Abstract
The Wnt signaling pathway plays key roles in development and adult homeostasis. Wnt proteins are secreted, lipid-modified glycoproteins. They can form morphogen gradients that are regulated at the level of protein secretion, diffusion, and internalization. These gradients can only exist if the hydrophobic Wnt proteins are prevented from aggregating in the extracellular environment. Heparan sulfate proteoglycans (HSPGs) are necessary for proper activity of Wnt proteins and influence their distribution along the morphogenetic gradient. In this study, we show that HSPGs are able to maintain the solubility of Wnt proteins, thus stabilizing their signaling activity. Our results suggest that the role of HSPGs is not only to concentrate Wnt molecules at the cell surface but also to prevent them from aggregating in the extracellular environment.
Keywords: HSPG, Wnt, FBS, aggregation, solubility
INTRODUCTION
The Wnt signaling pathway is a key player in embryonic development and adult tissue homeostasis (for reviews on the Wnt signaling pathway, see (Clevers, 2006; Huang and He, 2008)). In Drosophila, Wingless (Wg) controls segment polarity during larval development and patterns the wing imaginal disc. In Xenopus, maternal Wnt11 initiates axis formation (Tao et al., 2005). In mouse, Wnt3−/− embryo lack primitive streak, mesoderm and node (Liu et al., 1999), Wnt1−/− mice harbor severe defects in brain development (McMahon and Bradley, 1990; Thomas and Capecchi, 1990), and Wnt5a−/− animals display defects in limb patterning (Yamaguchi et al., 1999). Wnts are secreted proteins that are modified by the addition of palmitic acid on the first conserved cysteine and palmitoleic acid on a highly conserved serine residue (Willert et al., 2003; Takada et al., 2006). While these modifications render Wnt proteins hydrophobic (and thus unlikely to diffuse freely through the extracellular aqueous environment), they act as morphogens (Strigini and Cohen, 2000) and signal to cell populations distant from their site of production. This apparent discrepancy implies that, in vivo, Wnt proteins might be chaperoned while traveling in the extracellular matrix (ECM) from the sending to the receiving cell.
Heparan Sulfate ProteoGlycans (HSPGs) are components of the ECM. They consist of a protein core with covalently attached heparan sulfate (HS) chains composed of copolymers of uronic acid and glucosamine sulfated at various positions. Members of the HSPG family were shown to act as a reservoir or modulator for several growth factors and signaling molecules (for reviews on HSPGs and HS, see (Bernfield et al., 1999; Whitelock and Iozzo, 2005)). Genetic screens in Drosophila have unraveled interactions between the Wg signaling pathway and HSPGs. Flies mutated in genes involved in HS synthesis such as sugarless, sulfateless, tout-velu, sister of tout-velu, and brother of tout-velu display wg phenotypes including loss of naked cuticle, decreased target gene expression and notches at the wing margin (Binari et al., 1997; Hacker et al., 1997; Haerry et al., 1997; Lin and Perrimon, 1999; Bornemann et al., 2004; Han et al., 2004; Takei et al., 2004). Furthermore, embryos harboring mutations in the proteoglycan protein cores dally and dally-like phenocopy Wingless loss-of-function (Lin and Perrimon, 1999; Tsuda et al., 1999; Baeg et al., 2001). In vertebrates, the Xenopus homolog of the heparan sulfate copolymerase tout-velu, XEXT1, is necessary for Wnt11-induced axis formation (Tao et al., 2005), and the sulfatase QSulf1 regulates Wnt-dependent embryo patterning in the quail (Dhoot et al., 2001).
The nature of the interactions between the Wnt signaling pathway and HSPGs is not clear, but several lines of evidence suggest that HSPGs influence Wg signaling through ligand stabilization. For instance, while Wg overexpression can rescue sugarless mutants (Hacker et al., 1997), Wg protein cannot be detected at the surface of sugarless embryos or sulfateless wing imaginal disc clones (Baeg et al., 2001; Pfeiffer et al., 2002). Similarly, overexpression of dally-like leads to sequestration of Wg at the cell surface (Baeg et al., 2001) but dally-like mutant wing imaginal disc clones accumulate Wg at their boundaries (Kirkpatrick et al., 2004). Finally, Wnt proteins have been shown to interact with HSPGs (Ai et al., 2003; Tao et al., 2005), in a dynamic interaction where the HS sulfatase QSulf1 can promote Wnt signaling in a cell-autonomous manner by lowering the affinity of the HS chains for Wnt proteins (Dhoot et al., 2001; Ai et al., 2003).
While there is no doubt that HSPGs stabilize Wnt proteins, the mechanism by which this stabilization is achieved remains elusive. In this study, we used purified proteins to demonstrate that HSPGs maintain the activity of Wnt3A by preventing its aggregation in the extracellular environment.
RESULTS
Serum stabilizes Wnt protein activity
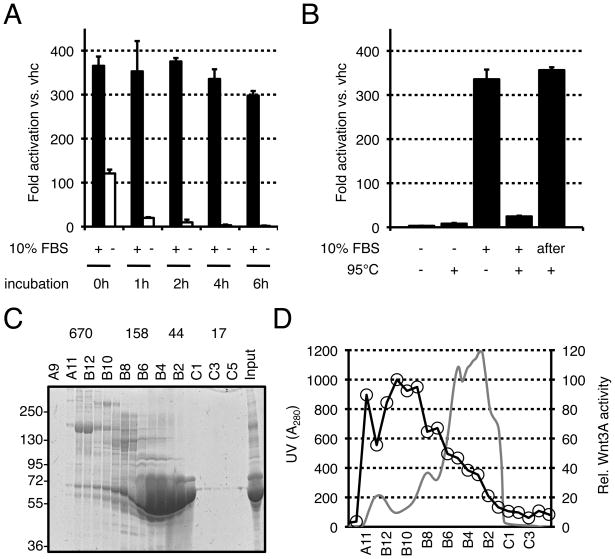
Because of their hydrophobicity, purified Wnt proteins are known to aggregate in solution unless stabilized by detergent or serum (Willert et al., 2003; Willert, 2008). To characterize the stabilizing property of serum, purified Wnt proteins were incubated in serum-free or serum-containing media for increasing length of time. Wnt activity was then analyzed on LS/L cells, which are mouse L cells that had been stably transfected with a Wnt-responsive luciferase reporter plasmid (Blitzer and Nusse, 2006). As shown in Fig. 1A, Wnt signaling activity was reduced by 3-fold immediately upon dilution in serum-free medium. Further incubation resulted in complete loss of activity. In contrast, the signaling activity of Wnt proteins was only marginally reduced after 6 hours of incubation in medium containing 10% fetal bovine serum (FBS). To eliminate the possibility that the stabilizing effect of FBS could be due to an inert chemical, serum-free and serum-containing media were heated at 95°C for 15 minutes, allowed to cool down at room temperature, and used in a Wnt reporter assay. Heat treatment completely ablated the stabilizing effect of FBS (Fig. 1B). This was not seen when the medium was heated prior to the addition of FBS (Fig. 1B, “after”), excluding a contribution of the medium itself. Altogether, these experiments indicate that the stabilizing activity of FBS is heat-labile and probably involves biomacromolecules. To characterize this activity, we separated the components of FBS according to their size by gel filtration (Fig. 1C) and analyzed the fractions for their ability to stabilize Wnt activity. As shown in Fig. 1D, the high molecular weight fractions were the most potent in this assay, indicating that the serous stabilizing activity was mediated by large molecules or complexes.
FIGURE 1. High-molecular weight fractions of fetal bovine serum stabilize Wnt activity.
(A) Wnt3A proteins loose their signaling activity in serum-free conditions. Wnt3A proteins were incubated in DMEM either in absence (−) or in presence (+) of 10% FBS for the indicated times. Wnt3A activity was then assayed on LS/L reporter cells. (B) The stabilizing activity of serum is heat-labile. DMEM with (+) or without (−) 10% FBS was heated at 95°C for 10 minutes and equilibrated to 37°C before addition of Wnt3A protein. The solutions were incubated for four hours and analyzed on LS/L cells. “after”: DMEM was heated at 95°C for 10 minutes and equilibrated at 37°C before addition of 10% FBS and Wnt3A proteins. (C) Size fractionation profile of FBS as analyzed by coomassie staining of an SDS-PAGE gel. The numbers above the gel represent the molecular weight of protein standards after gel filtration chromatography as well as the corresponding fractions (A9-C5). The molecular weight markers for the gel are on the left side. All molecular weights are in kD. (D) Comparison between the Absorbance at 280nm (gray line) and the Wnt3A-sustaining activity (black line with circles) of the FBS fractions. Wnt3A proteins were incubated in the various fractions for 1 hour and assayed on LS/L cells.
Heparan Sulfate Proteoglycans stabilize Wnt protein activity
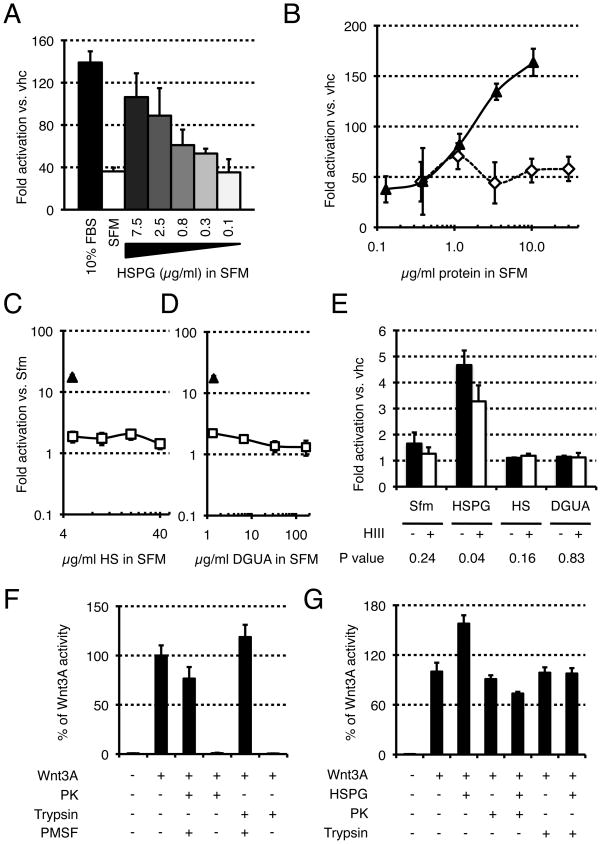
Since heparan sulfate proteoglycans are present in serum and have been shown to influence the activity and extracellular distribution of Wnt molecules, we specifically asked whether HSPGs could stabilize Wnt protein activity. When added to serum-free medium, HSPGs could stabilize Wnt activity in a dose-dependent manner (Fig. 2A). This stabilization could not be achieved by the control protein BSA (Fig. 2B) or by the individual HSPGs components heparan sulfate (Fig. 2C) and D-glucuronic acid (Fig. 2D), suggesting that it is mediated by intact HSPG molecules. In support of this, the stabilizing effect of HSPGs was significantly decreased upon incubation with Heparitinase III, an enzyme that selectively cleaves heparan sulfate chains (Fig. 2E). To examine whether proteolytic digestion of the core protein of HSPG would affect Wnt protein stabilization, we first showed that both trypsin and proteinase K were active under the conditions used (as seen by loss of Wnt3A activity), and that addition of the protease inhibitor phenylmethanesulphonyl fluoride (PMSF) prior to Wnt3A protected the protein from degradation and maintained its activity (Fig. 2F). When HSPGs were incubated with either protease prior to the addition of PMSF, they lost their ability to stabilize Wnt3A activity (Fig. 2G), suggesting that the core protein of HSPGs is also important for their stabilizing effect. Altogether, these experiments demonstrate that HSPGs can stabilize Wnt signaling activity in solution, and suggest that this action is accomplished by intact HSPG molecules.
FIGURE 2. HSPGs stabilize Wnt3A activity in serum-free conditions.
(A) Wnt3A proteins were incubated for one hour with complete medium (10% FBS), DMEM (SFM), or DMEM containing the indicated amount of HSPG and assayed on LS/L cells. (B) BSA does not stabilize Wnt3A. Wnt3A proteins were incubated with HSPGs (solid line, black triangles) or BSA (dashed line, white diamonds) for four hours and assayed on LS/L cells. Heparan sulfate (C) or D-glucuronic acid (D), two components of HSPGs, do not stabilize Wnt3A. Wnt3A proteins were incubated with HSPGs (triangle) or either heparan sulfate (C) or D-glucuronic acid (D) (both in solid line, white squares) for four hours and assayed on LS/L cells. (E) The stabilization activity of HSPG is sensitive to Heparitinase III. Wnt3A proteins were incubated for four hours in DMEM (alone or supplemented with 10 μg/mL HSPGs, 5 μg/mL HS, or 1.4 μg/mL DGUA) in absence (−) or presence (+) of Heparitinase III and assayed on LS/L cells. P values are calculated using the Student’s t-Test (two-tailed, homoscedastic). (F) Wnt3A activity is not impaired by Proteinase K (PK) or trypsin in presence of PMSF. Trypsin or PK were incubated for one hour, then added to Wnt3A solutions in absence or presence of PMSF, incubated for one hour, and assayed on LS/L cells. (G) HSPG core protein is important for the stability of Wnt3a. HSPGs were initially treated with either proteinase K (PK) or trypsin. After 2 hours, PMSF was added; the reaction was incubated for 10 minutes, incubated in DMEM containing Wnt3A or vehicle for 1 hour, and assayed on LS/L cells.
Heparan Sulfate Proteoglycans act by preventing Wnt protein aggregation
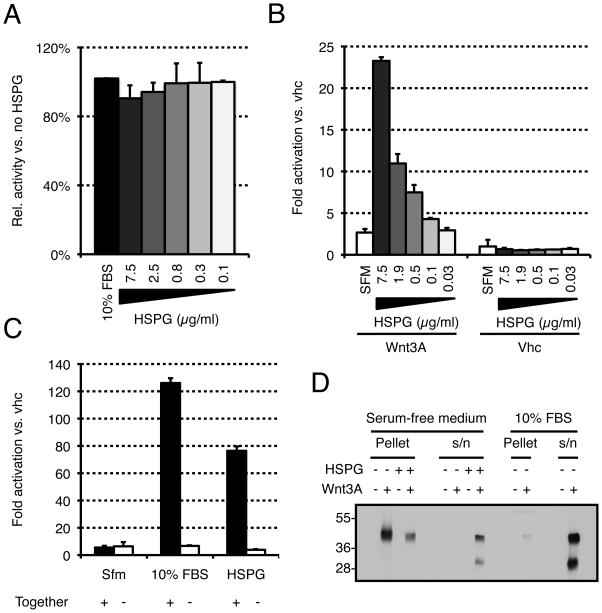
To better understand the mechanism by which HSPGs stabilize Wnt activity, we first checked their effect in presence of serum. As shown in Fig. 3A, addition of HSPGs had no effect on Wnt signaling activity when the proteins were incubated in serum-containing medium, suggesting that HSPGs do not signal independently to the Wnt pathway. To confirm this, HSPGs were added to Wnt reporter cells in presence or absence of Wnt proteins (Fig. 3B). From the results of this experiment, it is obvious that HSPGs cannot activate the pathway on their own. Therefore, they must act in concert with Wnt proteins. HSPGs could act either by stabilizing Wnt activity in the medium or by sensitizing cells to Wnt signals. To discriminate between these two possibilities, HSPGs and Wnt proteins were incubated either separately (−) or together (+) in serum-free medium (Fig. 3C). When incubated separately, all activity was lost, suggesting that HSPGs do not act by sensitizing the cells to lower amounts of Wnt protein but rather by stabilizing the protein, or its activity, in solution. When 10% FBS was used instead of HSPGs, the same effect was observed, indicating that HSPGs and serum are likely to stabilize the activity of Wnt3A via a similar mechanism. To determine whether HSPGs were acting at the level of protein stability or activity, we incubated Wnt proteins with or without HSPGs in serum-free medium and then spun the solutions for one hour at 16,000 × g. The pellet was directly resuspended in sample buffer while the supernatant was incubated overnight with blue sepharose beads that were then washed and resuspended in sample buffer (labeled s/n). In absence of HSPGs, Wnt proteins aggregated and were exclusively found in the pellet (Fig 3D). In contrast, the majority of Wnt proteins were found in the supernatant upon incubation with HSPGs. The same was true when the experiment was conducted in presence of 10% FBS (Fig. 3D). This clearly indicates that HSPGs, like serum, exert their stabilizing effect by preventing Wnt proteins from aggregating in aqueous environments.
FIGURE 3. HSPGs stabilize Wnt3A activity by preventing aggregation of Wnt3A proteins.
(A). HSPGs do not increase Wnt3A signaling in presence of serum. Wnt3A proteins were incubated in complete medium (10% FBS) supplemented with various amounts of HSPGs for one hour and assayed on LS/L cells. (B) HSPGs do not activate Wnt3A signaling in absence of Wnt3A protein. Wnt3A proteins or vehicle (vhc) were incubated with DMEM supplemented with various amounts of HSPGs for one hour and assayed on LS/L cells. (C) Serum or HSPGs do not act by sensitizing cells to Wnt3A. (−) Wnt3A proteins were incubated alone in DMEM for four hours and added to the same volume of DMEM (Sfm), complete medium (10% FBS), or 20 μg/ml HSPGs in DMEM (also incubated for four hours), and then assayed on LS/L cells. (+) Wnt3A proteins were incubated in DMEM, complete medium, or 10 μg/ml HSPGs in DMEM for four hours and assayed on LS/L cells. (D) HSPGs and serum act by preventing Wnt3A aggregation. Wnt3A proteins were incubated in various media for four hours at room temperature and pelleted at 16,000 × g. The pellet (protein aggregates) was resuspended in SDS-PAGE buffer while the supernatant (s/n, soluble proteins) was concentrated with blue sepharose beads and resuspended in SDS-PAGE buffer.
DISCUSSION
Heparan Sulfate Proteoglycans (HSPGs) play crucial roles during development. They are associated with the cell surface and extracellular matrix of a wide range of cells of vertebrate and invertebrate tissues, and are essential cofactors in cell-matrix processes, cell-cell recognition systems and receptor-growth factor interactions. Increasing amount of data demonstrate that HSPGs play crucial roles in modulating a wide variety of signaling pathways (for a review, see (Whitelock and Iozzo, 2005)). These large molecules influence the activity and extracellular distribution of Wnt molecules but the precise mechanism by which they act remains elusive. Their known presence in serum (Dziadek et al., 1985) and our observation that high molecular weight serous fractions were enriched for a Wnt stabilizing activity prompted us to specifically ask whether HSPGs could stabilize Wnt protein activity in solution. Our results clearly demonstrate that purified HSPGs can maintain the activity of purified Wnt proteins in solution. This specific activity is likely to depend on intact HSPG molecules since it was sensitive to proteolysis and HS chains cleavage. Furthermore, the observed effect of HSPGs on Wnt3A could not be mimicked by heparan sulfate, D-glucuronic acid, or the control protein bovine serum albumin. HSPGs could act either by sensitizing the cells to lower levels of Wnt proteins, a mechanism used by proteins such as R-spondin (Binnerts et al., 2007), or by stabilizing the activity of Wnt proteins at the protein level. We found the latter to be true as incubation with Wnt proteins was essential for HSPGs to exert their stabilizing function. We further demonstrated that HSPGs act by preventing the aggregation of Wnt ligands that normally occurs in an aqueous environment. This is most likely achieved through direct interaction between proteoglycans and Wnt proteins, as such an interaction has been observed between glypican1 and XWnt8 (Ai et al., 2003). It will be interesting for future studies to address the specificity of the interactions between different HSPG family members and Wnt proteins.
The role of HSPGs in the regulation of the Wnt pathway has been extensively studied, notably during the patterning of the Drosophila wing imaginal disc where HSPGs have been shown to influence Wg distribution (see introduction). In vertebrates, experiments performed on Xenopus animal cap explants have shown that heparinase treatment led to inhibition of Wnt-induced mesodermal markers as well as impaired mesoderm formation, a phenotype that could be rescued by exogenous HSPGs (Itoh and Sokol, 1994). Similarly, the membrane-anchored HSPG knypek (XGly4) has been shown to potentiate XWnt11-induced convergent extension movements during gastrulation (Topczewski et al., 2001), and both the glycosyl transferase XEXT1 and the sulfatase XtSulf1 have been shown to be required for XWnt11-induced axis formation (Tao et al., 2005; Freeman et al., 2008). In mouse, knockout of glypican-3 perturbs the balance between the Wnt/β-catenin and the Wnt/planar cell polarity pathways (Song et al., 2005).
While all these studies extend the observation that HSPGs modulate the Wnt signaling pathway to vertebrates, experiments performed in Xenopus have revealed that additional layers of complexity exist in the developing embryo. For example, the syndecan XSyn4 was shown to bind directly to Frizzled-7 (xFz7) and Disheveled (xDsh) to activate the Wnt/planar cell polarity pathway (Munoz et al., 2006), and the extracellular sulfatase XtSulf1, which has an important role in the post synthetic remodeling of the HS chains and therefore generates diversity among HSPGs, was recently shown to favor the Wnt/β-catenin signaling pathway by facilitating the interaction between XWnt11 and LRP6 (Freeman et al., 2008). Thus, HSPGs can also regulate Wnt signaling through direct interactions with receptors and components of the Wnt signal transduction machinery.
Wnt proteins have been shown to associate with lipoprotein particles (Panakova et al., 2005; Neumann et al., 2009), which themselves interact with HSPGs (Eugster et al., 2007). It is tempting to speculate that HSPGs mediate the interaction between Wnt proteins and lipoprotein particles to ensure proper spreading of Wnt ligands in the extracellular environment. Finally, Dally-like has been shown to be required for Wg to transcytose from the apical to the basolateral side of wing imaginal discs in order to permit long-range signaling (Gallet et al., 2008). All these mechanisms are likely to act in concert with the stabilizing effect of HSPGs on Wnt ligands described in this study to ensure proper control of Wnt signaling during development.
EXPERIMENTAL PROCEDURES
Cells, chemicals and antibodies
LS/L cells were obtained by stable transfection of mouse L cells with the plasmids pSuperTOPFlash (Veeman et al., 2003) and pEF1/Myc-His/lacZ (Invitrogen). Cells were cultured in DMEM supplemented with 10 % fetal bovine serum (Omega Scientific) and 1 % penicillin-streptomycin-glutamine (Gemini Bio-products). Heparan Sulfate Proteoglycans (HSPG), Heparan Sulfate (HS) and D-Glucuronic Acid (DGUA) (respectively H4777, H7640, and G5269) were obtained from Sigma-Aldrich. Heparinase III (50–120) was obtained from IBEX Technologies Inc. Wnt3A protein was detected by western blotting using a rabbit antibody developed in the laboratory (Willert et al., 2003) at 1:1000. Trypsin (T1426) was obtained from Sigma-Aldrich and Proteinase K from Roche.
Wnt purification and serum fractionation
The Wnt3A purification is a modification of Willert et al. (Willert et al., 2003) using Drosophila S2 cells that stably express the Wnt3A protein. The medium was conditioned for 7 days, filtered through a 0.2μM pore filter (Nalgene), adjusted to 1% Triton X-100 (Sigma), and applied to a blue (Cibacron blue) Sepharose HP column (Amersham Biosciences) previously equilibrated in binding buffer (150 mM KCl, 20 mM Tris-HCl, 1% CHAPS, pH 7.5). After washing with binding buffer, bound proteins were eluted in a single step with elution buffer (1.5 M KCl, 20 mM Tris-HCl, 1% CHAPS, pH 7.5). The trailing fractions of the eluate (after the main protein peak), enriched for Wnt3A proteins, were combined, concentrated using Centriprep-30 filter units (Millipore), and fractionated on a HiLoad 26/60 Superdex 200 column (Amersham Biosciences) in gel filtration buffer (PBS, 0.5 M NaCl, 1% CHAPS, pH 7.6). The peak fractions were diluted 3.65 times with PB supplemented with 1% CHAPS, and ran through a HiTrap Chelating column (Amersham Biosciences) previously loaded with copper and washed with binding buffer II (20mM NaH2PO4, 500 mM NaCl, 10 mM imidazole, 1% CHAPS, pH7.6). Bound proteins were eluted with increasing amount of elution buffer II (binding buffer II with 500 mM imidazole). Concentration of Wnt3A proteins was evaluated at 50 μg/ml by Coomassie staining using Bovine Serum Albumin as standard.
For serum fractionation, 8 ml of Fetal Bovine Serum (Omega Scientific) were fractionated on a HiLoad 26/60 Superdex 200 column (Amersham Biosciences) in DMEM. 10 ml fractions were collected, supplemented with 1% penicillin-streptomycin-glutamine, and filtered through 0.2 μM Acrodisc filters (Pall corp.).
Reporter assay
Wnt3A protein activity was assayed on LS/L reporter cells, mouse L cells that had been stably transfected with the Wnt-responsive luciferase reporter plasmid pSuperTOPFlash (Veeman et al., 2003), as well as a constitutive LacZ expression construct for normalization (pEF1/Myc-His/lacZ, Invitrogen). 50’000 LS/L cells/well were seeded in a 96-well plate in 100 μl of serum-free (Fig. 1) or complete (Figs 2 and 3) medium. Six hours later, the Wnt3A activity of the various samples was measured by adding 200 μl of solution to each well. Alternatively, the LS/L medium was replaced by the solution to analyze (Figs 1A and B). The next day, luciferase and β-galactosidase activities were measured with the dual-light combined reporter gene assay system (Applied Biosystems) with a Centro LB960 luminometer (Berthold) according to the manufacturer’s instructions. β-galactosidase activity was used to normalize the values, which are represented with their standard deviation.
Wnt incubation
Unless otherwise noted, all incubations were performed at 37°C. 250 ng/ml (Figs 1A and B), 500 ng/ml (Figs 1D to 3B, Fig. 3C, “+”) or 1 μg/ml (Fig. 3C, “−“) of Wnt3A proteins were incubated under various conditions (detailed in the figure legends). Following incubation, the solutions were analyzed for Wnt3A activity as described above. All media were supplemented with 1%PSQ).
Heparitinase III assay
Wnt3A (500 ng/ml final) was incubated for four hours at 37°C in DMEM/1%PSQ (alone or supplemented with 10 μg/ml HSPGs, 5 μg/ml HS, or 1.4 μg/ml DGUA). Where indicated, 1 μL Heparinase III (EC number 4.2.2.8, Catalog number #50–120, Ibex Pharmaceuticals Inc.) was added to the tubes at the beginning of incubation.
Protease assay
Proteinase K (35 ng/μl) or trypsin (35 ng/μl) were incubated in DMEM for one hour. The reactions were diluted five fold in DMEM in the presence of different combinations of Wnt3A (250ng/ml), PMSF (1mM) and vehicle. After one hour of incubation, the solutions were assayed for Wnt activity on LS/L reporter cells. When HSPGs were subject to proteolytic digestion, Proteinase K or trypsin (both at 35 ng/μl) were incubated with 100 μg/ml HSPGs in DMEM for two hours, then mixed at 1:1 with DMEM supplemented with 1 mM PMSF, incubated 10 minutes on ice, and diluted five fold in DMEM supplemented with 1mM PMSF and 250 ng/ml Wnt3A or vehicle. After one hour of incubation, the solutions were assayed for Wnt activity on LS/L reporter cells. As a positive control, 250 ng/ml Wnt3A were incubated with 10 μg/ml HSPGs and 1mM PMSF for one hour and further assayed for Wnt activity on LS/L reporter cells.
Wnt solubilization assay
Wnt3A proteins (500 ng/ml final) were incubated with or without 10 μg/ml HSPGs in serum-free medium at room temperature. 4 hours later, the solutions were pelleted at 16,000 × g for 1 hour at 4°C using a table-top centrifuge. The pellet was resuspended in SDS-PAGE buffer while the supernatant was mixed with blue sepharose beads and incubated o/n at 4°C. The beads were washed with binding buffer and resuspended in SDS-PAGE buffer. As a control, Wnt3A was incubated in complete medium and processed identically.
Acknowledgments
GRANT INFORMATION
Grant sponsor: Howard Hughes Medical Institute; Grant sponsor: California Institute of Regenerative Medicine; Grant number: RC1-00133-1; Grant sponsor (to CF): Swiss National Science Foundation; Grant number: PA00A3-115379. Grant sponsor (to SJH): The German Research foundation.
These studies were supported by the Howard Hughes Medical Institute, the California Institute of Regenerative Medicine (RC1-00133-1), the Swiss National Science Foundation (C. Fuerer) and the German Research foundation (SJ. Habib). We thank Amanda Mikels for establishing the LS/L cell line.
References
- Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, 3rd, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development. 2004;131:1927–1938. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- Dziadek M, Fujiwara S, Paulsson M, Timpl R. Immunological characterization of basement membrane types of heparan sulfate proteoglycan. EMBO J. 1985;4:905–912. doi: 10.1002/j.1460-2075.1985.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster C, Panakova D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Freeman SD, Moore WM, Guiral EC, Holme AD, Turnbull JE, Pownall ME. Extracellular regulation of developmental cell signaling by XtSulf1. Dev Biol. 2008;320:436–445. doi: 10.1016/j.ydbio.2008.05.554. [DOI] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Hacker U, Lin X, Perrimon N. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–3573. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Heslip TR, Marsh JL, O’Connor MB. Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development. 1997;124:3055–3064. doi: 10.1242/dev.124.16.3055. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Sokol SY. Heparan sulfate proteoglycans are required for mesoderm formation in Xenopus embryos. Development. 1994;120:2703–2711. doi: 10.1242/dev.120.9.2703. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Dimitroff BD, Rawson JM, Selleck SB. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell. 2004;7:513–523. doi: 10.1016/j.devcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- Neumann S, Coudreuse DY, van der Westhuyzen DR, Eckhardt ER, Korswagen HC, Schmitz G, Sprong H. Mammalian Wnt3a is released on lipoprotein particles. Traffic. 2009;10:334–343. doi: 10.1111/j.1600-0854.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Song HH, Shi W, Xiang YY, Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem. 2005;280:2116–2125. doi: 10.1074/jbc.M410090200. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 1999;400:276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Iozzo RV. Heparan sulfate: a complex polymer charged with biological activity. Chem Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Willert KH. Isolation and application of bioactive Wnt proteins. Methods Mol Biol. 2008;468:17–29. doi: 10.1007/978-1-59745-249-6_2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]