Abstract
The limited success of immunogold labeling for pre-embedding immunocytochemistry of neuronal antigens is largely attributed to poor penetration of large (5–20 nm) colloidal gold particles. We examined the applicability of using silver intensification of 1 nm colloidal gold particles non-covalently bound to goat anti-rabbit immunoglobulin (1) for single labeling of a rabbit antiserum against the catecholamine synthesizing enzyme, tyrosine hydroxylase (TH), and (2) for immunogold localization of rabbit anti-TH simultaneously with immunoperoxidase labeling of a mouse monoclonal antibody against the opiate peptide, leucine-enkephalin (LE). Vibratome sections were collected from acrolein fixed brains of adult rats. These sections were immunolabeled without use of freeze-thawing or other methods that enhance penetration, but damage ultrastructure. By light microscopy, incubations in the silver intensifier (Intense M, Janssen) for less than 10 min at room temperature resulted in a brownish-red reaction product for TH. This product was virtually indistinguishable from that seen using diaminobenzidine reaction for detection of peroxidase immunoreactivity. Longer incubations produced intense black silver deposits that were more clearly distinguishable from the brown immunoperoxidase labeling. However, by light microscopy, the gold particles seen by electron microscopy were most readily distinguished from peroxidase reaction product with shorter silver intensification periods. The smaller size of gold particles with shorter periods of silver intensification also facilitated evaluation of labeling with respect to subcellular organdies. Detection of the silver product did not appear to be appreciably changed by duration of post-fixation in osmium tetroxide. In dual-labeled sections, perikarya and terminals exhibiting immunogold-silver labeling for TH were distinct from those containing immunoperoxidase labeling for LE. These results (1) define the conditions needed for optimal immunogold-silver labeling of antigens while maintaining the ultrastructural morphology in brain, and (2) establish the necessity for controlled silver intensification for light or electron microscopic differentiation of immunogold-silver and peroxidase reaction products and for optimal subcellular resolution.
Keywords: Tyrosine hydroxylase, Enkephalin, Double labeling, Silver enhancement, Catecholamine
Introduction
Immunolabeling with colloidal gold was introduced by Faulk and Taylor (1971) and was considerably advanced by the demonstration of the formation of controlled sizes of gold particles by Frens (1973). The gold particles were non-covalently coupled to IgG, streptavidin or Fc-binding proteins for immunolabeling (In t Veld, 1989). The applicability of immunogold labeling with and without silver intensification has been particularly well documented in peripheral tissues (Danscher, 1981; Holgate et al., 1983; Krenacs et al., 1989; Zelechowska and Mandeville, 1989). The most notable successes of this method for studies of the central nervous system have been in the electron microscopic localization of GABA, L-glutamate and other small molecules whose conformations are well preserved even after embedding in plastic (Somogyi and Hodson, 1985; Ottersen, 1987). However, many other larger neuronal antigens such as the catecholamine synthesizing enzyme, tyrosine hydroxylase (TH) (Nagatsu et al., 1964) show little immunoreactivity in central axons following plastic embedding (Pickel et al., 1981; Leranth and Pickel, 1989). The requirement for optimal localization of TH and other enzymes before, rather than after embedding may indicate that the tertiary structure of the protein is altered by the fixation, dehydration or heating so as to become unrecognizable by the antibodies (Leranth and Pickel, 1989). Limited labeling of TH has been achieved in large, presumably dopaminergic axons within the plastic embedded sections of the intermediate lobe of the pituitary (Vuillez et al., 1987). More successful immunogold localization of antiserum against TH was shown in large dendrites in the hypothalamus immunolabeled with 5 nm gold particles prior to plastic embedding (Van den Pol, 1986). In Van den Pol’s study, penetration of the gold was facilitated by rapid freezing of blocks of tissue fixed with low (0.1%) concentrations of glutaraldehyde. Reports have indicated that smaller colloidal gold particles were found to give more optimal labeling probably due to their greater penetration (Van den Pol, 1986; Pickel et al., 1986b; Ellis et al., 1988). The difficulty of visualizing these small particles was partially overcome by use of a gold-catalyzed silver reduction procedure (Danscher, 1981; Van den Pol, 1986; In t Veld, 1989). The reaction was, however, sometimes spurious showing non-specific silver precipitates within the tissue. Moreover, the reaction was difficult to control based on visual cues due to its light sensitivity (Massari et al., 1988). The availability of smaller 1 nm colloidal gold probes and light insensitive silver intensifiers (Janssen) now suggest that this labeling procedure may be a highly reproducible and sensitive immunocytochemical marker (In t Veld, 1989; Dankner and Spector, 1989). In perfusion-fixed brain tissue, we sought to determine the conditions under which the 1 nm colloidal gold probes and light insensitive silver intensifier might be used to achieve: (1) cellularly and subcellularly selective localization of antisera; (2) optimal preservation of ultrastructure; and (3) differentiation from immunoperoxidase labeling of a second antiserum within the same section. The antibodies were raised in different species against TH and LE. These antisera were chosen based largely on their known widespread distributions in brain and their earlier localization by immunoautoradiographic and immunoperoxidase methods (Pickel et al., 1986a, 1989).
Materials and methods
Primary and secondary antisera
Polyclonal antiserum against tyrosine hydroxylase (TH) was produced in rabbits by a previously described method (Joh and Ross, 1983). The enzyme, purified from bovine adrenal medulla, was judged specific for TH by the demonstration that (1) with Western blotting and immunostaining, the antibody bound to a single band of protein corresponding to the molecular weight of TH (60 000 Da); and (2) the antibody specifically inhibited the catalytic activity of TH in crude homogenates of rat brain (Joh and Ross, 1983). The 1 nm colloidal gold adsorbed goat anti-rabbit IgG (AuroProbe One) and silver enhancement kit (IntenSE M) was obtained from Janssen Pharmaceutical (Piscataway, NJ). Monoclonal antibody against Leu-enkephalin (LE) was obtained from Sera Labs. (Westbury, NY). This antibody was produced and characterized using standard methods for small peptides (Cuello, 1982). Adsorption and immunodot-blotting with specific opioid peptides has shown that the mouse anti-LE antibody recognizes primarily Leu-enkephalin with some cross-reactivity against Met-enkephalin and dynorphin fragments (Milner et al., 1989). Goat anti-mouse IgG and mouse peroxidase-antiperoxidase (PAP) were obtained from Boehringer Mannheim (Indianapolis, IN). Biotinylated horse anti-mouse IgG and peroxidase avidin–biotin complex (ABC, Elite kit) was obtained from Vector Laboratories (Burlingame, CA).
Tissue preparation
Adult male Sprague–Dawley rats (150–200 g) were anesthetized with pentobarbital (50 mg/kg intraperitoneally) and perfused through the ascending aorta with (1) 10 ml heparinized saline, (2) 50 ml of 3.75% acrolein (glass distilled for use in electron microscopy and purchased from Electron Microscopy Sciences, Fort Washington, Pennsylvania) and 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (King et al., 1983), and (3) 200 ml 2% paraformaldehyde in the same buffer. The duration of the perfusion–fixation was 6 min. The brains were sliced into 2–4-mm slabs. These were post-fixed in the 2% paraformaldehyde for 1 h then stored in 0.1 M phosphate buffer for a short period prior to sectioning. The sections were cut in the coronal plane at 30–40 μm thickness using a vibratome.
The sections were treated with 1% sodium borohydride in 0.1 M phosphate buffer for 30 min and washed thoroughly in consecutive rinses with buffer. The borohydride treatment has been found to be particularly beneficial for immunolabeling of brain tissue after acrolein fixation (King et al., 1983; Leranth and Pickel, 1989).
Single immunogold-siloer labeling
The sections were rinsed in 0.1 M Tris buffered saline (pH 7.6) and incubated for 18–24 h in a pre-determined optimal dilution of 1 : 2000 of the TH-antiserum in Tris buffer. Following three 5-min washes in 0.1 M phosphate-buffered saline (PBS, pH 7.4), sections were placed in 0.2% gelatin-PBS in 0.8% BSA for 5 min and then incubated in goat anti-rabbit IgG bound to 1 nm colloidal gold (Janssen, AuroProbe one) diluted 1 : 50 in 0.1% gelatin-PBS, BSA for 2–3 h. Finally, the sections were processed through three 5-min washes in PBS; ten min fixation in 2% glutaraldehyde in PBS; and three 5-min washes in PBS. Prior to silver intensification, the buffer salts were removed by 3 brief, 1-min rinses in distilled water. These were then incubated with IntenSE M (Janssen) for 2–14 min while observing the appearance of the immunolabeling through a dissecting microscope. The reaction was terminated by transferring the sections through two brief rinses in distilled water and 2 rinses in 0.1 M phosphate buffer. All incubations and washes were at room temperature, except for one series of experiments where incubation with the silver intensifier was carried out at 4°C to test the effects of temperature on the duration of the intensification reaction.
Dual immunogold and immunoperoxidase labeling
Pre-incubations, washes and dilutions were prepared as described above in phosphate buffered saline (PBS) then in 0.2% gelatin-PBS in 0.8% BSA. For dual labeling, sections were incubated overnight with two primary antibodies, the rabbit anti-TH (1 : 2000) and mouse monoclonal anti-LE (1 : 50). These then were processed for immunolabeling with secondary antibodies using one of 2 protocols outlined below. The first combines avidin-biotin peroxidase complex (ABC) labeling (Hsu et al., 1981) with immunogold silver. In Protocol II mouse anti-LE antibody was identified by the double bridged (Ordronneau et al., 1981) peroxidase-antiperoxidase (PAP) labeling method of Sternberger (1979). For both the ABC and PAP labeling, the peroxidase product was demonstrated by incubation of sections for 6 min in 3,3′-diaminobenzidine (0.04 g/100 ml) and 0.003% hydrogen peroxide. All incubations and washes were carried out with continuous agitation. Each wash was for 5 min in PBS or Tris-saline except for the necessary rinses in distilled water before and after silver intensification (Table I).
TABLE I.
INCUBATION SEQUENCE
| Protocol I | Protocol II |
|---|---|
| (1) Horse anti-mouse biotinylated IgG (30 min at 1 : 400 dilution) ⇓ 2 × wash | (1) Goat anti-mouse IgG (30 min at 1 : 50 dilution) ⇓ 2 × wash |
| (2) Avidin-biotin peroxidase complex (30 min at 1 : 100) ⇓ 2 × wash | (2) Mouse peroxidase- anti-peroxidase (30 min at 1 : 100) ⇓ 2 × wash |
| (3) Diaminobenzidine and H2O2 (6 min) ⇓ 2 × wash | Repeat 1&2 above ⇓ 2 × wash Steps 3–6 of Protocol I |
| (4) Gold-goat anti-rabbit IgG (2 h at 1 : 50) ⇓ 2 × wash | |
| (5) Glutaraldehyde (2% for 10 min) ⇓ 2 × wash | |
| (6) IntenSE-M silver (SI) |
Microscopic preparation
The immunolabeled sections were rinsed in phosphate buffer and mounted on slides for light microscopic examination. The effects of osmification on detectability of the silver reaction product was evaluated by both light and electron microscopy. The fixation in 2% osmium tetroxide was varied from 20–90 min at room temperature. These were washed in phosphate buffer and either mounted on slides for light microscopy or dehydrated and flat embedded in Epon 812 between 2 sheets of Aclar plastic (Musurovsky and Bunge, 1968). Ultrathin sections were collected from the outer surface of the plastic embedded tissue using an LKB-ultramicrotome. These sections were examined using a Philips 301 electron microscope.
Controls
Controls for non-specific silver reactivity included the omission of incubations in (1) either or both primary antisera or (2) colloidal gold-anti IgG.
Controls for cross-reactivity of the two antibodies used in the dual labeling experiment included: (1) omission of either of the primary antisera, (2) omission of the colloidal gold incubation step, (3) omission of the peroxidase immuno-labeling technique, (4) assuring that the distsributions were consistent with those expected for each antiserum when singly labeled.
Results
Light microscopy
Silver intensification of the 1 nm colloidal gold particles showed highly selective localization of TH in the major catecholaminergic cell groups in the brainstem as well as fine varicose axons extending into many known dopaminergic (Anden et al., 1965) and noradrenergic terminal fields in the cortex, caudate nucleus, and hippocampal formation. In sections labeled only with TH by the immunogold method, silver intensification for 8 and 10 min resulted in a brown product remarkably similar to the diaminobenzidine product for horseradish peroxidase. This is illustrated for the A2 catecholamine cell group in the medial nuclei of the solitary tracts (mNTS) (Fig. 1A–D). At longer incubation times in the silver, these immunogold labeled profiles became black and color distinct from peroxidase reaction. Thus differential labeling by light microscopy is highly dependent on the duration of the silver intensification reaction. The reaction was also shown to proceed much more slowly at colder temperatures. At 4°C, light microscopic detection of the immunogold silver product for TH in catecholaminergic neurons was first seen after 30 min incubation in the silver intensifier. The light microscopic detection of the silver reaction product was not altered by short versus long incubations in osmium tetroxide. The background of the tissue was sufficiently light (Fig. 1D) to allow clear distinction of immunoreactive cells from the neuropil even after 90 min osmification.
Fig. 1.
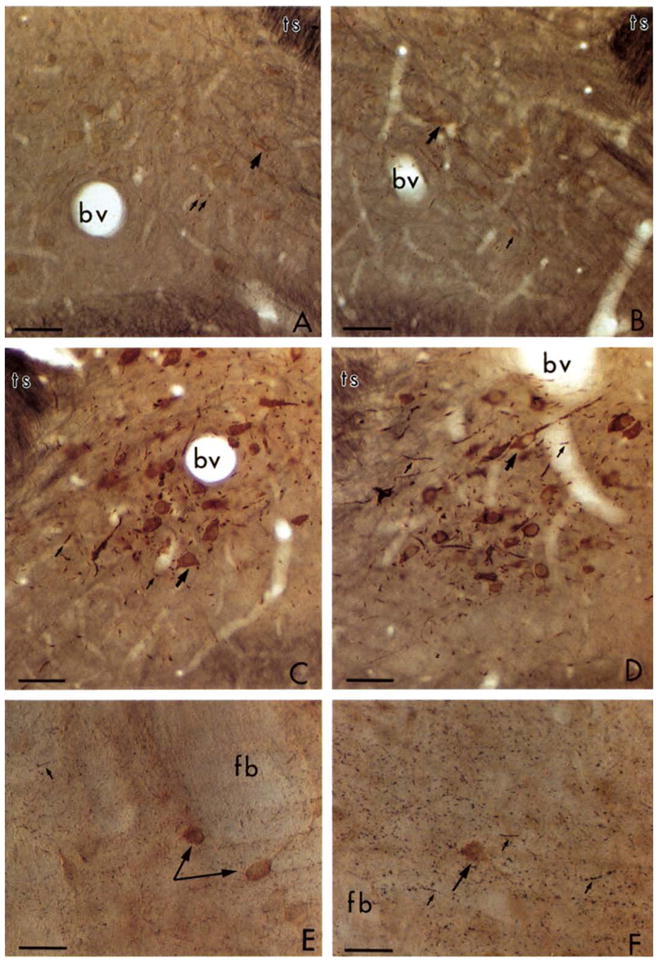
Light micrographs showing immunogold labeling for tyrosine hydroxylase (TH). A–D: photomicrographs of 4 coronal sections through the medial nucleus of the solitary tract that were sequentially incubated in rabbit TH-antiserum and goat anti-rabbit IgG bound to 1 nm gold particles. A and B were silver intensified for 8 min while C and D were for 10 min. A and C were post-fixed for 20 min and B and D for 90 min in 2% osmium tetroxide. There is no notable diminution of the immunogold labeling with longer postfixation periods. E–F: photomicrographs from sections that were dually labeled for TH using immunogold and for LE by the ABC method through the caudate nucleus. Incubations in the silver intensifier were 12 min in E and 14 min in F. The brown peroxidase reaction for LE in the perikarya (large arrows) appears distinct from the black immunosilver labeled axons (small arrows) in F. bv, blood vessel; ts, tractus solitarius; fb, fiber bundle. Bar = 50 μm.
In sections dually labeled for TH by the immunogold silver method and for LE by the ABC method, longer intensification periods in the silver greatly facilitated differentiation of silver versus peroxidase labels. This is illustrated by sections through the adult rat striatum in Fig. 1E and F. In these sections, the peroxidase labeling is seen in isolated neuronal perikarya; whereas immunogold silver labeling for TH appears as small black aggregates in the surrounding neuropil (Fig. 1F). This distribution would be consistent with the localization of TH in axons of the nigrostriatal system (Anden et al., 1965). These aggregates of silver were not seen over bundles of myelinated axons or perikarya, or in sections processed without TH antiserum.
Comparison of ABC and double-bridged PAP
Both the ABC and double bridged PAP method gave easily recognized peroxidase labeling for the monoclonal LE antibody at a 1:50 dilution. The product formed using the ABC method appeared somewhat more intense than that seen with the double-bridged PAP method. Thus the results from the ABC method were chosen for illustration (Figs. 1E, F and 5).
Fig. 5.
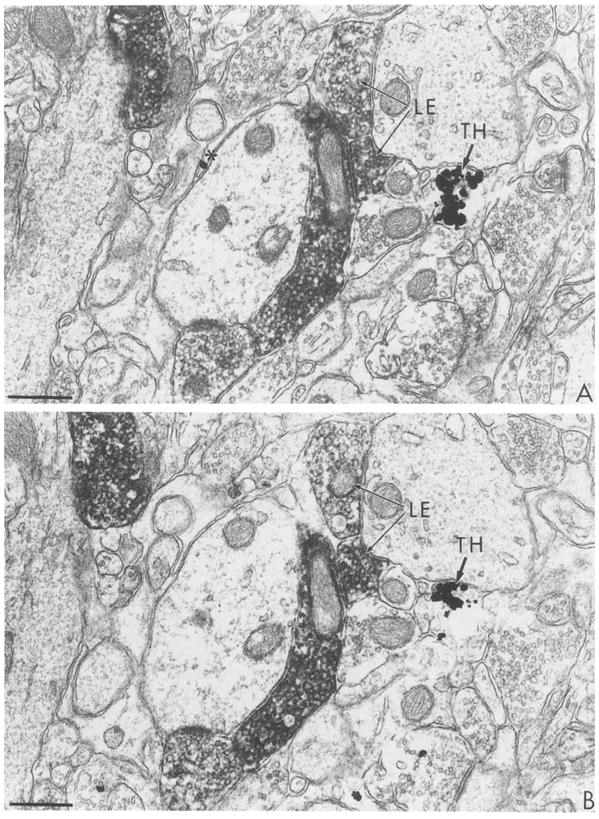
Electron micrographs showing serial sections (A and B) immunolabeled with combined ABC for LE and gold-silver for TH. The tissue was postfixed 20 min in OsO4. Peroxidase reaction product is localized in axon terminals containing LE. Silver particles indicative of TH-labeling also are seen in an axon terminal (TH). Silver enhancement time was 6 min at room temperature. Bar = 0.5 μm.
Subcellular localization
In neuronal perikarya of catecholaminergic neurons, silver-enhanced gold particles appeared preferentially associated with rough endoplasmic reticulum (Fig. 2). Further quantitative evaluation is necessary to establish the precise relation between the immunogold label and endoplasmic reticulum or other organdies. Electron microscopic visualization of gold particles with respect to organdies was optimal with incubations in the silver intensifier that were just below light microscopic detectability, usually 3–6 min at room temperature (Fig. 2). Longer silver incubations insure light microscopic visualization, but obscures subcellular localization due to the large size of the intensified particles. In dendrites of catecholaminergic neurons, clusters of gold-silver aggregates labeling TH also appeared to be associated with membranous organelles (Fig. 3A and B).
Fig. 2.
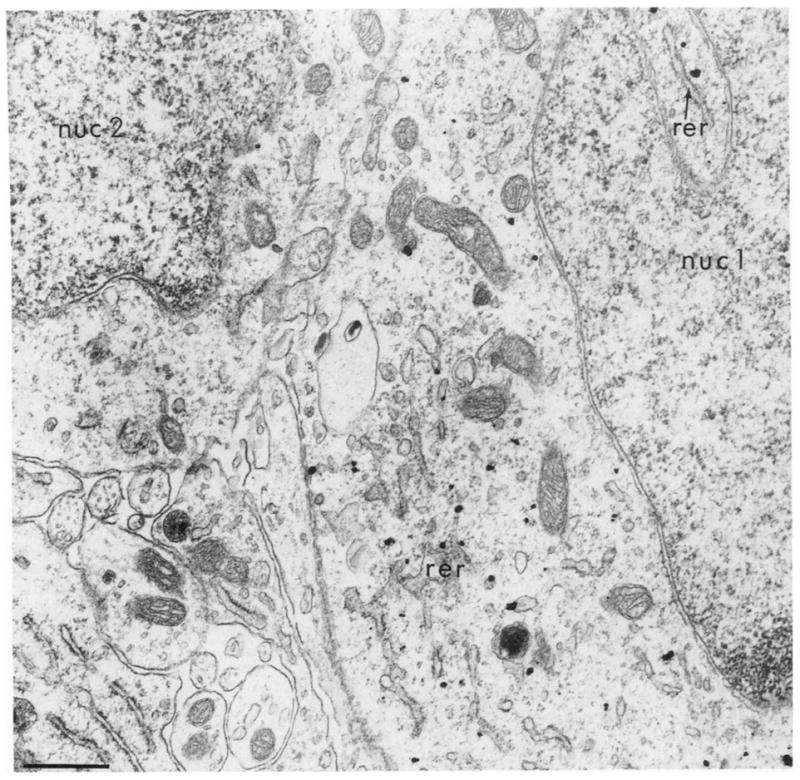
Ultrastructural localization of immunogold silver labeling for TH in a neuronal perikaryon in the medial nucleus of the solitary tract with 4 min silver intensification. The gold particles are exclusively localized within the cytoplasm of the neuron whose nucleus (nuc 1) is shown. The particles appear preferentially associated with the outer saccule of the rough endoplasmic reticulum (rer). The cytoplasm of another cell with nucleus (nuc 2) is unlabeled. The tissue was post-fixed 20 min in 2% OsO4. Bar = 0.3 μm.
Fig. 3.
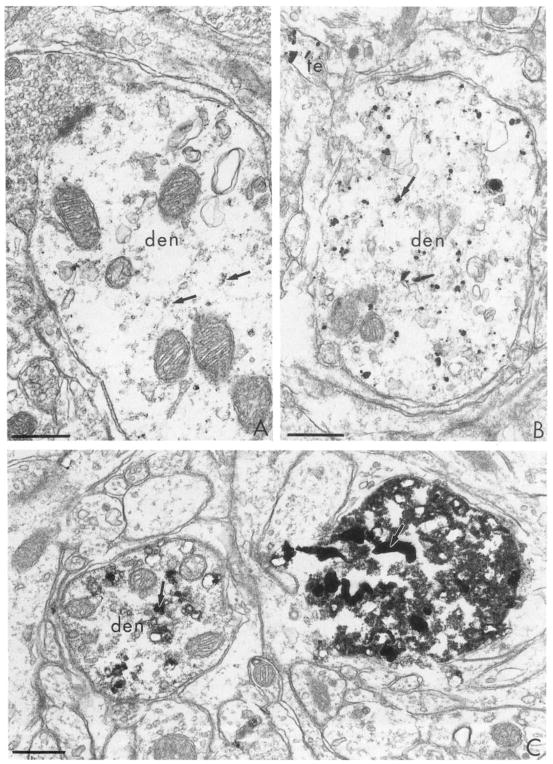
Ultrastructural localization of immunogold silver labeling for TH in processes, presumably dendrites (den) in the medial nucleus of the solitary tract. Silver intensification for 2, 4 and 14 min in A–C, respectively. Small clusters of gold particles are barely detectable in A and assume larger and somewhat irregular shapes in B and C. The intensely labeled process to the right of the plate in C appears homogeneously electron dense resembling peroxidase immunoreactivity. Arrows in C indicate an even denser product resembling autoradiographic silver grains. A and B were from tissues incubated 20 min and C from tissue postfixed 60 min in 2% OsO4. Bar = 0.4 μm throughout.
Temperature and duration of silver incubation
Extended periods of incubations in the silver intensifier at 4°C provided more flexibility with regard to the timing of the reaction over a 30–5-min period. However, the ultrastructure was damaged by this extended incubation period. Thus, the remaining study was carried out at room temperature. Incubation in silver intensifier ranging from 8–14 min at room temperature produced gradations in the observed density of TH immunoreactivity in dendrites of the A2-catecholaminergic cell group (Dahlstrom and Fuxe, 1965). At 2 min, clusters of gold-silver particles were barely detected at 20 000 × magnification. By 4 min, these particles became at least double in diameter (Fig. 3B). The gold-silver aggregates seen after 4 min intensification were also more irregular in shape. They assumed rod-like as well as the more typical round appearance (Figs. 3B and 4A). At incubation times of 12 min or longer, the reaction product became more uniformly electron dense and often resembled peroxidase product (Fig. 3C or 4B). The density of gold-silver particles within the labeled profiles also varied within the same section. The labelled profiles in Fig. 3C appear equidistant from the surface, but show considerable differences in density of the reaction product. The shorter incubation in silver generally necessitated collection of sections from more superficial portions of the tissue. This region has maximal access to antisera, gold and silver enhancers. However, in the most superficial sections taken from the surface of immunolabeled vibratome sections, the ultrastructural morphology is less optimal (Fig. 4A) than in sections taken at a greater depth (Fig. 4B). This is presumably due to the extended incubations in antisera, the silver solution itself and the washes in unbuffered solutions that allow swelling of the tissue. Thus optimal conditions for recognizing labeled profiles appeared to be within areas away from the surface of the tissue that had been incubated for longer periods in the silver intensifier.
Fig. 4.
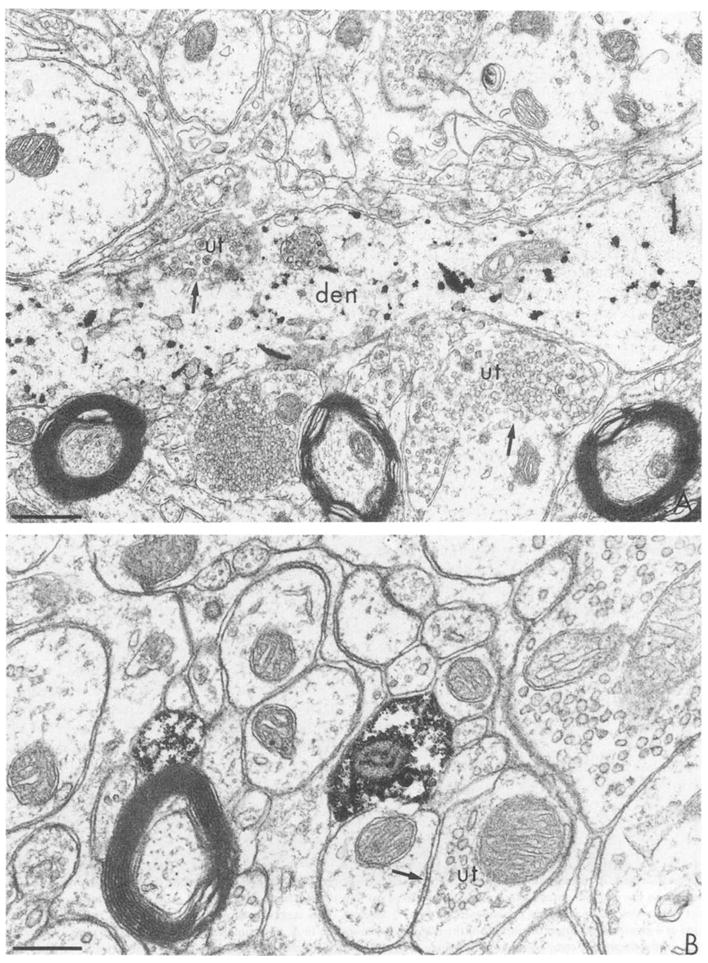
Electron micrographs showing immunogold-silver labeling for TH in the medial nucleus of the solitary tract. A: after a 6 min silver intensification, individual, but irregular, silver clusters are seen in a longitudinally sectioned dendrite (den). This section was collected from the extreme outer surface of tissue postfixed 20 min in 2% OsO4. B: after a 14 min silver intensification, the silver product has coalesced to resemble peroxidase (see Fig. 5) labeling. This section was postfixed for 60 min in 2% OsO4 and was collected at a greater depth from the surface of the tissue than the section in A. ut = unlabeled axon terminal, arrows indicate regions of membrane for comparison of morphology obtained at different post-fixation times. Bars: A, 0.6 μm; B, 0.3 μm.
Post-fixation in osmium tetroxide
Loss of silver product was difficult to examine systematically by electron microscopy. However, the labeling was easily seen after up to 90 min post-fixation in 2% osmium tetroxide. Greater preservation of lipid bilayers after at least 1 h post-fixation in osmium was most readily seen in neuronal and glial plasmalemmas and in myelin layers (Fig. 4B). The longer postfixation as well as greater depth from the surface (see above) probably account for the greater integrity of the cytological features of the neuropil shown in Fig. 4B vs. 4A.
Dual-labeling electron microscope
At appropriately chosen silver incubation times, clearly defined differential labeling of TH and LE was evident even in distal terminal fields as shown in the caudate nucleus (Fig. 5). Differentiation between isolated, presumably non-specific grains and selective accumulations of gold-silver product was facilitated by analysis of serial sections (Fig. 5A and B). However, single silver deposits were sometimes transected and thus also appeared in serial sections. The peroxidase reaction product for LE and immunogold labeling for TH were seen in axon terminals in contact with unlabeled dendrites in the striatum. The somewhat longer silver incubation time of 6 min facilitated maximal detection of both labels in several serial sections at some distance from the surface of the tissue. However, the synaptic vesicles within the TH-labeled terminal were obscured by the reaction product. This incubation period would be most advantageous in examining relative frequencies of different types of synaptic interactions, but is not useful for demonstrating associations of the immunogold labeling with specific organelles due to the excessive density of the reaction product.
Discussion
We have established that (1) intense immunogold-silver labeling of TH can be achieved while maintaining ultrastructural integrity of brain tissue before plastic embedding, (2) gold-silver and peroxidase product are distinct only under restricted silver incubations that differ for light and electron microscopy, (3) pre-embedding immunogold shows subcellular localization of TH in neuronal perikarya.
(1) Ultrastructural preservation
The intact membranes and preservation of ultrastructure in this study are largely attributed to fixation by aortic arch perfusion with acrolein followed by post-fixation in glutaraldehyde and osmium tetroxide.
Acrolein
We have shown that 1 nm gold particles intensified with a light insensitive silver developer can be detected in acrolein perfused brain tissues. This fixative was chosen based on its capacity to rapidly cross-link proteins (King et al., 1983). The fixative preserves the structure of cell membranes while maintaining immunoreactivity for both TH and LE. However, immunogold silver labeling can also be used following fixation with 4% paraformaldehyde for light microscopy or 4% paraformaldehyde plus 0.1% glutaraldehyde for electron microscopy (unpublished observations). These latter fixatives are particularly useful for localization of choline acetyltransferase (Leranth and Pickel, 1989).
Glutaraldehyde
The preservation of ultrastructure in the present study also was improved by post-fixation with 2% glutaraldehyde for 10 min following the diaminobenzidine reaction and preceding silver intensification. This procedure minimized the damage attributed to disruption of extracellular spaces during the exposure to unbuffered water solutions before, during, and after the silver intensification.
Osmium tetroxide
We detected no notable loss of silver product over a 20–90-min incubation in 2% osmium tetroxide. This was somewhat surprising due to the known instability of silver complexes (Basbaum, 1989) and the manufacturer’s (Janssen) warning regarding likely loss of silver during osmification. To circumvent the expected loss of silver Basbaum (1989) used the IntenSE M silver intensification of colloidal gold particles after osmification. The major disadvantage of this method is the loss of ability to visually evaluate the progress of the silver reaction due to the darkness of the tissue after osmification.
The ultrastructural preservation seen in the present study also most likely reflects the absence of freezing or detergents commonly used to enhance penetration. Additionally, the greater penetration of smaller gold particles (Lackie et al., 1985) made it possible to collect sections at greater depth from the surface where the cellular membranes were less damaged. The 1 nm colloidal gold particles in this study had more limited penetration than those of the peroxidase product, but were readily detected even in small distal axons and terminals near the surface of the tissue. With larger 5 and 10 nm particles, immunolabeling was seen almost exclusively in large dendrites and perikarya even in more permeabilized frozen tissue (Van den Pol, 1986). The more readily detected labeling in these profiles probably reflects the transport of immunoreagents within cells whose plasmalemma was partially removed by the vibratome.
Combining the first two primary antibodies reduced the time the sections were in buffer by about 24 h, thus also helping to maintain more intact preservation of cellular morphology. This approach is possible only if the two primary antisera are produced in separate species. Dual labeling with two antisera from the same species has been achieved by essentially covering the first series of bound immunoreagents with the immunosilver (Van den Pol, 1986) or diaminobenzidine products (Sternberger, 1979). Alternatively, we have used two immunocytochemical methods with different sensitivities. In this case, one antiserum is used at a dilution that is not recognized by the less sensitive method (Pickel et al., 1986a). With the increasing availability of polyclonal and monoclonal antibodies, these extreme and less reliable means of differentiation can usually be avoided by obtaining antisera prepared in separate species.
(2) Differential light and electron microscopic labeling with peroxidase and gold-silver markers
Our results confirmed earlier reports (Hsu et al., 1981) that the ABC method has greater sensitivity than most other peroxidase methods, including double-bridged PAP. This difference is most important for detecting antigens such as LE that may be present in relatively limited amounts in neuronal perikarya (Pickel et al., 1989). However, at appropriately chosen silver intensifications, both ABC and PAP products are easily distinguished from immunosilver. Thus, the method of choice for dual labeling largely depends on the antigen and availability of secondary markers. By light microscopy, gold-silver and peroxidase reaction products differ only after incubations in the silver intensifier that are sufficiently long to permit distinction of the black silver product. This period may vary from 10–15 min at room temperature to 30–45 min at 4°C. Sections examined at shorter incubation times or colder temperatures show non-distinguishable brown products for both markers. Other dual immunoautoradiographic and immunoperoxidase labeling methods do not have the same possibilities for erroneous conclusions (Pickel et al., 1986a). However, unlike autoradiography, the present method yields a more rapid assessment of light microscopic distributions of immunoreactive neurons. Provided appropriate silver intensification times are chosen, this is a valid dual light microscopic labeling method for detection of antigens particularly in regions containing perikarya immunolabeled for one marker and terminals labeled for a second marker. Labels in two separate populates of perikarya also are easily distinguished although not illustrated with photomicrographs in this report. However, co-existance of 2 markers within the same cells cannot readily be seen by light microscopy.
For electron microscopy, we have conversely established that less extensive (shorter and/or lower temperature) silver incubations provide labeling that is most easily distinguished from peroxidase product. The shorter silver incubations at room temperature were generally found to be advantageous in terms of ultrastructural preservation. The differential requirements for light and electron microscopic differentiation of peroxidase and gold-silver reactions make it necessary to prepare separate sets of sections with varying durations of silver intensification depending on the method of analysis.
(3) Subcellular localization of antigens
The apparent immunogold-silver localization of TH in association with the rough endoplasmic reticulum in the A2-group of catecholaminergic neurons (Moore, 1982) supports the concept that this enzyme is synthesized in the neuronal soma (Nagatsu et al., 1964). The rough or ribosomal endoplasmic reticulum is part of the endomembrane system within neurons whose function is involved with the synthesis of proteins such as neurotransmitter synthesizing enzymes (Broadwell and Cataldo, 1983). These results differ from earlier studies with peroxidase labeling of TH that was seen as diffuse distribution throughout the soma and dendrites with little, if any, organelle association (Pickel, 1985, 1986). This difference probably reflects both diffusion of the DAB reaction product and levels of detectability. However, lower levels of TH that may have been more diffusely distributed in the cytoplasm also may not have been detected with the short silver incubations. Additionally, quantitative methods are needed to further evaluate the degree to which the gold particles were associated with endoplasmic reticulum versus other organelles.
These results demonstrate the applicability of using immunogold silver-labeling for identity of subcellular localization of antigens before thin sectioning. The findings extend the now well documented resolution of immunogold for labeling subcellular organelles in plastic and frozen ultra-thin sections (Andre et al., 1987; Ottersen, 1987; De Biasi and Rustioni, 1988; Leranth and Pickel, 1989; Merighi et al., 1989). In comparison to post-embedding, the pre-embedding method is more limited by false negatives that may be attributed to inadequate penetration. However, the post-embedding method has other sources of false negatives attributed largely to loss of antigenicity or limited immunological interactions in plastic embedded sections (Leranth and Pickel, 1989). Both techniques require controls for non-specific immunologic interactions. Thus both pre- and post-embedding methods are useful primarily for positive identification of subcellular labeling.
Acknowledgments
This research was supported by NIH grants (HL18974 and EY08055), NIMH (MH00078 and MH40342), NIDA(DA04600). We are also indebted to Dr. Tong H. Joh for provision of the TH antiserum, and to Adam Starr for his technical assistance.
References
- Anden NE, Dahlstrom A, Fuxe K, Larsson K. Further evidence for the presence of nigrostiatal dopamine neurons in the rat. Am J Anat. 1965;116:329–334. doi: 10.1002/aja.1001160117. [DOI] [PubMed] [Google Scholar]
- Andre D, Vuillon Cacciuttolo G, Bosler O. GABA nerve endings in the rat red nucleus combined detection with serotonin terminals using dual immunocytochemistry. Neuroscience. 1987;23:1095–1102. doi: 10.1016/0306-4522(87)90184-9. [DOI] [PubMed] [Google Scholar]
- Basbaum AI. A rapid and simple silver enhancement procedure for ultrastructural localization of the retrograde tracer WGA apo HRP-Av and its use in double-label studies with post-embedding immunocytochemistry. J Histochem Cytochem. 1989;37:1811–1815. doi: 10.1177/37.12.2479673. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Cataldo AM. The neuronal endoplasmic reticulum: Its cytochemistry and contribution to the endomembrane system. J Histochem Cytochem. 1983;31:1077–1088. doi: 10.1177/31.9.6309951. [DOI] [PubMed] [Google Scholar]
- Cuello AC. Monoclonal antibodies in neuroanatomical research. In: Chan-Palay V, Palay SL, editors. Cytochemical Methods in Neuroanatomy. Alan R. Liss, Inc; New York, NY: 1982. pp. 151–164. [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1965;232:1–55. [PubMed] [Google Scholar]
- Dankner WM, Spector SA. Applications of immunogold-silver enhancement: testing of monoclonal anti-bodies and detection of human cytomegalovirus in histologic specimens. Am J Anat. 1989;185:310–313. doi: 10.1002/aja.1001850224. [DOI] [PubMed] [Google Scholar]
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electron microscopy. Histochemistry. 1981;71:81–92. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA. 1988;85:7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis IO, Bell J, Bancroft JD. An investigation of optimal gold particle size for immunohistological immunogold and immunogold-silver staining to be viewed by polarized incident light (EPI polarization) microscopy. J Histochem Cytochem. 1988;36:121–124. doi: 10.1177/36.1.3335767. [DOI] [PubMed] [Google Scholar]
- Faulk WP, Taylor GM. An immunocolloid method for the electron microscope. Immunochemistry. 1971;8:1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–22. [Google Scholar]
- Holgate CS, Jackson P, Cowen PN, Bird CC. Immunogold-silver staining: new method of immunostaining with enhanced sensitivity. J Histochem Cytochem. 1983;31:938–944. doi: 10.1177/31.7.6189883. [DOI] [PubMed] [Google Scholar]
- Hsu S, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled anti-body (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- In t Veld PA. Immunogold-silver labeling in histology. Am J Anat. 1989;185:321–326. doi: 10.1002/aja.1001850226. [DOI] [PubMed] [Google Scholar]
- Joh TH, Ross ME. Preparation of catecholamine-synthesizing enzymes as immunogens for immunohistochemistry. In: Cuello AC, editor. Immunohistochemistry. Methods in Neuroscience, IBRO Handbook. Chichester: 1983. pp. 121–138. [Google Scholar]
- King JC, Lechan RM, Kugel G, Anthony ELP. Acrolein: a fixative for immunocytochemical localization of peptides in the central nervous system. J Histochem Cytochem. 1983;31:62–68. doi: 10.1177/31.1.6187805. [DOI] [PubMed] [Google Scholar]
- Krenacs T, Laszik Z, Dobo E. Application of immunogold-silver staining and immunoenzymatic methods in multiple labelling of human pancreatic Langerhans islet cells. Acta Histochem (Jena) 1989;85:79–85. doi: 10.1016/S0065-1281(89)80102-3. [DOI] [PubMed] [Google Scholar]
- Lackie PM, Hennessy RJ, Hacker GW, Polak JM. Investigation of immunogold-silver staining by electron microscopy. Histochemistry. 1985;83:545–550. doi: 10.1007/BF00492458. [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborszky L, editors. Tract Tracing II. Plenum Publishing Co; New York, NY: 1989. pp. 129–172. [Google Scholar]
- Massari VJ, Chan J, Chronwall B, O’Donohue TL, Oertel WD, Pickel VM. Neuropeptide Y in the rat nucleus accumbens: ultrastructural localization in aspiny neurons receiving synaptic input from GABAergic terminals. J Neurosci Res. 1988;19:171–186. doi: 10.1002/jnr.490190202. [DOI] [PubMed] [Google Scholar]
- Masurovsky ER, Bunge RP. Fluoroplastic coverslips for long-term nerve tissue culture. Stain Technol. 1968;43:161–165. doi: 10.3109/10520296809115061. [DOI] [PubMed] [Google Scholar]
- Merighi A, Polack JM, Fumagalli G, Theodosis DT. Ultrastructural localization of neuropeptides and GABA in rat dorsal horn: a comparison of different immunogold labeling techniques. J Histochem Cytochem. 1989;37:529–540. doi: 10.1177/37.4.2564404. [DOI] [PubMed] [Google Scholar]
- Milner TA, V, Pickel M, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines. J Neurosci. 1989;9:2114–2130. doi: 10.1523/JNEUROSCI.09-06-02114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. Catecholamine neuron systems in brain. Ann NY Acad Sci. 1982;12:321–327. doi: 10.1002/ana.410120402. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Levit M, Udenfriend S. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Ordronneau P, Lindstrom PBM, Petrusz P. Four unlabeled antibody bridge techniques. A comparison. J Histochem Cytochem. 1981;29:1397–1404. doi: 10.1177/29.12.7033366. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res. 1987;69:167–174. doi: 10.1007/BF00247039. [DOI] [PubMed] [Google Scholar]
- Pickel VM. Ultrastructure of central catecholaminergic neurons. In: Panula P, Paivarinta H, Soinila S, editors. Neurohistochemistry: Modern Methods and Applications. Vol. 16. Alan R. Liss, Inc; New York, NY: 1986. pp. 397–423. [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225:373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Milner TA. Autoradiographic detection of (125I)-secondary antiserum: a sensitive light and electron microscopic labeling method compatible with peroxidase immunocytochemistry for dual localization of neuronal antigens. J Histochem Cytochem. 1986a;34:707–718. doi: 10.1177/34.6.2422251. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Ganten D. Dual peroxidase and colloidal gold labeling study of angiotensin converting enzyme and angiotensin-like immunoreactivity in the rat subfornical organ. J Neurosci. 1986b;6:2457–2469. doi: 10.1523/JNEUROSCI.06-08-02457.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Milner TA. Ultrastructural basis for interactions between central opioids and catecholamines. II. Nuclei of the solitary tracts. J Neurosci. 1989;9:2519–2535. doi: 10.1523/JNEUROSCI.09-07-02519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Hodson AJ. Antisera to γ-aminobutyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem. 1983;33:249–257. doi: 10.1177/33.3.2579124. [DOI] [PubMed] [Google Scholar]
- Sternberger LA. Immunocytochemistry. Wiley and Sons Inc; New York: 1979. [Google Scholar]
- Van den Pol AN. Tyrosine hydroxylase immunoreactive neurons throughout the hypothalamus receive glutamate decarboxylase immunoreactive synapses: a double pre-embedding immunocytochemical study with particulate silver and HRP. J Neurosci. 1986;6:877–891. doi: 10.1523/JNEUROSCI.06-03-00877.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillez P, Perez SC, Stoeckel ME. Colocalization of GABA and tyrosine hydroxylase immunoreactivities in the axons innervating the neurointermediate lobe of the rat pituitary: an ultrastructural immunogold study. Neurosci Lett. 1987;79:53–58. doi: 10.1016/0304-3940(87)90671-9. [DOI] [PubMed] [Google Scholar]
- Zelechowska MG, Mandeville R. Immunogold and immunogold/silver staining in the ultrastructural localization of target molecules identified by monoclonal antibodies. Anticancer Res. 1989;9:53–57. [PubMed] [Google Scholar]