Abstract
Plasmacytoid dendritic cells (pDC) have both stimulatory and regulatory effects on T cells. pDCs are a major CNS-infiltrating DC population during experimental autoimmune encephalomyelitis (EAE), but unlike myeloid DCs (mDC) have a minor role in T cell activation and epitope spreading. We show that depletion of pDCs during either the acute or relapse phases of EAE resulted in exacerbation of disease severity. pDC depletion significantly enhanced CNS but not peripheral CD4+ T cell activation, as well as IL-17 and IFN-γ production. Moreover, CNS pDCs suppressed CNS mDC-driven production of IL-17, IFN-γ and IL-10 in an IDO-independent manner. The data demonstrate that pDCs play a critical regulatory role in negatively regulating pathogenic CNS CD4+ T cell responses highlighting a new role for pDCs in inflammatory autoimmune disease.
Keywords: Autoimmune disease, T lymphocytes, dendritic cells, T cell activation, antigen presentation, immunoregulation
Introduction
Experimental autoimmune encephalomyelitis (EAE), is a widely employed model for multiple sclerosis (MS) (1) that is initiated and driven by myelin-specific CD4+ T cells producing IFN-γ and TNF (Th1) and IL-17 (by Th17) in the CNS (2,3). Disease progression in relapsing EAE (R-EAE) is characterized by ‘epitope spreading’ where new T cell responses develop to myelin epitopes distinct from the priming epitope (4). Our recent evidence indicates peripherally-derived myeloid DCs (mDCs) prime naïve CD4+ T cells in the CNS inducing a Th17 dominant phenotype (5,6). In contrast, CNS plasmacytoid DCs (pDCs) are inefficient at inducing the proliferation of and cytokine production by naïve and activated myelin specific CD4+ T cells, although the pDCs can ingest myelin proteins (6).
Enhanced numbers of activated pDCs have been described in MS, and activated pDCs associated with Sjögren's syndrome lupus and psoriasis (7-11). It was therefore important to understand the role of pDCs in MS/EAE pathogenesis. Using a monoclonal antibody (anti-mPCDA-1) to deplete pDCs from the CNS following R-EAE induction, we show that pDC depletion caused the rapid exacerbation of EAE severity in both the primary acute and relapse phases. Mechanistically, pDC depletion did not affect the frequency of myelin specific CD4+ T cells in peripheral lymphoid organs, but markedly enhanced CNS CD4+ T cell activation, as well as IL-17 and IFN-γ production. Moreover, CNS pDCs suppressed CNS mDC-driven production of IL-17, IFN-γ and IL-10 in an IDO-independent manner.
Materials and Methods
Mice
Female SJL/J mice were purchased from Harlan Sprague Dawley (SJL/JCrHsd) or Taconic (SJL/JCrNtac). Mice were housed and cared for according to Northwestern University ACUC approved protocols.
Induction of EAE
As previously reported using 50μg PLP139-151 (6).
Peptides & Antibodies
PLP139-151 (HSLGKWLGHPDKF) synthesized to > 95% purity by Genemed Synthesis, Inc. Conjugated antibodies were purchased from BD Pharmingen or eBioscience.
Depletion of plasmacytoid DCs
Mice received intraperitoneal injections of 250 μg anti-mPDCA-1 (clone JF05-1C2, rat IgG2b; Miltenyi Biotec), or purified rat IgG2b (eBioscience) every other day for 4 treatments.
Isolation of cells from secondary lymphoid tissues and CNS
As previously reported (6).
Flow cytometric analysis and gating
For analysis of cytokines, CNS cells were cultured for 4 hours in R10 media (6) plus golgi-stop™ (BD) according to manufacturers recommendations. Cells were stained with 5-6 color antibody cocktails. Amine-reactive, fixable live/dead viability dye was used according to manufacturers instructions (Molecular Probes, Invitrogen) and dead cells excluded. Data was acquired on an LSR II cytometer (BD), and analyzed using FACS DIVA (BD) or Flow Jo (Treestar Inc.) software.
CD4+ T cell activation assay
Cell populations were flow sorted as described in (6) to > 98% purity. Sorted populations were >95% pure. 2×104 APCs were co-cultured with 105 CD4+ T cells from the CNS or spleen for 96 h with R10 media, 5 μg/ml antiCD3 and 200 μM 1 methyl-D-tryptophan (Aldrich Chemical Company, Milwaukee), prepared according to (12). Carrier solution alone was added as a control. Cells were assessed for viability by flow cytometry as described above, and cytokines in the supernatants assessed by cytokine bead array for levels of IL-10, IL-17, and IFN-γ, according to manufacturers instructions (Upstate).
ELISPOT assays
ELISPOTS were performed as previously described (13), with 106 cells plus 10 μM PLP139-151.
Immunohistochemistry
Immunohistochemistry was performed on 6 μm thick frozen cerebellar and lumbar spinal cord sections from PBS-perfused mice as previously described (13). Staining was analyzed using a Leica DM5000B fluorescent microscope and Advanced SPOT software.
Statistical Analysis
Differences between groups were determined using an unpaired Students t test.
Results and Discussion
Anti-mPDCA-1 mAb efficiently and specifically depletes CNS-infiltrating pDCs during EAE
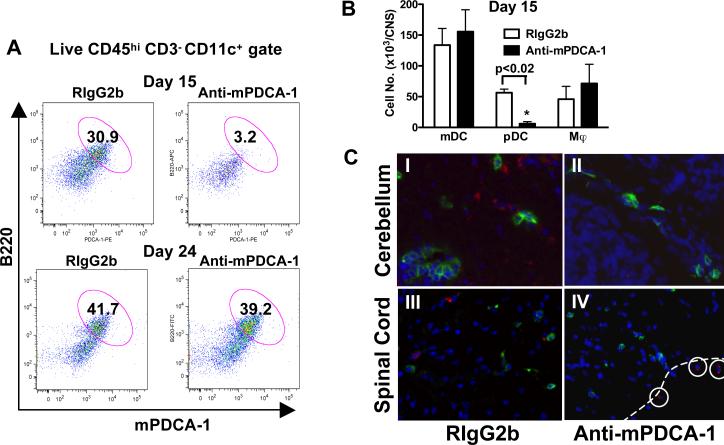
pDCs are a minor subset of DC in the secondary lymphoid tissues of most mouse strains (14), comprising 23% of DCs, and 1.15% of cells in the LNs of SJL/J mice (data not shown). Strikingly, CNS infiltrates during EAE contain 37.7% pDCs, 5.4% of the total CNS mononuclear cells (6). To investigate the role of pDCs during EAE, anti-mPDCA-1 mAb (15) was used to deplete pDCs during EAE onset. One day after pDC depletion, pDCs were depleted from LNs (not shown) (15) and CNS (92.4 ± 0.9% of CNS pDCs) (Fig. 1a,b). During the relapse phase of EAE, nine days following pDC depletion, the number of CNS pDCs returned to control levels in pDC depleted animals (Fig. 1a), and, importantly, anti-mPDCA-1 treatment did not affect the numbers of CNS mDCs or macrophages (Fig. 1b). Following pDC depletion a few mPDCA-1+ cells remained in the meningeal area of the CNS, however no mPDCA-1+ pDCs were detected in the parenchyma of the spinal cord or cerebellum (Fig. 1c). Inefficient clearing of pDCs from the blood vessel-rich areas of the CNS correlated with low level mPDCA-1 expression on blood CD11c+ B220+ pDCs (data not shown).
Figure 1. anti-mPDCA-1 efficiently and specifically depletes CNS pDCs during EAE.
(a) SJL mice were treated with anti-mPDCA-1 or isotype-matched control (Rat IgG2b) mAb on days 8-14 after EAE induction. One day (day 15) and 9 days after pDC depletion (day 24), the effect of treatment on gated live CNS DCs (CD45highCD3−CD11c+) was determined. (b) Day 15 analysis of CNS CD11b+ mDCs, CD11b−B220+ pDCs, and CD11b+CD11c− macrophages (Mϕ). Data are mean ± SEM of 4 animals per group representative of 5 experiments. p value determined by Mann-Whitney. (c) Day 15 immunohistochemical analysis of (a), cerebellum (I and II) and lumbar spinal cord (III and IV) stained for mPDCA-1 (red), CD4 (green) and DAPI (blue). Dotted line shows the meningeal edge of spinal cord tissue. mPDCA-1+ pDCs are circled. Magnifications ×200 (I and II) and ×100 (III and IV) are representative of 3 mice.
pDCs regulate the severity of EAE
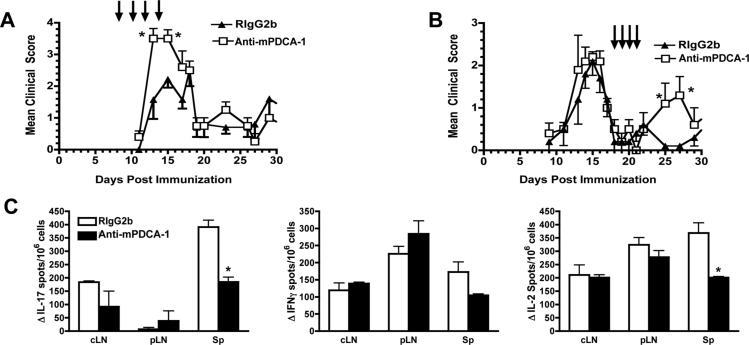
The depletion of pDCs at the onset of R-EAE resulted in a significant exacerbation of peak clinical disease (Fig. 2a). Clinical scores in pDC-depleted mice returned to control levels 2-3 days after the last anti-mPDCA-1 mAb treatment, consistent with a report showing that pDC numbers recover 3-5 days following anti-mPDCA-1 depletion (15). That pDC depletion caused an immediate increase in clinical severity, with a rapid return to control levels with pDC reconstitution suggests that pDCs have a direct, acute regulatory effect on CNS autoimmune disease. pDCs were then depleted during the primary relapse of EAE, leading to enhanced EAE and the abrogation of secondary remission (Fig. 2b).
Figure 2. CNS pDC depletion increases the severity of acute and relapsing R-EAE.
(a) Clinical EAE course following anti-mPDCA-1 or isotype control (RIgG2b) mAb treatment (arrows). Mean clinical scores ± SEM of 5-7 mice per treatment group, 1 of 5 experiments. (b) Treatment of EAE during remission (days 18-21). Mean clinical scores + SEM of 5 mice per treatment group, 1 of 2 experiments. *Mean clinical scores are significantly different from controls (p < 0.05; Mann-Whitney). c) Day 15 ELISPOTS for IFN-γ, IL-17, and IL-2 in response to PLP139-151 with cells from the cervical LN (cLN), inguinal LN (pLN) and spleen (Sp). Mean ± SEM of 3 animals, 1 of 3experiments. *Values significantly different, p < 0.01; un-paired Students t test.
The depletion of pDCs could have affected the priming of pathogenic T cells in the periphery (16), explaining the clinical outcome of pDC depletion (Fig 2a,b). However, the frequency of IL-17, IFN-γ and IL-2-producing CD4+ T cells specific for the immunizing peptide in the LNs, one day following the final mPCDA-1 mAb injection was unchanged compared with controls (Fig. 2c). In fact, PLP139-151-specific Th17 cells were significantly reduced in the spleens of pDC depleted mice, which may be reflective of reduced EAE severity (3), not enhanced severity as observed in the clinical experiments.
pDCs modulate the activation and frequency of Th1 and Th17 cells in the CNS
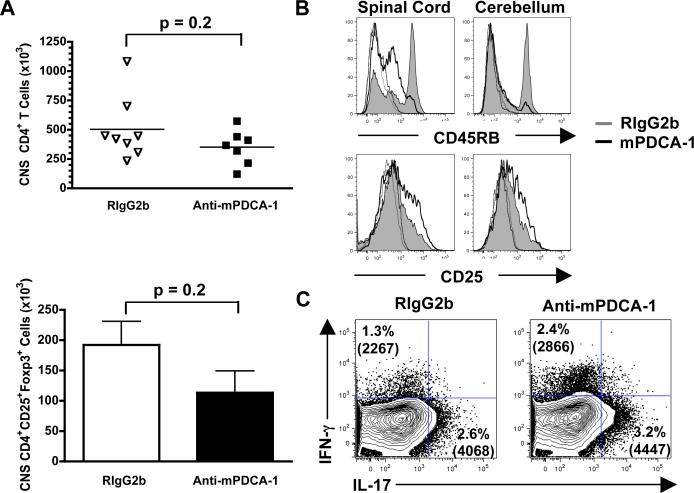
Clinical EAE generally correlates with the number and activation status of CNS-infiltrating effector CD4+ T cells, and inversely with Treg numbers (17). We found that pDC depletion did not significantly affect the numbers of CNS CD4+ T cells and Foxp3+ CD4+ Tregs (p = 0.2; Fig. 3a). We previously showed that CNS pDCs isolated during EAE poorly activate both naïve and activated myelin-specific T cells in comparison to mDCs (6). Because there was little change in the T cell numbers it is unlikely that the primary function of pDC during EAE is to prime and expand CD4+ T cells in the CNS.
Figure 3. pDCs regulate CNS CD4+ T cell activation and IFN-γ and IL-17 production.
(a) At day 15 after EAE induction, and treatment with RIgG2b or mPDCA-1 days 8-14, the numbers of CNS CD45+CD3+CD4+ T cells and CD4+FoxP3+CD25+ Tregs were enumerated. Data points for CD4+ T cells represents mice from 2 experiments, and the bars indicate the number of FoxP3+ T cells ± SEM of 4 animals in 2 experiments. p values determined by Mann-Whitney. (b) CD45RB and CD25 expression by CNS CD4+ T cells at day 15. Isotype control staining is shown in dotted lines of corresponding colors. Representative of 4 animals from 2 experiments. (c) Intracellular cytokine analysis of CNS CD4+ T cells isolated from 2 mice at day 15. The percentage of IFN-γ and IL-17 staining cells within the viable CD45hiCD3+CD4+ gate and the mean fluorescence intensities are indicated in parentheses. Results representative of 3 experiments.
Strikingly however, in contrast to peripheral responses, CNS CD4+ T cells were highly activated and produced more IL-17 and IFN-γ in the absence of pDCs. Following pDC depletion, CNS CD4+ T cells were significantly more activated than controls as assessed by down-regulation of CD45RB and upregulation of CD25 (Fig. 3b). Endogenous production of inflammatory cytokines by CNS CD4+ T cells was determined by incubating CNS isolates, which contain pathogenic T cells and DCs presenting endogenous myelin peptides (6), with golgi-stop for 4 h, then analyzing accumulated cells expressing IFN-γ and IL-17. The frequency of CNS Th17 cells was increased by an average of 1.6 ± 0.38 fold, and Th1 cells by 1.6 ± 0.27 fold, and more cytokine per cell (enhanced mean fluorescence intensity) was produced in pDC depleted mice (Fig. 3c). Thus, CNS pDCs promote the accumulation of CD4+ T cells and Tregs in the target organ, but strongly modulate the activation status of CD4+ T cells and, importantly, the frequency of CNS Th1 (IFN-γ) and Th17 (IL-17) cells.
CNS pDCs actively suppress IL-17, IFN-γ, and IL-10 production by CNS CD4+ T cells, in an IDO-independent manner
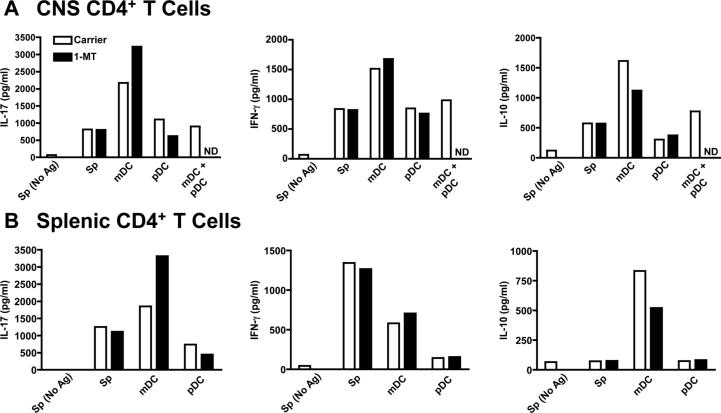
We next sought to determine the mechanism by which pDCs regulate CNS CD4+ T cell activation and cytokine production. pDCs have been implicated in inducing T cell anergy through IFN-α and IL-10 production (18), or TGF-β (19). Using quantitative PCR, we have previously shown that CNS pDCs expressed low levels of TGF-β transcripts (6), and similar levels of IFN-α4 mRNA (not shown) compared with other CNS APCs. In addition, IL-10 levels were lower in CNS pDC - CNS T cell co-cultures (Fig. 4a) and CNS pDCs stimulated with CD40 ligand (not shown) compared with CNS mDCs. Based on the low production by CNS pDCs, it is unlikely that TGF-β, IL-10, or IFN-α4 are a dominant pathway for pDC suppression of CD4+ T cells in the CNS.
Figure 4. pDCs actively suppress mDC supported IFN-γ, IL-17 and IL-10 production by CNS CD4+ T cells.
On day 14, 105 CD4+ T cells from the CNS (a) or spleen (b) were cultured with 2x104 irradiated splenic APCs (Sp), or sorted CNS pDCs, mDCs or a 1:1 mixture of pDCs and mDCs in the absence (white bars) or presence (black bars) of 1-MT. The amount of secreted IFN-γ, IL-17 and IL-10 in the culture supernatants was determined 4 days later. The results are representative of 3 experiments where 15-20 perfused mice were pooled. ND, not done.
pDCs are known to produce the T cell inhibitor indolamine 2,3-dioxygenase (IDO) in response to interferons (12,20). To determine if CNS pDCs produce IDO that suppresses CD4+ T cells during EAE, CNS pDCs and mDCs isolated during acute R-EAE were co-cultured with CD4+ T cells from the CNS and spleen of the same animals +/- 1-MT. pDCs were poor stimulators of CNS CD4+ T cell survival and expansion ex vivo and addition of 1-MT had little effect on CNS T cell viability in the presence of pDCs or mDCs (not shown). In agreement with our previously published work (6), CNS mDCs supported the highest levels of IL-17, IFN-γ and IL-10 production by both splenic and CNS-derived T cells (Fig. 4.). The IDO inhibitor 1-MT enhanced mDC-induced CD4+ T cell IL-17 and IFN-γ secretion, and decreased IL-10 production. However, 1-MT had no affect on cytokine production in CD4+ T cells cultured with CNS pDCs. Most profoundly, when CNS pDCs were co-cultured with CNS mDC and CNS T cells, production of IL-17, IFN-γ and IL-10 was significantly suppressed (Fig. 4a). These results indicate that CNS pDCs regulate CD4+ T cell cytokines in an active manner that dominates that of mDCs-driving Th17 cells in the CNS (6), and regulation is via an IDO-independent pathway. IFN-β modulates IFNγ and IL-17 production by human PBMCs (21), thus pDC production of IFN-β (22) may play a primary role in pDC modulation of Th1/Th17 activation during R-EAE concordant with the observation that IFN-β treatment is therapeutic in both EAE and MS (23,24). We are currently investigating the role of IFN-β production by pDCs during EAE.
In summary, we demonstrate an acute, dominant regulatory role for pDCs in CNS autoimmune disease. pDC depletion results in exacerbated EAE, but once pDCs return to normal levels, relapse severity returns to control levels. Normal relapses suggest the priming of naïve T cells in the CNS is unaffected. pDCs suppress mDC-dependent induction/expansion of CNS Th17 and Th1 cells (Fig. 4a). pDC suppression of T cell cytokine production is IDO-independent, but not due to killing of T cells, because in CNS mDC/pDC co-cultures, CNS CD4+ T cells have enhanced viability (not shown). These data support a dominant regulatory role for pDCs during EAE in that pDCs recruited to the CNS limit pathology by regulating T cell activation and cytokine production. Treatments that support and expand regulatory pDCs may therefore be attractive therapies for T cell-mediated autoimmune diseases.
Acknowledgments
We thank Matt Degutes (NWU) for technical assistance, James Marvin (NWU) for cell sorting, Dr. Xunrong Luo (NWU) and Drs. Qizhi Tang and Jeff Bluestone (UCSF) for helpful comments and discussion.
Abbreviations used in this paper
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- mDC
myeloid dendritic cells
- pDC
plasmacytoid dendritic cells
- PLP
proteolipid protein
Footnotes
This work was supported in part by U.S. Public Health Service National Institutes of Health Research Grant NS-030871, National Multiple Sclerosis Society (NMSS) Research Grant RG 3793-A-7, NMSS Postdoctoral Fellowship Grant (FG 1563 A-1 to SLB), and a grant from the Myelin Repair Foundation.
References
- 1.Hohlfeld R, Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. U. S. A. 2004;101(Suppl 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 4.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 5.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 7.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 8.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 10.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 12.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J. Immunol. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- 14.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 15.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami N, Lassmann S, Li Z, Odoardi F, Ritter T, Ziemssen T, Klinkert WE, Ellwart JW, Bradl M, Krivacic K, Lassmann H, Ransohoff RM, Volk HD, Wekerle H, Linington C, Flugel A. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez L, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 19.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 20.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 21.Meyers JA, Mangini AJ, Nagai T, Roff CF, Sehy D, van Seventer GA, van Seventer JM. Blockade of TLR9 agonist-induced type I interferons promotes inflammatory cytokine IFN-gamma and IL-17 secretion by activated human PBMC. Cytokine. 2006;35:235–246. doi: 10.1016/j.cyto.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin. Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Rudick RA, Ransohoff RM, Lee JC, Peppler R, Yu M, Mathisen PM, Tuohy VK. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology. 1998;50:1294–1300. doi: 10.1212/wnl.50.5.1294. [DOI] [PubMed] [Google Scholar]
- 24.Tuohy VK, Yu M, Yin L, Mathisen PM, Johnson JM, Kawczak JA. Modulation of the IL-10/IL-12 cytokine circuit by interferon-beta inhibits the development of epitope spreading and disease progression in murine autoimmune encephalomyelitis. J. Neuroimmunol. 2000;111:55–63. doi: 10.1016/s0165-5728(00)00384-2. [DOI] [PubMed] [Google Scholar]