito-RoGFP (C) were superfused under controlled O2 conditions. Effects of hypoxia on [Ca2+]i in PAMSC and SASMC assessed by YC2.3 (D). Values are means ± S.E., n=6 cover slips, 4-10 cells/cover slip. *p<0.05 compared to hypoxia (A-C) and *p<0.05 compared to normoxic baseline (D).
Discussion
In this study we genetically targeted a reporter of redox state to subcellular compartments. The expression of this sensor in vascular smooth muscle cells reveals important differences in protein thiol redox state between the cytosol, IMS and the mitochondrial matrix. Under normoxic conditions we found that the cytosol is the most reduced and the mitochondrial matrix is the most highly oxidized. The inter-membrane space demonstrates an intermediate level of protein thiol oxidation.
We previously used a fluorescence resonance energy transfer redox sensor (HSP-FRET) to detect increases in oxidant stress in the cytosol of cultured PASMC during hypoxia 7. Those measurements provided evidence of an increase in ROS signaling during hypoxia, but slow re-folding of the HSP-FRET protein after removal of the oxidant stress hindered our ability to calibrate its redox state during the experiment. Moreover, attempts to target its expression to the mitochondria were unsuccessful, impeding its usefulness for assessing sub-cellular redox signaling. By contrast, RoGFP’s ratiometric nature, and its successful targeting to distinct subcellular compartments, make it a useful probe for the molecular dissection of redox changes in response to hypoxia. The high level of basal oxidation detected by RoGFP in the matrix is consistent with a large literature identifying that compartment as the primary site of ROS production in the cell 24. During hypoxia, the matrix undergoes a reductive shift. The decrease in oxidation during hypoxia suggests that ROS generation in the matrix arises from the non-specific, [O2]-dependent generation of superoxide at a number of sites, such that a decrease in O2 availability decreases ROS production in a non-specific manner. In contrast to the mitochondrial matrix, the cytosol and the IMS demonstrate an oxidative shift during hypoxia. The increase in oxidant stress in the IMS and the cytosol occurs despite the decrease in [O2], and therefore appears to reflect a regulated increase in the generation of ROS in response to physiological hypoxia. Nevertheless, the oxidant signal we detect is subtle and it produces a relatively small change in protein thiol oxidation state.
To our knowledge, this is the first report comparing redox conditions in the cytosol, IMS and matrix compartments, and their responses to hypoxia. If mitochondria are the source of the oxidant signaling in the IMS and cytosol during hypoxia, the most likely site(s) of ROS generation could be within the IMS, at the outer membrane, or at the outer surface of the inner mitochondrial membrane. Importantly, the site of ROS generation cannot be in the matrix because hypoxia decreased oxidant stress in that compartment. We cannot exclude the possibility that ROS generation originates in the cytosol during hypoxia and diffuses into the IMS, as the oxidation response in these two compartments developed simultaneously. Weissmann et al. recently demonstrated a role for non-phagocytic NADPH oxidase function during the acute phase of HPV, and suggested that NADPH oxidase may contribute to the generation of ROS during hypoxia 25. It is therefore possible that NADPH oxidase, or an alternative system, contributes to the hypoxia-induced ROS generation that affects both the cytosol and IMS. We favor the conclusion that the mitochondrial electron transport chain is the principal source of hypoxia-induced ROS signals, based on previous studies using mitochondrial inhibitors, mitochondria-deficient π0 cells, and RNAi suppression of the electron transport chain 6-8, 26-32. Interestingly, Rathore et al. recently reported that hypoxia increases mitochondria-derived ROS in PASMC, which leads to the activation of NAD(P)H oxidase through PKCε activation, suggesting that both mitochondria and cytosolic oxidant systems may contribute to the overall response 28.
Our findings conflict directly with the model of HPV by Michelakis, Archer and Weir, who find that hypoxia-induced decreases in mitochondrial ROS generation lead to redox-dependent alterations in Kv channel activation in the plasma membrane, thereby triggering depolarization and subsequent contraction 2, 4, 33-36. One possible explanation for this conflict relates to their use of lucigenin chemiluminescence to assess oxidant stress. Our data reveal that redox responses can differ markedly among subcellular compartments. The unknown distribution of lucigenin between the intracellular and extracellular spaces, and its possible accumulation in mitochondria, cytosol, sarcoplasmic reticulum or other intracellular compartments, makes it difficult to interpret data obtained when the luminescence responses from all these compartments are recorded as a single signal. Our data demonstrate that, during hypoxia, oxidation increases slightly in some compartments while it decreases significantly in others. Therefore, it is likely that the lucigenin signal reports a decrease during hypoxia because the collective oxidant stress across all of these compartments undergoes a net decrease. However, that combined signal would not detect important increases in oxidant stress in specific compartments where oxidant signaling acts as a critical mediator of cellular responses.
For PASMC, hypoxia causes an increase in [Ca2+]i and vasoconstriction 37-48, while for SASMC, the result is a decrease in [Ca2+]i and vasodilation 4, 49, 50. Michelakis et al. reported that ROS generation increases during hypoxia in systemic arterial tissue, but decreases in pulmonary arteries, leading them to conclude that tissue-specific differences in mitochondrial function explain their opposite responses 4. Those findings were contradicted by Mehta et al., who detected a decrease in hypoxia-induced ROS generation in both PASMC and coronary artery smooth muscle cells 5. Our comparison of PASMC and SASMC revealed that both cell types exhibit increases in cytosolic ROS signaling during hypoxia, yet the functional responses were dissimilar, with PASMC exhibiting an increase in [Ca2+]i while SASMC showed a decrease (Fig. 5). Our results suggest that the differing physiological responses to hypoxia in the two types of vascular smooth muscle must therefore be mediated by cell-specific expression of downstream signaling pathways, allowing the same upstream oxidant signal to activate different tissue-specific responses to a hypoxic stimulus. This conclusion is supported by a growing number of studies that implicate hypoxia-induced increases in ROS signaling in mediating diverse tissue-specific responses across a wide range of cell types 6-8, 31, 51-58.
The ability of cytosolic catalase to attenuate the hypoxia-induced oxidation of the Cyto-RoGFP sensors is consistent with previous studies 7, 8. However, the over-expression of mitochondrial catalase had no effect on the hypoxia-induced decrease in oxidation of Mito-RoGFP (Fig. 4B). One possibility is that Mito-RoGFP is oxidized primarily by superoxide in the matrix. If so, then over-expression of mitochondrial catalase might not affect Mito-RoGFP oxidation because catalase degrades H2O2 but not superoxide. Alternatively, ROS production in the matrix may be largely absent during hypoxia. If so, then the hypoxia-induced decrease in Mito-RoGFP oxidation would reflect the rate at which the protein di-thiols are re-reduced by matrix reductases. If matrix H2O2 formation is minimal during hypoxia, then the rate of Mito-RoGFP oxidation would be minimal and mitochondrial catalase over-expression would not affect its redox state. The fact that cytosolic catalase over-expression attenuated the hypoxia-induced increase in cytosolic oxidant signaling underscores the importance of H2O2 signaling in that response.
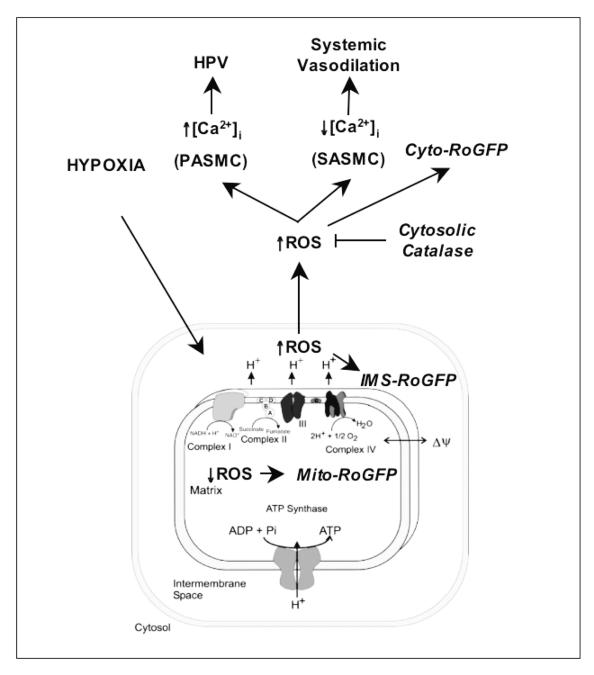
In summary, these results are consistent with a model for HPV in which hypoxia augments oxidant signaling by H2O2 in the cytosol and IMS, while it decreases oxidant stress in the mitochondrial matrix (Fig. 6). The cytosolic ROS signal triggers an increase in cytosolic calcium, which mediates PASMC contraction. Smooth muscle cells from systemic arteries demonstrate a similar pattern of oxidant signaling, indicating that the differences between PASMC and SASMC in terms of their response to hypoxia cannot be attributed to differences in mitochondrial function.
Figure 6.
Model depicting spatial differences in hypoxia-induced ROS signaling.
Novelty and Significance.
What is known:
The mitochondria of pulmonary arterial smooth muscle cells function as the O2 sensor underlying HPV by triggering a change in ROS signaling.
Conflicting reports from various groups have shown that mitochondrial ROS signaling either increases or decreases in response to hypoxia.
Attempts to resolve the question of whether ROS levels increase or decrease during hypoxia have been hindered by the technical limitations of the tools used to assess changes in oxidant stress.
New information contributed by this study:
A novel, redox-sensitive, ratiometric fluorescent protein sensor RoGFP was used to assess the effects of hypoxia on redox signaling in cultured pulmonary artery smooth muscle cells.
For the first time, genetic targeting sequences were used to express RoGFP in the cytosol, the mitochondrial matrix, and the mitochondrial intermembrane space, allowing assessment of oxidant signaling in distinct sub-cellular compartments.
The results are consistent with a model in which hypoxia induces the release of ROS from the outer surface of the inner mitochondrial membrane, thereby eliciting an increase in oxidant signaling in the intermembrane space. This ROS signal subsequently diffuses into the cytosol, where it elicits an increase in Ca2+, thereby activating HPV.
In the lung, alveolar hypoxia triggers constriction of small pulmonary arteries in a response known as hypoxic pulmonary vasoconstriction (HPV). While this response has been studied extensively, the underlying mechanism of cellular oxygen sensing is not established and the signaling pathways that link the O2 sensor to the contractile response have been a focus of debate. Our model proposes that hypoxia triggers the release of reactive oxygen species (ROS) signals from the outer surface of the inner mitochondrial membrane; these oxidants initially affect the intermembrane space and subsequently enter the cytosol where they trigger increases in cytosolic calcium. To test this we measured redox status in the mitochondrial matrix, the intermembrane space, and the cytosol of pulmonary artery myocytes during hypoxia, using targeted expression of a thiol redox-sensitive fluorescent protein sensor, RoGFP. Consistent with the model, we detected increases ROS signaling in the intermembrane space and the cytosol during hypoxia, while oxidant stress in the mitochondrial matrix decreased. The increase in cytosolic ROS signaling is then required for the subsequent increase in ionized calcium. These findings reveal that hypoxia activates compartmentalized sub-cellular redox signaling, and that increases in cytosolic ROS are required for activating HPV.
Supplementary Material
Acknowledgments
We thank Dr. Jingxiang Bai (Mount Sinai School of Medicine, NY) for providing recombinant adenoviruses containing catalase and mitochondria-targeted catalase; and Dr. Christopher Rhodes (Pacific Northwest Research Institute, Seattle, WA) for the adenovirus expressing the YC2.3 FRET sensor. We also thank Dr. Jotham R. Austin, II and Janice Wang (The University of Chicago, Chicago, IL) for their assistance with the electron microscopy studies.
Sources of Funding Supported by NIH Grants HL66315, HL35440 and HL079650 (to P.T.S), American Heart Association Grant 0235457Z (to G.B.W.), and NIH Grant NS056313 (to J.D.M.).
Nonstandard Abbreviations and Acronyms
- Cyto-RoGFP
cytosolic RoGFP
- DCF
dihydrodichlorofluorescein diacetate
- DHE
dihydroethidium
- DTT
dithiothreitol
- EPR
electron paramagnetic resonance
- GPD
glycerol phosphate dehydrogenase
- GSH
reduced glutathione
- HPV
hypoxic pulmonary vasoconstriction
- IMS
mitochondrial inter-membrane space
- IMS-RoGFP
mitochondrial inter-membrane space RoGFP
- Mito-RoGFP
mitochondrial matrix RoGFP
- NAC
N-acetyl-L-cysteine
- NO
nitric oxide
- PASMC
pulmonary arterial smooth muscle cells
- ROS
reactive oxygen species
- SASMC
systemic arterial smooth muscle cells
- tBH
tert-butyl hydroperoxide
- π0
mitochondria-deficient cells
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol. 1989;67:1903–1911. doi: 10.1152/jappl.1989.67.5.1903. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 3.Mohazzab KM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol. 1994;267:L823–831. doi: 10.1152/ajplung.1994.267.6.L823. [DOI] [PubMed] [Google Scholar]
- 4.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 5.Mehta JP, Campian JL, Guardiola J, Cabrera JA, Weir EK, Eaton JW. Generation of oxidants by hypoxic human pulmonary and coronary smooth-muscle cells. Chest. 2008;133:1410–1414. doi: 10.1378/chest.07-2984. [DOI] [PubMed] [Google Scholar]
- 6.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 7.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 8.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 9.Spasojevic I, Liochev SI, Fridovich I. Lucigenin: redox potential in aqueous media and redox cycling with O−2 production. Arch Biochem Biophys. 2000;373:447–450. doi: 10.1006/abbi.1999.1579. [DOI] [PubMed] [Google Scholar]
- 10.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 11.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 12.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 13.Cannon MB, Remington S James. Redox-sensitive green fluorescent protein: probes for dynamic intracellular redox responses. A review. Methods Mol Biol. 2009;476:50–64. doi: 10.1007/978-1-59745-129-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Lohman JR, Remington SJ. Development of a family of redox-sensitive green fluorescent protein indicators for use in relatively oxidizing subcellular environments. Biochemistry. 2008;47:8678–8688. doi: 10.1021/bi800498g. [DOI] [PubMed] [Google Scholar]
- 15.Jiang K, Schwarzer C, Lally E, Zhang S, Ruzin S, Machen T, Remington SJ, Feldman L. Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol. 2006;141:397–403. doi: 10.1104/pp.106.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 17.Brown LJ, MacDonald MJ, Lehn DA, Moran SM. Sequence of rat mitochondrial glycerol-3-phosphate dehydrogenase cDNA. Evidence for EF-hand calcium-binding domains. J Biol Chem. 1994;269:14363–14366. [PubMed] [Google Scholar]
- 18.Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 20.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 22.Bai J, Cederbaum AI. Overexpression of catalase in the mitochondrial or cytosolic compartment increases sensitivity of HepG2 cells to tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2000;275:19241–19249. doi: 10.1074/jbc.M000438200. [DOI] [PubMed] [Google Scholar]
- 23.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 25.Weissmann N, Zeller S, Schafer RU, Turowski C, Ay M, Quanz K, Ghofrani HA, Schermuly RT, Fink L, Seeger W, Grimminger F. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- 26.Weissmann N, Ebert N, Ahrens M, Ghofrani HA, Schermuly RT, Hanze J, Fink L, Rose F, Conzen J, Seeger W, Grimminger F. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol. 2003;29(6):721–732. doi: 10.1165/rcmb.2002-0217OC. [DOI] [PubMed] [Google Scholar]
- 27.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Guzy RD, Mack MM, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal. 2007;9:1317–1328. doi: 10.1089/ars.2007.1708. [DOI] [PubMed] [Google Scholar]
- 31.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol London. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. Faseb J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 34.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer SL, Weir EK, Reeve HL, Michelakis E. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv Exp Med Biol. 2000;475:219–240. doi: 10.1007/0-306-46825-5_21. [DOI] [PubMed] [Google Scholar]
- 36.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 37.Morio Y, McMurtry IF. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- 38.Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res. 2001;89:77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- 39.Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L318–325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- 40.Eu JP, Xu L, Stamler JS, Meissner G. Regulation of ryanodine receptors by reactive nitrogen species. Biochem Pharmacol. 1999;57:1079–1084. doi: 10.1016/s0006-2952(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 41.Evans AM, Dipp M. Hypoxic pulmonary vasoconstriction: cyclic adenosine diphosphate-ribose, smooth muscle Ca2+ stores and the endothelium. Respir Physiolo Neurobiol. 2002;132:3–15. doi: 10.1016/s1569-9048(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 42.Kumasaka S, Shoji H, Okabe E. Novel mechanisms involved in superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum linked to cyclic ADP-ribose stimulation. Antioxid Redox Signal. 1999;1:55–69. doi: 10.1089/ars.1999.1.1-55. [DOI] [PubMed] [Google Scholar]
- 43.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L848–858. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- 45.Sweeney M, Yuan JX. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respir Res. 2000;1:40–48. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol London. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Pacheco FR, Caramelo C, Castilla MA, Deudero JJ, Arias J, Yague S, Jimenez S, Bragado R, Alvarez-Arroyo MV. Mechanism of vascular smooth muscle cells activation by hydrogen peroxide: role of phospholipase C gamma. Nephrol Dial Transplant. 2002;17:392–398. doi: 10.1093/ndt/17.3.392. [DOI] [PubMed] [Google Scholar]
- 48.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y, Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Barneo J, Pardal R, Montoro RJ, Smani T, Garcia-Hirschfeld J, Urena J. K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Respir Physiol. 1999;115:215–227. doi: 10.1016/s0034-5687(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 51.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 52.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 55.Pastukh V, Ruchko M, Gorodnya O, Wilson GL, Gillespie MN. Sequence-specific oxidative base modifications in hypoxia-inducible genes. Free Radic Biol Med. 2007;43:1616–1626. doi: 10.1016/j.freeradbiomed.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 56.Lavani R, Chang WT, Anderson T, Shao ZH, Wojcik KR, Li CQ, Pietrowski R, Beiser DG, Idris AH, Hamann KJ, Becker LB, Vanden Hoek TL. Altering CO2 during reperfusion of ischemic cardiomyocytes modifies mitochondrial oxidant injury. Crit Care Med. 2007;35:1709–1716. doi: 10.1097/01.CCM.0000269209.53450.EC. [DOI] [PubMed] [Google Scholar]
- 57.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.