Abstract
Intercellular adhesion molecule 1 (ICAM-1) mediates binding and entry of major group human rhinoviruses (HRVs). Whereas the entry pathway of minor group HRVs has been studied in detail and is comparatively well understood, the pathway taken by major group HRVs is largely unknown. Use of immunofluorescence microscopy, colocalization with specific endocytic markers, dominant negative mutants, and pharmacological inhibitors allowed us to demonstrate that the major group virus HRV14 enters rhabdomyosarcoma cells transfected to express human ICAM-1 in a clathrin-, caveolin-, and flotillin-independent manner. Electron microscopy revealed that many virions accumulated in long tubular structures, easily distinguishable from clathrin-coated pits and caveolae. Virus entry was strongly sensitive to the Na+/H+ ion exchange inhibitor amiloride and moderately sensitive to cytochalasin D. Thus, cellular uptake of HRV14 occurs via a pathway exhibiting some, but not all, characteristics of macropinocytosis and is similar to that recently described for adenovirus 3 entry via αv integrin/CD46 in HeLa cells.
Human rhinoviruses (HRVs), members of the family Picornaviridae that represent a major cause of the common cold, essentially utilize two different receptor types for host cell attachment. The 12 minor group HRVs, exemplified by HRV2, bind low-density lipoprotein receptor (LDLR), LDLR-related protein (LRP) (20), and very-LDLR (VLDLR) (29) and are internalized via the well-characterized clathrin-dependent endocytic pathway (44); however, these ligands, like others, can switch to different entry portals when the clathrin-dependent pathway is blocked (4). Once the virus arrives in endosomal carrier vesicles or late endosomes, uncoating (i.e., the release of the viral RNA genome) is triggered by the acidic pH (35, 39).
The 87 major group HRVs, exemplified by HRV14, bind intercellular adhesion molecule-1 (ICAM-1). Following entry, uncoating is triggered by ICAM-1 itself (3), but the low endosomal pH facilitates this process (37). Based on inhibition of infection by the dominant negative (DN) dynamin-2 mutant dynK44A, it was proposed that HRV14 also follows a clathrin-dependent pathway in HeLa-H1 cells (9). However, ICAM-1 lacks a clathrin localization signal and even functions as a viral receptor when its cytoplasmic tail is replaced with a glycosylphosphatidylinositol (GPI) anchor (45). Furthermore, dynamin has also been shown to be involved in pathways other than clathrin-mediated endocytosis (CME), such as caveolae- and lipid raft-dependent entry, as a function of ligand and cell type (reviewed in references 30 and 34). Additionally, dynamin might play a role in formation and closure of circular pinocytic ruffles (31). More recently, a specific entry pathway for ICAM-1 ligands into human umbilical vein endothelial cells was identified and termed “cam-mediated endocytosis”; uptake was found to be triggered upon binding of multivalent ligands, such as immunoconjugates and immunobeads, and to occur independently from clathrin and caveolin. Inhibition by amiloride, actin depolymerization, and protein kinase C inhibitors pointed to macropinocytosis (33). So far, it is not known whether these findings are relevant to the entry pathway of HRVs via ICAM-1 as the uptake kinetics was significantly dependent on particle size. For all these reasons, involvement of clathrin in HRV14 uptake is questionable. Accordingly, we explored entry of HRV14 via ICAM-1 and compared the results with the well-characterized clathrin-dependent entry pathway of HRV2 (44). Employing pharmacological compounds, specific DN inhibitors, immunofluorescence, and electron microscopy, we demonstrate that HRV14 enters rhabdomyosarcoma ICAM-1-expressing (RD-ICAM) cells via a pathway independent of clathrin, caveolin, and flotillin.
MATERIALS AND METHODS
Cells and viruses.
Rhabdomyosarcoma cells stably transfected with human ICAM-1 (RD-ICAM), a kind gift of Darren Shafren (University of Newcastle, Australia), were cultured as previously described (25, 36). HeLa-H1 cells were grown in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. For infection, Dulbecco's modified Eagle's medium (DMEM) supplemented with 30 mM MgCl2, 2% FCS, and antibiotics (infection medium) was used. HRVs were originally obtained from ATCC. All experiments were conducted with plaque-purified virus.
Antibodies and reagents.
Mouse anti-HRV14, rabbit anti-HRV14, and monoclonal mouse anti-HRV2 (8F5) (19) were produced by following standard protocols. Rabbit anti-caveolin and rabbit anti-flotillin-1 antibodies were purchased from Santa Cruz Biotechnology. Secondary antibodies labeled with Texas Red or with Alexa Fluor 488 or Alexa Fluor 591 were from Jackson Laboratories and Molecular Probes, respectively. Alexa Fluor 568-transferrin, Alexa Fluor 488-cholera toxin B (CtxB), and lysine-fixable fluorescein isothiocyanate (FITC)-dextran (70 kDa) were purchased from Molecular Probes. The following drugs were purchased from Sigma if not stated otherwise: chlorpromazine dissolved in phosphate-buffered saline (PBS; 5 μg/ml), Filipin dissolved in methanol (10 μg/ml), and methyl-β-cyclodextrin (MßCD) dissolved in PBS (5 mM). Amiloride, cytochalasin D, and dynasore were dissolved in dimethyl sulfoxide (DMSO) and used at final concentrations of 5 mM, 10 μg/ml, and 80 μM, respectively.
Drug treatment.
RD-ICAM cells were seeded as two parallel sets either in 24-well plates or on coverslips. Cells were preincubated in the presence of the selected drug for 30 min and infected with HRV2 or HRV14 at 300 50% tissue culture infective doses (TCID50)/cell in serum-free infection medium. After an additional incubation for 30 min at 34°C, one set of samples was fixed and prepared for indirect fluorescent microscopy. The second set was harvested in 50 μl of sample buffer at 3 h postinfection (p.i.) for assessment of eukaryotic translation initiation factor 4GI (eIF4GI) cleavage via Western blotting. Drugs were present throughout the incubation.
Western blot analysis.
Proteins from whole-cell lysates from each drug treatment were separated on an SDS-6% polyacrylamide gel and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% milk powder in PBS-T (PBS containing 0.1% Tween 20), incubated with rabbit anti-eIF4GI (1:8,000) kindly donated by R. Rhoads, Louisiana State University, followed by horseradish peroxidase (HRP)-labeled anti-rabbit antibody (1:20,000; Jackson Laboratories), and eIF4GI and its cleavage products were visualized by using an ECL chemiluminescence detection system (Thermoscientific). Cleavage of eIF4GI was quantified with ImageJ (http://rsbweb.nih.gov/ij/) software by using scans of the X-ray films.
Uptake of FITC-dextran.
RD-ICAM cells were grown in 24-well plates up to 90% confluence and incubated with serum-free medium for 4 h at 37°C. Lysine-fixable FITC-dextran at 1 mg/ml was added in the presence or absence of 5 μg/ml purified HRV14 and 100 ng/ml epidermal growth factor (EGF) (Sigma), respectively, and incubated for 10 min. Cells were washed with cold PBS, detached with trypsin at 4°C, collected, and resuspended in PBS containing 7% FCS. In each experiment, the mean fluorescence of 10,000 cells was determined in a BD FACSCalibur.
Confocal fluorescence microscopy.
Cells were grown on glass coverslips until 40 to 50% confluent. After cells were washed with serum-free medium, they were challenged with HRV2 or HRV14 at 300 TCID50/cell as specified in the figure legends. Unbound virus was removed by three washings with PBS++ (PBS with 1 mM MgCl2 and 1 mM CaCl2). Cells were fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 for 5 min. After samples were quenched with 50 mM NH4Cl for 10 min, unspecific binding sites were blocked in 1% goat serum either for 1 h at room temperature or overnight at 4°C. Cells were incubated for 1 h at room temperature with primary antibody (1:500) and washed three times with PBS++, followed by secondary antibody (1:400), all diluted in 1% goat serum. Nuclei were stained with 0.1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI). For assessment of colocalization, cells were infected in the presence of 10 μg/ml Alexa Fluor 568-transferrin, 1 μg/ml Alexa Fluor 488-CtxB, and 500 μg/ml lysine-fixable FITC-dextran (70 kDa). After the times specified on the figures, unbound material was removed, and cells were fixed. For better distinction between internal and external virus, differential staining before and after permeabilization of the cells was carried out with antibodies conjugated to different dyes. Coverslips were mounted in Mowiol and viewed in a Carl Zeiss LSM510-Meta confocal laser scanning microscope (LSM). Stacks were spaced by 0.5 μm. Contrast of the images was adjusted using the LSM image browser, and figures were labeled with Adobe Photoshop CS3 software.
Transfection.
The plasmids pEGFP-C3, encoding the clathrin light chain fused to green fluorescent protein (GFP-clathrin), myc-tagged pCMV-amphi-SH3 (where amphi-SH3 is the Src homology 3 domain of amphiphysin), originally donated by E. Greene (Laboratory of Cell Biology, Bethesda, MD) and H. T. McMahon (Laboratory of Molecular Biology, Cambridge, United Kingdom), respectively, and pCMV-AP180 (44) were kind gifts from Luc Snyers. Cells were seeded on coverslips in 24-well plates and grown to 30% confluence. One microgram of plasmid DNA was mixed with 50 μl of OptiMem (Gibco). Separately, 3 μl of Lipofectamine 2000 (Invitrogen) was mixed with 50 μl of OptiMem and incubated for 5 min at room temperature; the two solutions were mixed gently and incubated for 20 min at room temperature. Cells were washed twice with antibiotic-free growth medium and laid down with 500 μl of the same medium. The mixture of plasmid DNA and Lipofectamine 2000 was applied onto the cells drop by drop with gentle rotation to avoid cell toxicity. Cells were incubated for 3 h with the mixture; then, the medium was replaced with fresh antibiotic-free growth medium. At 24 h posttransfection cells were infected with HRV14 and processed for immunofluorescence microscopy. For amphi-SH3 and AP180-C (the C-terminal domain of AP180), anti-myc antibody was used to verify expression. Viral entry and replication were monitored after 30 min and 7 h by using HRV14 at 300 and 5 TCID50/cell, respectively.
Electron microscopy.
RD-ICAM cells were seeded on Aclar coverslips in 24-well plates and grown to almost 100% confluence. Cells were washed and infected with HRV2 or HRV14 at 10,000 TCID50/cell. After incubation at 34°C for the times given on Fig. 7, unbound virus was removed, and cells were fixed with 2% freshly prepared glutaraldehyde for 1 h at room temperature. Cells were washed three times with 0.1 M Sorensen buffer (pH 7.3) and incubated for 1 h in 2% osmium tetroxide in the same buffer. After cells were washed, they were dehydrated with ascending concentrations of ethanol (40, 60, 80, 95, and 100%; kept over a molecular sieve). Cells were incubated with Epon-100% ethanol (EtOH; 1:1) for 30 min and twice with Epon only, embedded in resin-filled beam capsule lids, and incubated at 60°C to polymerize for 2 days. Sections of 70 nm were prepared, stained with 2% uranyl acetate and lead citrate for 10 and 5 min, respectively, and viewed under an FEI Morgagni 100 kV electron microscope equipped with an 11-megapixel charge-coupled-device (CCD) camera.
FIG. 7.
Electron microscopy of virus entry. RD-ICAM cells grown on coverslips were infected with HRV14 at 10,000 TCID50/cell for the times indicated at 34°C. Cells were fixed and stained with OsO4, embedded in resin, and cut into 70-nm slices. They were viewed at a magnification of ×35,000. For a control, cell slices depicting HRV2 entry in coated pits and vesicles are shown. Selected areas from the middle panels are shown at higher magnification in the right panels. Note the absence of HRV14 in clathrin-coated vesicles (upper panels), whereas HRV2 is clearly seen in clathrin-coated pits and vesicles (lower panels). Areas of interest are indicated with arrows. Bar, 350 nm.
RESULTS
Virus entry via ICAM-1 is clathrin independent.
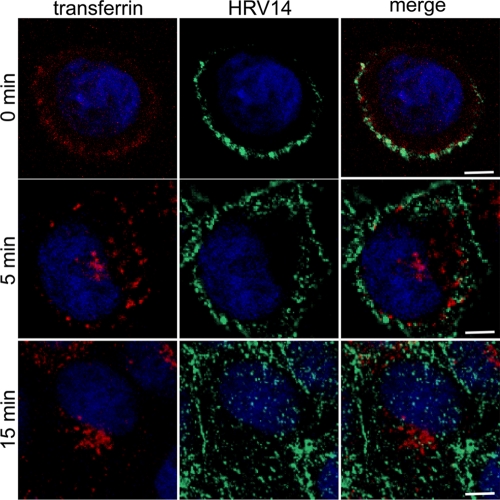
Although inhibition of HRV14 replication in HeLa cells expressing a DN mutant of dynamin-2 has been investigated (9), the clathrin dependence of uptake per se has not been analyzed. We thus first cointernalized HRV14 with human transferrin, a marker for CME. RD-ICAM cells were incubated with virus at 4°C in the presence of Alexa Fluor 568-transferrin for 1 h; cells were washed, shifted to 34°C, and fixed at 0, 5, and 15 min of incubation. Confocal microscopy revealed no colocalization at the plasma membrane (Fig. 1, 0 min). At 5 min after the temperature shift, most of the transferrin was internalized and accumulated in early endosomes and in the perinuclear region while HRV14 largely remained at the cell periphery. At 15 min virtually all transferrin had accumulated in the perinuclear region, whereas HRV14 was evenly distributed throughout the cell body, with a substantial fraction remaining at or close to the cell surface. The uptake kinetics of HRV14 is slow (26), and we consistently observed this plasma membrane accumulation even in HeLa cells that express less ICAM-1 than RD-ICAM cells. Nevertheless, HRV14 was unequivocally internalized, as also seen by differential staining prior to and after permeabilization (data not shown). These data clearly demonstrate that transferrin and HRV14 localize to distinct plasma membrane subdomains and enter with different kinetics, making clathrin-mediated endocytosis for HRV14 highly unlikely. On the other hand, HRV2, used as a control, completely overlapped with transferrin at the plasma membrane and in early endosomes (44; also data not shown) although their intracellular pathways separated thereafter (6).
FIG. 1.
HRV14 does not colocalize with transferrin. RD-ICAM cells were incubated with HRV14 at 300 TCID50/cell in the presence of 10 μg/ml Alexa Fluor 568-transferrin for 60 min at 4°C. Unbound material was washed away, and cells were shifted to 34°C. At the times indicated, cells were fixed and stained for virus with type-specific mouse antiserum followed by Alexa Fluor 488-labeled secondary antibody. Colocalization was assessed by confocal fluorescence microscopy. In the lower panels (15 min) 10 confocal slices were combined. Bar, 10 μm.
A more direct proof for clathrin-independent entry via ICAM-1 was obtained by using a GFP-tagged clathrin light chain. RD-ICAM cells expressing GFP-clathrin were challenged with virus and fixed at 5 and 15 min p.i. In accordance with the results shown above, GFP-clathrin did not colocalize with HRV14 at any time (data not shown).
DN inhibitors of clathrin-mediated endocytosis do not modify HRV14 entry via ICAM-1.
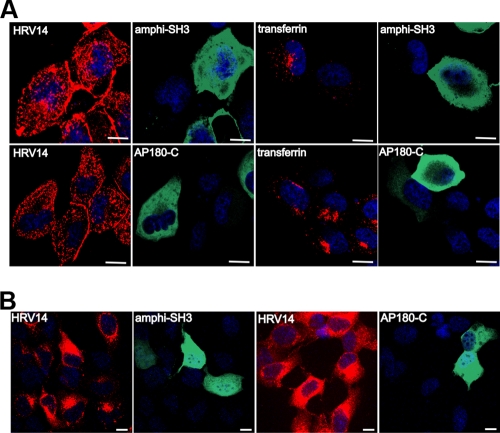
DN mutants of key proteins of the clathrin-dependent pathway have been shown to potently interfere with the normal functions of the respective wild-type proteins and hence specifically inhibit clathrin-mediated endocytosis. The SH3 domain of amphiphysin (amphi-SH3) interferes with recruitment of dynamin to coated pits and has been shown to inhibit receptor-mediated endocytosis of HRV2, transferrin, and epidermal growth factor, among many other ligands (44, 49). Thus, we made use of this DN inhibitor to confirm our data of the colocalization experiment and rule out the possible involvement of clathrin in HRV14 entry. Accordingly, RD-ICAM cells were transfected with myc-tagged amphi-SH3, followed by infection with HRV14. Confocal microscopy revealed no difference in entry in the presence or absence of the expression of amphi-SH3 (Fig. 2A, top row). amphi-SH3 was shown to prevent self-assembly of dynamin rings, which impedes constriction of clathrin-coated pits but does not affect clathrin assembly by itself (38). Therefore, to rather more directly assess clathrin function, we also used the C-terminal domain of AP180 (AP180-C), which affects clathrin-mediated endocytosis without interfering with dynamin function (11). Again, overexpression of DN AP180-C failed to modify entry of HRV14 (Fig. 2A, bottom row); in contrast, transferrin uptake was prevented by both DN inhibitors. In order to exclude the improbable possibility that the virus observed followed an abortive pathway, we also monitored viral replication after infection at a much lower multiplicity of infection ([MOI] 5 TCID50/cell). As shown in Fig. 2B, cells strongly overexpressing the DN protein mutants replicated HRV14 to a degree indistinguishable from untransfected cells. This not only proves that the clathrin-dependent endocytosis machinery is dispensable for HRV14 entry but also excludes any effect of the DN mutants on productive viral entry and replication.
FIG. 2.
DN inhibitors of the clathrin-dependent pathway have no effect on either entry or replication of HRV14. RD-ICAM cells were transfected with DN myc-tagged amphi-SH3 and AP180-C, as indicated, and challenged with HRV14 at 300 TCID50/cell for 30 min at 34°C (continuous internalization) (A) and at 5 TCID50/cell for 7 h (B). Virus was detected with type-specific antiserum and Texas Red-labeled secondary antibody, whereas expression of the DN inhibitors was assessed by using mouse anti-myc monoclonal antibody, followed by Alexa Fluor 488-labeled secondary antibody; samples were processed and viewed as described in the legend of Fig. 1. One confocal slice through the cell body is shown. Transferrin was used as a control. Bar, 10 μm.
HRV14 does not colocalize with CtxB.
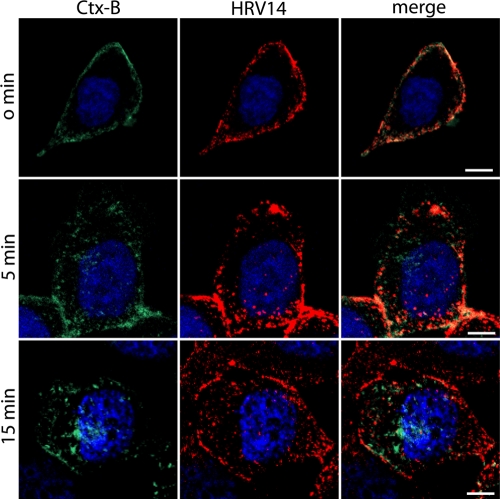
The entry pathway involving caveolin is the second well-characterized endocytic route. Thus, the eventual contribution of caveolin was evaluated by assessing a possible colocalization of virus with cholera toxin subunit B (CtxB). CtxB is a widely accepted marker of this pathway although it has also been shown to enter via other routes, such as by flotillin- (14) and clathrin-dependent endocytosis, in various cell types (16, 46). RD-ICAM cells were incubated with HRV14 in the presence of Alexa Fluor 488-CtxB for 60 min at 4°C, washed, and shifted to 37°C for the times indicated in Fig. 3. Confocal microscopy showed some overlap of HRV14 with CtxB at the cell surface at 0 min, but after being shifted to 34°C for 5 min, HRV14 and CtxB separated from each other. CtxB moved to the Golgi apparatus and concentrated at the perinuclear region at 15 min after the temperature shift while the HRV14 signal remained spread throughout the cytosol. These results indicate that HRV14 and CtxB enter with different kinetics and use different pathways to reach different destinations within the cell.
FIG. 3.
HRV14 does not colocalize with CtxB. RD-ICAM cells were incubated with HRV14 at 300 TCID50/cell and 1 μg/ml Alexa Fluor 488-CtxB for 60 min at 4°C. Unbound material was washed away, and cells were shifted to 34°C. At the times indicated, cells were fixed and stained for virus with type-specific mouse antiserum followed by Texas Red-labeled secondary antibody. Colocalization was assessed by confocal fluorescence microscopy as described in the legend of Fig. 1. Bar, 10 μm.
Further confirmation by more direct means was obtained by staining caveolin-1 and virus with appropriate antibodies. HRV14 did not colocalize with caveolin-1 (data not shown). These results definitely exclude the involvement of caveolin-1 in viral entry and intracellular trafficking via ICAM-1.
Entry via ICAM-1 is flotillin-1 independent.
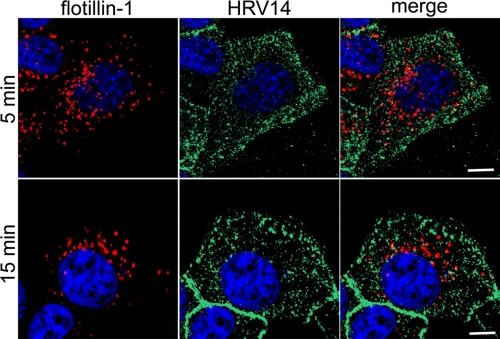
Flotillin-1 and -2 have been described as markers of a clathrin- and caveolin-independent pathway (14). Having excluded entry of HRV14 via clathrin- and caveolin-dependent routes, we wanted to investigate whether flotillin-1 was involved. Again, by using fluorescence microscopy, no colocalization of flotillin-1 and HRV14 was seen (Fig. 4). Therefore, it is very unlikely that this virus follows a flotillin-1-dependent route when accessing the cell via ICAM-1.
FIG. 4.
Flotillin-1 does not colocalize with HRV14. RD-ICAM cells were infected with HRV14 at 300 TCID50/cell for 5 and 15 min at 34°C (continuous internalization), unbound virus was removed, and the cells were fixed. Flotillin-1 was stained with rabbit anti-flotillin-1 antibody followed by Texas Red-conjugated secondary antibody. Virus was detected with type-specific antibody, followed by Alexa Fluor 488-labeled secondary antibody. Cells were processed and viewed as described in the legend of Fig. 1. One single confocal slice is shown. Bar, 10 μm.
Effect of pharmacologic inhibitors on entry of HRV2 and HRV14.
Because of the overexpression of ICAM-1 in RD-ICAM cells, large amounts of virus are taken up by the cell. However, only a fraction might undergo productive uncoating. Therefore, using a functional assay (by measuring translation of the uncoated viral RNA), we studied the effect of various drugs that have been frequently employed to specifically inhibit selected endocytic pathways. In order to avoid exposure of the cells to the chemicals for extended times, as is required to detect virus production, we made use of cleavage of the translation initiation factor eIF4GI as a reporter assay. Upon virus uncoating, the viral RNA is released into the cytoplasm and translated into a polyprotein; autocatalytic processing sets free the viral proteinase 2A, which efficiently cleaves eIF4GI into fragments detectable even at 3 h p.i. RD-ICAM cells were preincubated with the selected drugs for 30 min, as indicated in Fig. 5A, challenged with HRV14 and HRV2, and collected after 3 h. As previously described for poliovirus (21), processing of eIF4GI was assessed via Western blot analysis. The extent of cleavage was quantified from three gels obtained from three independent experiments (Fig. 5B).
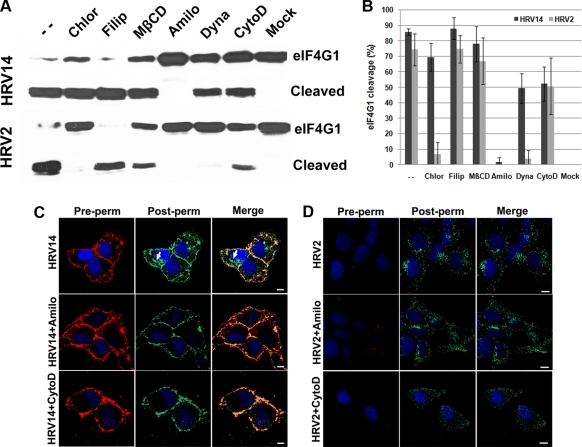
FIG. 5.
Inhibition profile of pharmacological inhibitors. Cells were grown in 24-well plates until 90% confluent. The drugs were added at the concentrations indicated in Materials and Methods and incubated for 30 min. (A) Then, virus was added at 300 TCID50/cell, and incubation continued for another 3 h. After removal of the supernatant, the cells were harvested in sample buffer, and proteins were separated on an SDS-6% polyacrylamide gel. Cleavage of eIF4GI was monitored via Western blotting with a rabbit antiserum, followed by HRP-conjugated anti-rabbit antibodies, and proteins were detected by chemiluminescence recorded on X-ray film. (B) Cleavage was quantified from scans of X-ray films from three independent experiments, including that shown in panel A, by using ImageJ software. Values are means ± standard deviations (SD). Note that the extent of cleavage under control conditions (i.e., absence of inhibitors) differed for the two virus types. (C) Cells were grown on coverslips until 70% confluent, preincubated with 5 mM amiloride and 10 μg/ml cytochalasin for 30 min as indicated, and challenged with HRV14 for 30 min at 34°C. Virus was stained and viewed as described in the legend of Fig. 1 but before (Pre-perm) and after (Post-perm) permeabilization by using secondary antibodies conjugated with Texas Red and Alexa Fluor 488, respectively. Note the strong intracellular staining of HRV14 in the controls (arrows) that is absent from amiloride-treated cells and substantially reduced in cytochalasin-treated cells. (D) For control purposes, the same experiment was carried out with HRV2. Bar, 10 μm.
Chlorpromazine causes the loss of coated pits from the plasma membrane (47) and has been extensively used to demonstrate clathrin-mediated endocytosis of viruses. Although chlorpromazine is known to have a number of side effects (22), it is still a good choice when entry of different ligands into the same cell type is studied. In agreement with the results above, eIF4GI cleavage upon HRV2 infection was prevented by the drug but only marginally reduced upon infection with HRV14.
Caveolin-mediated endocytosis is associated with lipid rafts. Removal or sequestering of cholesterol profoundly inhibits entry of CtxB and other ligands internalized via this route (42). Filipin destroys the rafts by intercalating between cholesterol molecules while methyl-ß-cyclodextrin (MßCD) sequesters cholesterol. Both lead to impairment of caveolin/lipid raft-dependent endocytosis. Neither Filipin nor MßCD significantly inhibited entry of HRV14 and HRV2. This confirms that caveolin/lipid rafts have no major importance in endocytosis of HRV2 and HRV14.
Amiloride, an inhibitor of the Na+/H+ exchanger, and cytochalasin D, which disrupts actin filaments, have both been shown to inhibit macropinocytosis. Although the mechanism of action of amiloride on macropinocytosis is still unclear, it has been employed at mM concentrations to study entry of various viruses and other ligands (32, 41, 48); cytochalasin, on the other hand, inhibits membrane ruffling and actin reorganization, which are required for plasma membrane engulfment of liquid and ligands. Amiloride completely inhibited cleavage of eIF4GI by both virus types. While these results suggest a macropinocytic pathway of HRV14, they disagree with the established clathrin dependence of HRV2 internalization (44). However, immunofluorescence microscopy unequivocally demonstrated that the drug did not prevent cell entry of HRV2 even at 5 mM (Fig. 5D), whereas eIF4GI cleavage was abrogated already at 200 μM amiloride (data not shown). This points to steps different from endocytosis, such as RNA release and/or polyprotein processing/translation, as targets of amiloride in HRV2 infection. Cytochalasin D exhibited a moderate effect (about 40% inhibition) on HRV14- and HRV2-mediated eIF4GI cleavage. Similar to amiloride, cytochalasin led to accumulation of HRV14 at the plasma membrane (Fig. 5C), whereas it apparently affected uptake of HRV2 only marginally (Fig. 5D). Note that plasma membrane staining of HRV2 was virtually absent because of the low steady-state concentration of the receptors on the membrane. Even though HRV14 entry in the presence of cytochalasin is below the detection limit of immunofluorescence microscopy, the cleavage assay demonstrated that enough virus entered the cells to exert a notable effect. Therefore, this functional assay is clearly more sensitive.
Finally, dynasore, an inhibitor of the GTPase activity of dynamin (28), virtually prevented eIF4GI cleavage when cells were infected with HRV2 but showed only about 40% inhibition in the case of HRV14 infection. This correlates with clathrin-dependent entry of HRV2. However, it disagrees with earlier findings of a strictly dynamin-dependent entry of HRV14 into HeLa-H1 cells (9) and indicates that the degree to which uptake of ICAM-1-bound ligands is dynamin dependent might be cell type specific.
The cleavage assay monitors viral entry via translation of the viral RNA without interference with later processes such as viral replication. Nevertheless, in order to lend more support to the above results, we also attempted to measure the number of cells that became infected in the presence of the drugs via staining of de novo synthesized viral protein and quantification by use of a TissueFAXS instrument. As pointed out above, this required maintaining the cells in the presence of the drugs for at least 7 h. Examination of the images taken by the instrument revealed that some of the compounds led to a more diffuse staining pattern than that of the controls (infected in the absence of drugs). As this strongly interfered with recognition of infected cells, these experiments did not produce meaningful results.
HRV14 uptake shows some, but not all, characteristics of macropinocytosis.
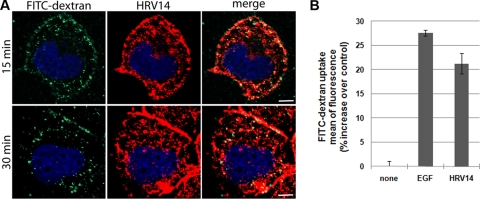
Based on the previous results, we concentrated on macropinocytosis as a candidate pathway for viral entry. RD-ICAM cells were challenged with HRV14 in the presence of 500 μg/ml of the fluid-phase marker FITC-dextran for 15 and 30 min at 34°C. Cells were fixed and examined by confocal fluorescence microscopy. Figure 6A shows substantial colocalization of HRV14 with FITC-dextran, which is considered a relatively specific marker of macropinocytosis (10). In most cell lines macropinocytosis is not constitutive but is induced by signaling events (23). We thus asked whether uptake of FITC-dextran was increased in RD-ICAM cells upon challenge with HRV14. Serum-starved cells were incubated with HRV14 and, as a control, with epidermal growth factor (EGF), together with FITC-dextran for 10 min. Fluorescence-activated cell sorter (FACS) analysis demonstrated a substantial stimulation of fluid-phase uptake by HRV14 and EGF (Fig. 6B).
FIG. 6.
HRV14 colocalizes with FITC-dextran and induces its uptake. (A) RD-ICAM cells were infected with HRV14 at 300 TCID50/cell (continuous internalization) in the presence of 500 μg/ml lysine-fixable FITC-dextran for the times indicated at 34°C, washed, and prepared for immunofluorescence microscopy as described in the legend of Fig. 1. Despite the strong signal of HRV14 because of the ICAM-1 overexpression, colocalization with FITC-dextran is evident. Bar, 10 μm. (B) Subconfluent RD-ICAM cells serum starved for 4 h were incubated with serum-free medium without EGF (no stimulation) or with 100 ng/ml EGF (control stimulation) and 5 μg/ml purified HRV14, together with 1 mg/ml 70-kDa FITC-dextran for 10 min at 37°C. Cells were washed with cold PBS and detached with trypsin at 4°C, and dextran uptake was determined by flow cytometry in a BD FACSCalibur. Percent increase of mean fluorescence of the cells related to the control is shown. Values are the means of three independent experiments ± SD.
Electron microscopy of virus entry.
The tubovesicular compartments involved in the diverse entry pathways exhibit characteristic morphological features, and clathrin-coated pits, caveolae, and macropinocytic membrane invaginations are distinguishable by electron microscopy. In addition, structures morphologically different from the three mentioned above have also been recently implicated in other uptake mechanisms (27). We thus studied the early events of HRV14 entry by electron microscopy. RD-ICAM cells were challenged with HRV14 for the times indicated in Fig. 7, and thin sections were prepared. As expected, HRV2, used as a control for clathrin-mediated endocytosis, accumulated in coated pits and vesicles at 5 min p.i. Conversely, many HRV14 virions appeared aligned in tubular structures like pearls on a string. This is very similar to the structures observed previously in HRV14-infected BHK cells transfected to express human ICAM-1 (15) and in adenovirus 3 (Ad3)-infected HeLa cells (1). Although apparently exploiting macropinocytosis, HRV14 thus enters via membrane invaginations very different from “conventional” macropinosomes.
HRV14 entry into HeLa cells.
The experiments described above were all carried out with RD cells stably transfected to express human ICAM-1. We chose this cell line because of its use in the elucidation of HRV8v uptake via heparan sulfate (A.G. Khan et al., unpublished data). However, in most of the previous work with rhinoviruses, HeLa-H1, a particularly susceptible substrain of HeLa cells (2), has been employed. Furthermore, this same cell line has also been used in experiments that suggest the clathrin-dependent endocytosis of HRV14 mentioned above (9). Therefore, we repeated some of the most significant experiments with this cell type. Virus uptake into HeLa cells was unaffected by the DN inhibitors amphi-SH3 and AP180-C. In electron microscopy, the virions did not appear as pearls on a string but, rather, were located to small, uncoated invaginations and short tubules. Similar virus-containing vesicles have been previously detected, but some coated structures were also seen (15). Therefore, although highly unlikely, clathrin-dependent endocytosis of HRV14 in HeLa cells cannot be excluded with absolute certainty. Unfortunately, the cleavage assay could not be employed with HeLa cells because proteolysis of eIF4GI, even at 4 h p.i., was only about 10% under experimental control conditions otherwise identical to those used for RD-ICAM cells. Therefore, partial inhibition could not be evaluated. Nevertheless, cleavage of eIF4GI in the presence of chlorpromazine was identical to that in the control in the absence of the drug. This is again evidence that clathrin does not play any role in HRV14 uptake into HeLa cells.
DISCUSSION
Our colocalization experiments strongly suggested that HRV14 entry is clathrin, caveolin, and flotillin independent in RD-ICAM cells. Markers of specific entry pathways not only lacked overlap with the virus but also exhibited significant differences from viral entry with respect to uptake kinetics. Antibody and nanobead binding of ICAM-1 exhibited similar properties, with slow entry into endothelial cells in a clathrin- and caveolin-independent manner (33). As viruses are intrinsically multivalent, they obviously behave similarly.
Involvement of clathrin in HRV14 uptake was ruled out using dominant negative inhibitors; both amphi-SH3 and AP180-C failed to modify HRV14 entry and replication. Entry via the caveolin-dependent pathway is also unlikely because of lack of inhibition by Filipin and MßCD. Furthermore, even though expression of caveolin in RD-ICAM cells was much lower than in HeLa-H1 cells, the efficiency of infection and virus replication was even better (data not shown). Although CtxB showed some colocalization with HRV14 at the plasma membrane, CtxB and HRV14 quickly separated and moved to different compartments in the cell.
The immunofluorescence results were further supported by using pharmacological inhibitors. In order to minimize pleiotropic effects, as are common for drugs blocking endocytosis pathways, we used cleavage of eIF4GI at 3 h p.i. as readout for successful entry and translation of the viral RNA in the cytoplasm. Viral replication is not required for this process because translation of the incoming RNA produces the proteinase 2A in sufficient amounts for cleavage of this cellular substrate (13). In this way, possible effects of the drugs on later steps, such as RNA synthesis and/or virus release (12, 18), can be excluded. Furthermore, there is no need to maintain the cells in the presence of the drug for extended times, thus avoiding interference by cytotoxic effects. Of all the drugs used, only amiloride completely blocked cleavage of eIF4GI, whereas cytochalasin D and dynasore inhibited by about 40%. Abolition of HRV14 entry by amiloride and reduction by cytochalasin suggest involvement of macropinocytosis in uptake via ICAM-1. This is supported by immunofluorescence microscopy which revealed HRV14 at or close to the membrane in the presence of either drug. Entry with characteristics of macropinocytosis was also corroborated by the colocalization of HRV14 with FITC-dextran, which is considered a relatively specific marker for this process (31), and by induction of dextran uptake at 10 min after viral challenge. Nevertheless, not all criteria for macropinocytosis were met (31); for example, the vesicles containing virus appeared definitely smaller than typical macropinosomes, and the inhibition by cytochalasin was only partial.
With respect to the dependence on dynamin, our results for HRV14 entry into RD-ICAM cells differ only quantitatively from previous observations on HRV14 entry into HeLa-H1 cells (9). Whereas inhibition by dynasore was only partial in RD-ICAM cells (our observations), in HeLa-H1 cells expression of DN dynK44A led to complete inhibition of viral replication, as monitored via indirect immunofluorescence microscopy. However, for the pathway exploited by antibodies against ICAM-1 and antibody-coated nanobeads into endothelial cells, a similar inhibition of about 50% by DN dynK44A was found (33). This might be explained in two ways: either dynamin dependence of this pathway is cell type specific, as already suggested by Bonazzi and coworkers (5) and demonstrated by Amstutz and colleagues for the two HeLa sublines HeLa-ATCC and HeLa-K (1), or it is correlated with the expression level of the receptor.
Electron microscopy of HRV14-infected HeLa cells revealed the virus in small vesicles and short tubules. However, when entering BHK cells transfected to overexpress human ICAM-1, HRV14 accumulated in long tubular structures with many virions threaded like pearls on a string (15). We obtained almost identical electron microscopy images from HRV14 internalized in RD-ICAM cells. These structures are reminiscent of Ad3 entering HeLa cells via macropinocytosis, as recently shown by using a number of complementary techniques (1). Similar tubular structures were also observed to contain GPI-anchored proteins (40); however, from the lack of inhibition by Filipin and MßCD, there is no indication for involvement of lipid rafts in HRV14 uptake into RD-ICAM cells. Even though the viruses accumulate in morphologically identical tubules, entry of Ad3 into HeLa-ATCC cells depends on C-terminal binding protein 1 of E1A (CtBP1), whereas HRV14 entry into RD-ICAM cells at least partially depends on dynamin 2. Amstutz and coworkers have not compared the two HeLa subtypes above with respect to the formation of these tubules. Therefore, it is not clear whether there is any correlation between dynamin dependence and the appearance of tubular structures. Interestingly, echovirus 1 (EV1) entering HeLa cells via integrin by macropinocytosis did not exhibit this pearl-on-a-string appearance (24).
Similar to the absence of reproduction in mouse cells (17), HRV14 failed to replicate in BHK cells transfected to express human ICAM-1, probably because specific host cell factors were absent (15). Conversely, RD-ICAM cells became productively infected, were lysed, and gave rise to viral progeny. This suggests that viral entry via these tubular structures is productive. Although infection by HRV14 and by other HRVs less stable at low pH is reduced but not prevented by bafilomycin because of the catalytic activity of ICAM-1, the process is facilitated at low pH (3, 37). It is therefore likely that HRV14 becomes further transferred from the tubular invaginations to acidic endosomes, where penetration into the cytosol occurs upon disruption of the vesicular membrane (43). As we did not observe any such tubules in the absence of HRV14, they might be induced by the virus's binding to ICAM-1 present in high concentrations in the RD-ICAM cells (this report) and in BHK-ICAM cells (15).
Entry of HRV2.
Although HRV2 was used merely as a control for clathrin-dependent endocytosis, we made some new findings with the pharmacological inhibitors. The endocytosis-blocking effect of chlorpromazine and dynasore was expected. However, we were surprised to find complete inhibition of eIF4GI cleavage by amiloride. The possibility that this drug affects entry can be excluded because immunofluorescence microscopy clearly showed that virus entered the cells in its presence. For coxsackievirus B3 and for HRV2, inhibition of RNA replication and of virus release, respectively, by amiloride has been suggested (12, 18). Our data, rather, point to inhibition of RNA release into the cytosol, RNA translation, or even inhibition of polyprotein processing. It will be interesting to find out which one of these is the target of amiloride. We also found only marginal inhibition by 5 mM MßCD in the cleavage assay, which contrasts with earlier findings indicating reduction of entry and accumulation of virus at the plasma membrane when the drug was present at 10 mM (44). In the absence of additional experimentation, we tentatively explain this by the difference in cell type used (i.e., HeLa-H1 cells in the previous study and RD-ICAM cells in the present work) and a strong concentration-dependent effect of drug action. In the case of HRV2, the presence of cytochalasin reduced viral entry and led to viral replication in small dots rather than in large areas (data not shown). Actin is also known to be involved in CME, and inhibition of its normal function might reduce HRV2 entry (8). The underlying mechanisms deserve further study.
Entry of the closely related poliovirus into HeLa cells occurs via a caveolin- and dynamin-independent pathway, whereas entry into brain microvascular endothelial cells depends on both components (7). We have now demonstrated a similar situation for HRV14. Entry of this virus into different cell types depends on dynamin to different degrees. Furthermore, by using HRV2 and HRV14, which bind different receptors and belong to species HRV-A and HRV-B, respectively, we show the existence of distinct entry pathways with different final destinations in identical cell types. Furthermore, the demonstration of the tubules involved in uptake of two different virus types (HRV14 and Ad3) raises the question of whether these structures are involved in a new type of viral cell entry mechanism. It will be interesting to study the mechanism of their induction.
Acknowledgments
This work was supported by the Austrian Science Foundation (FWF) (grant P18693-B09); A.G.K. obtained a fellowship from the Higher Education Commission of Pakistan.
We thank Irene Gösler for virus preparation, Günter Resch, Marlene Brandstetter, and Lisa Königsmaier for help with the electron microscope, and Tim Skern for critically reading the manuscript.
Footnotes
Published ahead of print on 3 February 2010.
REFERENCES
- 1.Amstutz, B., M. Gastaldelli, S. Kalin, N. Imelli, K. Boucke, E. Wandeler, J. Mercer, S. Hemmi, and U. F. Greber. 2008. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 27:956-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arruda, E., C. E. Crump, B. S. Rollins, A. Ohlin, and F. G. Hayden. 1996. Comparative susceptibilities of human embryonic fibroblasts and HeLa cells for isolation of human rhinoviruses. J. Clin. Microbiol. 34:1277-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, N., E. Prchla, M. Schwab, D. Blaas, and R. Fuchs. 1999. Human rhinovirus HRV14 uncoats from early endosomes in the presence of bafilomycin. FEBS Lett. 463:175-178. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 5.Bonazzi, M., S. Spano, G. Turacchio, C. Cericola, C. Valente, A. Colanzi, H. S. Kweon, V. W. Hsu, E. V. Polishchuck, R. S. Polishchuck, M. Sallese, T. Pulvirenti, D. Corda, and A. Luini. 2005. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7:570-580. [DOI] [PubMed] [Google Scholar]
- 6.Brabec, M., D. Blaas, and R. Fuchs. 2006. Wortmannin delays transfer of human rhinovirus serotype 2 to late endocytic compartments. Biochem. Biophys. Res. Commun. 348:741-749. [DOI] [PubMed] [Google Scholar]
- 7.Coyne, C. B., L. Shen, J. R. Turner, and J. M. Bergelson. 2007. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cureton, D. K., R. H. Massol, S. Saffarian, T. L. Kirchhausen, and S. P. Whelan. 2009. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5:e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcone, S., E. Cocucci, P. Podini, T. Kirchhausen, E. Clementi, and J. Meldolesi. 2006. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 119:4758-4769. [DOI] [PubMed] [Google Scholar]
- 11.Ford, M. G., B. M. Pearse, M. K. Higgins, Y. Vallis, D. J. Owen, A. Gibson, C. R. Hopkins, P. R. Evans, and H. T. McMahon. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291:1051-1055. [DOI] [PubMed] [Google Scholar]
- 12.Gazina, E. V., D. N. Harrison, M. Jefferies, H. Tan, D. Williams, D. A. Anderson, and S. Petrou. 2005. Ion transport blockers inhibit human rhinovirus 2 release. Antiviral Res. 67:98-106. [DOI] [PubMed] [Google Scholar]
- 13.Glaser, W., and T. Skern. 2000. Extremely efficient cleavage of eIF4G by picornaviral proteinases L and 2A in vitro. FEBS Lett. 480:151-155. [DOI] [PubMed] [Google Scholar]
- 14.Glebov, O. O., N. A. Bright, and B. J. Nichols. 2006. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 8:46-54. [DOI] [PubMed] [Google Scholar]
- 15.Grunert, H. P., K. U. Wolf, K. D. Langner, D. Sawitzky, K. O. Habermehl, and H. Zeichhardt. 1997. Internalization of human rhinovirus 14 into HeLa and ICAM-1-transfected BHK cells. Med. Microbiol. Immunol. 186:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Hansen, G. H., S. M. Dalskov, C. R. Rasmussen, L. Immerdal, L. L. Niels-Christiansen, and E. M. Danielsen. 2005. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 44:873-882. [DOI] [PubMed] [Google Scholar]
- 17.Harris, J. R., and V. R. Racaniello. 2003. Changes in rhinovirus protein 2C allow efficient replication in mouse cells. J. Virol. 77:4773-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison, D. N., E. V. Gazina, D. F. Purcell, D. A. Anderson, and S. Petrou. 2008. Amiloride derivatives inhibit coxsackievirus B3 RNA replication. J. Virol. 82:1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewat, E. A., and D. Blaas. 1996. Structure of a neutralizing antibody bound bivalently to human rhinovirus 2. EMBO J. 15:1515-1523. [PMC free article] [PubMed] [Google Scholar]
- 20.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blaas. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. U. S. A. 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irurzun, A., and L. Carrasco. 2001. Entry of poliovirus into cells is blocked by valinomycin and concanamycin A. Biochemistry 40:3589-3600. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov, A. I. 2008. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 440:15-33. [DOI] [PubMed] [Google Scholar]
- 23.Jones, A. T. 2007. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J. Cell. Mol. Med. 11:670-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karjalainen, M., E. Kakkonen, P. Upla, H. Paloranta, P. Kankaanpaa, P. Liberali, G. H. Renkema, T. Hyypia, J. Heino, and V. Marjomaki. 2008. A Raft-derived, Pak1-regulated entry participates in α2β1 integrin-dependent sorting to caveosomes. Mol. Biol. Cell 19:2857-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan, A. G., J. Pichler, A. Rosemann, and D. Blaas. 2007. Human rhinovirus type 54 infection via heparan sulfate is less efficient and strictly dependent on low endosomal pH. J. Virol. 81:4625-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonberg-Holm, K., and B. D. Korant. 1972. Early interaction of rhinoviruses with host cells. J. Virol. 9:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundmark, R., G. J. Doherty, M. T. Howes, K. Cortese, Y. Vallis, R. G. Parton, and H. T. McMahon. 2008. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr. Biol. 18:1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macia, E., M. Ehrlich, R. Massol, E. Boucrot, C. Brunner, and T. Kirchhausen. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839-850. [DOI] [PubMed] [Google Scholar]
- 29.Marlovits, T. C., C. Abrahamsberg, and D. Blaas. 1998. Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection. J. Virol. 72:10246-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayor, S., and R. E. Pagano. 2007. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer, J., and A. Helenius. 2009. Virus entry by macropinocytosis. Nat. Cell Biol. 11:510-520. [DOI] [PubMed] [Google Scholar]
- 32.Misinzo, G., P. Meerts, M. Bublot, J. Mast, H. M. Weingartl, and H. J. Nauwynck. 2005. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J. Gen. Virol. 86:2057-2068. [DOI] [PubMed] [Google Scholar]
- 33.Muro, S., R. Wiewrodt, A. Thomas, L. Koniaris, S. M. Albelda, V. R. Muzykantov, and M. Koval. 2003. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 116:1599-1609. [DOI] [PubMed] [Google Scholar]
- 34.Nabi, I. R., and P. U. Le. 2003. Caveolae/raft-dependent endocytosis. J. Cell Biol. 161:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neubauer, C., L. Frasel, E. Kuechler, and D. Blaas. 1987. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology 158:255-258. [DOI] [PubMed] [Google Scholar]
- 36.Newcombe, N. G., P. Andersson, E. S. Johansson, G. G. Au, A. M. Lindberg, R. D. Barry, and D. R. Shafren. 2003. Cellular receptor interactions of C-cluster human group A coxsackieviruses. J. Gen. Virol. 84:3041-3050. [DOI] [PubMed] [Google Scholar]
- 37.Nurani, G., B. Lindqvist, and J. M. Casasnovas. 2003. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J. Virol. 77:11985-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen, D. J., P. Wigge, Y. Vallis, J. D. A. Moore, P. R. Evans, and H. T. McMahon. 1998. Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. EMBO J. 17:5273-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabharanjak, S., P. Sharma, R. G. Parton, and S. Mayor. 2002. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2:411-423. [DOI] [PubMed] [Google Scholar]
- 41.Schneider, B., C. Schueller, O. Utermoehlen, and A. Haas. 2007. Lipid microdomain-dependent macropinocytosis determines compartmentation of Afipia felis. Traffic 8:226-240. [DOI] [PubMed] [Google Scholar]
- 42.Schnitzer, J. E., P. Oh, E. Pinney, and J. Allard. 1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127:1217-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schober, D., P. Kronenberger, E. Prchla, D. Blaas, and R. Fuchs. 1998. Major and minor-receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 72:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyers, L., H. Zwickl, and D. Blaas. 2003. Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis. J. Virol. 77:5360-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staunton, D. E., A. Gaur, P. Y. Chan, and T. A. Springer. 1992. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J. Immunol. 148:3271-3274. [PubMed] [Google Scholar]
- 46.Torgersen, M. L., G. Skretting, B. van Deurs, and K. Sandvig. 2001. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114:3737-3747. [DOI] [PubMed] [Google Scholar]
- 47.Wang, L. H., K. G. Rothberg, and R. G. W. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West, M. A., M. S. Bretscher, and C. Watts. 1989. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wigge, P., Y. Vallis, and H. T. McMahon. 1997. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr. Biol. 7:554-560. [DOI] [PubMed] [Google Scholar]