Abstract
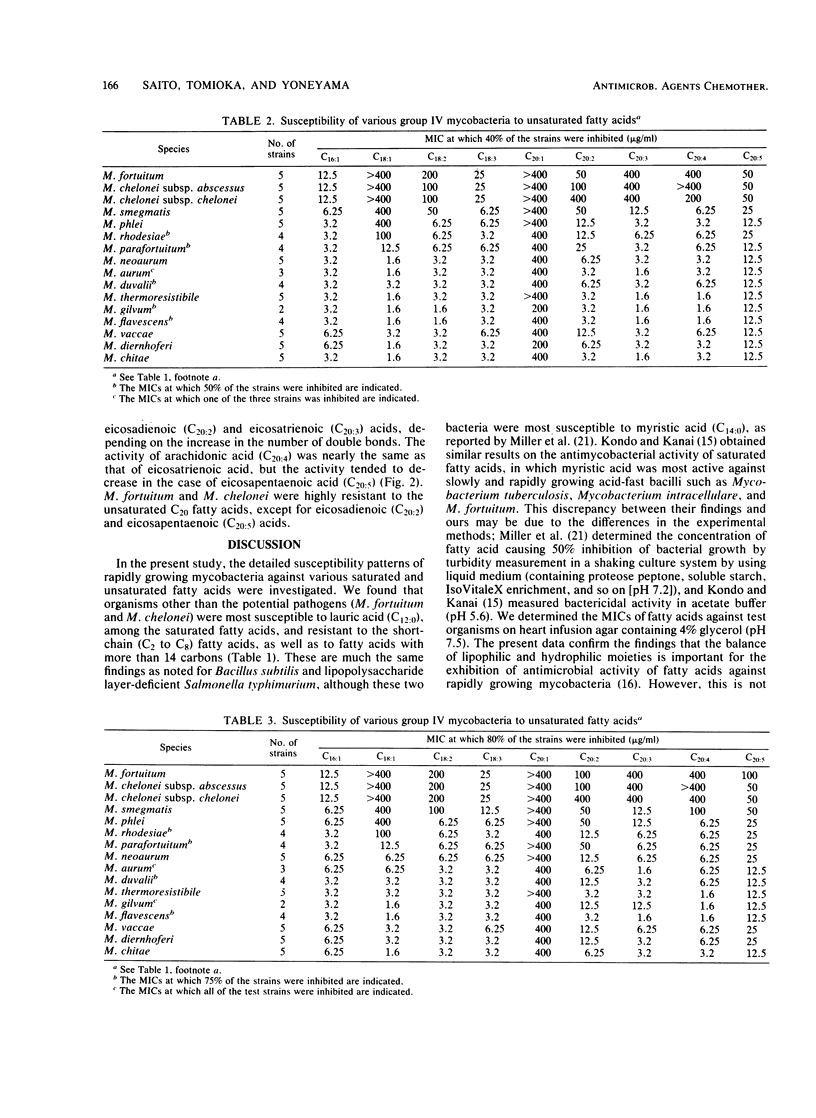
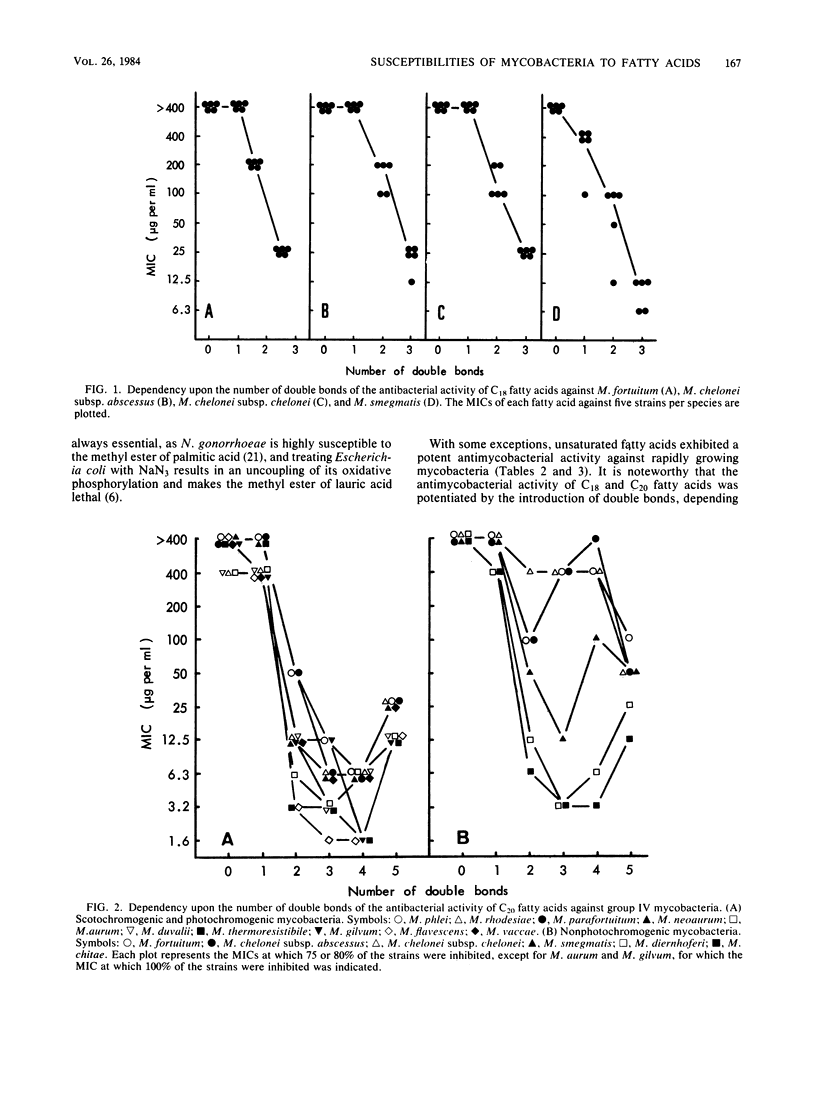
Seventy-one strains of 15 species of rapidly growing mycobacteria were studied for their susceptibilities to fatty acids with 2 to 20 carbons by the agar dilution method at pH 7.0. Most mycobacteria other than potential pathogens (Mycobacterium fortuitum and Mycobacterium chelonei) were resistant to saturated fatty acids, except for lauric acid (C12:0) (MIC, 6.25 to 25 micrograms/ml) and capric acid (C10:0) (MIC, 50 to 100 micrograms#ml). M. fortuitum and M. chelonei were substantially insusceptible to these fatty acids. Unsaturated fatty acids with 16 to 20 carbons, except for C20:5, were highly toxic to group IV mycobacteria other than M. fortuitum, M. chelonei, Mycobacterium smegmatis, and Mycobacterium phlei, these being highly resistant to all the unsaturated acids, except for C16:1, C18:3, and C20:5. Introduction of double bonds to C16 to C20 fatty acids caused a marked increase in their activities that depended on the increase in the number of double bonds, at least up to three or four. M. fortuitum and M. chelonei were more resistant to the unsaturated fatty acids (particularly to C20:3 and C20:4) than the other group IV mycobacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOENICKSE R., JUHASZ E. BESCHREIBUNG DER NEUEN SPECIES MYCOBACTERIUM VACCAE N. SP. Zentralbl Bakteriol Orig. 1964 Feb;192:133–135. [PubMed] [Google Scholar]
- BOJALIL L. F., CERBON J., TRUJILLO A. Adansonian classification of mycobacteria. J Gen Microbiol. 1962 Jun;28:333–346. doi: 10.1099/00221287-28-2-333. [DOI] [PubMed] [Google Scholar]
- Butcher G. W., King G., Dyke K. G. Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J Gen Microbiol. 1976 Jun;94(2):290–296. doi: 10.1099/00221287-94-2-290. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Daneo-Moore L. Effects of fatty acids on lysis of Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1122–1126. doi: 10.1128/jb.141.3.1122-1126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay J. P., Farías R. N. Inhibitory action of a non-metabolizable fatty acid on the growth of Escherichia coli: role of metabolism and outer membrane integrity. J Bacteriol. 1977 Dec;132(3):790–795. doi: 10.1128/jb.132.3.790-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON R. E., SMITH M. M. Rapidly growing, acid fast bacteria. I. Species' descriptions of Mycobacterium phlei Lehmann and Neumann and Mycobacterium smegmatis (Trevisan) Lehmann and Neumann. J Bacteriol. 1953 Jul;66(1):41–48. doi: 10.1128/jb.66.1.41-48.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B., Paton A. M., Thompson J. K. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J Appl Bacteriol. 1971 Dec;34(4):803–813. doi: 10.1111/j.1365-2672.1971.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway D. L., Dyke K. G. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979 Nov;115(1):233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- Hemsworth G. R., Kochan I. Secretion of antimycobacterial fatty acids by normal and activated macrophages. Infect Immun. 1978 Jan;19(1):170–177. doi: 10.1128/iai.19.1.170-177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978 Nov;37(13):2759–2764. [PubMed] [Google Scholar]
- Kanai K., Kondo E. Antibacterial and cytotoxic aspects of long-chain fatty acids as cell surface events: selected topics. Jpn J Med Sci Biol. 1979 Jun;32(3):135–174. doi: 10.7883/yoken1952.32.135. [DOI] [PubMed] [Google Scholar]
- Kanai K., Kondo E. Phospholipase A2-induced antimycobacterial activity in the membrane fraction obtained from peritoneal exudate cells of guinea pigs. Jpn J Med Sci Biol. 1980 Apr;33(2):87–101. doi: 10.7883/yoken1952.33.87. [DOI] [PubMed] [Google Scholar]
- Kondo E., Kanai K. The lethal effect of long-chain fatty acids on mycobacteria. Jpn J Med Sci Biol. 1972 Feb;25(1):1–13. doi: 10.7883/yoken1952.25.1. [DOI] [PubMed] [Google Scholar]
- Kondo E., Kanai K. The relationship between the chemical structure of fatty acids and their mycobactericidal activity. Jpn J Med Sci Biol. 1977 Aug;30(4):171–178. doi: 10.7883/yoken1952.30.171. [DOI] [PubMed] [Google Scholar]
- Kondo E., Kanai K. [Lipolytic enzyme activities of phagocytic cells as affected by the presence of tubercle bacilli (author's transl)]. Kekkaku. 1982 Jan;57(1):1–7. [PubMed] [Google Scholar]
- Kubica G. P., Baess I., Gordon R. E., Jenkins P. A., Kwapinski J. B., McDurmont C., Pattyn S. R., Saito H., Silcox V., Stanford J. L. A co-operative numerical analysis of rapidly growing mycobacteria. J Gen Microbiol. 1972 Nov;73(1):55–70. doi: 10.1099/00221287-73-1-55. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B. How macrophages kill tubercle bacilli. J Med Microbiol. 1983 Feb;16(1):1–12. doi: 10.1099/00222615-16-1-1. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruul H., Wetherall B. L., McDonald P. J. Bactericidal activity of a granule extract from human polymorphonuclear leukocytes against Bacteroides species. Infect Immun. 1983 Sep;41(3):1373–1375. doi: 10.1128/iai.41.3.1373-1375.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Watanabe T. H2O2-releasing function of macrophages activated with various mycobacteria based on wheat germ agglutinin and phorbol myristate acetate triggering. J Reticuloendothel Soc. 1981 Mar;29(3):193–204. [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):516–524. doi: 10.1128/jb.111.2.516-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford J. L., Gunthorpe W. J. A study of some fast-growing scotochromogenic mycobacteria including species descriptions of Mycobacterium gilvum (new species) and Mycobacterium duv alii (new species). Br J Exp Pathol. 1971 Dec;52(6):627–637. [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M., Mizuno S., Gane N. F., Mills A., King L. Mycobacterium rhodesiae sp. nov. A new species of rapid-growing scotochromogenic mycobacteria. Jpn J Microbiol. 1971 Sep;15(5):407–416. doi: 10.1111/j.1348-0421.1971.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Tsukamura M. Mycobacterium chitae: a new species. Jpn J Microbiol. 1967 Mar;11(1):43–47. doi: 10.1111/j.1348-0421.1967.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Toyama H., Mizuno S. [Mycobacterium parafortuitum (a new species)]. Igaku To Seibutsugaku. 1966 Apr 10;70(4):232–235. [PubMed] [Google Scholar]
- Tsukamura M., Tsukamura S. [Mycobacterium aurum; a new species]. Igaku To Seibutsugaku. 1966 May 10;72(5):270–273. [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


