Abstract
Regulatory T cells (T-reg) represent a major roadblock to the induction of anti-tumor immunity through vaccine approaches. TGF-β is a cytokine implicated in the generation and maintenance of T-reg cells, as well as their suppressive function. These experiments examined whether the generation of tumor-sensitized T-reg cells was TGF-β dependent and evaluated whether TGF-β produced by T-reg cells blocked the priming of tumor-specific T cells in vaccinated reconstituted-lymphopenic mice (RLM). We show that tumor-sensitized T-reg cells (CD25+/FoxP3+) obtained from tumor-bearing mice block the generation of tumor-specific T cells in RLM. Strikingly, this suppression is absent if tumor-sensitized T-reg cells are acquired from tumor-bearing mice expressing the dominant-negative TGFβRII in T cells. This loss of suppression was a result of the crucial role of TGF-β in generating tumor-sensitized T-reg cells, and not due to the insensitivity of naïve or tumor-primed effector T cells to the direct suppressive influence of TGF-β. We conclude that blocking TGF-β in a tumor-bearing host can inhibit the induction of highly suppressive tumor-sensitized T-reg. These data suggest that an integrative strategy combining “upfront” T-reg ablation followed by vaccination and TGF-β blockade may limit generation of new tumor-sensitized T-regs and improve the generation of therapeutic immune responses in patients with cancer.
Keywords: Tumor Immunity, Tolerance/Suppression/Anergy, Cytokines, T Cells, Adoptive immunotherapy
INTRODUCTION
It is evident from pre-clinical studies that tumor cells can be recognized by the immune system (1). Current research has focused on improving tumor-vaccine strategies in order to elicit more potent anti-tumor immune responses (2). However, the promising results from the pre-clinical data have not translated into significant clinical benefit for cancer patients. It appears that multimodal therapy concepts have to be developed and tested in vivo. Recently, the combination of CTLA-4 blockade, GM-CSF stimulated vaccination and adoptive T cell transfer into lymphopenic hosts were shown to overcome tumor induced immune suppression (3). Therefore it is of great interest to elucidate the molecular and cellular mechanisms that are in place to block effective tumor-vaccine responses to adjust and improve multimodal anti-tumor vaccine strategies.
Regulatory T cells (T-reg) are implicated as part of the tumor-induced immune suppressive network (4). Regulatory T cells are critical mediators of tolerance against self and foreign antigens (5, 6). An increased frequency of T-reg cells has been observed in patients with various types of cancer (7–9) suggesting a role for T-reg cells in the development and/or progression of human malignancies. T-reg cells can be divided into natural and adaptive subpopulations (10). Natural T-reg cells are phenotypically characterized as CD4+CD25+Foxp3+ T cells (11) and acquire this phenotype in the thymus (12). In contrast, adaptive T-reg cells acquire the same phenotype in the periphery (13–15). Evidence for the generation of adaptive Foxp3+ T-reg cells outside the thymus was derived from the observation that chronic and low-dose antigen presentation leads to the generation of adaptive Foxp3+ T-reg cells (16–18). Interestingly, in a model system using cancer cells expressing hemagglutinin (HA), peptide vaccination against HA led to increased vaccine-specific T-reg cells (19). These data suggest that vaccination against self-antigens may induce tolerance rather than productive immune responses. Investigators have tried to minimize the immune suppressive effects of T-reg cells by attempting to eliminate them. Recently, in a pre-clinical model the depletion of T-reg before adoptive transfer into a lymphopenic host resulted in statistically improved tumor rejection (20). Already in a clinical study, the administration of a recombinant IL-2 diphtheria toxin conjugate before vaccination with tumor RNA-transfected dendritic cell vaccines resulted in significantly improved priming of tumor-specific T cell responses and reduced absolute numbers of T-reg cells in renal cell cancer patients (21).
Multiple investigators have shown that transforming growth factor β (TGF-β) is a crucial mediator of tumor-induced immune suppression (reviewed by (22)). TGF-β is a pleiotropic immune modulator, which influences the function of different cells of the immune system including antigen presenting cells (APC), natural killer (NK) cells, B and T cells (23). TGF-β can prevent the development of Th1 (24) and Th2 (25) responses by blocking T-bet and GATA-3, respectively. TGF-β is also known to regulate the maintenance and induction of T-reg cells (26). It was shown in TGF-β1 knockout mice that TGF-β was not required for the generation of natural T-reg cells in the thymus, but it was necessary for their maintenance in the periphery (27). TGF-β has also been shown to be a key cytokine for the induction of adaptive T-reg cells (13). CD4+CD25− T cells can be induced ex vivo to become Foxp3+ T cells with in vivo and in vitro suppressive capacity when they are stimulated in the presence of TGF-β (13, 28, 29). Two animal models have demonstrated a crucial role for TGF-β in the T-reg cell mediated prevention of autoimmunity. In these models, adoptive transfer of T-reg cells could prevent autoimmunity only if they were co-transferred with T cells that had an intact intracellular TGF-β signaling pathway (30, 31).
Other models suggest that T-reg cells mediate suppression by both TGF-β dependent and independent mechanisms. In vitro suppression of proliferation can be achieved in T cells insensitive to TGF-β (32) indicating the dispensability of TGF-β. However, there are also data from both in vitro and in vivo experiments which show that T cells insensitive to TGF-β cannot be suppressed by T-reg cells (31, 33).
Work by Gorelik and colleagues, using mice whose CD4+ and CD8+ cells had a genetically impaired TGF-β receptor, demonstrated that blocking TGF-β allowed immune-mediated tumor rejection (34). However, it is unclear whether TGF-β is used by T-reg cells to mediate suppression and/or is important for the generation of tumor sensitized T-reg cells that mediate suppression (35). Already anti-sense oligonucleotides are being employed in clinical trials to block TGF-β either at the tumor-vaccine site (36, 37) or in the tumor microenvironment (38). Here we have attempted to further define the role of TGF-β in both the generation of tumor-sensitized T-reg cells and the mediation of immune suppression. We used a preclinical model where the generation of tumor-sensitized T-reg cells is separated from the priming of tumor-specific effector T cells and the in vivo evaluation of therapeutic activity. The effect of TGF-β was modeled by using transgenic mice that express the dominant-negative TGFβR type II (dnTGFβRII) (39) in CD8+ and CD4+ cells (40). In these transgenic mice CD4+ and CD8+ cells have only a minimal sensitivity to TGF-β, because the receptor complex formed by the TGFβRI and the dnTGFβRII impairs TGF-β signaling due to the truncated intracellular signaling domain of the dnTGFβRII. Results of these studies document that TGF-β is critical for the generation of tumor-sensitized adaptive T-reg cells that are efficient suppressors of effective T cell priming. Further, we show that tumor-sensitized T-reg cells can mediate effective suppression of a therapeutic immune response through a mechanism that is independent of TGF-β’s effect on T cells.
MATERIALS AND METHODS
Mice, Tumor Cell Lines and Experimentally Established Pulmonary Metastases
Female C57BL/6J (B6) mice were purchased from Charles River (Wilmington, MA). Female dominant-negative TGFβR type II (dnTGFβRII) mice (40) were kindly provided by Richard Flavell, Department of Immunobiology, Yale School of Medicine, New Haven, CT and were bred with C57BL/6J mice. Offspring were typed by PCR for the integration of the dnTGFβRII. Foxp3GFP/GFP mice (11) were kindly provided by Alexander Rudensky, Department of Immunology, University of Washington School of Medicine, Seattle, WA and crossed to dominant-negative TGFβR type II mice to generate male dnTGFβRII Foxp3−GFP/0 and female dnTGFβRII Foxp3GFP/− mice. Recognized principles of laboratory animal care were followed (Guide for the Care and Use of Laboratory Animals, National Research Council, 1996).
D5 is a poorly immunogenic subclone of the spontaneously arising B16BL6 melanoma. D5 cells secrete 1.5 ng TGF-β/ml/106cells/24 h. D5-G6 is a stable clone of D5 that was transduced with a murine GM-CSF retroviral MFG vector (41). D5-G6 cells secrete 40–60 ng GM-CSF/ml/106 cells/24 h. All tumor cells were cultured as described previously (42). In brief, we used complete medium (CM), which consisted of RPMI 1640 (BioWhittaker,Walkersville, Maryland) supplemented with β-ME (Aldrich, Milwaukee, Wisconsin), 10% FBS (Life Technologies, Grand Island, New York), NEAA, sodium pyruvate and L-glutamine. Cell lines were maintained in T-75 or T-150 culture flasks in a 5% CO2 incubator at 37 °C.
Mice were depleted of NK cells by i.p. injection of 500 µg NK1.1. antibody purified from the hybridoma HB-191 (ATCC) 24 hours before injection of D5 cells. Pulmonary metastases were established in WT and dnTGFβRII mice by tail vein injections with 0.15 × 106 and 0.5 × 106 D5 tumor cells, respectively. Fifteen days after i.v. injection tumor-bearing mice (TBM) were sacrificed by CO2 narcosis and their spleen cells were used to reconstitute lymphopenic recipients.
Lymphopenia, Reconstitution, Vaccination, and in Vitro Activation and Expansion
Lymphopenia was induced by sub-lethal irradiation of mice with 500R (Gammacell 3000, MDS Nordion, Ottawa, Canada). The reconstitution with spleen cells, vaccination, and in vitro activation and expansion was performed as described previously (43). Briefly, 20 million spleen cells from female mice were used to reconstitute irradiated female mice. These reconstituted lymphopenic mice (RLM) were vaccinated with D5-G6 tumor cells. Four aliquots of 1 × 106 tumor cells each were injected into both the fore and hind flanks. Eight days after vaccination, two enlarged inguinal, axillary, and scapular tumor vaccine draining lymph nodes (TVDLN) were collected, and single cell suspensions were prepared. The TVDLN cells were cultured at 1 × 106 cells/ml in CM in 24-well plates with 5 µg/ml 2c11 antibody (anti-CD3) and 2.5 µg/ml anti-CD28 mAb. After 2 days of activation, the T cells were harvested and subsequently expanded in CM containing 60 IU/ml IL-2 (Chiron Co., Emeryville, CA) in tissue culture bags or 6-well plates for 2 or 3 additional days. These in vitro activated and expanded cells are referred to as effector T cells (T-eff). The specific modifications for the individual experiments are described in the figure legends and are depicted in figure 1A, 3A and 5A.
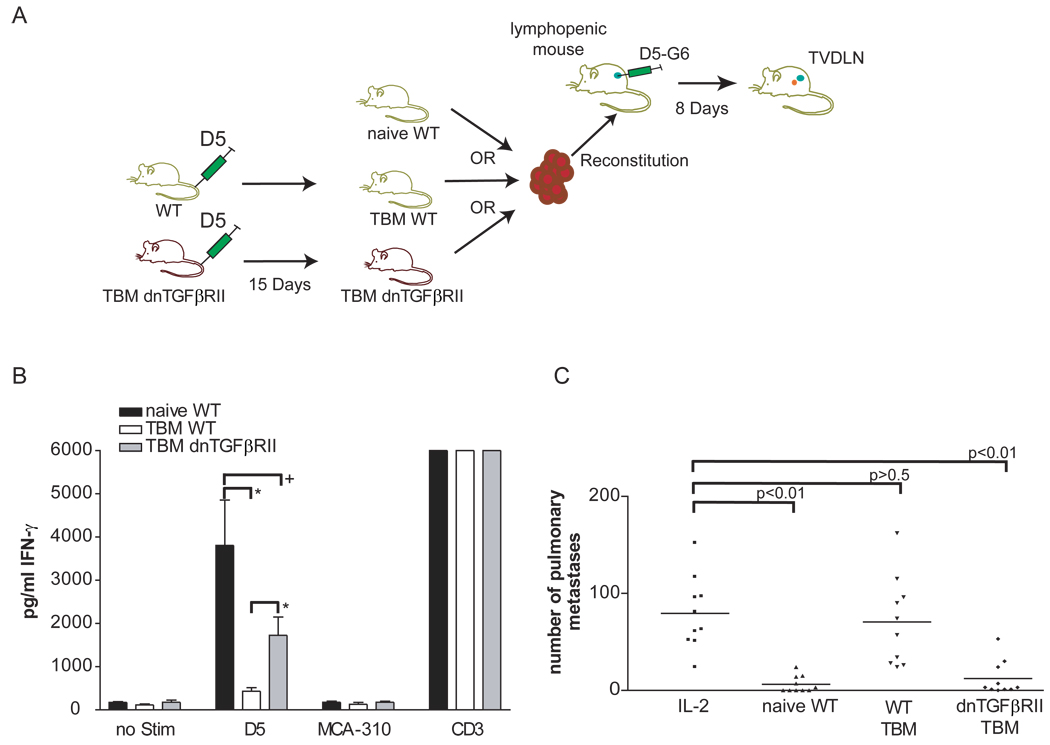
Figure 1. Therapeutic tumor-specific effector T cells can be generated from tumor-bearing dnTGFβRII spleen cells.
(A) 20 million spleen cells were used from either female naïve WT, WT TBM or dnTGFβRII TBM to reconstitute female mice sublethally irradiated with 500R. These reconstituted lymphopenic mice (RLM) were vaccinated with the D5-G6 tumor vaccine. Four aliquots of 1 × 106 tumor cells each were subcutaneously injected into both the fore and hind flanks. Eight days after vaccination, two enlarged inguinal, auxillary, and scapulary tumor vaccine draining lymph nodes (TVDLN) were collected. (B) IFN-γ was quantified by ELISA after effector T cells were restimulated with the tumor target D5 or the unrelated, syngeneic tumor target MCA-310. As a negative control effector T cells were cultured alone; as a positive control effector T cells were stimulated with plate-bound anti-CD3 (5 independent experiments), (*) p<0.02, (+) p>0.05. (C) Therapeutic efficacy was determined following transfer of effector T cells into mice bearing experimental pulmonary metastases that were established 3 days before T-cell transfer. Effector T cells generated from RLM reconstituted with spleen cells from either naïve WT mice (naïve WT), tumor-bearing WT mice (WT TBM), or tumor-bearing dnTGFβRII mice (dnTGFβRII TBM) were adoptively transferred. All groups received 90,000 IU IL-2 i.p. every 24 h for four days following adoptive transfer. A control group received IL-2 only and no effector T cells (IL-2) (data represents two combined experiments).
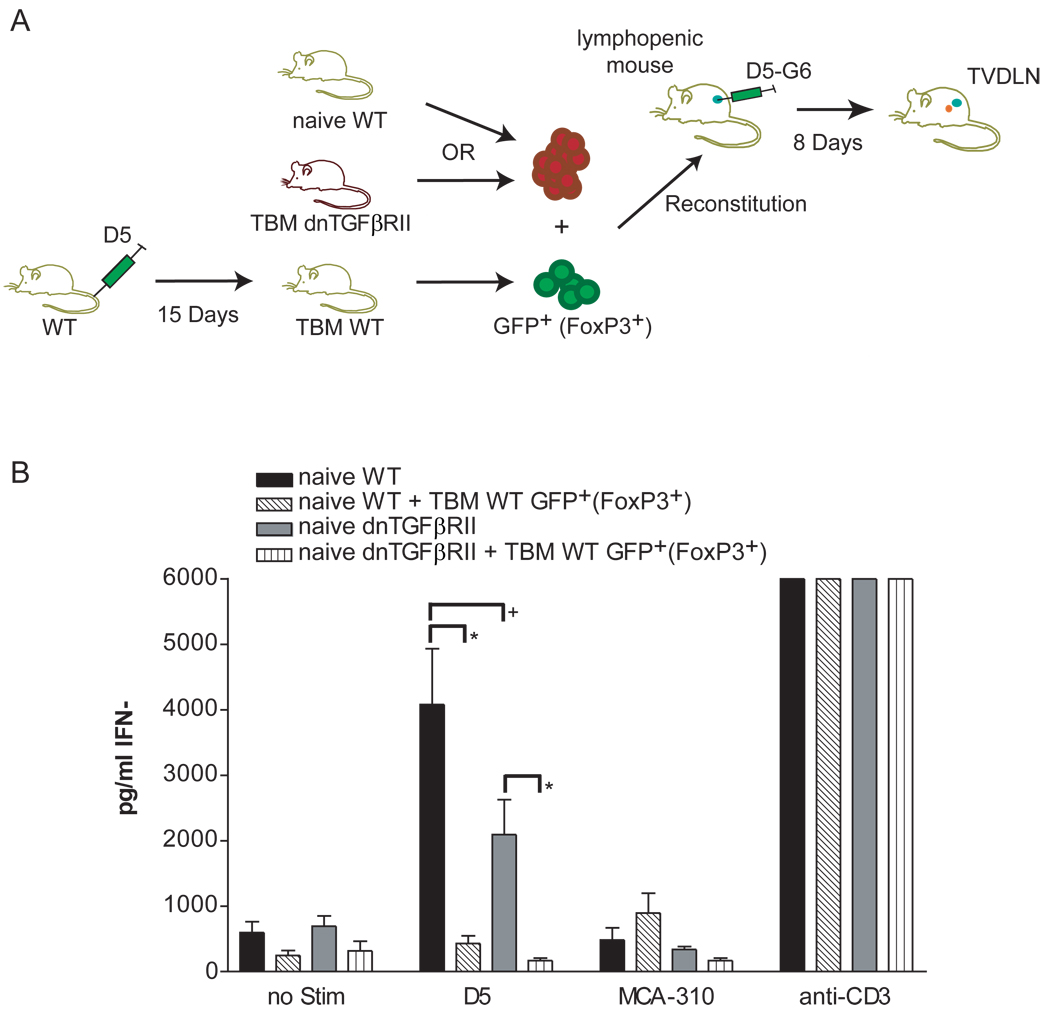
Figure 3. TGF-β is not necessary for tumor-induced T-reg immune suppression in reconstituted lymphopenic mice (RLM).
(A) 20 million spleen cells were used from either female naïve WT or naïve dnTGFβRII to reconstitute female mice sublethally irradiated with 500R. Additionally, two groups of RLM were reconstituted with 2×106 either WT naïve or dnTGFβRII naïve spleen cells and 3×105 GFP+ (FoxP3+) cells sorted from WT spleens of 15 days TBM. Reconstituted lymphopenic mice (RLM) were vaccinated with the D5-G6 tumor vaccine. Four aliquots of 1 × 106 tumor cells each were subcutaneously injected into both the fore and hind flanks. Eight days after vaccination, two enlarged inguinal, auxillary, and scapulary tumor vaccine draining lymph nodes (TVDLN) were collected. (B) IFN-γ was measured by ELISA after effector T cells were re-stimulated with the tumor target D5 or the unrelated, syngeneic tumor target MCA-310. As a negative control effector T cells were cultured alone; as a positive control effector T cells were stimulated with plate-bound anti-CD3 (3 of 4 independent experiments). Effector T cells from RLM reconstituted with either 20×106 naïve WT spleen alone (naïve WT) or with the addition of 3×105 GFP+FoxP3+ cells from WT tumor-bearing mice (naïve WT + TBM WT GFP+) or effector T cells were generated from RLM that were reconstituted with 20×106 naïve dnTGFβRII spleen (naïve dnTGFβRII) alone or with the addition of 3×105 GFP+FoxP3+ cells WT tumor-bearing mice (naïve dnTGFβRII + TBM WT GFP+), (*) p< 0.03, (+) p>0.05.
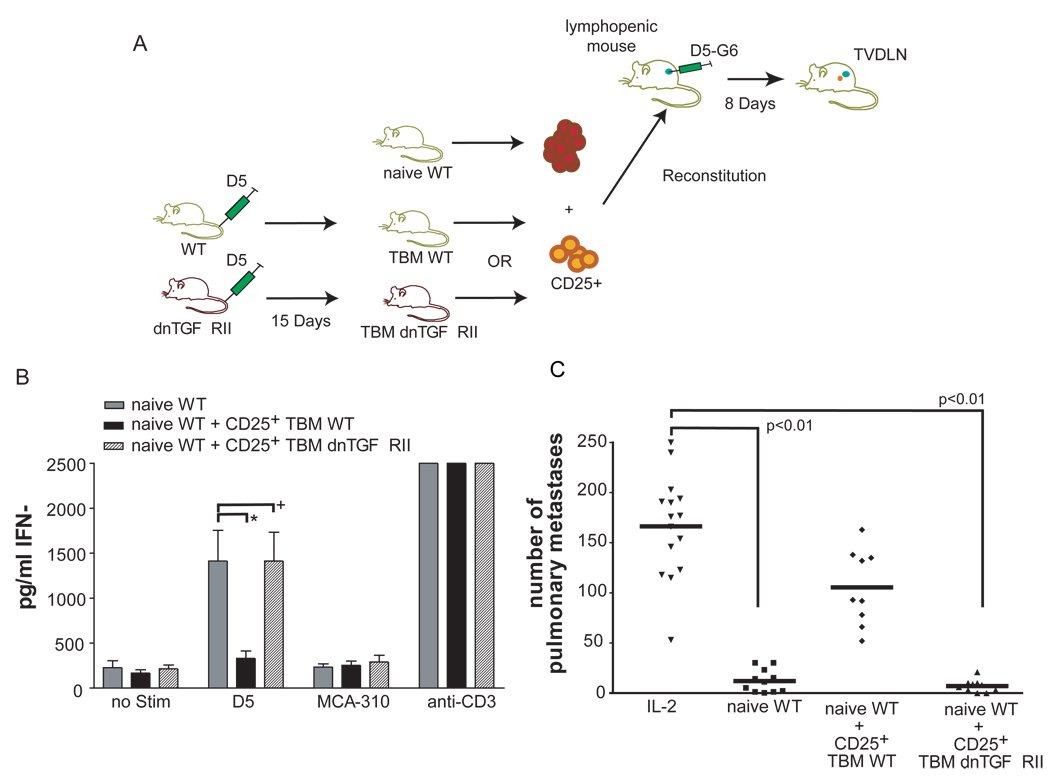
Figure 5. T-reg cells from mice insensitive to TGF-β do not suppress the priming of tumor-specific T cells.
(A) 20 million spleen cells were used from female naïve WT to reconstitute female mice sublethally irradiated with 500R. One group additionally received 1 × 106 CD25+ purified cells from WT TBM the other group received 1 × 106 CD25+ purified cells from WT dnTGFβRII. Reconstituted lymphopenic mice (RLM) were vaccinated with the D5-G6 tumor vaccine. Four aliquots of 1 × 106 tumor cells each were subcutaneously injected into both the fore and hind flanks. Eight days after vaccination, two enlarged inguinal, auxillary, and scapulary tumor vaccine draining lymph nodes (TVDLN) were collected. (B) IFN-γ was assessed by ELISA when effector T cells were re-stimulated with the tumor target D5 or the unrelated, syngeneic tumor target MCA-310. As a negative control effector T cells were cultured alone, as a positive control T-eff were stimulated with plate-bound anti-CD3 (3 independent experiments). Effector T cells were generated from RLM reconstituted with either 20×106 naïve WT spleen alone (naïve WT) or with the addition of either 1×106 CD25+ cells of WT tumor-bearing mice (naïve WT + TBM WT CD25+) or 1×106 CD25+ cells from dnTGFβRII tumor-bearing mice (naïve WT + TBM dnTGFβRII CD25+), (*) p< 0.02, (+) p>0.05. (C) Therapeutic efficacy was determined following transfer of effector T cells into mice bearing experimental pulmonary metastases, which were established 3 days before T-cell transfer. Effector T cells were generated from RLM reconstituted with spleen cells from naïve WT mice (naïve WT), or WT mice spleen cells mixed with either CD25+ from WT TBM (naïve WT + CD25+ WT TBM) or with CD25+ from dnTGFβRII TBM (naïve WT + CD25+ dnTGFβRII TBM). All groups received 90,000 IU IL-2 i.p. every 24 h for four days following adoptive transfer. A control group received IL-2 only and no effector T cells (IL-2) (data represents two combined individual experiments).
Induction of Foxp3+ T cells
To induce expression of Foxp3 in Foxp3− cells spleen cells from FoxP3GFP/GFP mice were sorted into a GFP− population (Foxp3−GFP−) and cultured at 4 × 106 cells/8 ml/well in CM in 6-well plates with 5 µg/ml 2c11 antibody (anti-CD3), 2.5 µg/ml anti-CD28 mAb, and 120 IU/ml IL-2 and various concentrations of recombinant human TGFβ-1 (eBioscience, San Diego, CA) for 48h.
Adoptive Immunotherapy
Effector T cells were washed twice in HBSS and injected i.v. in C57BL/6 female mice, in which pulmonary metastases were established 3 days earlier by tail vein injection of 0.3 × 106 D5 tumor cells. Starting on the day of T-cell infusion, mice received 90,000 IU IL-2 i.p. once per day for 4 days. Animals were sacrificed 13 days after tumor injection by CO2 narcosis. Lungs were removed and fixed in Fekete’s solution. The number of macroscopic pulmonary metastases was counted blinded, and metastases that were too numerous to count accurately were assigned a value of 250.
Measurement of cytokines
After in vitro activation and expansion, effector T cells were washed, resuspended in CM and IL-2 (60 IU/ml), and seeded at 1 × 106/2 ml/well in a 48-well plate. The cells were either cultured without further stimulation (negative control) or stimulated with 1 × 105 D5 tumor cells, MCA-310 tumor cells (syngeneic fibrosarcoma, specificity control), or immobilized anti-CD3 (positive control). Supernatants were harvested after 24 h and assayed for the release of IFN-γ by ELISA using commercially available reagents (IFN-γ, BD Biosciences Pharmingen, San Diego, CA). The concentration of cytokines in the supernatant was determined by regression analysis.
Fluorescence-activated Cell Sorter and Cell Purification
All samples were acquired using Summit 4.2 software on a Dako Cyan ADP Flow Cytometer equipped with three diode lasers (488, 635, and 407 nm), and modified with optimal bandpass and dichroic filters (Dako, Fort Collins, CO). FITC-antiCD3, PE-antiFoxp3, APC-Alexa750-antiCD4, APC-antiCD25 mAb were purchased from eBioscience, San Diego, CA. GFP+ and GFP− cells were sorted from single cell suspensions of spleen cells in HBSS without calcium or magnesium and 0.05% FBS using a MoFlo instrument (Dako, Fort Collins, CO). The purity of the sorted cells was determined immediately after sorting and was usually 95–98% for the cell populations of interest. CD25+ cells were purified from spleen cell suspensions in HBSS without calcium or magnesium. First APC-antiCD25 mAb (eBioscience, San Diego, CA) was used to stain CD25+ cells followed by a second magnetic-bead-linked-antiAPC Ab (Miltenyi Biotec, Auburn, CA). Cell purification was performed in accordance with manufacture’s instructions.
RESULTS
Tumor-specific effector T cells can be generated from tumor-bearing dnTGFβRII spleen cells
We have previously reported that vaccination of lymphopenic mice reconstituted with naïve WT spleen cells (RLM) augments priming of tumor-specific T cells that secrete IFN-γ when restimulated with specific tumor in vitro and can mediate significantly increased therapeutic activity (43, 44). We have also observed that this RLM strategy is ineffective when cells from 8–15 day tumor-bearing mice (TBM) are used to reconstitute lymphopenic mice (Poehlein et al, manuscript submitted). Importantly this suppressive effect could be eliminated by removing the CD25+ population from TBM spleen cells. Further, adding back CD25+ cells from TBM spleen blocked the generation of tumor-specific effector T cells by naïve T cells (Poehlein et al., manuscript submitted).
Given the significant role of TGF-β described in the context of T-reg cells, we posited that TGF-β played a critical role in mediating the immune suppression observed in vaccinated RLM that were reconstituted with spleen cells from TBM. To test this hypothesis we examined whether T cells insensitive TGF-β would exhibit the immune suppressive effects that WT mice exhibited when exposed to systemic tumor burden. Spleen cells from 15-day tumor-bearing dnTGFβRII or wt mice were transferred into lymphopenic recipients that were vaccinated on the same day with D5-G6 and 8 days later tumor-vaccine draining lymph nodes (TVDLN) were harvested (figure 1A). Consistent with observations of Poehlein et al., effector T cells generated from mice reconstituted with TBM spleen cells failed to secrete substantial amounts of tumor-specific IFN-γ (figure 1B). In contrast, TGF-β-insensitive effector T cells, generated from RLM reconstituted with dnTGFβRII TBM spleen cells, secreted statistically significantly higher amounts of tumor-specific IFN-γ than effector T cells generated from RLM reconstituted with WT TBM splenocytes. The difference between the amounts of IFN-γ secreted by effector T cells generated from RLM reconstituted with dnTGFβRII TBM spleen cells and effector T cells generated from RLM reconstituted with naïve WT spleen cells was not statistically different. Evaluation of therapeutic efficacy revealed a similar profile. Adoptive transfer of effector T cells generated from WT TBM failed to mediate significant regression of established pulmonary metastases compared to mice receiving IL-2 alone (figure 1C). In contrast, effector T cells generated from RLM receiving dnTGFβRII TBM spleen cells mediated a statistically significant reduction of pulmonary metastases.
Frequency of polyclonal CD4+CD25+Foxp3+ T cells is not altered either by systemic tumor burden or by insensitivity to TGF-β
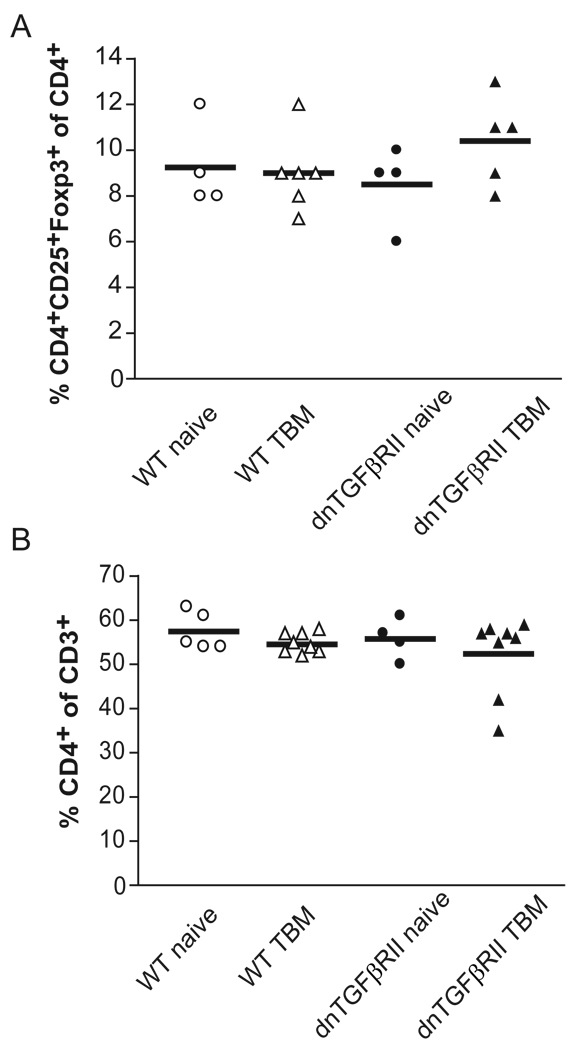
Since our previous data demonstrated that CD25+ cells were responsible for suppression mediated by WT TBM splenocytes, we questioned if dnTGFβRII TBM spleen cells lacked CD3+CD4+CD25+FoxP3+ T-reg cells. Evaluation of the frequency of T-reg cells in the spleen of WT and dnTGFβRII mice revealed that both naïve and TBM spleen cell populations contained comparable numbers of T-reg cells (figure 2C). To exclude the possibility that there was skewing of the CD3+ T cell compartment we measured CD3+CD4+ T cells. No significant difference was observed between these groups (figure 2A). Additionally, there was no significantly difference in activated T cells between dnTGFβRII and WT mice by measuring CD3+CD4+CD25+ cells (figure 2B).
Figure 2. The frequency of Foxp3+T regulatory cells in naïve or tumor-bearing mice (TBM) was unaltered by insensitivity to TGF-β.
Spleen cells from tumor-free and tumor-bearing WT and dnTGFβRII mice were analyzed by flow cytometry (at least 3 independent experiments per group).
Regulatory T cells suppress the priming of T cells insensitive to TGF-β
Since TGF-β insensitive spleen cells from TBM could be primed to become effector T cells with therapeutic activity and the frequency of total Foxp3+ T cells was not altered by tumor burden and insensitivity to TGF-β, we questioned whether T-reg cells from TBM were unable to suppress the priming of tumor-specific dnTGFβRII T cells in the RLM model. To test this hypothesis we attempted to prime naïve dnTGFβRII spleen cells in the presence of Foxp3+ T-reg cells from WT TBM. If TGF-β was the primary mechanism of suppression in this model, the tumor-sensitized T-reg cells from WT mice would be unable to suppress the generation of tumor-specific effector T cells from dnTGFβRII mice. Lymphopenic mice were reconstituted with either naïve WT or naïve dnTGFβRII spleen cells together with sorted GFP+ T-reg cells from spleens of female WT Foxp3GFP/− TBM (figure 3A). Effector T cells generated from RLM reconstituted with WT naïve spleen cells could be primed to become tumor-specific T cells and the addition of GFP+Foxp3+ T-reg cells from WT TBM to the naïve spleen cells used in the RLM model effectively eliminated the tumor-specific IFN-γ response (figure 3B). Similarly, effector T cells generated from RLM reconstituted with naïve dnTGFβRII spleen cells also showed a tumor-specific IFN-γ response that, while lower, was not statistically different than the response of effector T cells generated from RLM reconstituted with WT naïve spleen cells. Unexpectedly, the addition of GFP+Foxp3+ T cells from WT TBM to naïve dnTGFβRII spleen cells at the time of reconstitution eliminated the tumor-specific IFN-γ response of the naïve dnTGFβRII T cells (figure 3B). Thus, we conclude that TGF-β signaling does not play an essential role in mediating the suppression of priming and/or effector T cell generation in this model and that there are redundant mechanisms, beside TGF-β, that can suppress the priming of tumor-specific effector T cells in RLM.
Insensitivity to TGF-β prevents induction of adaptive Foxp3+ T regulatory cells in vitro
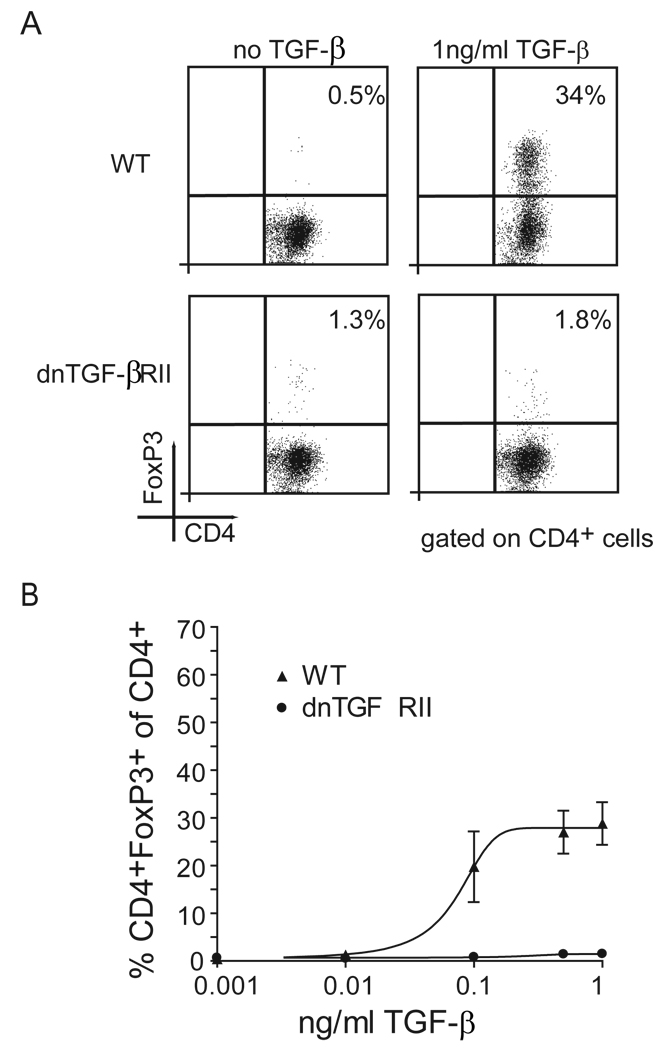
Since dnTGFβRII T cells could be suppressed by tumor-sensitized WT T-reg cells, we next asked whether the absence of suppression observed in figure 1C was because dnTGFβRII mice failed to generate tumor-induced T-reg cells. Further, we reasoned that since the insensitivity to TGF-β did not alter the frequency of the Foxp3+ T-cell compartment that this compartment consists primarily of natural T-reg cells. We also questioned whether we missed a difference between T-reg cells from WT and dnTGFβRII mice by measuring total Foxp3+ cells. Recently it was published that naïve Foxp3− T cells converted into Foxp3+ cells in the presence of TGF-β (45, 46). Based on this observation we hypothesized that Foxp3− cells from dnTGFβRII mice could not convert into Foxp3+ adaptive T-reg cells. To examine this hypothesis naïve male WT or dnTGFβRII Foxp3GFP0/+ spleen cells were sorted for GFP−(FoxP3−) cells and cultured with anti-CD3 mAb, anti-CD28 mAb, IL-2 and 1 ng/ml recombinant human TGF-β. Sort purity was 98% for GFP− spleen cells (data not shown). As hypothesized, the frequency of CD4+Foxp3+ cells increased significantly when WT spleen cells were cultured in the presence of TGF-β (figure 4A). Furthermore, the de novo induction of Foxp3 expression by TGF-β in WT cells was dose dependent. (figure 4B). In contrast, dnTGFβRII T cells failed to express Foxp3 even when cultured with the highest concentration of TGF-β.
Figure 4. TGF-β induces adaptive T-reg cells in vitro.
(A) Sorted GFP− spleen cells from naïve WT and dnTGFβRII mice were activated with anti-CD3, anti-CD28, and IL-2 in presence or absence of 1 ng/ml TGF-β. (B) Naïve and dnTGFβRII GFP− spleen cells were activated with anti-CD3, anti-CD28, and IL-2 in the presence or absence of different concentrations of TGF-β (2 independent experiments).
CD25+ cells insensitive to TGF-β from tumor-bearing mice do not suppress the priming of tumor-specific T cells
Since the in vitro data demonstrated that FoxP3− cells from dnTGFβRII mice could not be induced to express FoxP3, we hypothesized that T-reg cells from dnTGFβRII TBM would contain only natural T-reg cells and no tumor-sensitized adaptive T-reg cells. Since there are no phenotypic markers that can separate natural from adaptive T-reg cells, one way is to identify them by their functional suppression of tumor-specific T cells in the RLM model. To test whether dnTGFβRII mice generate tumor-sensitized adaptive T-reg cells that can suppress the generation of tumor-specific effector T cells we reconstituted lymphopenic mice with naïve spleen cells together with CD25+ cells from either WT TBM or dnTGFβRII TBM mice (figure 5A).
As shown previously, effector T cells generated from RLM reconstituted with WT naïve spleen cells could be primed to become tumor-specific T cells as demonstrated by their ability to secrete IFN-γ when re-stimulated with tumor (figure 5B). Whereas the addition of CD25+ cells from WT TBM essentially eliminated the tumor-specific IFN-γ response. In line with that observation therapeutic efficacy was significantly reduced by the addition of CD25+ cells from WT TBM to the naïve WT spleen cells used in vaccinated RLM (5C). In contrast, the addition of CD25+ cells from TBM dnTGFβRII at the time of reconstitution did not significantly reduce the tumor-specific IFN-γ response (figure 5B) or the therapeutic efficacy of effector T cells generated from these mice (figure 5C). Thus, we conclude that spleen cells from TBM that are insensitive to TGF-β do not contain tumor-sensitized adaptive T-reg cells, because in vitro these dnTGFβRII cells can not be induced to express Foxp3 and phenotypical T-reg cells from tumor-bearing dnTGFβRII mice fail to suppress the generation of tumor-specific effector T cells in the RLM model.
DISCUSSION
Regulatory T cells are considered to be an important component of the immunosuppressive environment caused by cancer (47, 48). Therefore it is important to understand mechanisms by which T-reg cells are induced and how they block anti-tumor immunity in order to develop multimodal therapy strategies that dampen the immune suppressive effects of T-reg cells. Our findings, as well as observations by others (reviewed in (49)) support the notion that TGF-β is a crucial molecule involved in the suppression of anti-tumor immunity. Secretion of TGF-β is not limited to our mouse model as multiple studies have demonstrated secretion of TGF-β by human tumors including tumors of the breast (50, 51), colon (52, 53) and pancreas (54). Furthermore TGF-β secretion is associated with metastatic spread and disease progression indicating it’s important role in human carcinogenesis (51–53). Our studies identified that TGF-β plays an essential role in the generation of tumor-sensitized T-reg cells, but it is not required as the suppressive molecule that blocks tumor-specific priming in the RLM model. We show that T-reg cells (Foxp3+ and CD25+ cells) obtained from WT TBM mediated immune suppression, while T-reg cells from dnTGFβRII bearing equivalent tumor burden were not suppressive. Furthermore, the suppressive mechanism used by tumor-sensitized T-reg cells was independent of TGF-βacting directly on the responding T cells. Therefore, we conclude that the lack of immune suppression observed in dnTGFβRII TBM is due to a deficiency of tumor-sensitized adaptive T-reg cells or the inability of tumor-sensitized natural T-reg to be activated by TGF-β in the tumor bearing host. We consider these findings to be clinically important, as we are investigating this RLM strategy in clinical trials with cancer patients (55). Our current protocol for reconstituting patients made lymphopenic using chemotherapy is to reinfuse an apheresis product depleted of T-reg cells. Given the observation that TGF-β is necessary for the de novo induction of tumor-sensitized T-reg cells, the blockade of TGF-β could be used to prevent the generation of new tumor-sensitized T-reg in reconstituted patients.
It could be argued that dnTGFβRII CD25+ cells from tumor-bearing mice contain effector T cells that could mediate the regression of pulmonary metastases upon adoptive transfer. This would be in accordance to what has be shown by Fahlen and colleagues in a colitis model (33). However, it is important to note that the ability to prime effectors from dnTGFβRII mice is lost if wt T-reg cells are cotransferred into RLM (figure 3). Thus, the susceptibility of dnTGFβRII T cells to T-reg mediated immune suppression is evident, and suggests that the critical element for priming dnTGFβRII tumor-specific T cells in the TBM is the lack of functional tumor-sensitized T-reg cells. In the same colitis model Fahlen and colleagues demonstrated that TGF-β is not the direct suppressor molecule used by T-reg cells. In that model T-reg cells from TGFβ-1−/− mice were suppressive. They concluded that there were other sources of TGF-β beside T-reg cells, because systemic blockade of TGF-β with an antibody resulted in the abrogation of the immune suppression. Our data are in agreement with the findings of Fahlen and suggest that mechanisms other than TGF-β secretion by T-reg cells can suppress the priming of tumor-specific T cells in vaccinated RLM. In line with our observation, Piccirillo et al. showed in vitro that CD4+CD25− dnTGFβRII T cells could be suppressed by WT CD25+ T cells and that WT CD4+ T cells could be suppressed by CD4+CD25+ TGF-b−/− T cells, indicating the dispensability of TGF-β for direct suppression of T cell priming (32). There are other possible mechanisms by which T-reg cells can mediate their suppressor function. For example, CTLA-4 expressed on T-reg cells can indirectly prevent activation of T cells by dampening the co-stimulatory signals provided by APCs (56) and IL-10 secreted by T-reg cells can mediate immune suppression (57, 58). Moreover, it is reported, that T-reg cells dampen the immune response by killing effector T cells through Granzyme B in a contact-dependent manner (59). Additionally, IL-35 has been introduced as a potential T-reg cell specific suppressor molecule (60).
The observation that insensitivity to TGF-β did not alter the frequencies of CD3+CD4+CD25+Foxp3+ T cells in naïve mice is in accordance with published data showing that naïve dnTGFβRII mice had the same level of Foxp3 RNA expression in CD4+CD25+ T cells as their WT littermates (33). Fahlen’s and our observation suggest that WT and dnTGFβRII mice generate the same amount of FoxP3+ T-reg cells. Tumor burden also did not lead to a measurable increase in the frequency of total T-reg cells after 15 days. This is in contrast to what has been reported in other clinical (7–9) and preclinical models (61). However, T cells from WT TBM spleens were suppressed from becoming tumor-specific effector T cells; and depletion of CD25+ cells from WT TBM spleen cells could reverse this suppression (Poehlein et al, manuscript in preparation). Additionally, this suppressive effect was mediated by the CD25+ fraction of TBM spleen cells, as the addition of small numbers of CD25+ cells from the spleen of a TBM abolished the priming of effector T cells with therapeutic efficacy in the RLM model. In addition we show here that sorted FoxP3+GFP+ cells from WT TBM were also potent suppressors of effector T cell priming. Together these observations argue for the generation of tumor-sensitized T-reg cells with strong immune suppressive capacity. However, phenotypically, there is no increase in the frequency or absolute number of FoxP3+ T cells in these TBM with highly functional suppressors. We have speculated that this may reflect a homeostatic mechanism that controls the total number of T reg cells at a certain level. Such a mechanism, while controlling the total number of T-reg cells, would allow for fluctuations in the T-reg repertoire. Further we postulate that tumors sensitize or induce T-reg from either natural existing T-reg cells or from the FoxP3-population (adaptive T-reg). Therefore, the difference between WT and dnTGFβRII mice seems to be the ability to generate a small population of adaptive tumor-sensitized T-regs, which are indistinguishable from natural T-regs. In an attempt to address this question we examined the ability of FoxP3− (GFP−) WT and dnTGFβRII T cells CD4+ T cells to convert to FoxP3+ T-reg cells. T cells were activated in vitro in the presence of TGF-β to induce FoxP3+ T cells as shown by others (45, 46). While WT Foxp3− T cells converted to FoxP3+ cells in the presence of TGF-β, FoxP3− dnTGFβRII T cells failed to express FoxP3.
These results indicate a T cell intrinsic mechanism by which activated T cells turn on Foxp3 in the presence of TGF-β. This in vitro data suggests that dnTGFβRII T cells are unlikely to generate adaptive T-reg cells. Since dnTGFβRII mice maintain levels of FoxP3+ T cells comparable to WT mice, we postulate that FoxP3+ population in dnTGFβRII mice are primarily natural T-reg cells that are not dependent on TGF-β for their generation. Given that they do not generate the potent suppressors of tumor-specific T cell, we consider that they lack tumor-sensitized adaptive T-reg cells. An alternative explanation is the possibility that TGF-β produced in the tumor bearing host activates WT natural T-reg cells to exert their immune suppressive capacity.
Taken together these data show that T cells insensitive to TGF-β can still be suppressed by WT tumor-sensitized T-reg cells, but that the generation of tumor-sensitized T-reg cells is dependent on TGF-β. If as we predict, tumor-sensitized T-reg cells are adaptive T-reg cells, our results argue that blockade of TGF-β prior to vaccination might reduce the highly suppressive tumor-sensitized T-reg cells that may be induced in the tumor-bearing host. This strategy may prevent induction of new tumor-sensitized T-regs as well as eliminate the role of TGF-β in maintaining adaptive T-reg cells in the periphery (27). Together, these data suggest a rationale for TGF-β blockade as an adjunct to cancer vaccines in patients enrolled on clinical immunotherapy trials.
Acknowledgments
This work was supported by RO1 CA80964, the Chiles Foundation, Robert W. Franz, the Providence Medical Foundation and the Murdock Trust.
REFERENCES
- 1.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 2.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 3.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 7.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 8.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 9.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 10.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 15.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 16.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, Zhang L, Gajewski TF. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 21.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 23.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 24.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 26.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 29.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 30.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 35.Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol. 2008;38:915–917. doi: 10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, DeVol E, Maples PB, Liu L, Chamberlin T, Shawler DL, Fakhrai H. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24:4721–4730. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 37.Fakhrai H, Mantil JC, Liu L, Nicholson GL, Murphy-Satter CS, Ruppert J, Shawler DL. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13:1052–1060. doi: 10.1038/sj.cgt.7700975. [DOI] [PubMed] [Google Scholar]
- 38.Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, Brawanski A, Proescholdt M, Schlaier J, Buchroithner J, Pichler J, Wurm G, Mehdorn M, Strege R, Schuierer G, Villarrubia V, Fellner F, Jansen O, Straube T, Nohria V, Goldbrunner M, Kunst M, Schmaus S, Stauder G, Bogdahn U, Schlingensiepen KH. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–212. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 39.Chen RH, Ebner R, Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-beta activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- 40.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 41.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 42.Hu HM, Winter H, Ma J, Croft M, Urba WJ, Fox BA. CD28, TNF receptor, and IL-12 are critical for CD4-independent cross-priming of therapeutic antitumor CD8+ T cells. J Immunol. 2002;169:4897–4904. doi: 10.4049/jimmunol.169.9.4897. [DOI] [PubMed] [Google Scholar]
- 43.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 44.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–2132. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 45.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 46.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 47.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119:254–264. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 50.Walker RA, Dearing SJ. Transforming growth factor beta 1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur J Cancer. 1992;28:641–644. doi: 10.1016/s0959-8049(05)80116-9. [DOI] [PubMed] [Google Scholar]
- 51.Dalal BI, Keown PA, Greenberg AH. Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am J Pathol. 1993;143:381–389. [PMC free article] [PubMed] [Google Scholar]
- 52.Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-beta1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer. 1999;85:554–561. doi: 10.1002/(sici)1097-0142(19990201)85:3<554::aid-cncr6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 53.Picon A, Gold LI, Wang J, Cohen A, Friedman E. A subset of metastatic human colon cancers expresses elevated levels of transforming growth factor beta1. Cancer Epidemiol Biomarkers Prev. 1998;7:497–504. [PubMed] [Google Scholar]
- 54.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI, Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 55.Ruttinger D, van den Engel NK, Winter H, Schlemmer M, Pohla H, Grutzner S, Wagner B, Schendel DJ, Fox BA, Jauch KW, Hatz RA. Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response. J Transl Med. 2007;5:43. doi: 10.1186/1479-5876-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 58.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 60.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 61.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25−lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]